Abstract
Purpose
The use of technology to implement cost-effective health care management on a large scale may be an alternative for diabetes management but needs to be evaluated in controlled trials. This study assessed the utility and cost-effectiveness of an automated Diabetes Remote Monitoring and Management System (DRMS) in glycemic control versus usual care.
Methods
In this randomized, controlled study, patients with uncontrolled diabetes on insulin were randomized to use of the DRMS or usual care. Participants in both groups were followed up for 6 months and had 3 clinic visits at 0, 3, and 6 months. The DRMS used text messages or phone calls to remind patients to test their blood glucose and to report results via an automated system, with no human interaction unless a patient had severely high or low blood glucose. The DRMS made adjustments to insulin dose(s) based on validated algorithms. Participants reported medication adherence through the Morisky Medication Adherence Scale-8, and diabetes-specific quality of life through the diabetes Daily Quality of Life questionnaire. A cost-effectiveness analysis was conducted based on the estimated overall costs of DRMS and usual care.
Findings
A total of 98 patients were enrolled (59 [60%] female; mean age, 59 years); 87 participants (89%) completed follow-up. HbA1c was similar between the DRMS and control groups at 3 months (7.60% vs 8.10%) and at 6 months (8.10% vs 7.90%). Changes from baseline to 6 months were not statistically significant for self-reported medication adherence and diabetes-specific quality of life, with the exception of the Daily Quality of Life–Social/Vocational Concerns subscale score (P = 0.04).
Implications
An automated system like the DRMS may improve glycemic control to the same degree as usual clinic care and may significantly improve the social/vocational aspects of quality of life. Cost-effectiveness analysis found DRMS to be cost-effective when compared to usual care and suggests DRMS has a good scale of economy for program scale up. Further research is needed to determine how to sustain the benefits seen with the automated system over longer periods.
Keywords: adherence, diabetes mellitus, technology
INTRODUCTION
Management of chronic diseases challenges health care systems by requiring frequent clinic visits, often for minor interventions and dose adjustments. This is particularly true for the management of diabetes in patients treated with insulin. Interventions aimed at improving glycemic control and medication adherence in diabetes are often costly and may not be feasible to carry out on a large enough scale to all patients.
A previous study reported that home telemonitoring with medication management by a nurse–practitioner led to a significantly greater improvement in glycemic control compared with that with monthly telephone calls (−1.7% vs −0.7%, respectively; P < 0.001) but needs significant human resources.1 A less hands-on approach using usual care plus Internet support in type 1 diabetic adolescents did not improve glycemia but resulted in significant changes in self-management (Cohen δ = 0.64; P = 0.02).2
A longer-term study using more comprehensive patient management was the IDEA Tel (Informatics for Diabetes Education and Telemedicine) study,3 which was a 5-year, randomized, controlled trial using telemedicine to monitor diabetes and blood pressure control in a population of Medicare beneficiaries. The intervention group had multiple technologies, including Web cameras for video conferencing with study staff, a glucometer and blood pressure machine that were connected via a telemedicine unit to report readings to study staff, and online educational pages. At the end of 5 years, there was a statistically significant, 25% reduction in hemoglobin A1c (HbA1c) compared with that in the usual care group, along with decreases in low-density lipoproteins and blood pressure.3 However, this intervention also required substantial resources.
Some systematic reviews of telemedicine-based interventions for diabetes management have reported insufficient evidence to prove reduction in HbA1c and cost-effectiveness 47. A major contributor to the lack of cost-effectiveness is that human intervention is still required in most systems.4 Another systematic review of data from 8 published randomized, controlled trials that included patients with both type 1 and type 2 diabetes reported “low-quality evidence” of small improvements in glycemic control with telemedicine compared with usual care.5
A Cochrane review analyzed 4 randomized, controlled trials of phone messaging as an intervention. Clinical measures (including HbA1c) showed no statistically significant difference between intervention and control participants. However, there was moderate-quality evidence of improved scores for self-management.6 Another review of telemedicine in diabetes reported an improvement in HbA1c with intervention in the short term (<6 months) compared with those with interventions that lasted at least 1 year. This study suggests that interventions may need to be modified over time to enhance patient adherence.7
The use of technology to implement cost-effective health care management on a large scale may be an alternative for diabetes management. However, so far technology has been used as an adjunct to provider management, mainly to obtain information from patients. Feedback and advice is then given, usually by telephone. Such interventions require significant human resources, and the response to patients is often delayed. Overall, the impact on glycemic control has been small5–7 and the cost-effectiveness difficult to quantify. What is needed is an automated response system that will respond immediately to patients’ input and provide advice that is compatible with guidelines on management of their disease.
