Abstract
IMPORTANCE
Behavioral change interventions have demonstrated short-term efficacy in reducing sexually transmitted infection (STI)/human immunodeficiency virus (HIV) risk behaviors; however, few have demonstrated long-term efficacy.
OBJECTIVE
To evaluate the efficacy of a telephone counseling prevention maintenance intervention (PMI) to sustain STI/HIV-preventive behaviors and reduce incident STIs during a 36-month follow-up.
DESIGN, SETTING, AND PARTICIPANTS
In a 2-arm randomized supplemental treatment trial at 3 clinics serving predominantly minority adolescents in Atlanta, Georgia, 701 African American adolescent girls aged 14 to 20 years received a primary treatment and subsequently received a different (supplemental) treatment (PMI) to enhance effects of the primary treatment.
INTERVENTIONS
Participants in the experimental condition (n = 342) received an adapted evidence-based STI/HIV intervention (HORIZONS) and a PMI consisting of brief telephone contacts every 8 weeks over 36 months to reinforce and complement prevention messages. Comparison-condition participants (n = 359) received HORIZONS and a time- and dose-consistent PMI focused on general health.
MAIN OUTCOMES AND MEASURES
The primary outcomes were percentage of participants with a laboratory-confirmed incident chlamydial infection and percentage of participants with a laboratory-confirmed gonococcal infection during the 36-month follow-up. Behavioral outcomes included the following: (1) proportion of condom-protected sexual acts in the 6 months and 90 days prior to assessments; (2) number of sexual episodes during the past 90 days in which participants engaged in sexual intercourse while high on drugs and/or alcohol; and (3) number of vaginal sex partners in the 6 months prior to assessments.
RESULTS
During the 36-month follow-up, fewer participants in the experimental condition than in the comparison condition had incident chlamydial infections (94 vs 104 participants, respectively; risk ratio = 0.50; 95%CI, 0.28 to 0.88; P = .02) and gonococcal infections (48 vs 54 participants, respectively; risk ratio = 0.40; 95%CI, 0.15 to 1.02; P = .06). Participants completing more telephone contacts had a lower risk of chlamydial infection (risk ratio = 0.95; 95%CI, 0.90 to 1.00; P = .05). Participants in the experimental condition reported a higher proportion of condom-protected sexual acts in the 90 days (mean difference = 0.08; 95%CI, 0.06 to 0.11; P = .02) and 6 months (mean difference = 0.08; 95%CI, 0.06 to 0.10; P = .04) prior to assessments and fewer episodes of sexual acts while high on drugs and/or alcohol (mean difference = −0.61; 95%CI, −0.98 to −0.24; P < .001).
CONCLUSIONS AND RELEVANCE
Sustaining the long-term impact of an STI/HIV intervention is achievable with brief, tailored telephone counseling.
TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT00279799
African American adolescents have been disproportionately affected by the human immunodeficiency virus (HIV) epidemic, accounting for 73% of adolescent HIV infections, with a diagnosis rate nearly 23 times greater than white adolescents.1 To confront this “national health crisis,”2 the US National HIV/AIDS Strategy emphasizes development and dissemination of effective prevention programs, particularly for those populations most adversely affected by the HIV epidemic.3
Recent reviews indicate that behavioral interventions are effective in enhancing shorter-term (ie, ≤12 months) adoption of sexually transmitted infection (STI)/HIV–preventive behaviors among adolescents, including African American girls.4,5 However, continuation of STI/HIV-preventive behaviors during longer periods (eg, >12 months) is less common. Across adolescent STI/HIV prevention trials, the median length of follow-up is 13 weeks.5 Furthermore, in the absence of maintenance strategies, changes in STI/HIV-preventive behaviors progressively diminish.6,7 Thus, development of innovative strategies to enhance maintenance of STI/HIV preventive behaviors remains a public health priority.5,8 One promising strategy is using telephone counseling maintenance interventions.
Historically, telephone counseling maintenance interventions have been used successfully to motivate diverse health behaviors such as smoking cessation, diet, and exercise.9,10Less frequently, they have been used as boosters to initial intervention content.11,12 Although telephone counseling interventions are promising, they have not been evaluated as a maintenance strategy within the context of adolescent STI/HIV prevention. Moreover, implementing an STI/HIV maintenance intervention during adolescence may be critical, as adolescents are particularly vulnerable to STI acquisition.
Our objective was to evaluate the efficacy of a maintenance intervention using brief telephone contacts to support STI/HIV-preventive behaviors and reduce STIs among African American adolescent girls.
