Abstract
Background/Aims
Several disorders of the gastrointestinal tract are associated with abnormal serotonin (5-HT) signaling or metabolism where the 5-HT3 and 5-HT4 receptors are clinically relevant. The aim was to examine the distribution of 5-HT3, 5-HT4, and 5-HT7 receptors in the normal human colon and how this is associated with receptor interacting chaperone 3, G protein coupled receptor kinases, and protein LIN-7 homologs to extend previous observations limited to the sigmoid colon or the upper intestine.
Methods
Samples from ascending, transverse, descending, and sigmoid human colon were dissected into 3 separate layers (mucosa, longitudinal, and circular muscles) and ileum samples were dissected into mucosa and muscle layers (n = 20). Complementary DNA was synthesized by reverse transcription from extracted RNA and expression was determined by quantitative or end point polymerase chain reaction.
Results
The 5-HT3 receptor subunits were found in all tissues throughout the colon and ileum. The A subunit was detected in all samples and the C subunit was expressed at similar levels while the B subunit was expressed at lower levels and less frequently. The 5-HT3 receptor E subunit was mainly found in the mucosa layers. All splice variants of the 5-HT4 and 5-HT7 receptors were expressed throughout the colon although the 5-HT4 receptor d, g, and i variants were expressed less often.
Conclusions
The major differences in 5-HT receptor distribution within the human colon are in relation to the mucosa and muscular tissue layers where the 5-HT3 receptor E subunit is predominantly found in the mucosal layer which may be of therapeutic relevance.
Keywords: Colon; G protein coupled receptor kinases; Ileum; Receptors, serotonin
Introduction
Most of the body serotonin stores are found in the gastrointestinal tract, where abnormalities in serotonin release, transport and metabolism are associated with dyspepsia, nausea and vomiting, coeliac disease, inflammatory bowel disease, and irritable bowel syndrome (IBS).1–5 Inflammation contributes to these disorders due to underlying inflammatory states in coeliac disease and inflammatory bowel disease and in part because it modulates serotonin levels.1,4,6
The serotonin (5-HT) 4 receptor is expressed in several different cell types in the human colon7–16 where it stimulates intestinal activity (ie, prokinetic action) and is a target for treating constipation predominant IBS (IBS-C) and chronic constipation. The 5-HT4 receptor partial agonist, tegaserod was used for treatment of IBS-C until withdrawn in 2007 as it was associated with rare adverse cardiovascular effects.1 Currently, the high affinity 5-HT4 receptor agonist, prucalopride is used to treat chronic or idiopathic constipation refractory to at least two other classes of laxatives in the European Union, Canada, and Australia.17 Intestinal 5-HT7 receptors are often located near 5-HT4 receptors where they augment 5-HT induced responses.11,18
5-HT3 receptor antagonists decrease colonic motility, secretion and nociception and are used to treat diarrhea predominant IBS (IBS-D).1,3 The 5-HT3 receptor is a ligand gated ion channel composed of 5 subunits that form homomeric (all 3A subunits) or heteromeric (mixture of 3A and 3B, 3C, 3D, or 3E subunits) functionally active channels.19–21 All of the human 5-HT3 receptor subunit genes are expressed in the gastrointestinal tract.22–26 Immunohistochemistry studies have pinpointed co-expression of A, B, C, D, and E subunits in enterocytes and also the nerves of the myenteric plexus24,27 indicating that heteromeric 5-HT3 receptors are likely to form in the human colon.
Although 5-HT4 and 5-HT7 receptor splice variants have been identified in separate studies in the small intestine and colon,22,28–30 no study has looked at their tissue distribution along the colon. However, one earlier study showed that the 5-HT3 receptor subunit transcript distribution differed in the sigmoid colon22 providing evidence that specific subunits may be potential targets. Therefore the aim of this study was to investigate the distribution of transcripts of 5-HT receptors and their associated proteins in the ileum and along the length of the colon. Membrane bound receptors are subject to strict processing to be positioned correctly and to regulate their responsiveness following activation. Homologous desensitization involving the G protein coupled receptor (GPCR) kinases (GRKs) down regulates surface expression of 5-HT4 receptors.31–33 Proteins such as protein LIN-7 (LIN7) homolog A to C of Caenorhabditis elegans (also known as Veli1-3 in mammals) are likely to be involved in processing 5-HT4 and 5-HT7 receptors to the plasma membrane.34–37 Receptor interacting chaperone 3 (RIC3) assists cell surface assembly of 5-HT3 receptors.38,39 Therefore, transcripts of RIC3, LIN7 homologs, and GRKs were measured as these proteins contribute to receptor processing. Although subtle differences in distribution of the receptors and associated proteins occurred along the colon, only the 5-HT3 receptor subunits exhibited any major differences in expression between the tissue layers which may be of therapeutic relevance.
Materials and Methods
Human Tissue
Full thickness specimens from different regions of the intestine of 24 patients (11 male and 13 female ranging in age from 50 to 88 years [median 73]) were collected immediately following surgical resection and transported to the laboratory in 4°C Krebs-Henseleit solution. The patients were undergoing surgical resection for colonic cancer and the pathologist indicated that the specimens obtained as far from the tumor as possible appeared to be disease free following gross visual examination. The mucosa and associated mesentery plus fat were removed before the longitudinal muscle bands (taeniae coli) were dissected and the remaining intertaenial tissue was cut into strips in the orientation of the circular smooth muscle. All tissue was stored in RNAlater® (Ambion, Austin Texas, USA) at −80°C. The project was approved by the Human Research Ethics Committee of the hospital and academic institute and all patients gave informed consent prior to surgery.