The Diabetes Remote Monitoring and Management System (DRMS, MedAdherence LLC, Norwalk, CT)* used in this study is a combination of mainly an automated system with minimal human interaction. It uses patients’ cell phones and landlines to deliver messages via text or automated voice. The system was designed to remind patients to check their blood glucose and report the readings as well as the medications they take, including insulin dose. It can also suggest adjustments to insulin doses using programmed validated algorithms.8 The DRMS can be tailored to customize recommended changes to self-management based on data entered by patients. Emergency backup human intervention is automatically available when glucose parameters are outside of recommended ranges. Providers can also monitor the progress of their patients through a Web-based portal.
In our study, we compared the DRMS system to standard care in a randomized, controlled trial in patients with uncontrolled diabetes on insulin, or patients with type 2 diabetes starting insulin. The aim of the study was to evaluate the hypothesis that an automated system like the DRMS is as effective in achieving improvement in glycemic control and management as is usual clinical care. Although the DRMS is capable of managing other modalities of care (eg, blood pressure monitoring, treatment adjustment, reminders of prescription refills, reminders of appointments, including eye examinations), for the purposes of this study, we focused exclusively on glycemic control.
PATIENTS AND METHODS
Study Design
Ninety-eight participants were recruited from the Tulane Medical Center and Southeast Louisiana Veterans Health Care System endocrinology clinics (New Orleans, Louisiana). Participants were randomized, after informed consent was obtained, to either the intervention (DRMS) group or the control group by using a random-number table and followed for 6 months. Both the intervention and control groups had the same visit schedule, 3 clinic visits at months 0, 3, and 6. The control group was advised to either adjust the dose of insulin themselves weekly9 or to make a call to the clinic or diabetes educator for dose adjustment. Participants randomized to the intervention group would communicate with DRMS at least once daily and more often if needed.
Patients with an HbA1c between 7.0% and 9.0%, over the age of 18 years, and currently taking or starting insulin were enrolled. Patients were excluded if they were on an insulin pump, had a history of a major cardiovascular event (eg, myocardial infarction, stroke) in the previous 6 months, or had hypoglycemia unawareness. All randomized participants had the same clinic schedule. At visit 1, day 0, participants were randomized to their group; had baseline HbA1c, weight, and blood pressure measurements taken; underwent a review of diabetes medications; and had medical history recorded. The participants also completed a Morisky Medication Adherence Scale-8 (MMAS-8) and a diabetes-specific Daily Quality of Life (DQoL) questionnaire. If the participant was randomized to the intervention group, he or she was instructed on how to use the system. The first contact from the DRMS was to confirm the telephone number. After participant confirmation, the DRMS began contacting the participant daily to ask whether he or she had checked his or her blood glucose. The control group had only clinic interventions by physicians and diabetes educators as usual, to improve their glycemic control.
At both 3 and 6 months, each participant returned to the research clinic for a study visit. Each participant had HbA1c, blood pressure, and weight measured; underwent a review of any changes in diabetes medications; and completed both the MMAS-8 and diabetes-specific DQoL questionnaires. If they were in the intervention group, we assessed whether there were any problems with the system. Also at 6 months the intervention group completed a survey evaluating the DRMS system.
Diabetes Remote Monitoring and Management System
An automated system like the DRMS uses technology (cell phones and landline phones) to minimize the use of human-based resources. The system employs rules-based technology to automate manual care and to monitor care plans, thus creating customized actions and interventions based on physician-defined parameters and the specific medical protocol.
The system reminds the patients to monitor blood glucose, take their medications, and alert providers when problems arise. There was an emergency safeguard put into place for when a participant submitted extreme values of blood glucose (<60 or >300 mg/dL); in these cases, the patient was immediately and automatically connected to the endocrinologist on call. We also modified the system to utilize a standard set of protocols for insulin dose adjustment10 or to be customized to better reflect the requirements of the individual physician.
For this study, the system was designed to contact the participant daily, either through text messaging or automated voice. From these messages, participants could either respond by submitting their blood glucose levels or respond at a later time. If a participant did not submit his or her blood glucose level at the initial contact, the DRMS would text or call again that same day to remind the participant to check his or her blood glucose. However, if a participant submitted a reading before the reminder, the system would not contact the participant on that day. Providers could monitor the progress of their patients through a Web-based, secure portal, and information could also be downloaded directly into electronic medical records.
