Abstract
Background/Aims
There are limited therapeutic options available for irritable bowel syndrome with diarrhea (IBS-D). We tested the effects of Atractylodes japonica rhizome, a perennial plant native to North Asia, on both upper and lower gastrointestinal (GI) motility in guinea pigs.
Methods
The extract of A. japonica rhizome was administered orally at different doses to test its effects on upper GI motility as determined from charcoal transit in native guinea pigs and in guinea pigs pretreated with thyrotropin-releasing hormone or mustard oil. Regarding its effect on lower GI motility, the removed guinea pig colon was suspended in a chamber containing Krebs-Henseleit solution and the transit time of artificial feces was measured with various dilutions of the extract. As for in vivo assay, weight and number of fecal pellets expelled were determined under the same drug preparation used in upper GI motility experiment.
Results
The extract of A. japonica rhizome had no significant effect on upper GI motility in either normal or altered physiological states. However, the extract increased colonic transit time in the in vitro model. In the fecal expulsion study, the cumulative weight and number of pellets did not differ significantly between the control group and groups treated with the extracts. In the animals pretreated in vivo with thyrotropin-releasing hormone, however, the weight and number of fecal pellets were significantly decreased in animals treated with 300 mg/kg and 600 mg/kg doses of extract.
Conclusions
Our findings suggest that the extract of A. japonica rhizome can be a potential agent for IBS-D.
Keywords: Atractylodes japonica, Diarrhea, Gastrointestinal motility, Guinea pigs, Irritable bowel syndrome
Introduction
Atractylodes japonica, a perennial plant native to North Asia, has commonly been used as a remedy for digestive disorders, particularly diarrhea. In preliminary animal experiments, an extract of A. japonica rhizome (SKI3246) significantly reduced visceral pain in visceral hypersensitivity rats model using trinitrobenzene sulfonic acid (TNBS) or acetic acid.1 The treatment also ameliorated castor oil-induced diarrhea in mice (unpublished data). These findings suggest that SKI3246 may potentially provide some relief for irritable bowel syndrome (IBS), especially when diarrhea is a principal component. To further explore this possibility, we assessed the effects of an A. japonica rhizome extract on gastrointestinal (GI) motility in guinea pigs, using in vitro and in vivo models.
Materials and Methods
Preparation of Animals
Adult male Hartley guinea pigs (250–350 g; Orient Bio, Inc, Seoul, Korea) were acclimated to their holding room (temperature controlled at 21 ± 1°C, 50 ± 10% humidity and 12-hour light/dark cycle). A standard guinea pig diet (7006; Teklad Guinea Pig Diet, Harlan Laboratories, Madison, WI, USA) and drinking water were provided ad libitum. Guinea pigs were deprived of food overnight before the experiment, but were allowed free access to water. Five to seven guinea pigs were used in each experimental group. All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals provided by the Animal Laboratory Ethics Committees of the Department of Laboratory Animal Medicine, Medical Research Center, Yonsei University College of Medicine.
Drugs and Chemicals
The following drugs and chemicals were used: charcoal (Sigma, St. Louis, MO, USA), mustard oil (MO) (Sigma), thyrotropin-releasing hormone (TRH) (Sigma), and extract of A. japonica rhizome, designated as SKI3246, from a pharmaceutical company (SKC, Seoul, Korea).1 Charcoal, MO, and SKI3246 were administrated orally with charcoal as the binding agent. The TRH was mixed in saline and injected subcutaneously at a total dose of 100 μg. Doses of drugs and chemicals used were based on the results of preliminary experiments as well as on previously published data.2,3
Experimental Design
Effect of SKI3246 on colonic transit time
Guinea pigs were killed by a blow to the occipital region of the head and severing the carotid arteries. A 10-cm segment of colon was removed and the lumen was flushed clean with Krebs-Henseleit (K-H) solution (pH 7.4; 118 mM NaCl, 4.8 mM KCl, 2.5 mM CaCl2, 1.2 mM KH2PO4, 1.5 mM MgSO4, 25 mM NaHCO3, 11 mM glucose). The colonic preparations were set up immediately as described below and allowed to equilibrate for at least 60 minutes before experiments began. The preparation was secured horizontally in a peristaltic bath containing K-H solution, which was maintained at 37°C in 95% O2 and 5% CO2. Oral and anal ends of the colonic preparation were cannulated to the inflow and outflow tubes, respectively.4,5
After the artificial feces (pellet) (12 mm × 4 mm) was inserted at the oral side of the isolated colon, and the oral and anal ends were tied securely to the respective tubes, K-H solution was pumped (0.25 mL/min) into the lumen of the preparation using a peristaltic pump (Masterflex 7523-30 with cartridge 3519-85; Cole-Palmer, Chicago, IL, USA) to induce peristalsis. The time it took the pellet to shift a distance of 2 cm was measured. Colonic transit time was assessed as the mean of pellet transit times through each of 4 consecutive colonic segments. The time required to move the first 2-cm distance was excluded from the analysis. After perfusion of control solution for periods of 60 minutes, the SKI3246 (50, 75, and 100 mg/mL) was applied to the bathing solution.