Methods
Participants
From June 1, 2005, to June 16, 2007, African American adolescent girls aged 14 to 20 years were recruited from 3 clinics providing sexual health services to predominantly minority adolescents in Atlanta, Georgia. An African American female recruiter approached adolescents in clinic waiting areas, described the study, solicited participation, and assessed eligibility. Eligibility criteria included self-identifying as African American, being aged 14 to 20 years at enrollment, and reporting at least 1 episode of unprotected vaginal sex in the past 6 months. Adolescents were excluded if they were married, pregnant, or attempting to become pregnant. Adolescents meeting inclusion criteria and interested in participating were scheduled to return to the clinic to complete informed consent procedures and baseline assessments and be randomized to trial conditions. Written informed consent was obtained from all adolescents. Parental consent was waived for those younger than 18 years owing to the confidential nature of clinic services. Of the 746 eligible adolescents, 701 (94.0%) enrolled, completed baseline assessments, and were randomized to study conditions (Figure 1). Participants were compensated for travel and childcare to complete assessments. Specifically, participants received $75 for completing the baseline assessment and group session, $20 for completing each of the 6-, 12-, 18-, 24-, 30-, and 36-month follow-ups, and $10 for each of the 18 individual telephone sessions. Cash payments for telephone sessions were made when the participant came for follow-up appointments. The Emory University Institutional Review Board approved all study protocols.
Figure 1. Participant Allocation CONSORT Flow Diagram.
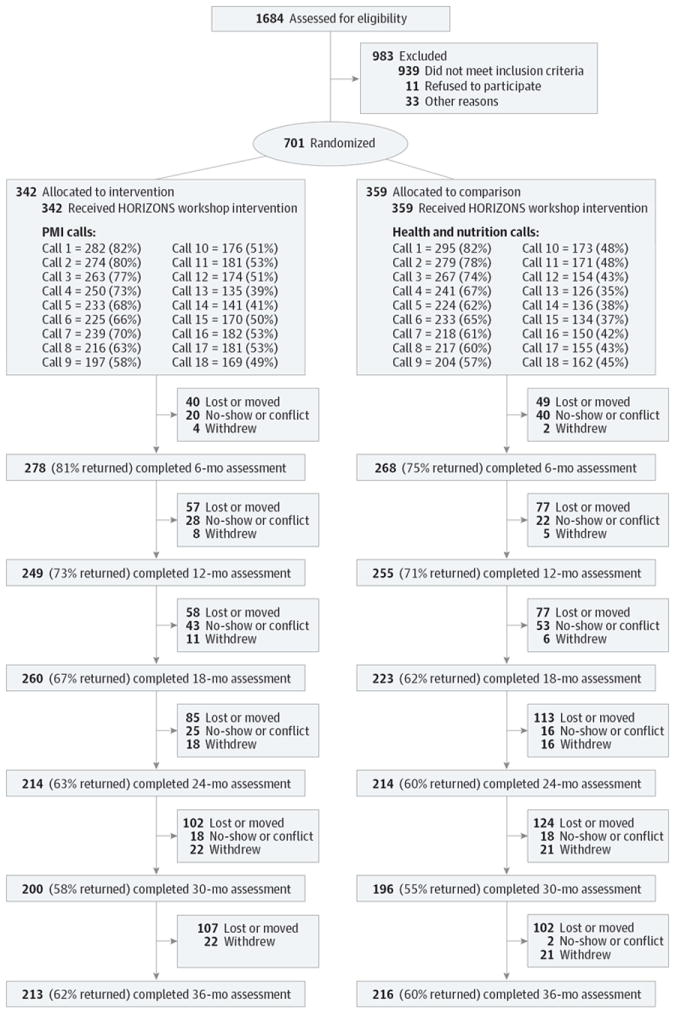
PMI indicates prevention maintenance intervention.
Study Procedures
Study Design
The study was a 2-arm randomized supplemental treatment trial in which participants received a primary treatment and subsequently received a different (supplemental) treatment to enhance effects of the primary treatment.13 Participants randomized to the experimental condition received as their primary treatment a Centers for Disease Control and Prevention (CDC)-defined evidence-based STI/HIV intervention for African American adolescent girls known as HORIZONS.14 The supplemental treatment was a newly developed prevention maintenance intervention (PMI), implemented following HORIZONS, consisting of brief, tailored telephone counseling administered every 8 weeks over 36 months (18 total telephone contacts). Participants randomized to the comparison condition also received HORIZONS as their primary treatment and a time- and dose-equivalent telephone counseling placebo intervention (general health promotion) designed to reduce the likelihood that effects of the PMI are attributable to differences in exposure to staff contact.