Statistical Analysis
All statistical analysis used intent-to-treat methodology, and all comparisons used a 2-tailed test at the 0.05 level of significance. Statistical analysis was conducted using SAS 9.2. Continuous variables are reported as means (95% CIs). Medication adherence was based on scores from the MMAS-8; patients were categorized as having either high, medium, or low adherence. High adherence was a score of 0, medium adherence was a score of 1 or 2, and low adherence was a score between 3 and 8. The diabetes-related DQoL had 3 subscales: Diabetes Impact, Social/Vocation Concerns, and Worries About Diabetes. The total score from the DQoL and the scores on each of the 3 subscales were analyzed. A validated prediction model of clinical characteristics was used to predict expected QALYs for diabetic patients and to evaluate the effect of the program on QALY improvement. The QALY prediction model used the variables age, sex, blood pressure, low-density lipoprotein, and HbA1c value.11 Predicted QALYs for patients in both the intervention group and the control group were calculated both at the baseline time and in the post-test period.
Cost Effectiveness Analysis
Cost-minimization analysis was conducted based on QALYs gained and cost differences between DRMS, usual care, and “ideal care” (eg, in a treat-to-target clinical trial using frequent dose titration, eg, the ATLANTUS study9) for diabetes management over a 1-year period. For this cost-minimization analysis, patients in the 3 groups were assumed, despite their different health care schedules, to have had the same health care utilizations, including their likelihood of developing complications, hospitalizations, and other health outcomes, including QALYs gained. The setup and maintenance costs for the DRMS were used to estimate the overall cost, which varied across different sizes of patient populations. The setup and maintenance fees for the DRMS in a 6-month period were dependent on the number of patients in the system. For this analysis, 4 different population sizes were used (350, 500, 750, and 1000 patients), and the cost per patient per month at each size were $15.60, $14.88, $13.56, and $12.52, respectively. Patients in the DRMS group had a level 4 visit on day 0, level 3 monthly visits in the first 3 months, and level 3 bimonthly visits thereafter. Participants randomized to DRMS were only monitored and communicated through the automated system.
Two separate comparisons for DRMS were conducted: the first one defined usual care by the estimates from Medical Expenditure Panel Survey Data (MEPS), a national representative database. Also DRMS data were compared to a “treat-to-target” clinical trial of insulin therapy in which frequent visits and telephone calls were used. This will be referred to as IDEAL CARE, which is a “treat-to-target” clinical trial of insulin therapy in which frequent visits and telephone calls were used.9,12 Unit cost was evaluated using standard costs and schedules of procedures for each health management plan. According to the Medicare reimbursement rates for Current Procedural Terminology (CPT) codes 99213 and 99214, the standard costs for level 4 visits are $79.29 and level 3 visits are $51.69.13 The costs for follow-up phone calls were estimated using the new Medicare reimbursement rate for telehealth management service, at minimum a 20-minute phone call (CPT code 99490), which was estimated to cost $33.43.14
The outpatient visit number was 11 (SE, 0.38) visits per year, which was estimated from the 2012 Medical Expenditure Panel Survey15 for diabetic patients on insulin. The estimated number of outpatient visits was equivalent to those in the 2006 Medical Expenditure Panel Survey16 and a similar survey conducted in Europe.17 Furthermore, it was assumed that all usual care patients had a level 4 visit (CPT code 99214) at day 0 (initial visit) and 10 level 3 visits (CPT code 99213) thereafter during 1 year. The cost of 16 telehealth services (a 20-minute phone call), which were conducted by a nurse weekly for 6 weeks after the initial physician visit, and monthly after week 6, were also included in the cost-effectiveness analysis.
For the ideal care group, in which 12 contacts were made by physician visits or telephone visits in 6 months, data were based on the information extracted from the ATLANTUS trial.9 For a 1-year period, 12 physician visits and 12 telephone consultation sessions were assumed to have taken place. In addition, the lower bound of health care utilization for the ideal care group was assumed to have been at least 8 visits in a 1-year period, based on the minimum physician visits of 4 visits in 6 months from the ATLANTUS trial.9
RESULTS
Of the 98 participants enrolled, the mean age was 59 years at the time of enrollment, 59 (60%) were female, 64 (65%) were black, and 79 (81%) had received a high school diploma or higher. Participants were on a variety of insulins; 32 (33%) were on long-acting insulin only, 10 (10%) were on short-acting insulin only, and 17 (17%) were on mixed insulin only. Thirty-nine participants (40%) were on a basal-bolus regimen, and 78 participants (80%) were taking other antidiabetic medications combined with insulin (Table I). In the DRMS group, 31 of 52 participants (60%) used phone calls to report into the system, whereas the rest used text messages exclusively. Overall, 87 participants (89%) completed follow-up. At the end of 3 months, the mean HbA1c in the intervention group was similar to that in the control group (7.60% vs 8.10%, respectively; P ¼ 0.25). Also at 6 months, DRMS was apparently as effective in HbA1c control as was usual care (8.1% vs 7.9%; P ¼ 0.78).