RNA Extraction
Approximately 18–25 mg excised tissue was homogenized using a PRO 200 homogenizer (PRO Scientific Inc, Oxford, CT, USA) and total RNA was extracted using RNeasy Fibrous kit (Qiagen, Chadstone Center, Victoria, Australia) and Turbo DNA-freeTM Kit (Life Technologies, Mulgrave, Victoria, Australia) was used to degrade any contaminating DNA. RNA was quantified pre and post-DNAse treatment with a Nanodrop 1000 using ND-1000 software (Thermo Scientific, Wilmington, DE, USA). The reverse transcription step was carried out using 1 μg of DNAse-treated RNA, SuperScript®III Reverse Transcriptase (Life Technologies) and oligo dT15 primers in a total reaction volume of 20 μL. Separate negative reverse-transcriptase controls were included for every reaction.
Polymerase Chain Reaction Conditions
In end-point polymerase chain reactions (PCR), the primers described in a prior study22 were used to amplify GRK, 5-HT4, and 5-HT7 receptor genes while the following primers were used to amplify LIN7A-C and glyceraldehyde dehydrogenase (GAPDH) genes. GAPDH (NM_002046.5) forward 5′-ACCACAGTCCATGCCATCAC-3′ (714–734) and reverse 5′-TCCACCACCCTGTTGCTGTA-3′ (1165-1146); LIN7A (NM_004664.2) forward 5′-CAGCTAGTGAAGGCCACTCC-3′ (486–505) and reverse 5′-GCAGCTGGTCTCCTCTTTTG-3′ (659-639); LIN7B (NM_022165.2) forward 5′-CAGCTTTATGACACGCTGGA-3′ (210–229) and reverse 5′-GATGACCCGGGAGATGT-3′ (413-397); and LIN7C (NM_018362.3) forward 5′-AACAGAAGAGGGCCTTGGAT-3′ (516-496) and reverse 5′-GCGGCTTTCAGCAGTTCTAC-3′ (321–340) which will produce products of 451 (for all 4 splice variants), 174, 203, and 195 bp respectively. It should be noted that the LIN7A primer set is within one exon. Amplifications were undertaken in a MyCycler thermal cycler (Bio-Rad Laboratories, Inc, Hercules, CA, USA) and a hot start involving 15 minutes at 94°C was made. To keep within an exponential range during amplification, 20 to 25 cycles were made with denaturation at 94°C, annealing at 55°C, and extension at 72°C all for 1 minute. Products were visualized following separation on agarose gels and staining with GelRedTM nucleic acid stain (Biotium, Hayward, CA, USA).
Quantitative PCR was undertaken using similar amplification conditions as described in a prior study22 except that the 30 μL reactions contained 1 μL cDNA, 0.5 μmol/L forward and reverse primers, 4 mmol/L MgCl2, and 2× SensiMix SYBR Green PCR Master Mix (Bioline, Sydney, NSW, Australia). The cDNA was amplified by 1 cycle at 95°C for 15 minutes followed by 36 cycles of 95°C for 15 seconds (denaturing), 55°C for 20 seconds (annealing), and 72°C for 25 seconds (extension) using a C1000TM Thermal Cycler and CFX96TM Real-Time System (Bio-Rad Laboratories). Melting point analysis indicated that only a single product was produced in each reaction and confirmed by preliminary runs with gel electrophoresis which also established that only one product of the predicted size was produced. Control runs of all PCR experiments contained non-template controls and negative reverse transcriptase controls consisting of RNA samples where reverse transcriptase was not added (such that no cDNA was produced) to test for DNA contamination.
Statistical Methods
Each quantitative PCR sample was analyzed in duplicate and efficiency of reactions was determined using linear regression of the Log (fluorescence) per cycle number data with the LinRegPCR program40 and ranged from 2.008 ± 0.006 (β-actin) to 1.877 ± 0.008 (5-HT3 receptor C subunit [HTR3C]). Expression data was calculated relative to β-actin and GAPDH and expressed as the following ratio: Ratio = (Efficiencyreference)Ct reference/(Efficiencysample)Ct sample, where Ct is the crossing point threshold of the sample for the amplified genes.
Relative expression data was further analyzed after log transformation using one-way ANOVA followed by Tukey’s multiple comparisons test. One-way ANOVA and Tukey’s multiple comparisons were used to analyze the number of patients expressing genes detected using end point PCR. The number of observations used to derive mean values is expressed by n and arithmetic mean values are given as mean ± SEM.
Results
Distribution of 5-HT3 Receptor Subunits in the Human Intestine
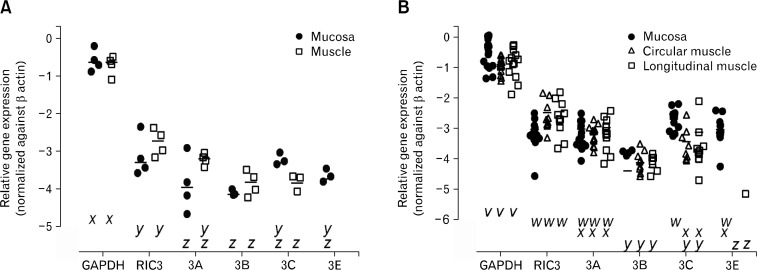
The distribution of 5-HT3 receptor subunits was examined using quantitative reverse transcript PCR on samples obtained from throughout the length of the colon and the ileum area of the small intestine. In all regions, expression was examined in both the mucosal and muscular tissue layers and reported relative to expression of β-actin. GAPDH was used as a second housekeeping gene for comparison with a previous study where the relative expression of the 5-HT3 receptors in the sigmoid colon was reported.22 Control RNA samples incubated without reverse transcriptase and then amplified with GAPDH primers were also undertaken to demonstrate that there was no DNA contamination of the RNA extractions (data not shown). GAPDH levels of expression were consistently and significantly higher than those of the 5-HT3 receptor subunits or RIC3 in both ileum and colon (P < 0.05 one-way ANOVA; Fig. 1) which is consistent with the previous study.22 The 5-HT3 receptor D subunit was consistently not detected in either ileum or colon samples which is in agreement with prior studies where transcripts of HTR3D were only evident at very low levels.23,25 In the ileum, RIC3 and 5-HT3 receptor A subunit transcripts were found in all samples with the other subunits being less prevalent (Fig. 1A). Transcript levels of RIC3 were significantly higher than 5-HT3 receptor B subunits in both mucosa and muscle layers and also the mucosa levels of the A subunit and the C subunit in the muscle layer. No significant differences were observed in the expression levels of the 5-HT3 receptor subunits with the major exception that the E subunit was only found in the mucosa layer (Fig. 1A).