Random assignment of participants to trial conditions was implemented subsequent to baseline assessment using well-defined concealment of allocation procedures.15 Prior to enrollment, one of us (R.J.D.) used a computer algorithm to generate a random allocation sequence and placed the sequence in opaque envelopes and staff executed treatment assignment subsequent to baseline assessment.
Intervention
Prior to implementing the trial, both conditions were pilot tested with adolescents recruited from the study clinics to assess feasibility, acceptability, and cultural appropriateness.
The primary treatment, HORIZONS, is a group-based intervention for African American adolescent girls designed to enhance STI/HIV-preventive attitudes, sexual negotiation and refusal skills, safer sex norms, and preventive behaviors. The efficacy of HORIZONS has been evaluated in a randomized trial; findings demonstrated significant reductions in chlamydial infections and sexual risk behaviors during a 12-month follow-up.14 In the current study, HORIZONS was implemented in a single group session by 2 trained African American female health educators with, on average, 7 or 8 participants per group.
The supplemental treatment was the telephone counseling PMI. The PMI was a 10-minute, health educator-administered telephone contact guided by a risk appraisal that identified participants’ STI/HIV risks and prioritized STI/HIV prevention strategies to reduce their risk.16 For the risk appraisal procedure, participants were asked to prioritize risk factors related to sexual risk behavior engagement (eg, partners resistant to using condoms). Health educators used the prioritized list to tailor telephone counseling strategies to address identified risk factors. For the general health promotion comparison condition, the telephone sessions focused on nutrition and physical activity goals set by the participant and barriers she encountered toward achieving her goals.
Data Collection
Data collection occurred at baseline, prior to randomization, and at 6, 12, 18, 24, 30, and 36 months following participation in the primary treatment, HORIZONS. At each assessment, participants provided self-collected vaginal swab specimens for STI assessment and completed an audio computer-assisted self-administered interview (ACASI).
Participants were trained, using an anatomical model, to self-collect vaginal swab specimens.17 Specimens were assayed for 2 bacterial pathogens, Chlamydia trachomatis and Neisseria gonorrhoeae, using the BD ProbeTec ET C trachomatis and N gonorrhoeae Amplified DNA Assay (Becton, Dickinson and Co).18 Participants with a positive test result were notified within approximately 5 days of testing, provided directly observed single-dose antimicrobial treatment and risk-reduction counseling per CDC recommendations, and encouraged to refer sex partners for treatment. The county health department was notified of these reportable STIs.
Subsequently, participants completed an ACASI that assessed their sociodemographic characteristics and STI/HIV-preventive behaviors.19 Behaviors were assessed for 3-month (ie, 90 days) and 6-month intervals preceding scheduled assessments using strategies to facilitate recall and enhance validity of self-report.20 To minimize assessment bias, ACASI monitors were blinded to participants’ treatment assignment.
Outcome Measures
Efficacy of the experimental condition was assessed using biological and behavioral outcomes.21
The 2 primary outcomes were percentage of participants detected with a laboratory-confirmed incident chlamydial infection and percentage detected with a laboratory-confirmed incident gonococcal infection during the 36-month follow-up.
Behavioral outcomes included the following: (1) proportion of condom-protected sexual acts (the number of times a condom was used during vaginal intercourse in the 6 months and 90 days prior to assessments [“Out of the xx times you’ve had vaginal sex, in the past 6 months/90 days, how many times did you use a condom?”] divided by the total number of intercourse occasions during the respective periods [“In the past 6 months/90 days, how many times have you had vaginal sex?”]); (2) number of sexual episodes during the past 90 days in which participants engaged in sexual intercourse while high on drugs and/or alcohol (“In the past 90 days, how many times did you have sex while high on alcohol or drugs?”); and (3) number of vaginal sex partners in the 6 months prior to assessments (“In the past 6 months, with how many guys have you had vaginal sex?”).
Statistical Analysis
We estimated a treatment effect of 20% reduction in incident chlamydial infections during the 36-month follow-up period. Using methods outlined by Rochon22 for repeated measurements and assuming a 20%correlation for within-person measurements, 80% participation at 6-, 12-, 18-, 24-, 30-, and 36-month assessments, and setting the type I error rate at .05 for a 2-tailed test, 700 participants were needed to detect the hypothesized reduction with 80% power.