Table I.
Demographic characteristics of the patients in this study of the utility of a remote monitoring system for diabetes control. Data are given as number (%) of patients.
| Characteristic | Intervention (n = 50) |
Control (n = 48) |
All Patients (N = 98) |
|---|---|---|---|
| Sex | |||
| Female | 34 (68) | 25 (52) | 59 (60) |
| Male | 16 (32) | 23 (48) | 39 (40) |
| Race | |||
| Black | 37 (74) | 27 (56) | 64 (65) |
| White | 12 (24) | 17 (36) | 29 (30) |
| Hispanic | 1 (2) | 0 | 1 (1) |
| Asian | 0 | 1 (2) | 1 (1) |
| Other | 0 | 3 (6) | 3 (3) |
| Education level | |||
| High school diploma | 20 (40) | 15 (31) | 35 (36) |
| College degree | 12 (24) | 20 (42) | 32 (33) |
| Less than high school | 12 (24) | 7 (15) | 19 (19) |
| Graduate degree | 5 (10) | 6 (13) | 11 (11) |
| Doctorate degree | 1 (2) | 0 | 1 (1) |
| Diabetes medications | |||
| Basal-bolus regimen | 18 (36) | 21 (44) | 39 (40) |
| Long-acting insulin only | 18 (36) | 14 (29) | 32 (33) |
| Mixed insulin only | 8 (16) | 9 (19) | 17 (17) |
| Short-acting insulin only | 6 (12) | 4 (8) | 10 (10) |
| Other antidiabetic medications + insulin | 38 (76) | 40 (83) | 78 (80) |
There were no adverse events reported by patients related to the system. There were 76 automated calls to the emergency physician for low and high blood glucose concentrations. However, on all of these occasions, the patients reported that they had made a mistake in their glucose monitoring or data entry. Patients reported that they had no symptoms and that current blood glucose concentrations were within the target range.
Overall improvements in self-reported medication adherence and in DQoL scores were not significant for DRMS when compared to those of the control group; however, only the difference between the DQoL–Social/Vocational Concerns subscale scores was statistically significant (9.63 vs 11.10, respectively; P ¼ 0.04).
None of the participants scored in the high medication adherence category; therefore, participants were categorized as having either medium (score of 1 or 2) or low adherence (score of 3–8). Medication adherence was measured by the percentage of participants above medium adherence. In the intervention group, 28.00% of participants were above medium adherence at the beginning of the study compared with 12.50% in the control group. At the end of 6 months, the intervention group increased to 37.21% and the control group increased to 20.51% (P ¼ 0.097) (Table II).
Table II.
Hemoglobin A1c, Morisky Scale, and DQoL questionnaire outcomes in this study of the utility of a remote monitoring system for diabetes control.
| Parameter | Intervention (n = 50) |
Control (n = 48) |
All Patients (N = 98) |
|---|---|---|---|
| HbA1c, median (SD), % | |||
| Baseline | 8.35 (68) | 8.30 (67) | 8.30 (67) |
| 3 mo | 7.60 (60) | 8.10 (65) | 7.80 (62) |
| 6 mo | 8.10 (65) | 7.90 (63) | 7.90 (63) |
| Adherence, % of patients above median | |||
| Baseline | 28.00 | 12.50 | 20.41 |
| 3 mo | 26.19 | 21.95 | 24.10 |
| 6 mo | 37.21 | 20.51 | 29.27 |
| DQoL–Impact Score, mean (95% CI) | |||
| Baseline | 43.74 (40.39–47.09) | 47.08 (44.23–49.94) | 45.38 (43.18–47.57) |
| 3 mo | 41.95 (38.60–45.30) | 44.63 (41.81–47.46) | 43.28 (41.11–45.45) |
| 6 mo | 42.09 (38.78–45.40) | 46.23 (42.41–50.05) | 44.06 (41.56–46.56) |
| DQoL–Worry: Social/Vocational, mean (95% CI) | |||
| Baseline | 9.72 (8.84–10.60) | 11.85 (10.42–13.29) | 10.77 (9.92–11.61) |
| 3 mo | 9.76 (8.71–10.81) | 10.54 (9.46–11.61) | 10.14 (9.41–10.88) |
| 6 mo | 9.63* (8.49–10.77) | 11.10 (9.77–12.43) | 10.33 (9.46–10.77) |
| DQoL–Worry: Diabetes Related, mean (95% CI) | |||
| Baseline | 7.96 (7.10–8.82) | 8.96 (8.15–9.76) | 8.45 (7.86–9.04) |
| 3 mo | 7.67 (6.73–8.60) | 8.10 (7.23–8.96) | 7.88 (7.25–8.51) |
| 6 mo | 7.53 (6.63–8.44) | 8.54 (7.54–9.53) | 8.01 (7.35–8.68) |
| DQoL–Total Score, mean (95% CI) | |||
| Baseline | 61.42 (57.03–65.81) | 67.90 (63.59–72.21) | 64.59 (61.50–67.68) |
| 3 mo | 59.38 (54.40–64.36) | 63.27 (59.08–67.45) | 61.30 (58.08–64.36) |
| 6 mo | 59.26 (54.48–64.03) | 65.87 (60.30–71.44) | 62.40 (58.76–64.03) |
DQoL = Daily Quality of Life questionnaire; HbA1c = glycosylated hemoglobin.
