Abstract
Exosomes have emerged as a novel mode of intercellular communication. Exosomes can shuttle bioactive molecules including proteins, DNA, mRNA, as well as non-coding RNAs from one cell to another, leading to the exchange of genetic information and reprogramming of the recipient cells. Increasing evidence suggests that tumor cells release excessive amount of exosomes, which may influence tumor initiation, growth, progression, metastasis, and drug resistance. In addition, exosomes transfer message from tumor cells to immune cells and stromal cells, contributing to the escape from immune surveillance and the formation of tumor niche. In this review, we highlight the recent advances in the biology of exosomes as cancer communicasomes. We review the multifaceted roles of exosomes, the small secreted particles, in communicating with other cells within tumor microenvironment. Given that exosomes are cell type specific, stable, and accessible from body fluids, exosomes may provide promising biomarkers for cancer diagnosis and represent new targets for cancer therapy.
Keyword: Exosomes, Intercellular communication, Cancer, Biomarker, Target
Introduction
Exosomes are small, lipid bilayer membrane vesicles of endocytic origin. Exosomes can be defined by several common characteristics, including size (50–100 nm in diameter), density (1.13–1.19 g/ml), morphology (“cup” or “dish” shaped in transmission electron microscopy), and certain enriched protein markers (tetraspanins, TSG101, Hsp70). Initially discovered as the garbage bags for removal of unwanted material from cells, the role of exosomes in immune response is gradually recognized as they function in antigen presentation. More recently, the researchers reveal that exosomes contain proteins and nucleic acids that are functional when transferred into recipient cells. Exosomes have been shown to act as shuttles between cells by transmitting signals (referred to as communicasomes). In this review, we highlight the recent advances in the roles of exosomes in cancer with an emphasis on the potential of exosomes as diagnosis biomarker and therapy target.
Biogenesis, release, and uptake of exosomes
Exosome formation is a fine-tuned process which includes four stages: initiation, endocytosis, multivesicular bodies (MVBs) formation, and exosome secretion [1]. Multivesicular bodies (MVBs) are endocytic structures formed by the budding of an endosomal membrane into the lumen of the compartment. After vesicular accumulation, the MVBs are either sorted for cargo degradation in the lysosome or released into the extracellular space as exosomes by fusing with the plasma membrane (Fig. 1). The mechanisms underlying the sorting of cargo into the intraluminal vesicles (ILVs) are not yet fully elucidated. Both endosomal sorting complex required for transport (ESCRT)-dependent and independent signals have been suggested to determine the sorting of exosomes [2]. The formation of exosomes has been shown to be controlled by the syndecan heparan sulfate proteoglycans and their cytoplasmic adaptor syntenin [3].
Fig. 1.
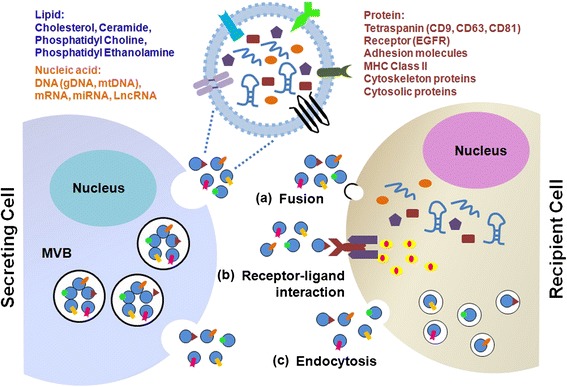
Biogenesis, release, structure, and uptake of exosomes. Exosomes are produced from the multivesicular bodies (MVBs) (also known as late endosomes). The membrane of the MVBs bulges inward to form exosomes. During this process, proteins (e.g., receptor, cytoplasmic proteins, tetraspanin), nucleic acids (e.g., DNA, mRNA, miRNA), and lipids (e.g., cholesterol, ceramide) are packed into exosomes in a cell type-dependent manner. MVBs fuse with the cellular membrane to release exosomes into the extracellular space. Several mechanisms have been suggested to mediate the uptake of exosomes, including a exosome fusion with the cellular membrane of the recipient cell, leading to the release of the exosomal cargo into the cytoplasm, b juxtracrine signaling through receptor-ligand interactions, c and endocytosis by phagocytosis
The Rab guanosine triphosphatases (GTPases) have been found to critically regulate exosome secretion. Ostrowski et al. have identified that Rab27a/b affects the size and localization of MVBs [4]. Hsu et al. suggest that Rab3 regulates MVBs docking to tethering at the plasma membrane [5]. The accumulation of intracellular Ca2+ results in increased exosome secretion [6]. In addition, intracellular and intercellular pH has been shown to affect exosome release. When the microenvironmental pH is low, exosome secretion and uptake by recipient cells increases [7]. There is evidence that oncogenes and tumor suppressors regulate exosome secretion in cancer [8]. Yu et al. demonstrate that p53-regulated protein tumor suppressor-activated pathway 6 (TSAP6) induces exosome secretion under stressed conditions [9, 10]. Heparanase is an enzyme with elevated level in cancer. Overexpression of heparanase promotes exosome secretion [11]. Intriguingly, exosomes from normal mammary epithelial cells inhibit exosome secretion by breast cancer cells, implicating a feedback control to maintain dynamic equilibrium [12].
Exosomes transfer information to the target cells through three main ways: (1) receptor-ligand interaction; (2) direct fusion with plasma membrane; (3) endocytosis by phagocytosis (Fig. 1). Although the specific receptors that mediate the uptake of exosomes have not been found, there are several proteins that may act as potential receptors for exosome uptake, such as Tim1/4 for B cells [13] and ICAM-1 for APCs [14]. The uptake of exosomes by direct plasma membrane fusion mode has not been well studied. Melanoma cells could take up exosomes by fusion and low pH facilitates this process [15]. Phagocytosis is an efficient way of exosome uptake. Phagocytic cells have a greater uptake of exosomes than non-phagocytic cells [16]. The uptake of exosomes by recipient cells is energy dependent [17]. Heparan sulfate proteoglycans (HSPGs) function as internalizing receptors of cancer cell-derived exosomes. Enzymatic depletion of cell-surface HSPG or pharmacological inhibition of endogenous proteoglycan biosynthesis significantly attenuates exosome uptake [18].
Structure and contents of exosomes
Exosomes consist of a lipid bilayer membrane surrounding a small cytosol (Fig. 1). The structured lipids not only mold the exosomes but are also involved in exosome function. In addition to lipids, nucleic acids and proteins have also been detected in exosomes. Thakur et al. demonstrate that double-stranded DNA is present in exosomes from cancer cells and reflects the mutational status of the originated cells [19]. Valadi et al. demonstrate that exosomes contain mRNA and miRNA [20]. Exosome-carried RNA can shuttle between cells and thus is called “exosomal shuttle RNA” (esRNA). The protein composition of tumor cell-derived exosomes has been well characterized for a number of cancers by using different proteomic methods. The most common proteins, mRNA, and miRNAs found in exosomes have been deposited in ExoCarta (www.exocarta.org). To date, 4563 proteins, 1639 mRNAs, and 764 miRNAs have been identified in exosomes from different species and tissues by independent examinations. The exosomal contents vary between different physiological and pathological conditions and original cell types. Moreover, the composition of exosomes can be distinct from the originated cells due to the selective sorting of the cargo into exosomes.
Isolation, detection, and analysis of exosomes
Exosomes have been isolated and characterized from distinct cells under normal and stressed conditions. At present, the most commonly used methods for exosome isolation include ultracentrifugation, combined with sucrose gradient, and the immune-bead isolation (e.g., magnetic activated cell sorting; MACS). There are many commercial kits available for the extraction of exosomes. Transmission electron microscopy (TEM), Western blot, and FACS are frequently used to characterize the isolated exosomes based on their biochemical properties (e.g., morphology, size, exosomal markers). There is a lack of the accurate method to determine the concentration of exosomes. The researchers have to rely on inaccurate measurements of protein concentration or nanoparticle tracking analysis. Quantitative RT-PCR, nucleic acid sequencing, Western blot, or ELISA are used for exosome RNA and protein identification. The International Society for Extracellular Vesicles (ISEV) has recently released minimal experimental requirements for definition of extracellular vesicles and their functions [21].
Roles of exosomes in cancer
Accumulating evidence indicates that exosomes play important roles in cancer. Exosomes transfer oncogenic proteins and nucleic acids to modulate the activity of recipient cells and play decisive roles in tumorigenesis, growth, progression, metastasis, and drug resistance (Fig. 2). Exosomes can act on various recipient cells. The uptake of exosomes may induce a persistent and efficient modulation of recipient cells. In this section, we will discuss about the roles of exosomes in cancer and the molecular mechanisms (Table 1).
Fig. 2.