Analyses of prespecified hypotheses were carried out using an intention-to-treat protocol with participants analyzed in their assigned treatment conditions irrespective of number of completed telephone contacts.13,23 Descriptive statistics summarized sociodemographic and study variables and bivariate analyses examined differences between conditions using t tests for continuous variables and χ2 tests for categorical variables. Variables in which differences approached statistical significance (P < .10) or in which differences were theoretically and empirically associated with study outcomes were included as covariates in multivariate models.
To assess intervention effects for the entire 36-month follow-up period, we estimated random effects (per person) and generalized estimating equation models (with exchangeable correlation) to control for within-subject correlated measurements.24 Fitted models were adjusted for the corresponding baseline measure and covariates to estimate adjusted risk ratios (RRs) for treatment effects on dichotomous biological outcomes and adjusted mean differences of treatment effects on continuous behavioral outcomes. Indicators for site and cohort were included to adjust for clustering; no time-dependent variables affected by treatment were included. The 95% confidence intervals for adjusted RRs and mean differences, and corresponding P values, were also computed.
Standard errors for adjusted (least-squares) means and mean differences were estimated from adjusted means of boot-strap samples drawn with replacement at the level of the participant.25 Percentage of relative change for continuous variables, defined as the difference between the adjusted means for each condition divided by the adjusted mean for the comparison condition, provides a common metric for the magnitude of change across different measures relative to the baseline measure.
To assess intervention effects specifically on the behavioral outcomes at the 36-month follow-up, the most rigorous assessment of the PMI’s efficacy in sustaining behavior change, adjusted means and mean differences for outcomes were calculated from an estimated linear regression model. Each of these models adjusted for the corresponding baseline measure for the specific outcome, sociodemographic variables observed to differ across study conditions, and theoretically or empirically relevant variables. All analyses were performed using Stata version 12 statistical software (StataCorp LP).
Results
Baseline
Of the 701 participants randomized, 342 were allocated to the experimental condition and 359 to the comparison condition. Importantly, for inclusion in analyses, participants must have completed at least 1 follow-up assessment. No differences were observed between the conditions in the number of participants completing at least 1 follow-up (P = .44), with 309 (90.4%) of the experimental group and 318 (88.6%) of the comparison group completing at least 1 follow-up and therefore included in analyses of the primary outcomes. No differences were observed for sociodemographic characteristics, STIs, or behavioral outcomes (Table 1).
Table 1.
Comparability Between Study Conditions at Baselinea
| Characteristic | Experimental (n = 342) | Comparison (n = 359) | P Value |
|---|---|---|---|
| Sociodemographic | |||
| Age, mean (SD), y | 17.55 (1.62) | 17.73 (1.72) | .15 |
| Graduated high school, No. (%) | 123 (36.0) | 151 (42.1) | .11 |
| Family aid index, mean (SD) | 0.88 (1.00) | 0.79 (0.96) | .16 |
| Poor neighborhood quality, mean (SD) | 0.68 (1.00) | 0.64 (1.00) | .59 |
| Employed, No. (%) | 119 (34.8) | 136 (37.9) | .40 |
| Relationship | |||
| Current boyfriend, No. (%) | 276 (80.7) | 281 (78.3) | .43 |
| Current relationship duration, mean (SD), mo | 14.24 (14.96) | 14.54 (4.83) | .81 |
| Perceived partner concurrency, No. (%) | 74 (28.9) | 65 (25.5) | .39 |
| Likert scale score of general age of sex partners, mean (SD)b | 3.78 (0.80) | 3.75 (0.77) | .56 |
| Psychosocial mediator, mean (SD) | |||
| Condom use self-efficacy | 16.90 (6.86) | 16.64 (7.10) | .63 |
| Communication self-efficacy | 20.61 (3.52) | 20.52 (3.62) | .75 |
| Communication frequency | 11.56 (4.23) | 12.26 (4.37) | .03 |
| Refusal self-efficacy | 24.61 (3.27) | 24.52 (3.52) | .70 |
| Fear of condom negotiation | 8.29 (3.62) | 8.23 (2.80) | .79 |
| Sexual behavior | |||
| Condom use, mean (SD), % | |||
| Past 90 d | 47.66 (37) | 48.37 (37) | .81 |
| Past 6 mo | 48.45 (36) | 49.22 (35) | .77 |
| Consistent condom use, No. (%) | |||
| Past 90 d | 55 (16.9) | 58 (17.1) | .95 |
| Past 6 mo | 36 (10.5) | 43 (12.0) | .54 |
| Positive result for sexually transmitted infection, No. (%) | |||
| Chlamydial | 66 (19.3) | 54 (15.0) | .14 |
| Gonococcal | 18 (5.3) | 26 (7.2) | .28 |
| Other factor | |||
| Douching, No. (%) | 151 (44.2) | 147 (40.9) | .39 |
| Depression, mean (SD) | 14.56 (6.30) | 15.15 (6.69) | .22 |
| Sexual adventurism, mean (SD) | 19.23 (4.35) | 19.25 (4.29) | .94 |
| Impulsivity, mean (SD) | 38.83 (7.86) | 38.60 (7.42) | .69 |
| History of abuse, No. (%) | |||
| Emotional | 198 (57.9) | 194 (54.0) | .30 |
| Physical | 137 (40.1) | 139 (38.7) | .72 |
| Tried, No. (%) | |||
| Alcohol | 271 (79.2) | 275 (76.6) | .40 |
| Marijuana | 244 (71.3) | 265 (73.8) | .46 |
All items have been successfully used by the study team in prior human immunodeficiency virus trials,13 and full-scale descriptions and corresponding assessment questions are available on request.