Figure 1.
Distribution of serotonin type 3 (5-HT3) receptor subunits and receptor interacting chaperone 3 (RIC3) transcripts in human intestinal tissue layers. (A) Comparison of the relative expression levels of transcripts of RIC3 and 5-HT3 receptor subunits in the mucosal and muscle tissue layers in the human ileum (n = 4). (B) Comparison of the relative expression levels of transcripts of RIC3 and 5-HT3 receptor subunits in the mucosal and muscle layers (circular and longitudinal) in tissue samples obtained from throughout the human colon (n = 16). Data are expressed as a ratio relative to β-actin as described in the Material and Methods section. Bars indicate the mean. 5-HT3 receptor D subunit (HTR3D) transcripts were not detected in any tissue tested. RIC3 and 5-HT3 receptor subunit transcripts are expressed at significantly lower levels than glyceraldehyde dehydrogenase (GAPDH) transcripts (P < 0.001 one-way ANOVA followed by Tukey’s multiple comparisons test) in all tissues. The letters above the x-axis (v, w, x, y, and z representing the highest to lowest level respectively) indicate that the transcripts are found at significantly different levels (P < 0.05 one-way ANOVA followed by Tukey’s multiple comparisons test) in the ileum or colon tissue layers (ie, transcripts in the different layers with an x underneath are expressed at the same level; x is the highest and z the lowest level in [A] while v is the highest and z the lowest level in [B]).
The colonic tissue was dissected into mucosa, circular and longitudinal muscular layers and a similar distribution of the specific transcripts occurred in the different areas (Supplementary Fig. 1). Therefore, the regional data was pooled to see if there were any differences in gene expression at the tissue layer level (Fig. 1B). Generally a similar distribution pattern of RIC3 and the 5-HT3 receptor subunits in the colonic tissue layers was observed to that seen in the ileum. However some variations were seen in 5-HT3 receptor subunit expression between the different tissue layers (Fig. 1B). The 5-HT3 receptor B subunit was expressed at significantly lower levels than RIC3 or the A subunits in all tissue layers. The mucosal levels of the C subunit were significantly higher than the B subunit in any tissue and the C subunit in the muscular layers. The 5-HT3 receptor E subunit transcripts were nearly always only detected in the mucosa samples at levels significantly greater than the B subunit (Fig. 1B). Only one longitudinal muscle sample showed any E subunit expression (Fig. 1B), so to determine if the 5-HT3 receptor E subunit was expressed in the muscle layers but below the reliable detection levels of the quantitative PCR conditions, the amplified PCR samples were run in gels (Supplementary Fig. 2). Only very low levels of expression were observed in two additional colon mucosa samples and two longitudinal muscle samples as well as an additional ileum muscle sample.
Distribution of 5-HT4 and 5-HT7 Receptors in the Human Intestine
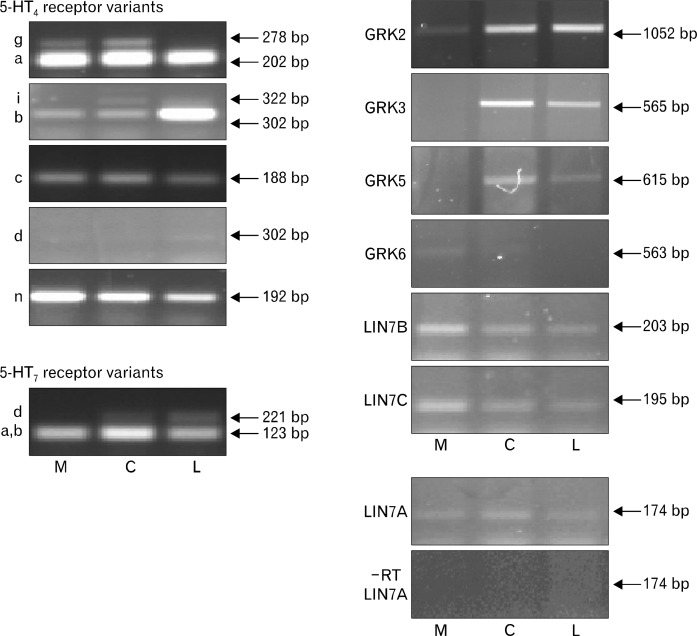
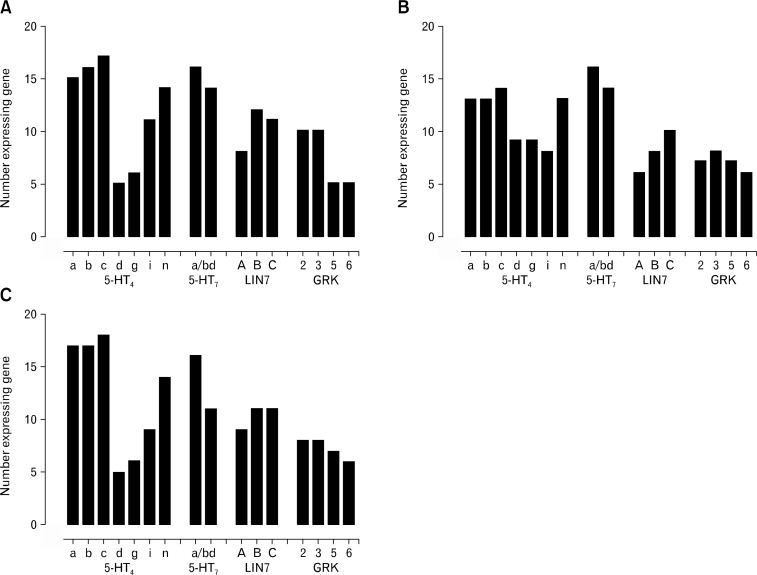
Adjacent tissue samples to those used in the analysis of the 5-HT3 receptor and RIC3 gene expression were used to examine the distribution of 5-HT4 and 5-HT7 receptors. Since several splice variants with overlapping 3’ coding regions exist for the 5-HT4 and 5-HT7 receptors, their distribution was examined using partial or nested PCR. GAPDH expression was used as a control for this analysis as it has previously been shown to be comparable between human colonic tissue layers11 (Fig. 1 and Supplementary Fig. 3). An example of the expression of different transcripts following end point RT-PCR analysis is shown for a descending colon sample (Fig. 2). The samples were obtained from ileum, ascending, transverse, descending and sigmoid colon and overall the distribution of transcripts appeared independent of the region of the colon and similar across the types of tissue layers (Supplementary Table 1). Therefore the number of patients expressing any of the genes was compared with the colon tissue layers (Fig. 3). One-way ANOVA indicates that the only differences are in the expression of the gene transcripts (P < 0.05) and not the tissue layer. For instance, 5-HT4 receptor d and g splice variants are significantly less likely to be detected in mucosa and