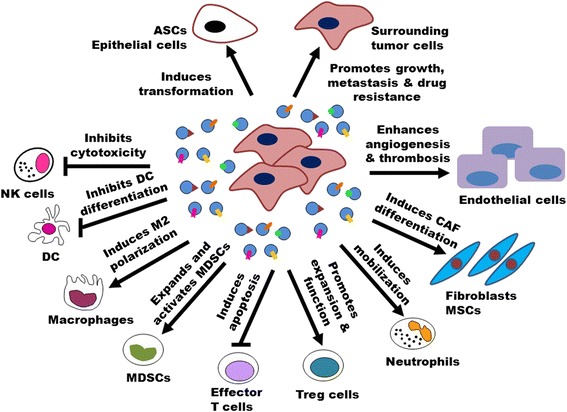
Roles of exosomes in cancer. Exosomes are critically involved in tumor initiation, growth, progression, metastasis, and drug resistance by transferring oncogenic proteins and nucleic acids. Tumor-derived exosomes can activate endothelial cells to support tumor angiogenesis and thrombosis. Tumor-derived exosomes can convert fibroblasts and MSCs into myofibroblasts to facilitate tumor angiogenesis and metastasis. Tumor-derived exosomes contribute to create an immunosuppressive microenvironment by inducing apoptosis and impairing the function of effector T cells and NK cells, inhibiting DC differentiation, expanding MDSCs, as well as promoting Treg cell activity. Tumor-derived exosomes can mobilize neutrophils and skew M2 polarization of macrophages to promote tumor progression. Moreover, tumor-derived exosomes can help tumor cells develop drug resistance by transferring multidrug-resistant proteins and miRNAs, exporting tumoricidal drugs, and neutralizing antibody-based drugs. In turn, exosomes from activated T cells, macrophages, and stromal cells can promote tumor metastasis and drug resistance
Table 1.
Overview on the function of exosomes in cancer
| Exosomal cargo | Secreting cell | Recipient cell | Function | Reference |
|---|---|---|---|---|
| EGFRvIII | Glioblastoma cells | Glioblastoma cells | Promotes tumor cell growth | [26] |
| Angiogenin, IL-8, VEGF | Glioblastoma cells | Endothelial cells | Promotes tube formation | [75] |
| ∆Np73 | Colon cancer cells | Colon cancer cells | Promotes tumor cell proliferation and therapy resistance | [27] |
| KRAS | Colon cancer cells (mutant KRAS) | Colon cancer cells (wild-type KRAS) | Enhances tumor cell growth | [97] |
| MET | Melanoma cells (highly metastatic) | Bone marrow progenitor cells | Promotes tumor growth and metastasis | [39] |
| HIF-1α | Nasopharyngeal carcinoma (NPC) cells (EBV-positive) | NPC cells (EBV-negative) | Promotes tumor cell migration and invasion | [37] |
| αvβ6 Integrin | Prostate cancer cells | Prostate cancer cells | Promotes tumor cell migration | [98] |
| Survivin | Cervical cancer cells | Cervical cancer cells | Inhibits genotoxic stress-induced apoptosis and promotes cell proliferation | [25, 99] |
| Wnt5a | Macrophages | Breast cancer cells | Enhances tumor cell invasion | [100] |
| Wnt3a | Diffuse large B-cell lymphoma side population (SP) cells | Neighboring non-SP cells | Modulates SP–non-SP transition and promotes tumor progression | [24] |
| FasL | Activated CD8+ T cells | Melanoma cells, lung cancer cells | Induces MMP9 expression and promotes lung metastasis | [43] |
| IL-6, CCL2, fibronectin | Multiple myeloma (MM) BM-MSCs | MM cells | Promotes tumor cell growth | [29] |
| Hsp72 | Murine thymoma, mammary carcinoma, colon carcinoma cells | MDSCs | Induces immunosuppression and enhances tumor growth | [63] |
| TF | Squamous cells, colon cancer cells | Endothelial cells | Promotes coagulation | [71] |
| CD39, CD73 | Bladder, colorectal, prostate, breast cancer cells | T cells | Induces adenosine production and inhibits T cell activation | [101] |
| TGF-β | Mesothelioma, prostate, bladder, colorectal, breast cancer cells | Fibroblasts | Induces myofibroblast differentiation and promotes tumor angiogenesis and growth | [66, 67] |
| TGF-β | Prostate cancer, gastric cancer | MSCs | Induces myofibroblast differentiation and promotes angiogenesis and invasiveness | [68, 102] |
| TGF-β | Pleural effusions of mesothelioma patients | NK cells, CD8+ T cells | Downregulates NKG2D expression and impairs cell killing activity | [103] |
| MICA*008 | Cervical cancer cells | NK cells | Decreases NKG2D expression and reduces NK cytotoxicity | [104] |
| TGF-β, PGE2 | Murine mammary adenocarcinoma cells | Bone marrow myeloid cells (CD11b+Ly6G+) | Induces MDSCs accumulation and immunosuppression | [61] |
| CCL20 | Nasopharyngeal carcinoma cells | Regulatory T cells | Recruits and induces Treg conversion | [59] |
| KIT | Mast cells | Lung cancer cells | Accelerates cell proliferation | [105] |
| KIT | Gastrointestinal stromal tumor (GIST) cells | Progenitor smooth muscle cells | Increases tumor invasiveness | [40] |
| Wnt11 | Fibroblasts | Breast cancer cells | Promotes tumor metastasis | [42] |
| MIF | Pancreatic cancer cells | Liver Kupffer cells | Promotes metastasis | [47] |
| Hsp70 | Renal cancer cells (murine Renca cell line) | MDSCs | Induces MDSCs activation and enhances tumor growth | [106] |
| Adrenomedullin | Pancreatic cancer cells | Adipocytes | Promotes lipolysis | [107] |
| S1P, CCL20, PGE2 | Enteropathogenic bacteria-stimulated intestinal epithelial cells | Th17 cells | Promotes the development of colon cancer | [108] |
| miR-9 | Lung cancer, melanoma, pancreatic cancer, glioblastoma, colorectal cancer cells | Endothelial cells | Induces tumor angiogenesis | [109] |
| miR-125b, 130b, 155 | Prostate cancer (PC) cells | PC patient adipose-derived stem cells (pASCs) | Induces neoplastic transformation | [22] |
| miR-135b | Multiple myeloma cells (under chronic hypoxia condition) | Endothelial cells | Enhances endothelial tube formation | [36] |
| miR-10b | Metastatic breast cancer cells | Mammary epithelial cells | Promotes cell migration | [110] |
| miR-92a | Chronic myeloid leukemia (CML) cells | Endothelial cells | Promotes cell migration and tube formation | [35] |
| miR-210 | CML cells (under hypoxia condition) | Endothelial cells | Promotes angiogenic activity | [34] |
| miR-223 | IL-4-activated macrophages | Breast cancer cells | Promotes cell invasion | [44] |
| miR-222 | Drug-resistant breast cancer cells | Drug-sensitive breast cancer cells | Transmits chemoresistance | [111] |
| miR-584, 517c, 378 | Hepatocellular carcinoma (HCC) cells | HCC cells | Promotes HCC cell growth and metastasis | [112] |
| miR-21, 29a | Lung cancer cells | Macrophages | Promotes tumor metastasis | [46] |
| miR-105 | Metastatic breast cancer cells | Endothelial cells | Destroys tight junction, induces vascular permeability, and promotes metastasis | [33] |
| Pre-miRNAs, RISC-loading complex | Breast cancer cells | Non-tumorigenic epithelial cells | Induces cell transformation | [23] |
| miR-24-3p, 891a, 106a-5p, 20a-5p, 1908 | Nasopharyngeal carcinoma | T cells | Promotes T cell dysfunction and tumor progression | [60] |
| miR-221, 222 | Gastric cancer tissue derived MSCs | Gastric cancer cells | Enhances tumor cell migration | [60] |
| miR-122 | Breast cancer cells | Lung fibroblasts, brain astrocytes, and neurons | Reprograms systemic energy metabolism and facilitates metastasis | [113] |
| miR-23b | Bladder cancer cells (cellular disposal by exosome release) | None | Acquires metastatic potential | [38] |
| miR-503 | Endothelial cells | Breast cancer cells | Impairs tumor cell growth | [114] |
| miR-140 | Preadipocytes | Ductal carcinoma in situ (DCIS) cells | Enhances tumorigenesis | [115] |
| miR-127, 197, 222, 223 | Bone marrow stromal cells | Breast cancer cells | Decreases cell proliferation and induces cell quiescence | [116] |
| TUC339 | Hepatocellular carcinoma (HCC) cells | HCC cells | Promotes tumor cell growth and inhibits cell adhesion | [81] |
| Linc-ROR | HCC cells | HCC cells | Reduces chemotherapy sensitivity | [82] |
Tumorigenesis
Normal cells are transformed into cancer cells in the process of tumorigenesis. Exosomes from malignant cells have shown the potential to induce normal cell transformation. For instance, prostate cancer cell-derived exosomes could induce neoplastic transformation of adipose-derived stem cells (ASCs) [22], which is associated with trafficking of oncogenic proteins (Ras superfamily of GTPases), mRNA (K-ras and H-ras), as well as miRNAs (miR-125b, miR-130b, and miR-155) by exosomes. In addition, Melo et al. suggest that breast cancer cell-derived exosomes contain precursor microRNAs (pre-miRNAs) associated with RNA-induced silencing complex (RISC)-loading complex proteins, which could induce a rapid and efficient silencing of mRNAs in nontumorigenic epithelial cells, resulting in transcriptome reprogramming and oncogenic transformation [23]. They further demonstrate that the exosomes from serum specimen from breast cancer patients but not those from healthy donors induce tumor formation in mice when co-injected with the nontumorigenic epithelial cells, suggesting a potential mechanism for exosome in tumorigenesis. Cancer is composed of heterogeneous cell populations. Side population (SP) cells are a sub-population of cells that exhibit stem cell-like characteristics and can be isolated in cancer by adapting the Hoechst33342 staining method. Koch et al. demonstrate that in diffuse large B-cell lymphoma, side population cells could export Wnt3a via exosomes to neighboring cells, thus modulating SP-non-SP transitions and maintaining population equilibrium [24]. Altogether, these findings indicate that exosomes may contribute to tumor development and uncontrolled tumor progression by acting as a mediator in the transformation of normal cells to malignant cells and a modulator for the balance between cancer stem cells (CSCs) and non-CSCs.