Effect of SKI3246 on upper gastrointestinal conditions induced by thyrotropin-releasing hormone or mustard oil
GI transit was measured using the charcoal transit assay. The charcoal mixture consisted of charcoal, barium and normal saline in a 1:2:6 mixture. The guinea pigs were fasted for more than 8 hours before the experiment. The TRH (100 μg/kg) was injected subcutaneously, and MO (10 mg/kg) was administered orally. After 30 minutes, SKI3246 (150, 300, and 600 mg/kg) was administered using an orogastric cannula. For the assay in the naive condition, SKI3246 was administered to the guinea pigs without pretreatment. After another 30 minutes, the guinea pigs received the charcoal mixture by intragastric administration. Upper GI transit was evaluated as the distance (cm) from the pylorus to the most distal point of migration of the charcoal mixture and expressed as a percentage (%) of the total distance traveled by the charcoal mix through the length of the small intestine (cm). The distance moved through the small intestine represents both gastric transit and small bowel transit. In this context, small intestine transit is used interchangeably with upper GI transit.6
Effect of SKI3246 on normal lower gastrointestinal motility and on motility following treatment with thyrotropin-releasing hormone or mustard oil
In the fecal pellet output assay, TRH (100 μg/kg) was injected subcutaneously or MO (10 mg/kg) was administered through an orogastric cannula. After 30 minutes, SKI3246 (150, 300, and 600 mg/kg) was administered through the cannula. SKI3246 (150, 300, and 600 mg/kg) was also administered to a group of guinea pigs without pretreatment. Each guinea pig was placed into an individual experimental cage, and both the weight and number of fecal pellets produced were measured and recorded at 1-hour intervals for the first 4 hours. As a result, a 4-hour cumulative fecal pellet output was measured for each guinea pig.
Statistical Methods
Non-parametric methods were used in all statistical analyses, since the number of experimental subjects per group was less than 10. The Wilcoxon signed rank test was used to analyze colonic transit time and the Mann-Whitney test was used in the upper GI transit analysis. Results of the fecal expulsion study were analyzed by the repeated measures ANOVA. If the spherocity assumption was not fulfilled on Mauchly’s test, then the Greenhouse-Geisser correction method was used. Statistical significance was determined at the 5% level. All data were analyzed using SPSS version 18.0 (SPSS Inc, Chicago, IL, USA).
Results
Effect of SKI3246 on Colonic Transit Time
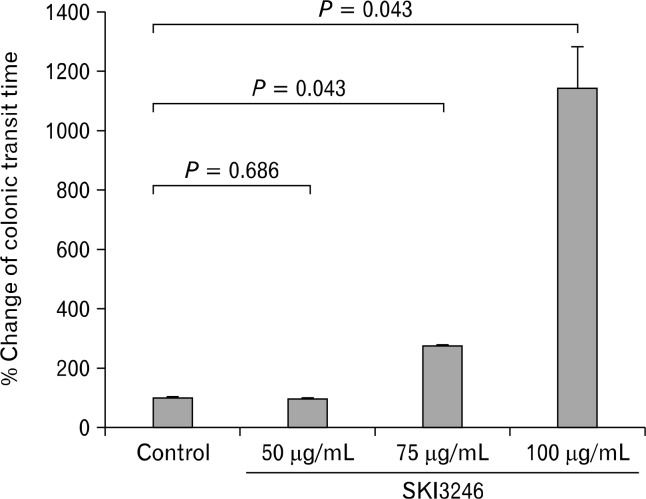
In comparison to control treatment, SKI3246 at the lowest concentration used (50 μg/mL), had no effect on colonic transit time. However, the mean colonic transit time was significantly increased at higher SKI3246 concentrations (2.8 times at 75 μg/mL and 11.4 times at 100 μg/mL, both groups P = 0.043), and the effect showed a dose-dependent tendency (Fig. 1).
Figure 1.
Effect of SKI3246 on colonic transit time in isolated colonic segments from guinea pigs (n = 5). The colonic transit time was significantly increased at higher concentrations of SKI3246 compared with the control group (75 μg/mL and 100 μg/mL, both groups P = 0.043) and the effect was dose-dependent (Wilcoxon signed rank test).