longitudinal muscle compared to the 4a, b, c, and n splice variants (P < 0.05 one-way ANOVA; Fig. 3). Like the 5-HT4 a, b, c, and n splice variants, the 5-HT7 receptor splice variants were widely expressed in all regions and tissues studied. Some minor differences are evident with respect to GRK distribution. For example, GRK6 is less likely to be expressed in the ileum or ascending and transverse colon compared to the descending and sigmoid colon (Supplementary Table). Both GRK5 and GRK6 are expressed less often in all of the tissue layers compared to the 5-HT7 receptor a/b variants and in the mucosa and longitudinal muscle than the 5-HT4 receptor a, b, c and n variants (P < 0.05, one-way ANOVA; Fig. 3). However, no differences in the frequency of expression were observed between the different GRK or LIN7 genes in the different tissue layers (P > 0.05, one-way ANOVA; Fig. 3).
Figure 2.
Reverse transcriptase polymerase chain reaction (RT-PCR) analysis of expression of protein LIN-7 homologs G protein coupled receptor kinase (GRK), serotonin type 4 (5-HT4) and 5-HT7 receptor gene products in the human descending colon of one patient. The products of predicted sizes are indicated by arrows and the size was correlated to 100 bp (molecular weight) markers run on 1.5% agarose gels stained with GelRed. A negative control is shown for the LIN7A sample as the primers are within one exon. Letters represent samples obtained from M (mucosa), C (circular muscle), and L (longitudinal muscle).
Figure 3.
Comparison of the number of patients expressing transcripts of G protein coupled receptor kinase (GRK), protein LIN-7 (LIN7) homologs, and 5-HT4 or 5-HT7 receptor splice variants in the mucosal (A), circular muscle (B), and longitudinal muscle (C) layers in tissue samples obtained from throughout the human colon (n = 16-18). Data are expressed as the number of patients where transcripts were detected. 5-HT4 receptor d and g splice variants in mucosa and longitudinal muscle, GRK5 and GRK6 (all tissue layers) were detected at significantly lower frequencies than 5-HT7 or 5-HT4 a, b, c, or n receptor splice variants (P < 0.05 one-way ANOVA followed by Tukey’s multiple comparisons test).
Discussion
Despite many similarities in its general functionality, the human intestinal tract exhibits considerable differences to small animal models such as mice, rats, and guinea pigs.41 Serotonin receptors for instance are widespread throughout the gastrointestinal tract in small animal models and humans but the proportion and type of receptors at particular regions are different.41–43 In addition, human 5-HT receptors are considerably different in their composition as evident by the diverse splice variants in 5-HT4 and 5-HT7 receptors and the additional 5-HT3 receptor subunits.1,19,44 The function and pharmacological responses of 5-HT receptors in the intestine also varies between small animal models and humans15,41,45,46 and therefore this study was undertaken to gain an insight to the distribution of 5-HT3, 5-HT4, and 5-HT7 receptors expressed in the human ileum and throughout the human colon. Although subtle differences in the regional distribution of the 5-HT receptors occurred, the main differences were between the mucosal and muscular tissue layers.
Quantitative and end point PCR were used to assess the distribution of 5-HT3, 5-HT4, and 5-HT7 receptors using samples obtained from surgical resection that appeared to be disease free following gross visual examination. Tissue samples were stored in RNAlater® at −80°C until use. Only samples that contained readily detectable levels of GAPDH (and β-actin for quantitative PCR) and were not contaminated by genomic DNA (no bands detected in the negative reverse transcript assays) were processed further to exclude the probability of undetectable expression due to storage-related RNA degradation (Supplementary Fig. 3). The robustness of the quantitative PCR was ensured by adhering to the MIQE guidelines47 and samples were run on gels to ensure that only the expected products were generated (Supplementary Fig. 2). The end point PCR studies were designed to ensure that the products were still within the linear amplification range. Where expression levels were low (eg, 5-HT4 receptor splice variant d; Fig. 2), independent observers confirmed the presence of detectable bands in the gels.