Tumor growth
The promoting effects of exosomes from distinct sources on tumor cell proliferation have been widely reported. Cancer cells uptake exosomes that contain survivin, an anti-apoptotic protein, to protect them from genotoxic stress-induced cell death [25]. Exosomes from serum of glioblastoma patients contain EGFRvIII mRNA, which stimulate the proliferation of human glioma cells through a self-promoting way [26]. Colon cancer cell-derived exosomes are enriched in ΔNp73 mRNA. The proliferation potential of target cells is greatly enhanced by incubation with ΔNp73-containing exosomes [27]. The interaction between tumor stromal cells and tumor cells also efficiently promote tumor growth. Exosomes from chronic myelogenous leukemia (CML) cells stimulate bone marrow stromal cells to produce IL-8, which in turn promote the growth of leukemia cells [28]. Bone marrow mesenchymal stromal cells (BM-MSCs) from multiple myeloma (MM) patients release exosomes that express increased levels of oncogenic proteins, cytokines, and adhesion molecules to facilitate the growth of MM cells [29]. Thus, exosomes from tumor cells and microenvironment could act coordinately to promote tumor growth.
Tumor angiogenesis
The formation of new blood vessels is required for tumor growth and progression. Proteomic analysis has revealed that abundant angiogenic factors are present in malignant mesothelioma-derived exosomes [30]. Exosome uptake induces upregulation of angiogenesis-related genes and results in enhanced endothelial cell proliferation, migration, and sprouting [31]. Exosomes derived from hypoxic glioblastoma cells are more potent to induce angiogenesis [32]. Exosomes from metastatic breast cancer cells contain miR-105. Exosome-mediated transfer of miR-105 degrades ZO-1 protein, disturbs tight junctions, and induces vascular permeability in distant organs [33]. Exosomal miR-92a from K562 leukemia cells targets integrin α5 to enhance endothelial cell migration and tube formation [34]. MiR-210 is significantly enriched in exosomes from hypoxic K562 cells, which promotes the angiogenic activity of endothelial cells [35]. Multiple myeloma cells grown under hypoxic condition produce more exosomes containing miR-135b, which directly suppresses FIH-1, an inhibitor of HIF-1, to enhance endothelial tube formation in endothelial cells [36]. Exosomes are critically involved in tumor angiogenesis by directly delivering angiogenic proteins into endothelial cells or modulating the angiogenic function of endothelial cells by exosomal miRNAs.
Tumor metastasis
Exosomes contribute to tumor metastasis by enhancing tumor cell migration and invasion, establishing pre-metastatic niche, and remodeling the extracellular matrix. EBV-positive nasopharyngeal carcinoma (NPC) cell-derived exosomes contain HIF-1α, which increases migration and invasiveness of EBV-negative NPC cells [37]. Metastatic cancer cells secrete increased level of miRNA with tumor-suppressor function, which may suggest another mechanism for the role of exosomes in metastasis [38]. The formation of pre-metastatic niche is a prerequisite for tumor metastasis. Exosomes from highly metastatic melanoma enhance the metastatic ability of primary tumors by converting bone marrow progenitor cells to a pro-vasculogenic and pre-metastatic phenotype via the MET receptor [39]. Gastrointestinal stromal tumor cells release exosomes containing protein tyrosine kinase to convert progenitor smooth muscle cells to a pre-metastatic phenotype [40]. Suetsugu et al. show that highly metastatic breast cancer cells can transfer their own exosomes to other cancer cells and normal lung tissue cells in vitro and in vivo by using fluorescent protein imaging method [41], which provides direct evidence for the involvement of exosomes from highly metastatic cancer cells in educating stromal cells. Luga and colleagues have shown that exosomes produced by stromal cells are taken up by breast cancer cells and are then loaded with Wnt11, which is associated with stimulation of the invasiveness and metastasis of the breast cancer cells [42]. Exosomes from activated CD8+ T cells promote cancer cell invasion and lung metastasis via the Fas/FasL pathway [43], which adds another layer of mechanism for the role of tumor-infiltrating lymphocytes in cancer metastasis. Exosome-mediated transfer of oncogenic microRNAs into cancer cells is associated with enhanced metastatic potential. IL-4-activated macrophage-derived exosomes transfer miR-223 to co-cultivated breast cancer cells, leading to increase of cell invasion [44]. Exosome-mediated delivery of miR-221/222 from MSCs to gastric cancer cells greatly enhances gastric cancer cell migration [45]. Fabbri et al. suggest that miRNAs in tumor-secreted exosomes can directly bind toll-like receptor (TLR) in immune cells to promote tumor metastasis [46]. Recently, Costa-Silva and colleagues demonstrate that MIF-containing exosomes from pancreatic ductal adenocarcinoma (PDAC) cells induce TGF-β production in liver Kupffer cells, which in turn upregulates fibronectin (FN) expression by hepatic stellate cells and enhances recruitment of bone marrow-derived cells, finally leading to the formation of liver pre-metastatic niche [47], suggesting a complicated network that involves cancer cells, stromal cells, and immune cells in exosome-initiated pre-metastatic niche formation. Intriguingly, Zomer et al. use the Cre-LoxP system to visualize extracellular vesicle (EV) exchange between tumor cells in living mice [48]. They show that the less malignant tumor cells that take up EVs released by malignant tumor cells display enhanced migratory behavior and metastatic capacity, indicating that the metastatic behavior can be phenocopied through extracellular vesicle exchange. Taken together, these findings reveal that the intercellular communication mediated by exosomes may be an important mechanism for tumor metastasis.
Tumor drug resistance
Exosomes contribute to the development of therapy resistance in tumor cells through a variety of mechanisms. Tumor-derived exosomes can transfer multi-drug resistance (MDR)-associated proteins and miRNAs to target cells [49, 50]. In addition, exosomes participate in the process of tumor resistance by mediating drug efflux. The drugs and their metabolites can be encapsulated and exported by exosomes [51, 52]. Melanosomal sequestration of cytotoxic drugs contributes to the intractability of malignant melanomas [53]. Moreover, exosomes may counteract the effect of antibody drugs by modulating their binding to tumor cells. Lymphoma exosomes carry CD20, which bind therapeutic anti-CD20 antibodies and protect target cells from antibody attack [54]. Exosomes from HER2-overexpressing breast cancer cells express active HER2 and can bind to the HER2 antibody trastuzumab to inhibit its activity [55]. Exosomes secreted by stromal cells also contribute to tumor drug resistance. BM-MSC-derived exosomes induce multiple myeloma cells resistant to bortezomib through the activation of several survival relevant pathways [56]. Therefore, exosomes released by cancer cells and stromal cells may have a potential to modulate sensitivity of cancer cells to distinct therapies.
Tumor immune escape
Initially reported as tumor-associated antigens and tumor immune response stimulators, the recent studies have shown that tumor-derived exosomes might rather perform immunosuppressive functions. Tumor exosomes block the differentiation of murine myeloid precursor cells into dendritic cells (DC) [57]. Tumor exosome-carried TGF-β1 skews IL-2 responsiveness in favor of regulatory T cells and away from cytotoxic cells [58]. Human nasopharyngeal carcinoma-derived exosomes recruit, expand, and regulate the function of regulatory T cells through CCL20 [59]. NPC cell-derived exosomes impair T cell function, which is associated with upregulated miRNAs in the exosomes [60]. Tumor cell-derived exosomes switch the differentiation of myeloid cells to myeloid-derived suppressor cells (MDSCs) and induce accelerated lung metastasis in a MyD88-dependent manner [61, 62]. Hsp72 on tumor-derived exosomes promotes the immunosuppressive activity of MDSCs via autocrine activation of IL-6/STAT3 pathway [63]. Breast cancer cell-derived exosomes simulate the activation of NF-κB and enhance the secretion of pro-inflammatory cytokines in macrophages [64]. Exosomes from human prostate cancer cells express ligands for NKG2D on their surface and downregulate NKG2D expression on natural killer (NK) and CD8+ T cells, leading to the impairment of their cytotoxic function [65]. Collectively, these data suggest that tumor-derived exosomes interfere on multiple levels with the immune system to drive tumor immune evasion.
Tumor-stroma interaction
Tumor stroma is believed to be critically involved in tumor development and progression. Webber et al. suggest that prostate cancer cells could trigger differentiation of fibroblasts into myofibroblasts through exosomal TGF-β [66]. In addition, prostate cancer exosomes triggered TGFβ1-dependent fibroblast differentiation resemble stromal cells isolated from cancerous prostate tissue [67], which accelerates tumor growth by supporting angiogenesis. MSCs function as precursors for tumor myofibroblast. The research from our lab suggests that tumor cell-derived exosomes could induce differentiation of human MSCs to carcinoma-associated fibroblasts (CAFs) [68]. Adipose tissue-derived MSCs treated with breast cancer-derived exosomes also display the characteristics of myofibroblasts [69]. Moreover, stromal communication with cancer cells modulates therapy response. Boelens et al. suggest that exosomes transferred from stromal cells to breast cancer cells constitute a juxtacrine NOTCH3 pathway to expand therapy-resistant tumor-initiating cells [70]. Luga et al. demonstrate that fibroblast-secreted exosomes mobilize autocrine Wnt-planar cell polarity (PCP) signaling to drive breast cancer cell invasion and metastasis [42]. Therefore, exosomes may mediate a reciprocal interplay between tumor cells and stromal cells to synergistically promote tumor progression.