Effects of SKI3246 on Normal Upper Gastrointestinal Transit and on Transit Conditions Induced by Thyrotropin-releasing Hormone or Mustard Oil
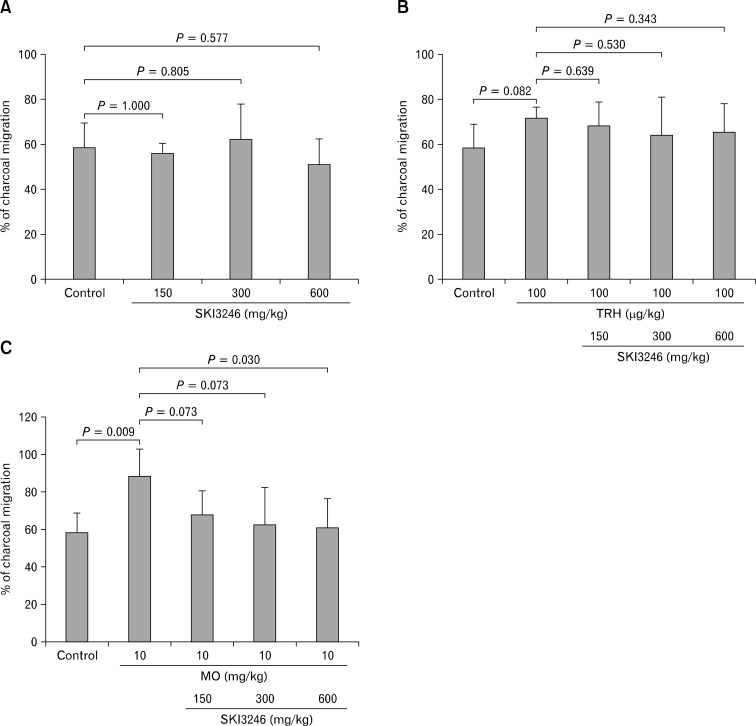
In guinea pigs receiving no pretreatment, SKI3246 had no effect on charcoal transit compared with the control group (150, 300, and 600 mg/kg, respectively) (Fig. 2A).
Figure 2.
Effect of SKI3246 on upper gastrointestinal (GI) transit time of guinea pigs. (A) Effect of SKI3246 on upper GI transit time of guinea pigs in the native condition (n = 6). SKI3246 had no effect on charcoal transit time at 3 dose levels as compared with the control group by Mann-Whitney test. (B) Effect of SKI3246 on upper GI transit time in TRH-pretreated guinea pigs (control groups, n = 6; TRH pretreatment control groups, n = 5; SKI3246 treated groups, n = 7). Guinea pigs pretreated with TRH (100 μg/kg) showed no difference in charcoal transit compared with the control group. As compared with the control group, SKI3246 showed no effect on charcoal transit at 3 different dose levels among groups pretreated with TRH. (C) Effect of SKI3246 on upper GI transit in mustard oil (MO)-pretreated guinea pigs (control groups, n = 6; MO pretreatment control groups, n = 5; SKI3246 treated groups, n = 7). Groups pretreated with MO (10 mg/kg) displayed significantly elongated charcoal transit compared with the control group (P = 0.009). Among MO-pretreated groups, SKI3246 had no effect on charcoal transit at lower doses compared with control group. At a higher dose (600 mg/kg), however, SKI3246 significantly shortened the charcoal transit (P = 0.030). The error bars indicate standard deviation.
In comparison to the control group, the groups treated with TRH (100 μg/kg) showed no difference in charcoal transit (Fig. 2B). Moreover, SKI3246 had no effect on charcoal transit at any of 3 dose levels compared with control groups (150, 300, and 600 mg/kg, respectively) among TRH-pretreated groups.
In comparison to the control groups, groups treated with MO (10 mg/kg) displayed significantly elongated charcoal transit (% of charcoal migration: 58.40 ± 10.84 vs 88.32 ± 15.26, respectively; P = 0.009) (Fig. 2C). Among MO-pretreated groups, SKI3246 had no effect on charcoal transit at lower doses (150 mg/kg and 300 mg/kg). At a higher dose (600 mg/kg), however, SKI3246 significantly shortened the charcoal transit (% of charcoal migraion: 88.32 ± 15.26 vs 60.82 ± 15.62, respectively; P = 0.030) (Fig. 2C).