The distribution of 5-HT3 receptor A subunits is in keeping with previous studies where this subunit was widespread and thought to reflect the distribution of functional 5-HT3 receptor subunits.11,22,23,25,48 The HTR3B gene which encodes for the canonical 5-HT3 receptor B subunit was expressed at lower levels than HTR3A in all regions of colon but at similar levels in the ileum (Fig. 1). The actual occurrence of HTR3B in human colon and ileum (Supplementary Fig. 1) was almost 50% less than the total occurrences of HTR3A and agrees with previous findings where lower levels but no distinct patterns of expression were observed.23,25,27 Interestingly, the expression and occurrence of the 5-HT3 receptor C subunit was similar to HTR3A while HTR3E was predominantly restricted to the mucosa confirming prior observations in the colon, small intestine and stomach.22,23,25,26,38,49 The mucosa plays a role in fluid transport, while the muscular layers are involved with motility so it is possible that physiological alterations in the different colon tissue layers may result in selective colon disease or dysfunction. Immunoreactants to both 5-HT3 receptor C and E subunits have been identified as being co-expressed within the human colon (enterocytes, myenteric plexus and muscular layer).24 Together these findings are suggestive that associations of 5-HT3 receptor A subunits occur with C and/or E subunits in the gastrointestinal tissues. In fact, genetic studies have revealed associations of HTR3C and HTR3E with several clinical conditions.50–53 Interestingly, a single nucleotide polymorphism in the 5-HT3 receptor E subunit that is associated with female IBS-D where the 5-HT3 receptor subunit is up-regulated by microRNA (miR-510) co-expressed with the E subunit in gut epithelium enterocytes.51
The 5-HT4 receptor splice variants have different preferences to ligands as shown by different potency and binding affinities for various 5-HT4 receptor agonists44,54–57 and their distribution may influence drug responses. 5-HT4 receptor transcripts have been identified in human mucosa biopsies obtained from the duodenum, ileum and colon and this mucosal expression contributes to luminal responses in model animal systems.10 There are no significant patterns in the expression of 5-HT4 or 5-HT7 receptors although the number of patients expressing the 5-HT4 receptor d and g splice variants is consistently lower (Fig. 3). The 5-HT4 receptor d splice variant in particular has been shown to have low expression levels before in different parts of the body such as the central nervous system.30 It would be of interest to learn if changes in distribution of 5-HT4 receptor splice variants occur in gastrointestinal disorders as the 5-HT4 receptor a and b splice variants are down regulated while the d variant is upregulated in adenomas.58 The 5-HT7 receptor splice variants in this study were expressed in all of the different tissue layers. The overall expression patterns of 5-HT4 and 5-HT7 receptors are in accordance with their known functional properties in the human colon to regulate ascending contraction and descending relaxation to generate peristaltic reflexes resulting in bowel movement.7–16,18 5-HT7 receptors are also found on macrophages and have been implicated as a potential target for treating intestinal inflammatory disorders.59,60
Serotonin induces desensitization (tachyphylaxis) at different rates and magnitudes in different tissues and this is the case with 5-HT4 receptor agonists as highlighted by prucalopride desensitizing pig atrium but not pig stomach 5-HT4 receptors.61 Different GRKs are associated with 5-HT4 receptor desensitization depending on the tissue type in the rat and significantly these GRKs are not necessarily those predicted by studies using recombinant proteins expressed in cell culture.32 GRK2 and GRK3 were relatively well distributed in all tissue layers while GRK5 was less common and GRK6 was absent in the ileum but present in the descending and sigmoid colon, so there may be regional and tissue layer variation in the GRKs involved in 5-HT4 receptor desensitization in the human colon. The distribution of LIN7 homologs in the human colon is of interest as LIN7C (Veli 3) has been shown to interact with the 5-HT4a receptor splice variant.35 In the human intestine, all 3 LIN7 homologues were present in the different tissue regions except the region of the transverse colon tissue layers. However, detection of the LIN7 homologues may be limited by their occurrence at pre-synaptic (axonal) and postsynaptic (dendritic) subcellular compartments.36
In conclusion, this study demonstrates that the major differences in the localization of 5-HT receptors within the human colon are in relation to the mucosa and muscular tissue rather than the region of the intestine. The fact that the study proteins are distributed along the intestine, only serves to emphasize how dominant the receptors and their associated proteins are, and so any dysfunction of one is likely to manifest seriously in clinical conditions. Only minor differences were observed in the distribution of 5-HT4 and 5-HT7 receptors and their splice variants. Differences in the distribution of 5-HT3 receptor subunits were evident with A, B, and C subunits occurring in all tissues whereas the E subunit was only significantly observed in the mucosal layer. The findings suggest that it may be possible to target 5-HT3 receptors in the mucosal or muscular layers if subunit specific molecules can be generated to cater for different colon diseases or dysfunction.
Footnotes
Note: To access the supplementary table and figures mentioned in this article, visit the online version of Journal of Neurogastroenterology and Motility at http://www.jnmjournal.org/, and at http://dx.doi.org/10.5056/jnm14157.
Financial support: None.
Conflicts of interest: None.
Author contributions: Ian M Coupar and Helen R Irving conceived and designed the study; Nor S Yaakob, Kenneth A Chinkwo, Navinisha Chetty, and Helen R Irving were involved in designing the experiments, acquiring, analysing, and interpreting data; Helen R Irving drafted the manuscript; and all authors were involved in critically revising the manuscript.
ORCID: Nor S Yaakob, http://orcid.org/0000-0003-4726-1972; Kenneth A Chinkwo, http://orcid.org/0000-0002-7369-1880; Navinisha Chetty, http://orcid.org/0000-0002-3524-4385; Helen R Irving, http://orcid.org/0000-0002-1514-0909.