P = 0.04 versus control.
During the 6-month study period, the mean QALY score in the intervention group increased from 13.9 to 14.1, while the mean QALY score in the control group increased from 13.85 to 14.0. This difference in improvement of QALY scores was not statistically or clinically significant (data not shown).
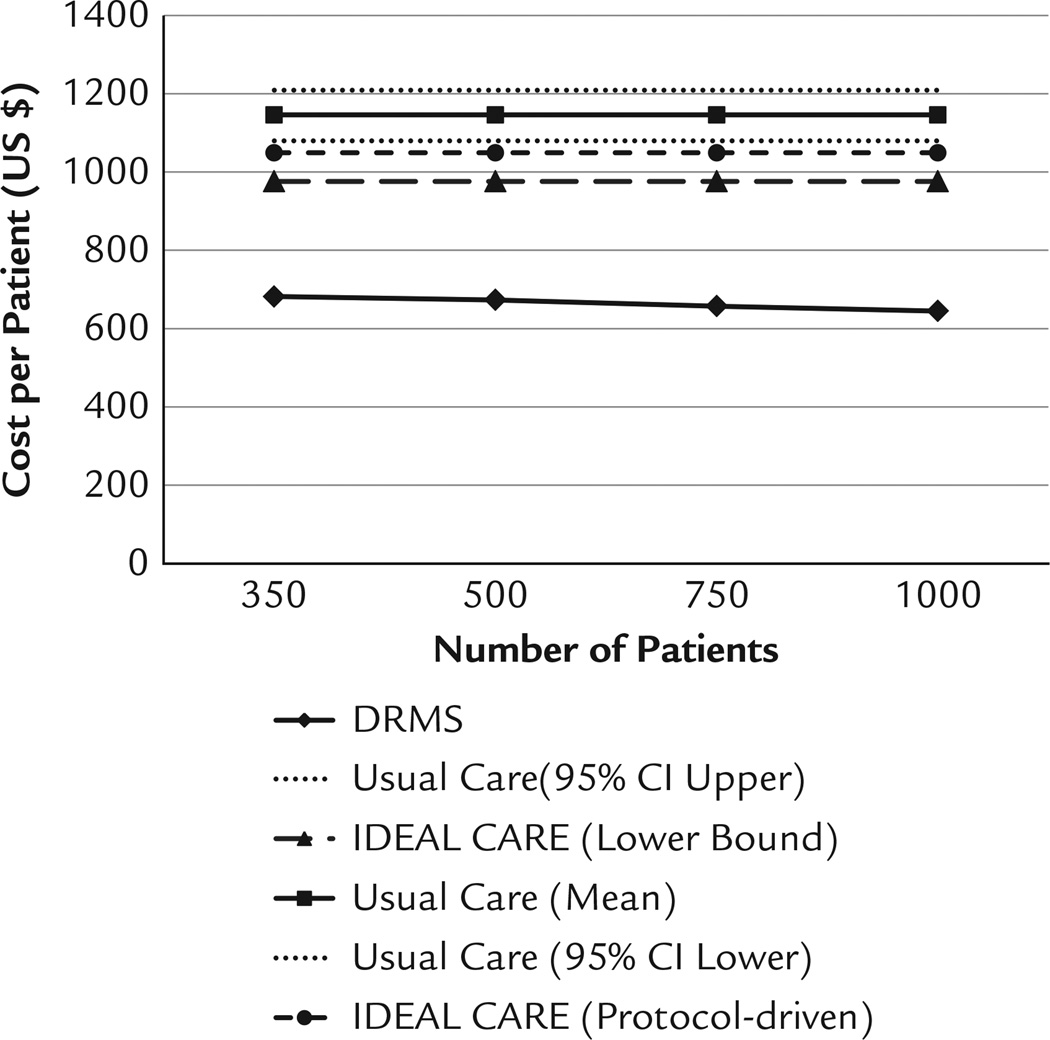
In the cost-effectiveness evaluation, the DRMS intervention was cost-effective when compared to usual care and was numerically but not statistically less costly than ideal care (Figure). Assuming an enrollment of 350 patients, an institution would pay $681.82 per patient per year for DRMS (including the cost of the setup of the system). The estimated cost for usual care was $1131.07 (95% CI, $1079.38–$1208.61), and the cost of ideal care varied from a minimum of $976 to a protocol-driven level of $1049.04 per patient per year. The cost of DRMS intervention was significantly less than that of usual care (P < 0.001) and was below the lower bound of that of ideal care.