Effect of SKI3246 on Lower Gastrointestinal Motility in the Native Condition and Conditions Induced by Thyrotropin-releasing Hormone or Mustard Oil
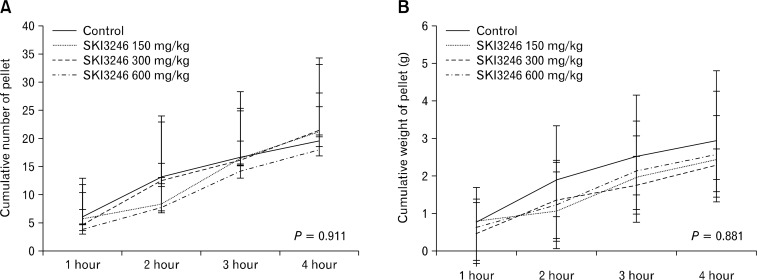
In the fecal expulsion test of guinea pigs in the native condition, cumulative number and weight of egested pellets did not differ among 4 groups, including the control group and groups treated with SKI3246 at 3 dose levels (150, 300, and 600 mg/kg) (Fig. 3).
Figure 3.
Effect of SKI3246 on lower gastrointestinal motility in the native guinea pigs. (A) Effect of SKI3246 on the cumulative number of egested pellets in guinea pigs in the native condition (control group, n = 7; treatment groups, n = 6). The cumulative number of egested pellets did not differ among the four groups, including a control group and groups treated with SKI3246 at 150, 300, and 600 mg/kg by the repeated measures ANOVA test or Greenhouse-Geisser correction method. There was no group effect (F = 0.054, P = 0.983), whereas time effect was present (F = 42.87, P < 0.001). (B) Effect of SKI3246 on cumulative weight of egested pellets in guinea pigs in the native condition (control group, n = 7; treatment groups, n = 6). The cumulative weight of egested pellets did not differ among the four groups, including a control group and groups treated with SKI3246 at 150, 300, and 600 mg/kg by the repeated measures ANOVA test or Greenhouse-Geisser correction method. There was no group effect (F = 0.235, P = 0.639), whereas time effect was present (F = 30.41, P < 0.001). The error bars indicate standard deviation.
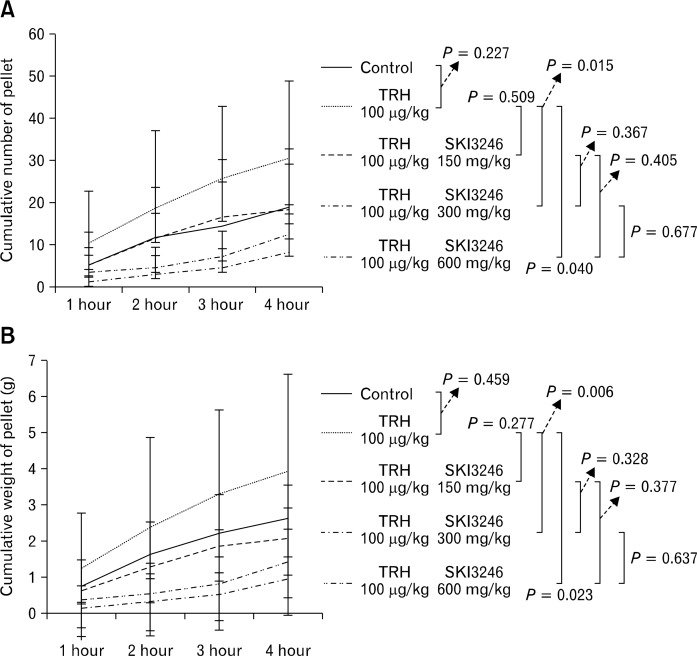
Cumulative number and weight of egested pellets did not differ between the control group and groups pretreated with TRH (100 μg/kg) (Fig. 4). In the fecal expulsion test of guinea pigs pretreated with TRH, cumulative number and weight of egested pellets did not differ between the control group and groups treated with low dose SKI3246 (150 mg/kg) (Fig. 4). However, the cumulative number and weight of egested pellets were significantly higher in groups that received higher doses of SKI3246 compared with the control group (P = 0.015 and P = 0.006, respectively at 300 mg/kg; P = 0.040 and P = 0.023, respectively at 600 mg/kg) (Fig. 4).
Figure 4.