References
- 1.Beattie DT, Smith JA. Serotonin pharmacology in the gastrointestinal tract: a review. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:181–203. doi: 10.1007/s00210-008-0276-9. [DOI] [PubMed] [Google Scholar]
- 2.Crowell MD. Role of serotonin in the pathophysiology of the irritable bowel syndrome. Br J Pharmacol. 2004;141:1285–1293. doi: 10.1038/sj.bjp.0705762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Ponti F. Drug development for the irritable bowel syndrome: current challenges and future perspectives. Front Pharmacol. 2013;4:7. doi: 10.3389/fphar.2013.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spiller R. Recent advances in understanding the role of serotonin in gastrointestinal motility in functional bowel disorders: alterations in 5-HT signalling and metabolism in human disease. Neurogastroenterol Motil. 2007;19(suppl 2):25–31. doi: 10.1111/j.1365-2982.2007.00965.x. [DOI] [PubMed] [Google Scholar]
- 5.Spiller R. Serotonin and clinical GI disorders. Neuropharmacology. 2008;55:1072–1080. doi: 10.1016/j.neuropharm.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 6.Spiller R, Garsed K. Infection, inflammation, and the irritable bowel syndrome. Dig Liver Dis. 2009;41:844–849. doi: 10.1016/j.dld.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Borman RA, Burleigh DE. Human colonic mucosa possesses a mixed population of 5-HT receptors. Eur J Pharmacol. 1996;309:271–274. doi: 10.1016/0014-2999(96)00466-9. [DOI] [PubMed] [Google Scholar]
- 8.Cellek S, John AK, Thangiah R, et al. 5-HT4 receptor agonists enhance both cholinergic and nitrergic activities in human isolated colon circular muscle. Neurogastroenterol Motil. 2006;18:853–861. doi: 10.1111/j.1365-2982.2006.00810.x. [DOI] [PubMed] [Google Scholar]
- 9.Cellek S, Thangiah R, Jarvie EM, Vivekanandan S, Lalude O, Sanger GJ. Synergy between 5-HT4 receptor activation and acetylcholinesterase inhibition in human colon and rat forestomach. Neurogastroenterol Motil. 2008;20:539–545. doi: 10.1111/j.1365-2982.2007.01062.x. [DOI] [PubMed] [Google Scholar]
- 10.Hoffman JM, Tyler K, Maceachern SJ, et al. Activation of colonic mucosal 5-HT4 receptor accelerates propulsive motility and inhibits visceral hypersensitivity. Gastroenterology. 2012;142:844–854. e4. doi: 10.1053/j.gastro.2011.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Irving HR, Tan YY, Tochon-Danguy N, et al. Comparison of 5-HT4 and 5-HT7 receptor expression and function in the circular muscle of the human colon. Life Sci. 2007;80:1198–1205. doi: 10.1016/j.lfs.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 12.Leclere PG, Prins NH, Schuurkes JA, Lefebvre RA. 5-HT4 receptors located on cholinergic nerves in human colon circular muscle. Neurogastroenterol Motil. 2005;17:366–375. doi: 10.1111/j.1365-2982.2005.00621.x. [DOI] [PubMed] [Google Scholar]
- 13.Mayer EA. Gut feelings: the emerging biology of gut:brain communication. Nat Rev Neurosci. 2011;12:453–466. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McLean PG, Coupar IM. Further investigation into the signal transduction mechanism of the 5-HT4-like receptor in the smooth muscle of human colon. Br J Pharmacol. 1996;118:1058–1064. doi: 10.1111/j.1476-5381.1996.tb15506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McLean PG, Coupar IM, Molenaar P. A comparative study of functional 5-HT4 receptors in human colon, rat ileum and rat oesophagus. Br J Pharmacol. 1995;115:47–56. doi: 10.1111/j.1476-5381.1995.tb16318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prins NH, Akkermans LM, Lefebvre RA, Schuurkes JA. 5-HT4 receptors on cholinergic nerves involved in contractility of canine and human large intestine longitudinal muscle. Br J Pharmacol. 2000;131:927–932. doi: 10.1038/sj.bjp.0703615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Camilleri M, Deiteren A. Prucalopride for constipation. Expert Opin Pharmacother. 2010;11:451–461. doi: 10.1517/14656560903567057. [DOI] [PubMed] [Google Scholar]
- 18.Prins NH, Briejer MR, Van Bergen PJ, Akkermans LM, Schuurkes JA. Evidence for 5-HT7 receptors mediating relaxation of human colonic circular smooth muscle. Br J Pharmacol. 1999;128:849–852. doi: 10.1038/sj.bjp.0702762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barnes NM, Hales TG, Lummis SC, Peters JA. The 5-HT3 receptor - the relationship between structure and function. Neuropharmacology. 2009;56:273–284. doi: 10.1016/j.neuropharm.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walstab J, Rappold G, Niesler B. 5-HT3 receptors: role in disease and target of drugs. Pharmacol Ther. 2010;128:146–169. doi: 10.1016/j.pharmthera.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 21.Yaakob N, Malone DT, Exintaris B, Irving HR. Heterogeneity amongst 5-HT3 receptor subunits: is this significant? Curr Mol Med. 2011;11:57–68. doi: 10.2174/156652411794474392. [DOI] [PubMed] [Google Scholar]
- 22.Chetty N, Coupar IM, Tan YY, Desmond PV, Irving HR. Distribution of serotonin receptors and interacting proteins in the human sigmoid colon. Neurogastroenterol Motil. 2009;21:551–558. e14–e15. doi: 10.1111/j.1365-2982.2008.01223.x. [DOI] [PubMed] [Google Scholar]