Figure.
Cost analysis of DRMS compared with the Usual Care and IDEAL CARE study.12 DRMS = Diabetes Remote Monitoring and Management System.
At the final visit, 44 participants in the DRMS group completed an evaluation survey. Of the 44, 36 participants (82%) said they liked the idea of their physicians communicating with them in this manner. In terms of glycemic and overall health improvements, 38 (86%) and 35 (80%), respectively, reported at least some noticed improvement. Over 40 participants (91%) thought this system could help to manage their diabetes long-term. However, 16 participants (36%) reported problems with the automated calls, and 24 participants (55%) reported that they had technical problems, such as hang-ups (data not shown).
DISCUSSION
Our study found that an automated system, DRMS, managed glycemic control just as well as did usual care. Furthermore, patients liked communication via this technology, were more adherent with their medications, and reported improvement in the social/vocational aspects of quality of life. Also there were no serious adverse events related to the system reported.
Based on a literature search, this is the first report of an automated system to improve glycemic control that can also be monitored remotely on the Internet and has built-in safety features to ensure medical contact when necessary. The results from this study are comparable to those from other studies involving telemonitoring as an intervention and are especially consistent with those from the Cochrane review and mHealth services in Veterans Affairs.6 The mHealth services in Veterans Affairs reported improvements with self-management but did not collect physiologic measurements.18 However, other studies—DiaTel Extension,1 TExT-MED (A Mobile Health Intervention for Inner City Patients With Poorly Controlled Diabetes: Proof-of-Concept of the TExT-MED Program),19 and the Shape Program (Adherence to Self-Monitoring via Interactive Voice Response Technology in an eHealth Intervention Targeting Weight Gain Prevention Among Black Women)20—have reported statistically significant differences in physiologic measures as well as in self-management.
These findings suggest the DRMS intervention is cost effective. Since the fixed costs of the system set up remains essentially unchanged, if the number of patients increase the cost per patient will decrease. For example if the number in the DRMS intervention increased to 1000 the cost would be $617.21 per patient per year, in comparison to $681.82 per patient with 350 total patients. Thus, it suggests DRMS has a good scale of economy for a program scale-up.
The automated system used in this study is easily scalable and can accommodate a large number of patients in a practice using it or across multiple clinical settings. Moreover, it may be more cost-effective, particularly when applied to large numbers of patients. Because the overall cost of DRMS decreases per patient as the patient load increases, the overall benefit of utilizing the DRMS may be even greater than that with usual care, in which the fixed cost per patient is constant. It therefore has considerable potential to decrease health care costs in similar patients.
This kind of automated system may also be useful in other health care management programs, such as blood pressure management or when adjusting or starting new medication. It can be used to remind patients of upcoming visits, to take medications, or to check blood glucose or blood pressure when needed. It also allows for more regular follow-up without the inconvenience of an in-person visit. This can ease the burden for not only the patient but also the clinic staff by eliminating the need for reminder phone calls done by staff. Instead, the staff will need only to monitor the system to ensure that patients have been contacted or have reported necessary measures. The system could also be adapted to include reminders of other aspects of diabetes care (eg, eye screening, appointments, and prescription refill reminders), although these were not tested in this study, which focused solely on glycemic control through insulin dose adjustments.
There were some limitations with this study and the system. The loss-to-follow-up percentage was ~11%; however, there were similar amounts of loss to follow-up in both the control group (6 participants) and the intervention group (5 participants). Therefore, the loss to follow-up was less likely to have introduced bias based on randomization to control or intervention (DRMS). Other limitations were focused on the technical aspects of the DRMS. Some participants reported issues with disconnections and with the system not recognizing their responses accurately. In patients using a conventional voice-response phone system, a patient’s accent may have hindered the system from accurately recognizing a verbal response, and background noise made a difference in the number of issues that the system had with recognizing responses. After participants removed any background noise, the system was able to record responses accurately and timely. Also, patients with hearing loss may not necessarily have benefited from the phone calls.