Tumor thrombosis
Tissue factor (TF) overexpression is closely associated with tumor progression. TF can get incorporated into tumor-derived exosomes. The hypercoagulable state in cancer patients may be partially influenced by the release of TF-bearing exosomes from tumor cells. Garnier et al. demonstrate that exosomes link the procoagulant status with metastatic phenotype in cancer. Induction of EMT changes in epithelial cancer cells results in the release of exosomes containing elevated level of tissue factor. Importantly, TF-rich exosomes can be transferred to endothelial cells and cause their exaggerated procoagulant conversion [71], suggesting that EMT influences tumor-vascular interaction through altered TF-containing exosomes. However, the exact roles of exosomes in tumor thrombosis and consequent impact on tumor growth, progression, and metastasis remain to be further explored.
Exosomes as cancer biomarkers and targets
The findings that exosomes play critical roles in almost all aspects of cancer provide opportunities for the development of exosomes as ideal diagnostic biomarkers and therapeutic targets. Exosome-shuttled proteins and nucleic acids have been suggested as novel diagnostic and prognostic indicators for a variety of cancers. Moreover, utilizing tumor-derived exosomes as vaccines and exosomes from distinct sources as carriers for drugs and small molecules have been proved to be effective in pre-clinical studies and clinical trials.
Exosomes as cancer diagnostic biomarkers
Exosomes are readily accessible in nearly all body fluids including blood, urine, saliva, and ascites. Exosomes contain bioactive molecules that reflect the pathological state of the originated cells, thus providing an enriched source of biomarkers (Table 2). The level of exosomes is elevated in the plasma of some cancer patients as compared to healthy controls. There is a positive correlation between the abundance of tumor exosomes and tumor stage in ovarian cancer patients [72]. Tumor is characterized by a specific miRNA profile. The majority of circulating microRNAs is concentrated in exosomes [73]. Exosomal miRNAs have been suggested as diagnostic and prognostic indicators for lung cancer, esophageal squamous cell carcinoma, prostate cancer, breast cancer, glioblastoma, ovarian cancer, and other cancer types [74–80]. Exosomal miRNAs are positively correlated with the stage and degree of cancer progression. In addition to miRNAs, long non-coding RNAs (LncRNAs) are also detected in exosomes [81, 82]. LncRNA from serum of gastric cancer patients is defined as a novel exosomal biomarker [83, 84].
Table 2.
Exosomes from distinct biofluids of cancer patients as biomarkers
| Exosomal cargos | Cancer types | Methods | Clinical value | Biofluids | References |
|---|---|---|---|---|---|
| CD34 | Acute myeloid leukemia (AML) | Immunoaffinity capture | Higher levels of CD34+ exosomes in AML patients | Plasma | [117] |
| EDIL-3/Del1 | Bladder cancer | Western blot | Elevated expression in patients with high-grade bladder cancer | Urine | [118] |
| miR-101, 372, 373 | Breast cancer | qRT-PCR | Highly expressed in breast cancer patients and elevated miR-373 expression in receptor-negative breast cancer patients | Serum | [119] |
| miR-21, 146a | Cervical cancer | qRT-PCR | Elevated expression in exosomes from cervical cancer patients than healthy controls and HPV(+) subjects | Cervicovaginal lavages | [120] |
| Let-7a, miR-1229, 1246, 150, 21, 223, 23a | Colon cancer | qRT-PCR | Highly expressed in exosomes from colon cancer patients | Serum | [121] |
| CD147, CD9 | Colon cancer | Exoscreen | Higher levels of CD147/CD9 double-positive extracellular vesicles in cancer patients than healthy controls | Serum | [122] |
| miR-17-92a cluster | Colon cancer | qRT-PCR | Elevated expression in cancer patients and higher levels predict poorer prognoses | Serum | [123] |
| miR-21 | Esophageal squamous cell carcinoma (ESCC) | qRT-PCR | Exosomal levels of miR-21 are significantly higher in patients with ESCC than those with benign diseases | Serum | [80] |
| LINC00152 | Gastric cancer | qRT-PCR | Elevated expression levels in gastric cancer patients than healthy controls | Plasma | [83] |
| EGFRvIII (mRNA) | Glioblastoma | Nested RT-PCR | Mutated EGFRvIII could be detected in exosomes from 7 of 25 glioblastoma patients but not that from 30 healthy subjects | Serum | [75] |
| miR-718 | Hepatocellular carcinoma (HCC) | qRT-PCR | Decreased expression of miR-718 in exosomes from HCC cases with recurrence after liver transplantation compared with those without recurrence | Serum | [124] |
| miR-21 | Hepatocellular carcinoma (HCC) | qRT-PCR | Higher exosomal levels in patients with HCC than those with hepatitis or healthy controls | Serum | [125] |
| miR-17-3p, 21, 106a, 146, 155, 191, 192, 203, 205, 210, 212, 214 | Lung cancer | miRNA array | Total exosome and miRNA levels are upregulated in lung cancer patients and these 12 miRNAs could be detected in exosomes | Plasma | [76] |
| LRG1 | Lung cancer | Western blot | Patients with non-small cell lung cancer have an increased LRG1 expression in exosomes compared to healthy controls | Urine | [126] |
| TYRP2, VLA-4, Hsp70, MET | Melanoma | Western blot, multiplex protein analysis | The levels of these 4 proteins are increased in exosomes from stage III and IV patients compared to stage I patients as well as healthy controls | Plasma | [39] |
| CD63, caveolin-1 | Melanoma | In-house sandwich ELISA (Exotest) | Melanoma patients have more CD63- and caveolin-1-positive exosomes compared to healthy controls | Plasma | [127] |
| Galectin-9 | Nasopharyngeal carcinoma (NPC) | Western blot | Exosomes from NPC patients but not that from healthy controls contain galectin-9 | Serum | [128] |
| Claudin-4 | Ovarian cancer | Western blot | Claudin-4 could be detected in exosomes from 32 of 63 ovarian cancer patients but only 1 of 50 healthy controls | Plasma | [129] |
| miR-21, 141, 200a, 200b, 200c, 203, 205, 214 | Ovarian cancer | miRNA array | The levels of these 8 miRNAs are elevated in exosomes from ovarian cancer patients compared to healthy controls and benign tumors | Serum | [72] |
| miR-1246, 4644, 3976, 4306 | Pancreatic cancer | qRT-PCR | Upregulated expression in pancreatic cancer patients compared to healthy controls | Serum | [130] |
| PTEN | Prostate cancer | Western blot | PTEN is exclusively expressed in exosomes of prostate cancer patients compared to healthy controls | Plasma | [131] |
| Survivin | Prostate cancer | Western blot, ELISA | Prostate cancer patients have more survivin-positive exosomes compared to healthy controls as well as patients with benign prostatic hyperplasia | Plasma | [132] |
| PSA, PSMA | Prostate cancer | Western blot | Detected in 20 of 24 exosomes from prostate cancer patients but not in healthy controls | Urine | [133] |
| miR-1290, miR-375 | Prostate cancer | qRT-PCR | Highly expressed in castration-resistant prostate cancer patients and their levels are significantly associated with poor overall survival | Plasma | [134] |
| LncRNA-p21 | Prostate cancer | qRT-PCR | Higher level of exosomal lncRNA-p21 in patients with prostate cancer than those with benign hyperplasia | Plasma | [135] |
Exosomes as cancer therapy targets
Exosome-based immunotherapy
Dendritic cell-derived exosomes (dexosomes) have been developed as immunotherapeutic anticancer agents [85]. Tumor peptide-pulsed DC-derived exosomes suppress growth of established murine tumors in a T cell-dependent manner [86]. Exosomes secreted by living tumor cells contain and transfer tumor antigens to dendritic cells and induce potent CD8+ T cell-dependent antitumor effects on mouse tumors [87]. Dexosomes have entered clinical trials for colorectal cancer, metastatic melanoma, and non-small cell lung cancer and have achieved modest therapeutic effects [88].
Exosome removal for cancer therapy
The removal of exosomes from advanced cancer patients is a novel strategy to treat cancer [89]. Exosome depletion by dimethyl amiloride (DMA) in mice restores the anti-tumor efficacy of cyclophosphamide (CTX) through the inhibition of MDSC functions. Amiloride, a drug used to treat high blood pressure, inhibits exosome formation and blunts MDSC suppressor functions in colorectal cancer patients [63]. The biotechnology company Aethlon Medical has developed an adjunct therapeutic method HER2osome, which is able to reduce tumor-secreted HER2 positive exosomes in the circulation and thus inhibit HER2-positive breast cancer progression. However, further work is needed to evaluate the clinical safety of such a treatment strategy based on exosome removal.
Exosomes as anti-cancer drug delivery vehicles
The use of exosomes as nucleic acid or drug delivery vehicles has gained considerable interest due to their excellent biodistribution and biocompatibility [90]. Exosome-mediated delivery of therapeutic short interfering RNA (siRNA) to the target cells has been tested. The exosome-delivered siRNA is effective at causing post-transcriptional gene silencing and inducing cell death in recipient cancer cells [91–93]. To improve drug delivery efficacy to tumors, the researchers have modified exosomes with targeting ligands such as iRGD-Lamp2b. The modified exosomes show highly efficient targeting to αV integrin-positive breast cancer cells, and intravenous injection of these exosomes obviously inhibits tumor growth [94]. In addition, exosomes have been utilized as effective vehicle for drug delivery [95]. Exosomes from MSCs have been tested as the vehicle to package and deliver active drugs such as paclitaxel [96].