Effect of SKI3246 on lower gastrointestinal motility in thyrotropin-releasing hormone (TRH) pretreated guinea pigs. (A) Effect of SKI3246 on the cumulative number of egested pellets in guinea pigs pretreated with TRH (control group, n = 7; treatment groups, n = 6). Among TRH-pretreated animals, the cumulative number of egested pellets did not differ between the control group and the group treated with the lowest dose of SKI3246 (150 mg/kg). However, the cumulative number of egested pellets in the groups that received higher doses of SKI3246 was significantly higher than that of the control group (300 mg/kg, P = 0.015 and 600 mg/kg, P = 0.040) by the repeated measures ANOVA test or Greenhouse-Geisser correction method. The group and time differences between subgroups are as follows: (1) Control vs TRH: group and time effect (F = 1.33, P = 0.274 and F = 27.59, P < 0.001), (2) TRH vs TRH + SKI3246 150 mg: group and time effect (F = 1.10, P = 0.319 and F = 15.24, P = 0.001), (3) TRH vs TRH + SKI3246 300 mg: group and time effect (F = 6.17, P = 0.032 and F = 21.96, P < 0.001), (4) TRH vs TRH + SKI3246 600mg: Group and time effect (F=4.27, P=0.066 and F=24.67, P < 0.001), (5) TRH + SKI3246 150 mg vs TRH + SKI3246 300 mg: group and time effect (F = 3.87, P = 0.077 and F = 6.43, P = 0.002), (6) TRH + SKI3246 150 mg vs TRH + SKI3246 600 mg: group and time effect (F = 1.75, P = 0.215 and F = 7.66, P = 0.011), (7) TRH + SKI3246 300 mg vs TRH + SKI3246 600 mg: group and time effect (F = 1.17, P = 0.305 and F = 11.75, P = 0.002). (B) Effect of SKI3246 on the cumulative weight of egested pellets in guinea pigs pretreated with TRH (control group, n = 7; treatment groups, n = 6). Among TRH-pretreated groups, the cumulative weight of egested pellets did not differ between the control group and the group given the lowest dose of SKI3246 (150 mg/kg). However, as compared to control values, the cumulative weight of egested pellets was significantly higher in groups that received SKI3246 at higher doses (300 mg/kg, P = 0.006; and 600 mg/kg, P = 0.023) by the repeated measures ANOVA test or Greenhouse-Geisser correction method. The group and time differences between subgroups are as follows: (1) Control vs TRH: group and time effect (F = 0.78, P = 0.397 and F = 27.80, P < 0.001), (2) TRH vs TRH + SKI3246 150 mg: group and time effect (F = 1.56, P = 0.240 and F = 18.12, P < 0.001), (3) TRH vs TRH + SKI3246 300 mg: group and time effect (F = 5.82, P = 0.037 and F = 22.71, P < 0.001), (4) TRH vs TRH + SKI3246 600 mg: group and time effect (F = 4.30, P = 0.065 and F = 24.37, P < 0.001), (5) TRH + SKI3246 150 mg vs TRH + SKI3246 300 mg: group and time effect (F = 4.15, P = 0.069 and F = 7.86, P = 0.007), (6) TRH + SKI3246 150 mg vs TRH + SKI3246 600 mg: group and time effect (F = 1.86, P = 0.203 and F = 9.13, P < 0.001), (7) TRH + SKI3246 300 mg vs TRH + SKI3246 600 mg: group and time effect (F= 0.847, P = 0.379 and F=14.15, P < 0.001). The error bars indicate standard deviation.
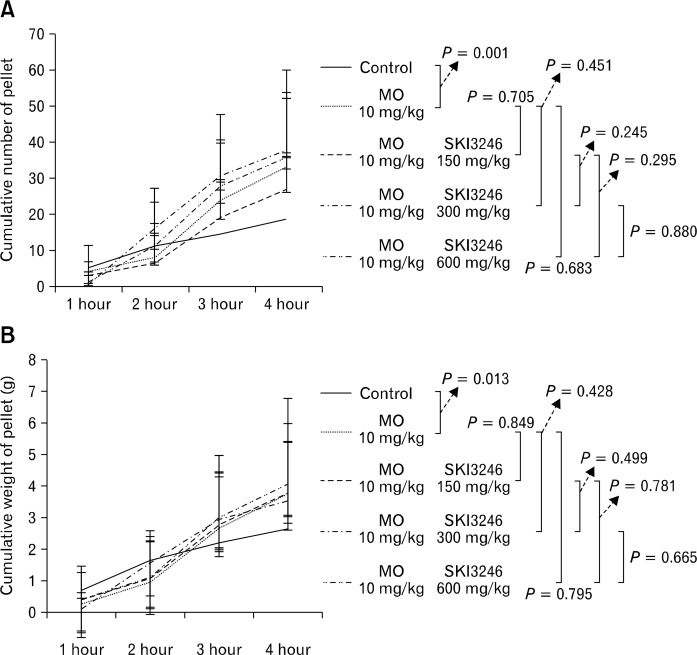
Cumulative number and weight of egested pellets were significantly higher in MO-treated groups than in control groups (P = 0.001 and P = 0.013, respectively; Fig. 5). Among groups pretreated with MO, cumulative number and weight of egested pellets did not differ between the control group and groups pre-treated with MO at three different doses of SKI3246 (Fig. 5).