Another limitation was that the DRMS was not well suited to more complex insulin-adjustment algorithms. The algorithms worked well to adjust evening doses of long-acting or mixed insulin based on fasting blood glucose levels. However, for patients using more complex insulin regimens with multiple injections, or those individualized to account for carbohydrate intake or prevailing blood glucose, it was difficult for the system to differentiate between the different insulins and to be clear to the participant about which insulin or dose to adjust. Management in patients using multiple injections of prandial insulin may have been more problematic than in those using only basal insulin.21
Another possible limitation of this study was that the parameters we used in the cost-effectiveness analysis were not the primary data collected directly from the study. If a major deviation existed between the contexts of the data we extracted and the study cohort, the estimation would have been imprecise. However, primary data were not collected in our study. To improve the estimations in our study and to minimize the deviations across contexts, all of the data we used in the cost-effectiveness analysis were generated from official statistics, well-established studies, and the standardized treatment arrangement used in Louisiana.
An interesting outcome was participants’ sense of accountability, which may also have been a study limitation. This was both a negative and positive factor. Knowing their blood glucose would be reviewed by someone in the medical practice may have made participants more conscious of whether their blood glucose was at goal. However, as a consequence, participants may have been reluctant to report blood glucose concentrations that were not at goal. Therefore, their adherence to reporting into the system may have been variable and may have accounted for some deterioration in glycemia after initial improvement. Interestingly, this occurred in the control group as well, emphasizing the challenges of maintaining good glycemic control in such populations. In those individuals who may not report levels, additional follow-up may be necessary to address their hesitation to report high blood glucose levels.
Further research is needed to evaluate this system for long-term sustainability and on a larger-scale intervention. We found in our study as time went on, the appeal of the system seemed to have waned. Adjustments and additions to the system could be used to increase the system’s appeal and improve adherence. Further enhancements to the system may help with long-term control. Clearly, a refinement is needed in the insulin-adjustment algorithm to help to differentiate basal long-acting insulin from rapidacting prandial insulin to allow for better management of prandial glucose with more appropriate prandial insulin adjustment. However, such adjustment is often challenging in current clinical practice as well. Even in the setting of clinical trials, it has been difficult to achieve optimal glycemic control using prandial insulin.22,23 Finally, it is important to adapt the system to include some novel ways to keep patients engaged in their own diabetes management beyond 3 months.
CONCLUSIONS
We conclude that it may be possible to use an automated remote, telephone-based system to manage glycemic control in patients attempting to improve control with insulin. The system could be scalable and cost-effective.
ACKNOWLEDGMENTS
Ms. Katalenich implemented the study and drafted the manuscript. Drs. Shi and Liu and H. Shao conducted statistical analysis and reviewed the manuscript. Ms. McDuffie and Dr. Thethi supervised study implementation and reviewed the manuscript. Dr. Carpio implemented the study and reviewed the manuscript. Dr. Fonseca designed the study, supervised study implementation, and reviewed/edited the manuscript.
This study was funded by a grant from Eli Lilly to Tulane University. There was no requirement to use Eli Lilly products in the study, and the sponsor had no role in the conduct of the study or in the analysis and interpretation of the data. This study was also supported in part by grant 1-U54-GM104940 from the National Institute of General Medical Sciences, National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Dr. Shi has received unrestricted research grants from Bristol-Myers Squibb, Cepheid, and Genentech. Ms. McDuffie has received consultant’s fees from Amgen and Novo Nordisk. Dr. Fonseca has received consultant’s fees from Abbott, AstraZeneca, Bristol-Myers Squibb, Boehringer-Ingelheim, Daiichi Sankyo, Eli Lilly, GlaxoSmithKline, Janssen, Novo Nordisk, Pamlabs, Sano., and Takeda. Tulane University Endocrinology has received grants and research support from Abbott, Asahi, Eli Lilly, EndoBarrier, Gilead, Novo Nordisk, and Sanofi.
Footnotes
Trademark of MedAdherence Inc (Norwalk, Connecticut).
CONFLICTS OF INTEREST
The authors have indicated that they have no other conflicts of interest with regard to the content of this article.