Conclusion
The rapid expansion of the number of published studies on exosomes clearly shows that research on exosomes and their functions is now a very exciting field. Exosomes are small particles with big roles and are emerging as major players in intercellular communication. Exosomes have been suggested as active transporters for proteins, DNA, mRNA, and non-coding RNAs. The roles of exosomes in cancer have been gradually realized. Although some reports have suggested anti-tumor roles of exosomes due to their potential to elicit immune response, most of the reports have revealed the various pro-tumor effects of exosomes, which is further supported by the observations that the level of circulating exosomes is increased in cancer patients and correlated with tumor progression. In this review, we discussed several aspects of exosome biology in cancer. Cancer cells communicate with the surrounding and distant cells via exosomes, which constitutes a bi-directional interaction network to synergistically promote cancer development, progression, metastasis, and drug resistance. However, the exact mechanisms mediating the complex roles of exosomes in cancer have not yet fully elucidated. Exosomes would be ideal biomarkers for cancer diagnosis and targeted therapy because they closely represent the state of their parental cells and are relatively stable in the circulation and could be easily collected from body fluids. The potential of exosomal contents for diagnostic and prognostic biomarkers have been investigated in various cancers. It is required to develop faster and more convenient methods for validating the proposed exosomal cargos as biomarkers in specimens from human cancer patients. The use of nanotechnology to load exosomes with small molecules or drugs for cancer therapy has also been exploited. Improvements in developing new strategies to obtain a large amount of exosomes from appropriate donor cells, efficiently introducing the therapeutic agents into exosomes, and optimizing the targeted delivery of exosomes to particular tissues will facilitate the use of exosomes as natural carrier in clinical therapy. Future studies of exosomes will not only shed lights on their roles in the pathogenesis of cancer but will open new avenues for cancer diagnosis and therapeutics.
Acknowledgements
We thank members of the Xu Lab for their excellent work and helpful discussions. This work was supported by the following: Major Research Plan of the National Natural Science Foundation of China (grant no. 91129718), the National Natural Science Foundation of China (grant no. 81201660), the Natural Science Foundation of the Jiangsu Province (grant no. BK20141303), Jiangsu Province’s Project of Scientific and Technological Innovation and Achievements Transformation (grant no. BL2012055), Jiangsu Province’s Outstanding Medical Academic Leader and Sci-tech Innovation Team Program (grant no. LJ201117), Jiangsu Province’s Qing Lan project, Foundation for Young Academic Leader of Jiangsu University, Starting Foundation for Senior Talents of Jiangsu University (grant no. 13JDG086).
Abbreviations
- APC
antigen-presenting cell
- ASCs
adipose-derived stem cells
- BM-MSCs
bone marrow mesenchymal stromal cells
- CAFs
carcinoma-associated fibroblasts
- CTX
cyclophosphamide
- Dexosomes
dendritic cell-derived exosomes
- DMA
dimethyl amiloride
- EMT
epithelial-mesenchymal transition
- ESCRT
endosomal sorting complex required for transport
- esRNA
exosomal shuttle RNA
- FIH-1
factor-inhibiting hypoxia-inducible factor 1
- GTPases
guanosine triphosphatases
- HIF-1
hypoxia-inducible factor 1
- HSPGs
heparan sulfate proteoglycans
- ICAM
intercellular adhesion molecule
- ILVs
Intraluminal vesicles
- LncRNA
long non-coding RNA
- MACS
magnetic activated cell sorting
- MDR
multi-drug resistance
- MDSCs
myeloid-derived suppressor cells
- MVBs
multivesicular bodies
- NPC
nasopharyngeal carcinoma
- PCP
planar cell polarity
- RISC
RNA-induced silencing complex
- siRNA
short interfering RNA
- SP
side population
- TEM
transmission electron microscopy
- TF
tissue factor
- TIM
T cell immunoglobulin and mucin domain molecule
- TLR
toll-like receptor
- TSAP6
tumor suppressor-activated pathway 6
Footnotes
Competing interests
The authors declare that they have no competing interest
Authors’ contributions
XZ and WRX were responsible for the conception and design of the manuscript. All authors participated in the drafting of the manuscript and approved its final version. All authors read and approved the final manuscript.
Contributor Information
Xu Zhang, Phone: +86 511 85038215, Email: xuzhang@ujs.edu.cn.
Xiao Yuan, Email: 28478018@qq.com.
Hui Shi, Email: 735594932@qq.com.
Lijun Wu, Email: 1983016304@qq.com.
Hui Qian, Email: lstmmmlst@163.com.
Wenrong Xu, Phone: +86 511 85038215, Email: icls@ujs.edu.cn.
References
- 1.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 2.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319(5867):1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 3.Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, et al. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14(7):677–685. doi: 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]
- 4.Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12(1):1–13. doi: 10.1038/ncb2000. [DOI] [PubMed] [Google Scholar]
- 5.Hsu C, Morohashi Y, Yoshimura S, Manrique-Hoyos N, Jung S, Lauterbach MA, et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J Cell Biol. 2010;189(2):223–232. doi: 10.1083/jcb.200911018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Savina A, Furlan M, Vidal M, Colombo MI. Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J Biol Chem. 2003;278(22):20083–20090. doi: 10.1074/jbc.M301642200. [DOI] [PubMed] [Google Scholar]
- 7.Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A, et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J Biol Chem. 2009;284(49):34211–34222. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu JL, May L, Lhotak V, Shahrzad S, Shirasawa S, Weitz JI, et al. Oncogenic events regulate tissue factor expression in colorectal cancer cells: implications for tumor progression and angiogenesis. Blood. 2005;105(4):1734–1741. doi: 10.1182/blood-2004-05-2042. [DOI] [PubMed] [Google Scholar]
- 9.Yu X, Harris SL, Levine AJ. The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res. 2006;66(9):4795–4801. doi: 10.1158/0008-5472.CAN-05-4579. [DOI] [PubMed] [Google Scholar]
- 10.Lespagnol A, Duflaut D, Beekman C, Blanc L, Fiucci G, Marine JC, et al. Exosome secretion, including the DNA damage-induced p53-dependent secretory pathway, is severely compromised in TSAP6/Steap3-null mice. Cell Death Differ. 2008;15(11):1723–1733. doi: 10.1038/cdd.2008.104. [DOI] [PubMed] [Google Scholar]
- 11.Thompson CA, Purushothaman A, Ramani VC, Vlodavsky I, Sanderson RD. Heparanase regulates secretion, composition, and function of tumor cell-derived exosomes. J Biol Chem. 2013;288(14):10093–10099. doi: 10.1074/jbc.C112.444562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riches A, Campbell E, Borger E, Powis S. Regulation of exosome release from mammary epithelial and breast cancer cells - a new regulatory pathway. Eur J Cancer. 2014;50(5):1025–1034. doi: 10.1016/j.ejca.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 13.Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450(7168):435–439. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- 14.Segura E, Nicco C, Lombard B, Veron P, Raposo G, Batteux F, et al. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood. 2005;106(1):216–223. doi: 10.1182/blood-2005-01-0220. [DOI] [PubMed] [Google Scholar]
- 15.Tian T, Wang Y, Wang H, Zhu Z, Xiao Z. Visualizing of the cellular uptake and intracellular trafficking of exosomes by live-cell microscopy. J Cell Biochem. 2010;111(2):488–496. doi: 10.1002/jcb.22733. [DOI] [PubMed] [Google Scholar]
- 16.Feng D, Zhao WL, Ye YY, Bai XC, Liu RQ, Chang LF, et al. Cellular internalization of exosomes occurs through phagocytosis. Traffic. 2010;11(5):675–687. doi: 10.1111/j.1600-0854.2010.01041.x. [DOI] [PubMed] [Google Scholar]
- 17.Escrevente C, Keller S, Altevogt P, Costa J. Interaction and uptake of exosomes by ovarian cancer cells. BMC Cancer. 2011;11:108. doi: 10.1186/1471-2407-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christianson HC, Svensson KJ, van Kuppevelt TH, Li JP, Belting M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc Natl Acad Sci U S A. 2013;110(43):17380–17385. doi: 10.1073/pnas.1304266110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thakur BK, Zhang H, Becker A, Matei I, Huang Y, Costa-Silva B, et al. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res. 2014;24(6):766–769. doi: 10.1038/cr.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 21.Lotvall J, Hill AF, Hochberg F, Buzas EI, Di Vizio D, Gardiner C, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3:26913. doi: 10.3402/jev.v3.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abd Elmageed ZY, Yang Y, Thomas R, Ranjan M, Mondal D, Moroz K, et al. Neoplastic reprogramming of patient-derived adipose stem cells by prostate cancer cell-associated exosomes. Stem Cells. 2014;32(4):983–997. doi: 10.1002/stem.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melo SA, Sugimoto H, O'Connell JT, Kato N, Villanueva A, Vidal A, et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell. 2014;26(5):707–721. doi: 10.1016/j.ccell.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koch R, Demant M, Aung T, Diering N, Cicholas A, Chapuy B, et al. Populational equilibrium through exosome-mediated Wnt signaling in tumor progression of diffuse large B-cell lymphoma. Blood. 2014;123(14):2189–2198. doi: 10.1182/blood-2013-08-523886. [DOI] [PubMed] [Google Scholar]