Figure 5.
Effect of SKI3246 on lower gastrointestinal motility in mustard oil (MO) pretreated guinea pigs. (A) Effect of SKI3246 on the cumulative number of egested pellets in guinea pigs pretreated with MO (control group, n = 7; treatment groups, n = 6). The cumulative number of egested pellets was significantly higher in the MO-pretreated groups than in the control group (P = 0.001). Among groups pretreated with MO, the cumulative number of egested pellets did not differ from the control value or from MO-pretreated groups given any dose of SKI3246 (P = 0.705, P = 0.451, and P = 683, at 150, 300, and 600 mg/kg, respectively, using repeated measures ANOVA test or Greenhouse-Geisser correction method). The group and time differences between subgroups are as follows: (1) Control vs MO: group and time effect (F = 0.65, P = 0.436 and F = 31.70, P < 0.001), (2) MO vs MO + SKI3246 150 mg: group and time effect (F = 0.39, P = 0.545 and F = 50.34, P < 0.001), (3) MO vs MO + SKI3246 300 mg: group and time effect (F = 0.38, P = 0.551 and F = 38.93, P < 0.001), (4) MO vs MO + SKI3246 600 mg: group and time effect (F = 0.10, P = 0.761 and F = 41.94, P < 0.001), (5) MO + SKI3246 150 mg vs MO + SKI3246 300 mg: group and time effect (F = 1.68, P = 0.224 and F = 40.50, P < 0.001), (6) MO + SKI3246 150 mg vs MO + SKI3246 600 mg: group and time effect (F = 1.25, P = 0.290 and F = 44.69, P < 0.001), (7) MO + SKI3246 300 mg vs MO + SKI3246 600 mg: group and time effect (F = 0.13, P = 0.729 and F = 35.98, P < 0.001). (B) Effect of SKI3246 on the cumulative weight of egested pellets in guinea pigs pretreated with MO (control group, n = 7; treatment groups, n = 6). The cumulative weight of egested pellets was significantly higher in the MO-pretreated groups than in the control group (P = 0.013). Among groups pretreated with MO, the cumulative weight of egested pellets did not differ significantly from control values and from values for groups receiving any dose of SKI3246 by the repeated measures ANOVA or Greenhouse-Geisser correction method. The group and time differences between subgroups are as follows: (1) Control vs MO: group and time effect (F = 0.26, P = 0.623 and F = 25.94, P < 0.001), (2) MO vs MO + SKI3246 150 mg: group and time effect (F = 0.12, P = 0.740 and F = 42.81, P < 0.001), (3) MO vs MO + SKI3246 300 mg: group and time effect (F = 0.06, P = 0.817 and F = 34.01, P < 0.001), (4) MO vs MO + SKI3246 600 mg: group and time effect (F = 0.04, P = 0.849 and F = 32.30, P < 0.001), (5) MO + SKI3246 150 mg vs MO + SKI3246 300 mg: group and time effect (F = 0.02, P = 0.895 and F = 60.93, P < 0.001), (6) MO + SKI3246 150 mg vs MO + SKI3246 600 mg: group and time effect (F = 0.04, P = 0.839 and F = 46.29, P < 0.001), (7) MO + SKI3246 300 mg vs MO + SKI3246 600 mg: group and time effect (F = 0.01, P = 0.950 and F = 35.97, P < 0.001). The error bars indicate standard deviation.
Discussion
In this study, we demonstrated that the extract of A. japonica rhizome, SKI3246, had no significant effect on the upper GI tract in the guinea pig either before or after exposure to TRH and MO. In the in vitro system, however, the extract induced an increase in colonic transit time, and in the in vivo study, SKI3246 affected lower GI tract activity, significantly decreasing the weight and number of fecal pellets following treatment with TRH.
Two models of altered gut functions were used for this study, one based on the action of TRH and the other on the irritant properties of mustard oil. MO, allyl isothiocyanate, the predominant aromatic constituent of mustard, horseradish, and wasabi, is a direct stimulant of small nerve fibers and a potent acute inflammatory irritant.7 Although the GI response to MO in guinea pig is not well-documented, the intra-colonic administration of MO in mice results in acute colitis, which may progress to an IBS-like acceleration of upper GI transit.7 In our guinea pig model, MO, significant elongated upper GI transit and increased the number and weight of fecal pellets. On the whole, SKI3246 did not affect either upper or lower GI transit in the MO-induced model, although at the highest dose it slowed upper GI transit. Although the pharmacological action of SKI3246 is not completely defined, this complex preparation has been shown its action through the neurokinin (NK) receptor antagonism, especially NK2 receptor.1 Meanwhile, it has been known that MO displays proinflammatory activity mainly via inducing plasma extravasation, which is principally due to the release of substance P and to NK1 receptor activation.7–10 These differences in mechanism between the 2 agents may explain in part the lack of GI motility response to SKI3246 in the MO-induced model in this study.