- 23.Holbrook JD, Gill CH, Zebda N, et al. Characterisation of 5-HT3C, 5-HT3D and 5-HT3E receptor subunits: evolution, distribution and function. J Neurochem. 2009;108:384–396. doi: 10.1111/j.1471-4159.2008.05775.x. [DOI] [PubMed] [Google Scholar]
- 24.Kapeller J, Möller D, Lasitschka F, et al. Serotonin receptor diversity in the human colon: expression of serotonin type 3 receptor subunits 5-HT3C, 5-HT3D, and 5-HT3E. J Comp Neurol. 2011;519:420–432. doi: 10.1002/cne.22525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niesler B, Frank B, Kapeller J, Rappold GA. Cloning, physical mapping and expression analysis of the human 5-HT3 serotonin receptor-like genes HTR3C, HTR3D and HTR3E. Gene. 2003;310:101–111. doi: 10.1016/S0378-1119(03)00503-1. [DOI] [PubMed] [Google Scholar]
- 26.Van Lelyveld N, Ter Linde J, Schipper ME, Samson M. Regional differences in expression of TPH-1, SERT, 5-HT3 and 5-HT4 receptors in the human stomach and duodenum. Neurogastroenterol Motil. 2007;19:342–348. doi: 10.1111/j.1365-2982.2006.00891.x. [DOI] [PubMed] [Google Scholar]
- 27.Michel K, Zeller F, Langer R, et al. Serotonin excites neurons in the human submucous plexus via 5-HT3 receptors. Gastroenterology. 2005;128:1317–1326. doi: 10.1053/j.gastro.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Blondel O, Gastineau M, Dahmoune Y, Langlois M, Fischmeister R. Cloning, expression, and pharmacology of four human 5-hydroxytryptamine4 receptor isoforms produced by alternative splicing in the carboxyl terminus. J Neurochem. 1998;70:2252–2261. doi: 10.1046/j.1471-4159.1998.70062252.x. [DOI] [PubMed] [Google Scholar]
- 29.Krobert KA, Bach T, Syversveen T, Kvingedal AM, Levy FO. The cloned human 5-HT7 receptor splice variants: a comparative characterization of their pharmacology, function and distribution. Naunyn Schmiedebergs Arch Pharmacol. 2001;363:620–632. doi: 10.1007/s002100000369. [DOI] [PubMed] [Google Scholar]
- 30.Medhurst AD, Lezoulac’h F, Fischmeister R, Middlemiss DN, Sanger GJ. Quantitative mRNA analysis of five C-terminal splice variants of the human 5-HT4 receptor in the central nervous system by TaqMan real time RT-PCR. Brain Res Mol Brain Res. 2001;90:125–134. doi: 10.1016/S0169-328X(01)00095-X. [DOI] [PubMed] [Google Scholar]
- 31.Grider JR. Desensitization of the peristaltic reflex induced by mucosal stimulation with the selective 5-HT4 agonist tegaserod. Am J Physiol Gastrointest Liver Physiol. 2006;290:G319–G327. doi: 10.1152/ajpgi.00326.2005. [DOI] [PubMed] [Google Scholar]
- 32.Nedi T, White PJ, Coupar IM, Irving HR. Tissue dependent differences in G-protein coupled receptor kinases associated with 5-HT4 receptor desensitization in the rat gastro-intestinal tract. Biochem Pharmacol. 2011;81:123–133. doi: 10.1016/j.bcp.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Rondé P, Ansanay H, Dumuis A, Miller R, Bockaert J. Homologous desensitization of 5-hydroxytryptamine4 receptors in rat esophagus: functional and second messenger studies. J Pharmacol Exp Ther. 1995;272:977–983. [PubMed] [Google Scholar]
- 34.Bohl J, Brimer N, Lyons C, Vande Pol SB. The stardust family protein MPP7 forms a tripartite complex with LIN7 and DLG1 that regulates the stability and localization of DLG1 to cell junctions. J Biol Chem. 2007;282:9392–9400. doi: 10.1074/jbc.M610002200. [DOI] [PubMed] [Google Scholar]
- 35.Joubert L, Hanson B, Barthet G, et al. New sorting nexin (SNX27) and NHERF specifically interact with the 5-HT4a receptor splice variant: roles in receptor targeting. J Cell Sci. 2004;117(Pt 22):5367–5379. doi: 10.1242/jcs.01379. [DOI] [PubMed] [Google Scholar]
- 36.Setou M, Nakagawa T, Seog DH, Hirokawa N. Kinesin super-family motor protein KIF17 and mLin-10 in NMDA receptor containing vesicle transport. Science. 2000;288:1796–1802. doi: 10.1126/science.288.5472.1796. [DOI] [PubMed] [Google Scholar]
- 37.Shelly M, Mosesson Y, Citri A, et al. Polar expression of ErbB-2/HER2 in epithelia: bimodal regulation by Lin-7. Dev Cell. 2003;5:475–486. doi: 10.1016/j.devcel.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Walstab J, Hammer C, Lasitschka F, et al. RIC-3 exclusively enhances the surface expression of human homomeric 5-hydroxytryptamine type 3A (5-HT3A) receptors despite direct interactions with 5-HT3A, -C, -D and -E subunits. J Biol Chem. 2010;285:26956–26965. doi: 10.1074/jbc.M110.122838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng A, McDonald NA, Connolly CN. Cell surface expression of 5-hydroxytryptamine type 3 receptors is promoted by RIC-3. J Biol Chem. 2005;280:22502–22507. doi: 10.1074/jbc.M414341200. [DOI] [PubMed] [Google Scholar]
- 40.Ramakers C, Ruijter JM, Deprez RH, Moorman AF. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci Lett. 2003;339:62–66. doi: 10.1016/S0304-3940(02)01423-4. [DOI] [PubMed] [Google Scholar]
- 41.Wouters MM, Farrugia G, Schemann M. 5-HT receptors on inter-stitial cells of Cajal, smooth muscle and enteric nerves. Neurogastroenterol Motil. 2007;19(suppl 2):5–12. doi: 10.1111/j.1365-2982.2007.00963.x. [DOI] [PubMed] [Google Scholar]