- 25.Khan S, Aspe JR, Asumen MG, Almaguel F, Odumosu O, Acevedo-Martinez S, et al. Extracellular, cell-permeable survivin inhibits apoptosis while promoting proliferative and metastatic potential. Br J Cancer. 2009;100(7):1073–1086. doi: 10.1038/sj.bjc.6604978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10(5):619–624. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 27.Soldevilla B, Rodriguez M, San Millan C, Garcia V, Fernandez-Perianez R, Gil-Calderon B, et al. Tumor-derived exosomes are enriched in DeltaNp73, which promotes oncogenic potential in acceptor cells and correlates with patient survival. Hum Mol Genet. 2014;23(2):467–478. doi: 10.1093/hmg/ddt437. [DOI] [PubMed] [Google Scholar]
- 28.Corrado C, Raimondo S, Saieva L, Flugy AM, De Leo G, Alessandro R. Exosome-mediated crosstalk between chronic myelogenous leukemia cells and human bone marrow stromal cells triggers an interleukin 8-dependent survival of leukemia cells. Cancer Lett. 2014;348(1–2):71–76. doi: 10.1016/j.canlet.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 29.Roccaro AM, Sacco A, Maiso P, Azab AK, Tai YT, Reagan M, et al. BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J Clin Invest. 2013;123(4):1542–1555. doi: 10.1172/JCI66517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park JE, Tan HS, Datta A, Lai RC, Zhang H, Meng W, et al. Hypoxic tumor cell modulates its microenvironment to enhance angiogenic and metastatic potential by secretion of proteins and exosomes. Mol Cell Proteomics. 2010;9(6):1085–1099. doi: 10.1074/mcp.M900381-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nazarenko I, Rana S, Baumann A, McAlear J, Hellwig A, Trendelenburg M, et al. Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation. Cancer Res. 2010;70(4):1668–1678. doi: 10.1158/0008-5472.CAN-09-2470. [DOI] [PubMed] [Google Scholar]
- 32.Kucharzewska P, Christianson HC, Welch JE, Svensson KJ, Fredlund E, Ringner M, et al. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci U S A. 2013;110(18):7312–7317. doi: 10.1073/pnas.1220998110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR, et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 2014;25(4):501–515. doi: 10.1016/j.ccr.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Umezu T, Ohyashiki K, Kuroda M, Ohyashiki JH. Leukemia cell to endothelial cell communication via exosomal miRNAs. Oncogene. 2013;32(22):2747–2755. doi: 10.1038/onc.2012.295. [DOI] [PubMed] [Google Scholar]
- 35.Tadokoro H, Umezu T, Ohyashiki K, Hirano T, Ohyashiki JH. Exosomes derived from hypoxic leukemia cells enhance tube formation in endothelial cells. J Biol Chem. 2013;288(48):34343–34351. doi: 10.1074/jbc.M113.480822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Umezu T, Tadokoro H, Azuma K, Yoshizawa S, Ohyashiki K, Ohyashiki JH. Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1. Blood. 2014;124(25):3748–3757. doi: 10.1182/blood-2014-05-576116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aga M, Bentz GL, Raffa S, Torrisi MR, Kondo S, Wakisaka N, et al. Exosomal HIF1alpha supports invasive potential of nasopharyngeal carcinoma-associated LMP1-positive exosomes. Oncogene. 2014;33(37):4613–4622. doi: 10.1038/onc.2014.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ostenfeld MS, Jeppesen DK, Laurberg JR, Boysen AT, Bramsen JB, Primdal-Bengtson B, et al. Cellular disposal of miR23b by RAB27-dependent exosome release is linked to acquisition of metastatic properties. Cancer Res. 2014;74(20):5758–5771. doi: 10.1158/0008-5472.CAN-13-3512. [DOI] [PubMed] [Google Scholar]
- 39.Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18(6):883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Atay S, Banskota S, Crow J, Sethi G, Rink L, Godwin AK. Oncogenic KIT-containing exosomes increase gastrointestinal stromal tumor cell invasion. Proc Natl Acad Sci U S A. 2014;111(2):711–716. doi: 10.1073/pnas.1310501111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suetsugu A, Honma K, Saji S, Moriwaki H, Ochiya T, Hoffman RM. Imaging exosome transfer from breast cancer cells to stroma at metastatic sites in orthotopic nude-mouse models. Adv Drug Deliv Rev. 2013;65(3):383–390. doi: 10.1016/j.addr.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 42.Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E, et al. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell. 2012;151(7):1542–1556. doi: 10.1016/j.cell.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 43.Cai Z, Yang F, Yu L, Yu Z, Jiang L, Wang Q, et al. Activated T cell exosomes promote tumor invasion via Fas signaling pathway. J Immunol. 2012;188(12):5954–61. doi: 10.4049/jimmunol.1103466. [DOI] [PubMed] [Google Scholar]
- 44.Yang M, Chen J, Su F, Yu B, Lin L, Liu Y, et al. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol Cancer. 2011;10:117. doi: 10.1186/1476-4598-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang M, Zhao C, Shi H, Zhang B, Zhang L, Zhang X, et al. Deregulated microRNAs in gastric cancer tissue-derived mesenchymal stem cells: novel biomarkers and a mechanism for gastric cancer. Br J Cancer. 2014;110(5):1199–1210. doi: 10.1038/bjc.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R, et al. MicroRNAs bind to toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci U S A. 2012;109(31):E2110–2116. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17(6):816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zomer A, Maynard C, Verweij FJ, Kamermans A, Schafer R, Beerling E, et al. In Vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell. 2015;161(5):1046–1057. doi: 10.1016/j.cell.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Corcoran C, Rani S, O'Brien K, O'Neill A, Prencipe M, Sheikh R, et al. Docetaxel-resistance in prostate cancer: evaluating associated phenotypic changes and potential for resistance transfer via exosomes. PLoS One. 2012;7(12):e50999. doi: 10.1371/journal.pone.0050999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wei Y, Lai X, Yu S, Chen S, Ma Y, Zhang Y, et al. Exosomal miR-221/222 enhances tamoxifen resistance in recipient ER-positive breast cancer cells. Breast Cancer Res Treat. 2014;147(2):423–431. doi: 10.1007/s10549-014-3037-0. [DOI] [PubMed] [Google Scholar]
- 51.Shedden K, Xie XT, Chandaroy P, Chang YT, Rosania GR. Expulsion of small molecules in vesicles shed by cancer cells: association with gene expression and chemosensitivity profiles. Cancer Res. 2003;63(15):4331–4337. [PubMed] [Google Scholar]
- 52.Safaei R, Larson BJ, Cheng TC, Gibson MA, Otani S, Naerdemann W, et al. Abnormal lysosomal trafficking and enhanced exosomal export of cisplatin in drug-resistant human ovarian carcinoma cells. Mol Cancer Ther. 2005;4(10):1595–1604. doi: 10.1158/1535-7163.MCT-05-0102. [DOI] [PubMed] [Google Scholar]
- 53.Chen KG, Valencia JC, Lai B, Zhang G, Paterson JK, Rouzaud F, et al. Melanosomal sequestration of cytotoxic drugs contributes to the intractability of malignant melanomas. Proc Natl Acad Sci U S A. 2006;103(26):9903–9907. doi: 10.1073/pnas.0600213103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aung T, Chapuy B, Vogel D, Wenzel D, Oppermann M, Lahmann M, et al. Exosomal evasion of humoral immunotherapy in aggressive B-cell lymphoma modulated by ATP-binding cassette transporter A3. Proc Natl Acad Sci U S A. 2011;108(37):15336–15341. doi: 10.1073/pnas.1102855108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ciravolo V, Huber V, Ghedini GC, Venturelli E, Bianchi F, Campiglio M, et al. Potential role of HER2-overexpressing exosomes in countering trastuzumab-based therapy. J Cell Physiol. 2012;227(2):658–667. doi: 10.1002/jcp.22773. [DOI] [PubMed] [Google Scholar]
- 56.Battke C, Ruiss R, Welsch U, Wimberger P, Lang S, Jochum S, et al. Tumour exosomes inhibit binding of tumour-reactive antibodies to tumour cells and reduce ADCC. Cancer Immunol Immunother. 2011;60(5):639–648. doi: 10.1007/s00262-011-0979-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu S, Liu C, Su K, Wang J, Liu Y, Zhang L, et al. Tumor exosomes inhibit differentiation of bone marrow dendritic cells. J Immunol. 2007;178(11):6867–6875. doi: 10.4049/jimmunol.178.11.6867. [DOI] [PubMed] [Google Scholar]
- 58.Clayton A, Mitchell JP, Court J, Mason MD, Tabi Z. Human tumor-derived exosomes selectively impair lymphocyte responses to interleukin-2. Cancer Res. 2007;67(15):7458–7466. doi: 10.1158/0008-5472.CAN-06-3456. [DOI] [PubMed] [Google Scholar]
- 59.Mrizak D, Martin N, Barjon C, Jimenez-Pailhes AS, Mustapha R, Niki T, et al. Effect of nasopharyngeal carcinoma-derived exosomes on human regulatory T cells. J Natl Cancer Inst. 2014;107(1):363. doi: 10.1093/jnci/dju363. [DOI] [PubMed] [Google Scholar]