In the in vitro experiments, SKI3246 significantly increased colonic transit time, in contrast to its effect in the intact guinea pig. As measured by the fecal expulsion test, SKI3246 did not affect GI motility in vivo at any dose level applied. These somewhat discrepant results might be due to physiologic counterbalance mechanism in in vivo condition, that might be impaired in in vitro isolated colonic segment unit. Moreover, although the control of intrinsic nervous system and smooth muscle response to the exogenous drug challenge are intact, control of extrinsic nervous system and GI hormonal regulation may be lacking in in vitro conditions.
TRH, an agent of the hypothalamic-pituitary axis, participates in central regulation of peripheral autonomic function. Administered centrally or peripherally, TRH may influence GI motility. Centrally administered TRH stimulates gastric motility in rats,11 small intestinal transit in rats,12 and colonic activity in rabbits.13 Administered intravenously, TRH stimulates gastric action potentials in dogs;14 and subcutaneously injected TRH may increase fecal pellet output in rats.15 The GI response to TRH administered subcutaneously in the guinea pig has not been studied in detail; however, one in vitro study showed that TRH stimulates motility of the gastric antrum, small intestine and taenia coli of the guinea pig.16 In our study, TRH increased upper GI transit and the number and weight of fecal pellet expulsion, although the effect did not reach statistical significance. In the altered guinea pig model induced by TRH, SKI3246 significantly decreased number and weight of fecal pellets at 300 mg/kg and 600 mg/kg doses; however, it did not affect upper GI transit in this model. With respect to the mechanism by which TRH may affect bowel function, it is reported that TRH acts centrally to induce cholinergic and serotonergic vagal stimulation of GI secretory and motor function.15 The effect of TRH also depends on endogenous 5-hydroxytryptamine (serotonin or 5-HT) release from the enterocromaffin cell and/or enteric serotonergic neurons in the intestine. Thus, the effect of SKI3246 on GI motility may also plausibly be related to blockade of cholinergic or serotonergic response, apart from the NK2 pathway.
In the in vitro assay, SKI3246 increased colonic transit time with a dose-dependent tendency. In contrast, its effect on fecal expulsion in the intact animal was not a dose-dependent manner, showing no difference between SKI3246 treatments at 300 mg/kg and 600 mg/kg. This finding implies that at least the dose of 300 mg/kg might be needed for substantial effect on altered lower GI condition by TRH. With respect to upper GI motility in the guinea pig, SKI3246 showed no effect in either the native or TRH-induced condition. At a high dose (600 mg/kg), however, SKI3246 inhibited transit in the upper GI tract pretreated by MO. These findings suggest that optimal dose determination will be an important consideration in future clinical trials of SKI3246, since our data imply that upper gastrointestinal side effects of SKI3246, including nausea or vomiting can occur at higher doses.
The condition induced by TRH in the guinea pig resembles conditions observed clinically, such as diarrhea or IBS with diarrhea, and SKI3246 significantly decreased bowel movement which was measured by the weight and number of fecal pellets. In addition to the effect on bowel motility, SKI3246 significantly reduces visceral pain in rats.1 The capability of SKI3246 to influence both bowel movement and visceral pain supports further investigation of SKI3246 as a treatment for IBS with diarrhea presenting with both pain and diarrhea.
In conclusion, the extract of A. japonica rhizome SKI3246 showed no significant effect on upper GI tract motility in guinea pigs, in either the native or altered conditions induced by MO or TRH. In the in vitro model, however, SKI3246 significantly increased colonic transit time. Effects of SKI3246 in vivo were largely confined to the lower GI tract, significantly decreasing the weight and number of fecal pellets in animals with induced diarrhea. These findings justify further investigation of SKI3246 for use in treating IBS with diarrhea as a prominent component. Moreover, further investigation of the pharmacologic action of SKI3246 on GI motility is also warranted.
Footnotes
Financial support: This study was supported by a research grant from SK Chemical Company, Ltd.
Conflicts of interest: None.
Author contributions: Jae Jun Park, acquisition of data, analysis and interpretation of data, drafting of manuscript, and critical revision; Nu Ri Chon, acquisition of data; Young Ju Lee, acquisition of data, analysis and interpretation of data, and critical revision; Hyojin Park, study conception and design, drafting of manuscript, and critical revision.