- 42.Kim HS. 5-Hydroxytryptamine4 receptor agonists and colonic motility. J Smooth Muscle Res. 2009;45:25–29. doi: 10.1540/jsmr.45.25. [DOI] [PubMed] [Google Scholar]
- 43.Liu M, Geddis MS, Wen Y, Setlik W, Gershon MD. Expression and function of 5-HT4 receptors in the mouse enteric nervous system. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1148–G1163. doi: 10.1152/ajpgi.00245.2005. [DOI] [PubMed] [Google Scholar]
- 44.Coupar IM, Desmond PV, Irving HR. Human 5-HT4 and 5-HT7 receptor splice variants: are they important? Curr Neuropharmacol. 2007;5:224–231. doi: 10.2174/157015907782793621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coupar IM, Irving HR, Manallack DT, et al. Assessment of the pharmacological properties of 5-methoxyindole derivatives at 5-HT4 receptors. J Pharm Pharmacol. 2012;64:1099–1106. doi: 10.1111/j.2042-7158.2012.01500.x. [DOI] [PubMed] [Google Scholar]
- 46.Vickery RG, Mai N, Kaufman E, et al. A comparison of the pharmacological properties of guinea-pig and human recombinant 5-HT4 receptors. Br J Pharmacol. 2007;150:782–791. doi: 10.1038/sj.bjp.0707154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bustin SA, Benes V, Garson JA, et al. The MIQE guidelines; minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 48.Boyd GW, Low P, Dunlop JI, et al. Assembly and cell surface expression of homomeric and heteromeric 5-HT3 receptors: the role of oligomerization and chaperone proteins. Mol Cell Neurosci. 2002;21:38–50. doi: 10.1006/mcne.2002.1160. [DOI] [PubMed] [Google Scholar]
- 49.Kerckhoffs AP, Ter Linde JJ, Akkermans LM, Samson M. Trypsinogen IV, serotonin transporter transcript levels and serotonin content are increasd in small intestine of irritable bowel syndrome patients. Neurogastroenterol Motil. 2008;20:900–907. doi: 10.1111/j.1365-2982.2008.01100.x. [DOI] [PubMed] [Google Scholar]
- 50.Goecke TW, Ekici AB, Niesler B, et al. Two naturally occurring variants of the serotonin receptor gene HTR3C are associated with nausea in pregnancy. Acta Obstet Gynecol Scand. 2010;89:7–14. doi: 10.3109/00016340903322727. [DOI] [PubMed] [Google Scholar]
- 51.Kapeller J, Houghton LA, Mönnikes H, et al. First evidence for an association of a functional variant in the microRNA-510 target site of the serotonin receptor-type 3E gene with diarrhea predominant irritable bowel syndrome. Hum Mol Genet. 2008;17:2967–2977. doi: 10.1093/hmg/ddn195. [DOI] [PubMed] [Google Scholar]
- 52.Rehnström K, Ylisaukko-oja T, Nummela I, et al. Allelic variants in HTR3C show association with autism. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:741–746. doi: 10.1002/ajmg.b.30882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schuhmacher A, Mössner R, Quednow BB, et al. Influence of 5-HT3 receptor subunit genes HTR3A, HTR3B, HTR3C, HTR3D and HTR3E on treatment response to antipsychotics in schizophrenia. Pharmacogenet Genomics. 2009;19:843–851. doi: 10.1097/FPC.0b013e3283313296. [DOI] [PubMed] [Google Scholar]
- 54.Irving HR, Tochon-Danguy N, Chinkwo KA, et al. Investigations into the binding affinities of different human 5-HT4 receptor splice variants. Pharmacology. 2010;85:224–233. doi: 10.1159/000280418. [DOI] [PubMed] [Google Scholar]
- 55.Mialet J, Berque-Bestel I, Eftekhari P, et al. Isolation of the serotoninergic 5-HT4e receptor from human heart and comparative analysis of its pharmacological profile in C6-glial and CHO cell lines. Br J Pharmacol. 2000;129:771–781. doi: 10.1038/sj.bjp.0703101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mialet J, Berque-Bestel I, Sicsic S, Langlois M, Fischmeister R, Lezoualc’h F. Pharmacological characterization of the human 5-HT4d receptor splice variant stably expressed in Chinese hamster ovary cells. Br J Pharmacol. 2000;131:827–835. doi: 10.1038/sj.bjp.0703641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mialet J, Fischmeister R, Lezoualc’h F. Characterisation of human 5-HT4d receptor desensitization in CHO cells. Br J Pharmacol. 2003;138:445–452. doi: 10.1038/sj.bjp.0705061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cartier D, Jégou S, Parmentier F, et al. Expression profile of serotonin4 (5-HT4) receptors in adrenocortical aldosteone-producing adenomas. Eur J Endocrinol. 2005;153:939–947. doi: 10.1530/eje.1.02051. [DOI] [PubMed] [Google Scholar]
- 59.Kim JJ, Bridle BW, Ghia JE, et al. Targeted inhibition of serotonin type 7 (5-HT7) receptor function modulates immune responses and reduces the severity of intestinal inflammation. J Immunol. 2013;190:4795–4804. doi: 10.4049/jimmunol.1201887. [DOI] [PubMed] [Google Scholar]
- 60.de las Casas-Engel M, Domínguez-Soto A, Sierra-Filardi E, et al. Serotonin skews human macrophage polarization through HTR2B and HTR7. J Immunol. 2013;190:2301–2310. doi: 10.4049/jimmunol.1201133. [DOI] [PubMed] [Google Scholar]
- 61.De Maeyer JH, Schuurkes JA, Lefebvre RA. Selective desensitization of the 5-HT4 receptor-mediated response in pig atrium but not in stomach. Br J Pharmacol. 2009;156:362–376. doi: 10.1111/j.1476-5381.2008.00007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.