REFERENCES
- 1.Stone RA, Rao RH, Sevick MA, et al. Active care management supported by home telemonitoring in veterans with type 2 diabetes: The DiaTel randomized controlled trial. Diabetes Care. 2010;33:478–484. doi: 10.2337/dc09-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mulvaney SA, Rothman RL, Wallston KA, et al. An internet-based program to improve self-management in adolescents with type 1 diabetes. Diabetes Care. 2010;33:602–604. doi: 10.2337/dc09-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shea S, Weinstock RS, Teresi JA, et al. A randomized trial comparing telemedicine case management with usual care in older, ethnically diverse, medically underserved patients with diabetes mellitus: 5 year results of the IDEATel study. J Am Med Inform Assoc. 2009;16:446–456. doi: 10.1197/jamia.M3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farmer A, Gibson OJ, Tarassenko L, Neil A. A systematic review of telemedicine interventions to support blood glucose self-monitoring in diabetes. Diabet Med. 2005;22:1372–1378. doi: 10.1111/j.1464-5491.2005.01627.x. [DOI] [PubMed] [Google Scholar]
- 5.Health Quality Ontario. Home telemonitoring for type 2 diabetes: An evidence-based analysis. Ont Health Technol Assess Ser. 2009;9:1–38. [PMC free article] [PubMed] [Google Scholar]
- 6.de Jongh T, Gurol-Urganci I, Vodopivec-Jamsek V, et al. Mobile phone messaging for facilitating self-management of long-term illnesses. Cochrane Database Syst Rev. 2012;12:CD007459. doi: 10.1002/14651858.CD007459.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marcolino MS, Maia JX, Alkmim MB, et al. Telemedicine application in the care of diabetes patients: Systematic review and meta-analysis. PLoS One. 2013;8:e79246. doi: 10.1371/journal.pone.0079246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: A patient-centered approach: Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35:1364–1379. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies M, Storms F, Shutler S, et al. ATLANTUS Study Group. Improvement of glycemic control in subjects with poorly controlled type 2 diabetes: Comparison of two treatment algorithms using insulin glargine. Diabetes Care. 2005;28:1282–1288. doi: 10.2337/diacare.28.6.1282. [DOI] [PubMed] [Google Scholar]
- 10.American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes care. 2014;37(suppl 1):S14–S80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 11.Schmittdiel J, Vijan S, Fireman B, et al. Predicted quality-adjusted life years as a composite measure of the clinical value of diabetes risk factor control. Med Care. 2007;45:315–321. doi: 10.1097/01.mlr.0000254582.85666.01. [DOI] [PubMed] [Google Scholar]
- 12.Strange P. Treat-to-target insulin titration algorithms when initiating long or intermediate acting insulin in type 2 diabetes. J Diabetes Sci Technol. 2007;1:540–548. doi: 10.1177/193229680700100412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.CMS. 2014 Medicare part B fee schedule. Louisiana: 2014. [Google Scholar]
- 14.CMS. 2015 Medicare part B fee schedule. Louisiana: 2015. [Google Scholar]
- 15.Agency for Healthcare Research and Quality. Medical expenditure panel survey (MEPS) [Accessed May 23, 2014]; http://www.ahrq.gov/research/data/meps/index.html. [PubMed]
- 16.Nichols L, Barton PL, Glazner J, McCollum M. Diabetes, minor depression and health care utilization and expenditures: A retrospective database study. Cost Eff Resour Alloc. 2007;5:4. doi: 10.1186/1478-7547-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levin A, Chaudhry MR, Djurdjev O, et al. Diabetes, kidney disease and cardiovascular disease patients. Assessing care of complex patients using outpatient testing and visits: Additional metrics by which to evaluate health care system functioning. Nephrol Dial Transplant. 2009;24:2714–2720. doi: 10.1093/ndt/gfp180. [DOI] [PubMed] [Google Scholar]
- 18.Aikens JE, Rosland AM, Piette JD. Improvements in illness self-management and psychological distress associated with telemonitoring support for adults with diabetes. Prim Care Diabetes. 2014;9:127–134. doi: 10.1016/j.pcd.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arora S, Peters AL, Agy C, Menchine M. A mobile health intervention for inner city patients with poorly controlled diabetes: Proof-of-concept of the TExT-MED program. Diabetes Technol Ther. 2012;14:492–496. doi: 10.1089/dia.2011.0252. [DOI] [PubMed] [Google Scholar]
- 20.Steinberg DM, Levine EL, Lane I, et al. Adherence to self-monitoring via interactive voice response technology in an eHealth intervention targeting weight gain prevention among black women: Randomized controlled trial. J Med Internet Res. 2014;16:e114. doi: 10.2196/jmir.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holman RR, Farmer AJ, Davies MJ, et al. Three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med. 2009;361:1736–1747. doi: 10.1056/NEJMoa0905479. [DOI] [PubMed] [Google Scholar]
- 22.Holman RR, Thorne KI, Farmer AJ, et al. Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med. 2007;357:1716–1730. doi: 10.1056/NEJMoa075392. [DOI] [PubMed] [Google Scholar]
- 23.Raz I, Wilson PW, Strojek K, et al. Effects of prandial versus fasting glycemia on cardiovascular outcomes in type 2 diabetes: The HEART2D trial. Diabetes Care. 2009;32:381–386. doi: 10.2337/dc08-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]