Participants reported the general age of their sex partners using a Likert scale from 1, indicating much younger than the participant (≥4 years younger), to 5, indicating much older than the participant (≥4 years older).
At baseline, adolescents reported a mean of 8.16 lifetime sex partners and 27.4 episodes of vaginal sex in the previous 6 months. The mean proportion of condom-protected sexual acts was 0.49 (past 6 months). Approximately 17.1% had a laboratory-confirmed chlamydial infection and 6.3% had a laboratory-confirmed gonococcal infection.
Dose of Primary and Supplemental Treatment
The primary treatment, HORIZONS, was received by all participants in the experimental and comparison conditions. The mean (SD) number of telephone contacts (dose) received by participants was 10.78 (5.44) in the experimental condition and 9.86 (5.22) in the comparison condition (P = .02). The minimum number of telephone contacts for each condition was 0 and the maximum was 18, with 2.6% receiving 0 calls and 9.6% receiving all 18 calls in the experimental condition and 2.2% receiving 0 calls and 5.8% receiving all 18 calls in the comparison condition. The interaction of dose by treatment group was not significant in any of the models.
Attrition
Attrition across assessments by condition is presented in Figure 1. Overall, 89.7% of participants completed at least 1 follow-up assessment. Differences in attrition were observed between study conditions at 6-month follow-up (P = .03), with higher retention in the experimental condition; no differences were observed at the 12-month (P = .73), 18-month (P = .12), 24-month (P = .23), 30-month (P = .34), or 36-month (P = .57) assessment. No differences were observed for sociodemographic characteristics at the 6-, 12-, 18-, 24-, 30-, or 36-month assessment. For each condition, no differences were observed on baseline variables for participants retained in the trial compared with those unavailable for follow-up.
Outcomes
Primary Outcomes
During the 36-month follow-up, 94 adolescents in the experimental condition and 104 in the comparison condition were detected with a chlamydial infection; 48 adolescents in the experimental condition and 54 in the comparison condition were detected with a gonococcal infection. Averaged across follow-up assessments, participants in the experimental condition, relative to participants in the comparison condition, were less likely to have incident chlamydial infections (RR = 0.50; 95% CI, 0.28-0.88; P = .02) and gonococcal infections (RR = 0.40; 95% CI, 0.15-1.02; P = .06) (Table 2). Participants in both conditions receiving more telephone contacts had a greater reduction in chlamydial infections (RR = 0.95; 95% CI, 0.90-1.00; P = .05). Thus, for every telephone contact completed there was an exponential reduction in incident chlamydial infection. Specifically, a participant completing k calls had 0.95k the original risk of infection (eg, completing 1 call reduced the risk of infection by 1 − 0.951 = 0.05, or 5%, and completing 4 calls reduced the risk by 1 − 0.954 = 0.185, or 18.5%). No effect of dose was observed for gonococcal infections.
Table 2.
Effects of the Experimental Intervention on Sexually Transmitted Infection Incidence During the Entire 36-Month Follow-up
| Incident Infection From Baseline to 36 moa | Participants, No. | Participants, %
|
GEE Model
|
||
|---|---|---|---|---|---|
| Unadjusted | Adjusted | ARR (95% CI)b | P Value | ||
| Chlamydial | |||||
|
| |||||
| Experimental | 94 | 9.33 | 8.52 | 0.50 (0.28-0.88) | .02 |
|
| |||||
| Comparison | 104 | 10.52 | 10.36 | ||
|
| |||||
| Gonococcal | |||||
|
| |||||
| Experimental | 48 | 3.91 | 3.33 | 0.40 (0.15-1.02) | .06 |
|
| |||||
| Comparison | 54 | 4.50 | 5.02 | ||
Abbreviations: ARR, adjusted risk ratio; GEE, generalized estimating equation.