- 60.Ye SB, Li ZL, Luo DH, Huang BJ, Chen YS, Zhang XS, et al. Tumor-derived exosomes promote tumor progression and T-cell dysfunction through the regulation of enriched exosomal microRNAs in human nasopharyngeal carcinoma. Oncotarget. 2014;5(14):5439–5452. doi: 10.18632/oncotarget.2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xiang X, Poliakov A, Liu C, Liu Y, Deng ZB, Wang J, et al. Induction of myeloid-derived suppressor cells by tumor exosomes. Int J Cancer. 2009;124(11):2621–2633. doi: 10.1002/ijc.24249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu Y, Xiang X, Zhuang X, Zhang S, Liu C, Cheng Z, et al. Contribution of MyD88 to the tumor exosome-mediated induction of myeloid derived suppressor cells. Am J Pathol. 2010;176(5):2490–2499. doi: 10.2353/ajpath.2010.090777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest. 2010;120(2):457–471. doi: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chow A, Zhou W, Liu L, Fong MY, Champer J, Van Haute D, et al. Macrophage immunomodulation by breast cancer-derived exosomes requires toll-like receptor 2-mediated activation of NF-kappaB. Sci Rep. 2014;4:5750. doi: 10.1038/srep05750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lundholm M, Schroder M, Nagaeva O, Baranov V, Widmark A, Mincheva-Nilsson L, et al. Prostate tumor-derived exosomes down-regulate NKG2D expression on natural killer cells and CD8+ T cells: mechanism of immune evasion. PLoS One. 2014;9(9):e108925. doi: 10.1371/journal.pone.0108925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Webber J, Steadman R, Mason MD, Tabi Z, Clayton A. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res. 2010;70(23):9621–9630. doi: 10.1158/0008-5472.CAN-10-1722. [DOI] [PubMed] [Google Scholar]
- 67.Webber JP, Spary LK, Sanders AJ, Chowdhury R, Jiang WG, Steadman R, et al. Differentiation of tumour-promoting stromal myofibroblasts by cancer exosomes. Oncogene. 2015;34(3):290–302. doi: 10.1038/onc.2013.560. [DOI] [PubMed] [Google Scholar]
- 68.Gu J, Qian H, Shen L, Zhang X, Zhu W, Huang L, et al. Gastric cancer exosomes trigger differentiation of umbilical cord derived mesenchymal stem cells to carcinoma-associated fibroblasts through TGF-beta/Smad pathway. PLoS One. 2012;7(12):e52465. doi: 10.1371/journal.pone.0052465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cho JA, Park H, Lim EH, Lee KW. Exosomes from breast cancer cells can convert adipose tissue-derived mesenchymal stem cells into myofibroblast-like cells. Int J Oncol. 2012;40(1):130–138. doi: 10.3892/ijo.2011.1193. [DOI] [PubMed] [Google Scholar]
- 70.Boelens MC, Wu TJ, Nabet BY, Xu B, Qiu Y, Yoon T, et al. Exosome transfer from stromal to breast cancer cells regulates therapy resistance pathways. Cell. 2014;159(3):499–513. doi: 10.1016/j.cell.2014.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Garnier D, Magnus N, Lee TH, Bentley V, Meehan B, Milsom C, et al. Cancer cells induced to express mesenchymal phenotype release exosome-like extracellular vesicles carrying tissue factor. J Biol Chem. 2012;287(52):43565–43572. doi: 10.1074/jbc.M112.401760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110(1):13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 73.Gallo A, Tandon M, Alevizos I, Illei GG. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS One. 2012;7(3):e30679. doi: 10.1371/journal.pone.0030679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang W, Chen Y. Circulating miRNAs in cancer: from detection to therapy. J Hematol Oncol. 2014;7(1):86. doi: 10.1186/s13045-014-0086-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rabinowits G, Gercel-Taylor C, Day JM, Taylor DD, Kloecker GH. Exosomal microRNA: a diagnostic marker for lung cancer. Clin Lung Cancer. 2009;10(1):42–46. doi: 10.3816/CLC.2009.n.006. [DOI] [PubMed] [Google Scholar]
- 77.Lau C, Kim Y, Chia D, Spielmann N, Eibl G, Elashoff D, et al. Role of pancreatic cancer-derived exosomes in salivary biomarker development. J Biol Chem. 2013;288(37):26888–26897. doi: 10.1074/jbc.M113.452458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nilsson J, Skog J, Nordstrand A, Baranov V, Mincheva-Nilsson L, Breakefield XO, et al. Prostate cancer-derived urine exosomes: a novel approach to biomarkers for prostate cancer. Br J Cancer. 2009;100(10):1603–1607. doi: 10.1038/sj.bjc.6605058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Corcoran C, Friel AM, Duffy MJ, Crown J, O'Driscoll L. Intracellular and extracellular microRNAs in breast cancer. Clin Chem. 2011;57(1):18–32. doi: 10.1373/clinchem.2010.150730. [DOI] [PubMed] [Google Scholar]
- 80.Tanaka Y, Kamohara H, Kinoshita K, Kurashige J, Ishimoto T, Iwatsuki M, et al. Clinical impact of serum exosomal microRNA-21 as a clinical biomarker in human esophageal squamous cell carcinoma. Cancer. 2013;119(6):1159–1167. doi: 10.1002/cncr.27895. [DOI] [PubMed] [Google Scholar]
- 81.Kogure T, Yan IK, Lin WL, Patel T. Extracellular vesicle-mediated transfer of a novel long noncoding RNA TUC339: a mechanism of intercellular signaling in human hepatocellular cancer. Genes Cancer. 2013;4(7–8):261–272. doi: 10.1177/1947601913499020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Takahashi K, Yan IK, Kogure T, Haga H, Patel T. Extracellular vesicle-mediated transfer of long non-coding RNA ROR modulates chemosensitivity in human hepatocellular cancer. FEBS Open Bio. 2014;4:458–467. doi: 10.1016/j.fob.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li Q, Shao Y, Zhang X, Zheng T, Miao M, Qin L, et al. Plasma long noncoding RNA protected by exosomes as a potential stable biomarker for gastric cancer. Tumour Biol. 2015;36(3):2007–2012. doi: 10.1007/s13277-014-2807-y. [DOI] [PubMed] [Google Scholar]
- 84.Wang J, Zhou Y, Lu J, Sun Y, Xiao H, Liu M, et al. Combined detection of serum exosomal miR-21 and HOTAIR as diagnostic and prognostic biomarkers for laryngeal squamous cell carcinoma. Med Oncol. 2014;31(9):148. doi: 10.1007/s12032-014-0148-8. [DOI] [PubMed] [Google Scholar]
- 85.Pitt JM, Charrier M, Viaud S, Andre F, Besse B, Chaput N, et al. Dendritic cell-derived exosomes as immunotherapies in the fight against cancer. J Immunol. 2014;193(3):1006–1011. doi: 10.4049/jimmunol.1400703. [DOI] [PubMed] [Google Scholar]
- 86.Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med. 1998;4(5):594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 87.Wolfers J, Lozier A, Raposo G, Regnault A, Thery C, Masurier C, et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7(3):297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 88.Tan A, De La Pena H, Seifalian AM. The application of exosomes as a nanoscale cancer vaccine. Int J Nanomedicine. 2010;5:889–900. doi: 10.2147/IJN.S13402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marleau AM, Chen CS, Joyce JA, Tullis RH. Exosome removal as a therapeutic adjuvant in cancer. J Transl Med. 2012;10:134. doi: 10.1186/1479-5876-10-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.van den Boorn JG, Dassler J, Coch C, Schlee M, Hartmann G. Exosomes as nucleic acid nanocarriers. Adv Drug Deliv Rev. 2013;65(3):331–335. doi: 10.1016/j.addr.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 91.Shtam TA, Kovalev RA, Varfolomeeva EY, Makarov EM, Kil YV, Filatov MV. Exosomes are natural carriers of exogenous siRNA to human cells in vitro. Cell Commun Signal. 2013;11:88. doi: 10.1186/1478-811X-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang Y, Li L, Yu J, Zhu D, Li X, Gu H, et al. Microvesicle-mediated delivery of transforming growth factor beta1 siRNA for the suppression of tumor growth in mice. Biomaterials. 2014;35(14):4390–4400. doi: 10.1016/j.biomaterials.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 93.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29(4):341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 94.Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35(7):2383–2390. doi: 10.1016/j.biomaterials.2013.11.083. [DOI] [PubMed] [Google Scholar]
- 95.Smyth T, Kullberg M, Malik N, Smith-Jones P, Graner MW, Anchordoquy TJ. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J Control Release. 2015;199:145–155. doi: 10.1016/j.jconrel.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pascucci L, Cocce V, Bonomi A, Ami D, Ceccarelli P, Ciusani E, et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. J Control Release. 2014;192:262–270. doi: 10.1016/j.jconrel.2014.07.042. [DOI] [PubMed] [Google Scholar]
- 97.Demory Beckler M, Higginbotham JN, Franklin JL, Ham AJ, Halvey PJ, Imasuen IE, et al. Proteomic analysis of exosomes from mutant KRAS colon cancer cells identifies intercellular transfer of mutant KRAS. Mol Cell Proteomics. 2013;12(2):343–355. doi: 10.1074/mcp.M112.022806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fedele C, Singh A, Zerlanko BJ, Iozzo RV, Languino LR. The alphavbeta6 integrin is transferred intercellularly via exosomes. J Biol Chem. 2015;290(8):4545–4551. doi: 10.1074/jbc.C114.617662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Khan S, Jutzy JM, Aspe JR, McGregor DW, Neidigh JW, Wall NR. Survivin is released from cancer cells via exosomes. Apoptosis. 2011;16(1):1–12. doi: 10.1007/s10495-010-0534-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Menck K, Klemm F, Gross JC, Pukrop T, Wenzel D, Binder C. Induction and transport of Wnt5a during macrophage-induced malignant invasion is mediated by two types of extracellular vesicles. Oncotarget. 2013;4(11):2057–2066. doi: 10.18632/oncotarget.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Clayton A, Al-Taei S, Webber J, Mason MD, Tabi Z. Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J Immunol. 2011;187(2):676–683. doi: 10.4049/jimmunol.1003884. [DOI] [PubMed] [Google Scholar]