References
- 1.Son HJ, Jung K, Park YH, et al. Inhibitory effects of SKI3246, the rhizome extract of Atractylodes japonica, on visceral hypersensitivity in experimental irritable bowel syndrome rat models. Arch Pharm Res. 2015;38:642–649. doi: 10.1007/s12272-014-0454-x. [DOI] [PubMed] [Google Scholar]
- 2.Kojima R, Doihara H, Nozawa K, Kawabata-Shoda E, Yokoyama T, Ito H. Characterization of two models of drug-induced constipation in mice and evaluation of mustard oil in these models. Pharmacology. 2009;84:227–233. doi: 10.1159/000236524. [DOI] [PubMed] [Google Scholar]
- 3.Park YM, Lee YJ, Lee YH, Kim TI, Park H. Effects of ramosetron on gastrointestinal transit of Guinea pig. J Neurogastroenterol Motil. 2013;19:36–41. doi: 10.5056/jnm.2013.19.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waterman SA, Tonini M, Costa M. The role of ascending excitatory and descending inhibitory pathways in peristalsis in the isolated guinea-pig small intestine. J Physiol. 1994;481(Pt 1):223–232. doi: 10.1113/jphysiol.1994.sp020433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ji SW, Park H, Chung JP, Lee SI, Lee YH. Effects of tegaserod on ileal peristalsis of guinea pig in vitro. J Pharmacol Sci. 2004;94:144–152. doi: 10.1254/jphs.94.144. [DOI] [PubMed] [Google Scholar]
- 6.Kimball ES, Wallace NH, Schneider CR, D’Andrea MR, Hornby PJ. Small intestinal cannabinoid receptor changes following a single colonic insult with oil of mustard in mice. Front Pharmacol. 2010;1:132. doi: 10.3389/fphar.2010.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kimball ES, Palmer JM, D’Andrea MR, Hornby PJ, Wade PR. Acute colitis induction by oil of mustard results in later development of an IBS-like accelerated upper GI transit in mice. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1266–G1273. doi: 10.1152/ajpgi.00444.2004. [DOI] [PubMed] [Google Scholar]
- 8.Amann R, Egger T, Schuligoi R. The tachykinin NK1 receptor antagonist SR140333 prevents the increase of nerve growth factor in rat paw skin induced by substance P or neurogenic inflammation. Neuroscience. 2000;100:611–615. doi: 10.1016/S0306-4522(00)00315-8. [DOI] [PubMed] [Google Scholar]
- 9.Inoue H, Asaka T, Nagata N, Koshihara Y. Mechanism of mustard oil-induced skin inflammation in mice. Eur J Pharmacol. 1997;333:231–240. doi: 10.1016/S0014-2999(97)01040-6. [DOI] [PubMed] [Google Scholar]
- 10.Louis SM, Jamieson A, Russell NJ, Dockray GJ. The role of substance P and calcitonin gene-related peptide in neurogenic plasma extravasation and vasodilatation in the rat. Neuroscience. 1989;32:581–586. doi: 10.1016/0306-4522(89)90281-9. [DOI] [PubMed] [Google Scholar]
- 11.Bond EF, Heitkemper MM, Gruver MK. Mediation of thyrotropin-releasing hormone induced gastric motility increases in developing rats. Eur J Pharmacol. 1992;217:127–135. doi: 10.1016/0014-2999(92)90831-N. [DOI] [PubMed] [Google Scholar]
- 12.Bond E, Heitkemper M. Thyrotropin-releasing hormone stimulates intestinal transit in young rats. Regul Pept. 1990;27:263–271. doi: 10.1016/0167-0115(90)90045-X. [DOI] [PubMed] [Google Scholar]
- 13.Smith JR, La Hann TR, Chesnut RM, Carino MA, Horita A. Thyrotropin-releasing hormone: stimulation of colonic activity following intracerebroventricular administration. Science. 1977;196:660–662. doi: 10.1126/science.404705. [DOI] [PubMed] [Google Scholar]
- 14.Morley JE, Steinbach JH, Feldman EJ, Solomon TE. The effects of thyrotropin releasing hormone (TRH) on the gastrointestinal tract. Life Sci. 1979;24:1059–1065. doi: 10.1016/0024-3205(79)90038-9. [DOI] [PubMed] [Google Scholar]
- 15.Miyata K, Kamato T, Nishida A, et al. Role of the serotonin3 receptor in stress-induced defecation. J Pharmacol Exp Ther. 1992;261:297–303. [PubMed] [Google Scholar]
- 16.Oouchi M, Ichikawa S. Thyrotropin-releasing hormone effects on guinea pig antrum. Gastroenterology. 1985;88(5 Pt 1):1126–1131. doi: 10.1016/s0016-5085(85)80070-6. [DOI] [PubMed] [Google Scholar]