Number of participants detected with an incident chlamydial or gonococcal infection at the 6-, 12-, 18-, 24-, 30-, or 36-month assessment (therefore, total comparison participants with ≥1 follow-up included in analyses, n = 318; total experimental participants with ≥1 follow-up included in analyses, n = 309).
Biological outcome adjusted by baseline variables: clinic, family aid index (receipt of government assistance), years in school, partner communication frequency, unprotected vaginal sex in the past 90 days, history of emotional and physical abuse, depression, perceived partner concurrency, corresponding baseline result, and dose of telephone contacts.
Behavioral Outcomes Across the 36-Month Follow-up Period
Averaged across all follow-up assessments, participants in the experimental condition reported a higher proportion of condom-protected sexual acts in the 6 months (mean difference = 0.08; 95% CI, 0.06 to 0.10; P = .04) and 90 days (mean difference = 0.08; 95% CI, 0.06 to 0.11; P = .02) prior to follow-up assessments (Figure 2). Participants in the experimental condition also reported fewer episodes of sex while high on drugs and/or alcohol in the 90 days prior to follow-up assessments (mean difference = −0.61; 95% CI, −0.98 to −0.24; P < .001) (Figure 2) but did not significantly differ in number of partners in the 6 months prior to follow-up assessments (mean difference = −0.24; 95% CI, −0.33 to −0.15; P = .86).
Figure 2. Overall Effects on the Primary Behavioral Outcomes During the 36-Month Follow-up Period.
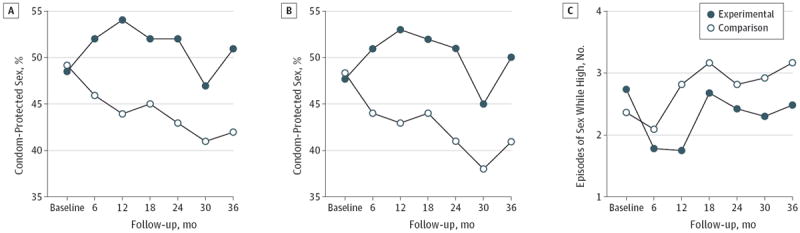
All figures represent adjusted numbers. A, Proportion of condom-protected sex in the 6 months prior to follow-up assessments. B, Proportion of condom-protected sex in the 90 days prior to follow-up assessments. C, Episodes of sex while high on drugs and/or alcohol in the 90 days prior to follow-up assessments.
Behavioral Outcomes at the 36-Month Follow-up Assessment
The 36-month follow-up assessment-specific analyses are presented in Table 3. For condom-protected sexual acts in both the 6 months and 90 days prior to assessment, significant differences were observed between conditions, with participants in the experimental condition reporting a significantly higher proportion of condom-protected sexual acts (P = .008 for the prior 90 days and P = .02 for the prior 6 months), fewer episodes of sex while high on drugs and/or alcohol in the prior 90 days (P = .01), and fewer vaginal sex partners in the prior 6 months (P = .046).
Table 3.
Effects of the Experimental Intervention on Behavioral Outcomes at the 36-Month Follow-up Assessment
| 36-mo Behavioral Outcome | Mean (SE)
|
Mean Difference (95% CI)b | Relative Change, % (95% CI)b | P Value | |||
|---|---|---|---|---|---|---|---|
| Experimental
|
Comparison
|
||||||
| Crude | Adjusteda | Crude | Adjusteda | ||||
| Proportion of condom use | |||||||
|
| |||||||
| Prior 90 d | 0.52 (0.04) | 0.50 (0.02) | 0.37 (0.04) | 0.41 (0.02) | 0.17 (0.05 to 0.29) | 0.23 (0.06 to 0.40) | .008 |
|
| |||||||
| Prior 6 mo | 0.52 (0.04) | 0.51 (0.02) | 0.39 (0.04) | 0.42 (0.02) | 0.14 (0.02 to 0.25) | 0.18 (0.03 to 0.33) | .02 |
|
| |||||||
| Episodes of sex while high on drugs and/or alcohol in prior 90 d, No. | 4.87 (1.39) | 2.49 (0.37) | 2.83 (0.81) | 3.18 (0.24) | 2.04 (0.45 to 3.63) | 0.42 (0.09 to 0.75) | .01 |
|
| |||||||
| Vaginal sex partners in prior 6 mo, No. | 1.49 (0.12) | 1.42 (0.03) | 1.60 (0.12) | 1.69 (0.03) | −0.11 (−0.22 to −0.00) | −0.07 (−0.15 to −0.00) | .046 |
Behavioral outcome adjusted by baseline variables: clinic, family aid index (receipt of government assistance), years in school, partner communication frequency, unprotected vaginal sex in the past 90 days, history of emotional and physical abuse, depression, perceived partner concurrency, corresponding baseline level of the outcome variable, and dose of telephone contacts.