- 102.Chowdhury R, Webber JP, Gurney M, Mason MD, Tabi Z, Clayton A. Cancer exosomes trigger mesenchymal stem cell differentiation into pro-angiogenic and pro-invasive myofibroblasts. Oncotarget. 2015;6(2):715–731. doi: 10.18632/oncotarget.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Clayton A, Mitchell JP, Court J, Linnane S, Mason MD, Tabi Z. Human tumor-derived exosomes down-modulate NKG2D expression. J Immunol. 2008;180(11):7249–7258. doi: 10.4049/jimmunol.180.11.7249. [DOI] [PubMed] [Google Scholar]
- 104.Ashiru O, Boutet P, Fernandez-Messina L, Aguera-Gonzalez S, Skepper JN, Vales-Gomez M, et al. Natural killer cell cytotoxicity is suppressed by exposure to the human NKG2D ligand MICA*008 that is shed by tumor cells in exosomes. Cancer Res. 2010;70(2):481–489. doi: 10.1158/0008-5472.CAN-09-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xiao H, Lasser C, Shelke GV, Wang J, Radinger M, Lunavat TR, et al. Mast cell exosomes promote lung adenocarcinoma cell proliferation—role of KIT-stem cell factor signaling. Cell Commun Signal. 2014;12:64. doi: 10.1186/s12964-014-0064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Diao J, Yang X, Song X, Chen S, He Y, Wang Q, et al. Exosomal Hsp70 mediates immunosuppressive activity of the myeloid-derived suppressor cells via phosphorylation of Stat3. Med Oncol. 2015;32(2):453. doi: 10.1007/s12032-014-0453-2. [DOI] [PubMed] [Google Scholar]
- 107.Sagar G, Sah RP, Javeed N, Dutta SK, Smyrk TC, Lau JS, et al. Pathogenesis of pancreatic cancer exosome-induced lipolysis in adipose tissue. Gut 2015, doi:gutjnl-2014-308350 [DOI] [PMC free article] [PubMed]
- 108.Deng Z, Mu J, Tseng M, Wattenberg B, Zhuang X, Egilmez NK, et al. Enterobacteria-secreted particles induce production of exosome-like S1P-containing particles by intestinal epithelium to drive Th17-mediated tumorigenesis. Nat Commun. 2012;6:6956. doi: 10.1038/ncomms7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhuang G, Wu X, Jiang Z, Kasman I, Yao J, Guan Y, et al. Tumour-secreted miR-9 promotes endothelial cell migration and angiogenesis by activating the JAK-STAT pathway. EMBO J. 2012;31(17):3513–3523. doi: 10.1038/emboj.2012.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Singh R, Pochampally R, Watabe K, Lu Z, Mo YY. Exosome-mediated transfer of miR-10b promotes cell invasion in breast cancer. Mol Cancer. 2014;13:256. doi: 10.1186/1476-4598-13-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen WX, Liu XM, Lv MM, Chen L, Zhao JH, Zhong SL, et al. Exosomes from drug-resistant breast cancer cells transmit chemoresistance by a horizontal transfer of microRNAs. PLoS One. 2014;9(4):e95240. doi: 10.1371/journal.pone.0095240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kogure T, Lin WL, Yan IK, Braconi C, Patel T. Intercellular nanovesicle-mediated microRNA transfer: a mechanism of environmental modulation of hepatocellular cancer cell growth. Hepatology. 2011;54(4):1237–1248. doi: 10.1002/hep.24504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fong MY, Zhou W, Liu L, Alontaga AY, Chandra M, Ashby J, et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat Cell Biol. 2015;17(2):183–194. doi: 10.1038/ncb3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bovy N, Blomme B, Freres P, Dederen S, Nivelles O, Lion M, et al. Endothelial exosomes contribute to the antitumor response during breast cancer neoadjuvant chemotherapy via microRNA transfer. Oncotarget. 2015;6(12):10253–10266. doi: 10.18632/oncotarget.3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gernapudi R, Yao Y, Zhang Y, Wolfson B, Roy S, Duru N, et al. Targeting exosomes from preadipocytes inhibits preadipocyte to cancer stem cell signaling in early-stage breast cancer. Breast Cancer Res Treat. 2015;150(3):685–695. doi: 10.1007/s10549-015-3326-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lim PK, Bliss SA, Patel SA, Taborga M, Dave MA, Gregory LA, et al. Gap junction-mediated import of microRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells. Cancer Res. 2011;71(5):1550–1560. doi: 10.1158/0008-5472.CAN-10-2372. [DOI] [PubMed] [Google Scholar]
- 117.Hong CS, Muller L, Boyiadzis M, Whiteside TL. Isolation and characterization of CD34+ blast-derived exosomes in acute myeloid leukemia. PLoS One. 2014;9(8):e103310. doi: 10.1371/journal.pone.0103310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Beckham CJ, Olsen J, Yin PN, Wu CH, Ting HJ, Hagen FK, et al. Bladder cancer exosomes contain EDIL-3/Del1 and facilitate cancer progression. J Urol. 2014;192(2):583–592. doi: 10.1016/j.juro.2014.02.035. [DOI] [PubMed] [Google Scholar]
- 119.Eichelser C, Stuckrath I, Muller V, Milde-Langosch K, Wikman H, Pantel K, et al. Increased serum levels of circulating exosomal microRNA-373 in receptor-negative breast cancer patients. Oncotarget. 2014;5(20):9650–9663. doi: 10.18632/oncotarget.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu J, Sun H, Wang X, Yu Q, Li S, Yu X, et al. Increased exosomal microRNA-21 and microRNA-146a levels in the cervicovaginal lavage specimens of patients with cervical cancer. Int J Mol Sci. 2014;15(1):758–773. doi: 10.3390/ijms15010758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ogata-Kawata H, Izumiya M, Kurioka D, Honma Y, Yamada Y, Furuta K, et al. Circulating exosomal microRNAs as biomarkers of colon cancer. PLoS One. 2014;9(4):e92921. doi: 10.1371/journal.pone.0092921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yoshioka Y, Kosaka N, Konishi Y, Ohta H, Okamoto H, Sonoda H, et al. Ultra-sensitive liquid biopsy of circulating extracellular vesicles using ExoScreen. Nat Commun. 2014;5:3591. doi: 10.1038/ncomms4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Matsumura T, Sugimachi K, Iinuma H, Takahashi Y, Kurashige J, Sawada G, et al. Exosomal microRNA in serum is a novel biomarker of recurrence in human colorectal cancer. Br J Cancer 2015, doi:bjc2015201 [DOI] [PMC free article] [PubMed]
- 124.Sugimachi K, Matsumura T, Hirata H, Uchi R, Ueda M, Ueo H, et al. Identification of a bona fide microRNA biomarker in serum exosomes that predicts hepatocellular carcinoma recurrence after liver transplantation. Br J Cancer. 2015;112(3):532–538. doi: 10.1038/bjc.2014.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang H, Hou L, Li A, Duan Y, Gao H, Song X. Expression of serum exosomal microRNA-21 in human hepatocellular carcinoma. Biomed Res Int. 2014;2014:864894. doi: 10.1155/2014/864894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li Y, Zhang Y, Qiu F, Qiu Z. Proteomic identification of exosomal LRG1: a potential urinary biomarker for detecting NSCLC. Electrophoresis. 2011;32(15):1976–1983. doi: 10.1002/elps.201000598. [DOI] [PubMed] [Google Scholar]
- 127.Logozzi M, De Milito A, Lugini L, Borghi M, Calabro L, Spada M, et al. High levels of exosomes expressing CD63 and caveolin-1 in plasma of melanoma patients. PLoS One. 2009;4(4):e5219. doi: 10.1371/journal.pone.0005219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Klibi J, Niki T, Riedel A, Pioche-Durieu C, Souquere S, Rubinstein E, et al. Blood diffusion and Th1-suppressive effects of galectin-9-containing exosomes released by Epstein-Barr virus-infected nasopharyngeal carcinoma cells. Blood. 2009;113(9):1957–1966. doi: 10.1182/blood-2008-02-142596. [DOI] [PubMed] [Google Scholar]
- 129.Li J, Sherman-Baust CA, Tsai-Turton M, Bristow RE, Roden RB, Morin PJ. Claudin-containing exosomes in the peripheral circulation of women with ovarian cancer. BMC Cancer. 2009;9:244. doi: 10.1186/1471-2407-9-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Madhavan B, Yue S, Galli U, Rana S, Gross W, Muller M, et al. Combined evaluation of a panel of protein and miRNA serum-exosome biomarkers for pancreatic cancer diagnosis increases sensitivity and specificity. Int J Cancer. 2015;136(11):2616–27. doi: 10.1002/ijc.29324. [DOI] [PubMed] [Google Scholar]
- 131.Gabriel K, Ingram A, Austin R, Kapoor A, Tang D, Majeed F, et al. Regulation of the tumor suppressor PTEN through exosomes: a diagnostic potential for prostate cancer. PLoS One. 2013;8(7):e70047. doi: 10.1371/journal.pone.0070047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Khan S, Jutzy JM, Valenzuela MM, Turay D, Aspe JR, Ashok A, et al. Plasma-derived exosomal survivin, a plausible biomarker for early detection of prostate cancer. PLoS One. 2012;7(10):e46737. doi: 10.1371/journal.pone.0046737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mitchell PJ, Welton J, Staffurth J, Court J, Mason MD, Tabi Z, et al. Can urinary exosomes act as treatment response markers in prostate cancer? J Transl Med. 2009;7:4. doi: 10.1186/1479-5876-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Huang X, Yuan T, Liang M, Du M, Xia S, Dittmar R, et al. Exosomal miR-1290 and miR-375 as prognostic markers in castration-resistant prostate cancer. Eur Urol. 2015;67(1):33–41. doi: 10.1016/j.eururo.2014.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Isin M, Uysaler E, Ozgur E, Koseoglu H, Sanli O, Yucel OB, et al. Exosomal lncRNA-p21 levels may help to distinguish prostate cancer from benign disease. Front Genet. 2015;6:168. doi: 10.3389/fgene.2015.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]