Mean difference and relative change of adjusted means.
Discussion
Sustaining the long-term impact of an adapted CDC-defined evidence-based STI/HIV intervention is achievable with brief, tailored telephone counseling. Implementing a PMI with a 36-month follow-up may be particularly relevant during adolescence, a developmental period characterized by high rates of STIs. To our knowledge, this is the first trial to evaluate the efficacy of a PMI using telephone counseling and the first trial to demonstrate reductions in chlamydial infections and maintenance of STI/HIV-preventive behaviors over 36 months.
As chlamydial infections are prevalent among adolescents26 and facilitate HIV transmission,27,28 even small decreases in incidence could result in reductions in Chlamydia-associated treatment costs and reductions in HIV morbidity29 and its associated treatment costs.30 Further, modeling studies suggest that reductions in incident chlamydial infections may be a promising surrogate marker for HIV incidence in prevention trials.31
Although the characteristics of effective telephone counseling interventions have not been determined by randomized trials, a review32 suggests that greater frequency and individually tailored messages33 may be effective for sustaining newly initiated health-promoting behaviors. In our study, participants receiving the PMI completed, on average, approximately 11 telephone contacts. Reductions in chlamydial infections were also associated with higher dose; participants across conditions receiving more telephone contacts had a greater reduction in chlamydial infections. Telephone counseling may have served as an external cue reminding participants of the need to practice safer sex, reinforcing use of preventive behaviors. Exposure to a telephone counseling PMI provided risk-specific prevention strategies and targeted participants’ prioritized barriers to maintaining STI/HIV-preventive behaviors. The number of completed telephone contacts may be attributable in part to using technology that is integral to adolescents’ lifestyle, permitting increased accessibility and flexibility in scheduling contacts.
This study has a number of methodological strengths. Foremost is the use of a methodologically rigorous randomized trial incorporating a time-matched placebo comparison condition. Another is the extended follow-up of 36 months. Third is the measurement of laboratory-confirmed bacterial infections to complement self-reported STI/HIV-preventive behaviors.20,34
This study is not without limitations. First, the findings may not be applicable to African American adolescent girls with different sociodemographic characteristics or STI/HIV risk profiles. Other methodological concerns are the reliability of self-reported outcomes and the fact that the study had increasing attrition across 36 months. Not with standing that attrition levels were substantial (and every effort in future studies should aim to decrease attrition), investigations determined that inference from analyses on imputed data did not differ from analyses on complete data. Given the study design, we cannot ascertain the effects of the maintenance intervention from those of the primary intervention. Future research should focus on ascertaining the efficacy of telephone counseling alone in achieving reductions in sexual risk behaviors and STIs among this population. Finally, while an increased proportion of condom-protected sexual acts may partially explain the difference in STIs, there is the possibility that other unmeasured variables (eg, partner risk factors, STI density in certain geographical or neighborhood settings) may also contribute to differences in STIs. This should be empirically tested in future studies.
Conclusions
Efficacious behavioral change interventions will remain a mainstay to reduce STI/HIV-associated morbidity among African American adolescents. To translate STI/HIV interventions from research to practice, the CDC has developed a systematic strategy to disseminate evidence-based behavioral interventions.35 However, it is unclear whether these interventions can sustain behavioral change for protracted periods. Telephone counseling PMIs offer a potentially cost-effective strategy to provide tailored prevention information and behavioral skills coaching to sustain STI/HIV-preventive behaviors.36,37 Thus, dissemination of efficacious telephone-delivered PMIs could be a valuable adjuvant to support the prevention impact of the CDC’s evidence-based interventions. Currently there are no STI/HIV PMIs available in the toolkit of prevention approaches.
New advances in mobile technology are transforming public health.36,37 Mobile telephones, unlike other technological innovations that are often unavailable to lower socioeconomic groups, are common among adolescents; 75% own a cellular telephone and 38% make daily calls.38 Moreover, African American adolescents’ use of mobile telephones is equal to or surpasses that of their white peers.38 Thus, the increasing availability of mobile telephones suggests that PMIs can capitalize on a technology that is rapidly being adopted and integrated into African American adolescents’ lifestyle to affect observed disparities in STI/HIV.39
Prevention maintenance strategies represent one effective and efficient approach for sustaining STI/HIV-preventive behaviors. Ultimately, curtailing the HIV epidemic among adolescents will depend on how quickly and efficiently we can translate research into practice and scale up prevention efforts into sustainable programs.
Acknowledgments
Funding/Support: This work was supported by grants 5R01 MH070537 from the National Institute of Mental Health and P30 AI050409 from the Center for AIDS Research, Emory University and by the Office of Behavioral and Social Science Research, National Institutes of Health.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr DiClemente had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: DiClemente, Wingood.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: DiClemente, Sales, Brown, Rose, Davis.
Critical revision of the manuscript for important intellectual content: Wingood, Sales, Brown, Rose, Lang, Caliendo, Hardin.
Statistical analysis: DiClemente, Lang, Hardin.
Obtained funding: DiClemente, Wingood.
Administrative, technical, or material support: DiClemente, Wingood, Brown, Rose, Davis, Caliendo.
Study supervision: Wingood, Rose.
Additional Contributions: We thank the many staff members who contributed their time and energy to this project. We particularly thank the young women who gave their time to participate in the project.
Conflict of Interest Disclosures: None reported.
References
- 1.Centers for Disease Control and Prevention. Diagnoses of HIV infection and AIDS among adolescents and young adults in the United States and 5 US dependent areas, 2006-2009. HIV Surveill Suppl Rep. 2012;17(2):1–46. [Google Scholar]
- 2.Sutton MY, Jones RL, Wolitski RJ, Cleveland JC, Dean HD, Fenton KA. A review of the Centers for Disease Control and Prevention’s response to the HIV/AIDS crisis among blacks in the United States, 1981-2009. Am J Public Health. 2009;99((S2)(suppl 2)):S351–S359. doi: 10.2105/AJPH.2008.157958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White House Office of National AIDS Policy. National HIV/AIDS Strategy for the United States. Washington, DC: White House Office of National AIDS Policy; 2010. [Google Scholar]
- 4.Chin HB, Sipe TA, Elder R, et al. Community Preventive Services Task Force. The effectiveness of group-based comprehensive risk-reduction and abstinence education interventions to prevent or reduce the risk of adolescent pregnancy, human immunodeficiency virus, and sexually transmitted infections: two systematic reviews for the Guide to Community Preventive Services. Am J Prev Med. 2012;42(3):272–294. doi: 10.1016/j.amepre.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Johnson BT, Scott-Sheldon LAJ, Huedo-Medina TB, Carey MP. Interventions to reduce sexual risk for human immunodeficiency virus in adolescents: a meta-analysis of trials, 1985-2008. Arch Pediatr Adolesc Med. 2011;165(1):77–84. doi: 10.1001/archpediatrics.2010.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiClemente RJ, Brown JL, Sales JM, Rose ES. Rate of decay in proportion of condom-protected sex acts among adolescents after participation in an HIV risk-reduction intervention. J Acquir Immune Defic Syndr. 2013;63(suppl 1):S85–S89. doi: 10.1097/QAI.0b013e3182920173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldman MB, Silapaswan A, Schaefer N, Schermele D. Is there life after DEBI? examining health behavior maintenance in the diffusion of effective behavioral interventions initiative. Am J Community Psychol. 2014;53(3-4):286–313. doi: 10.1007/s10464-014-9629-3. [DOI] [PubMed] [Google Scholar]
- 8.Stanton B. Adolescent human immunodeficiency virus prevention: what we have accomplished and what still needs to be done. Arch Pediatr Adolesc Med. 2009;163(12):1162–1163. doi: 10.1001/archpediatrics.2009.226. [DOI] [PubMed] [Google Scholar]
- 9.Jeffery RW, Sherwood NE, Brelje K, et al. Mail and phone interventions for weight loss in a managed-care setting: Weigh-To-Be one-year outcomes. Int J Obes Relat Metab Disord. 2003;27(12):1584–1592. doi: 10.1038/sj.ijo.0802473. [DOI] [PubMed] [Google Scholar]
- 10.Marshall AL, Owen N, Bauman AE. Mediated approaches for influencing physical activity: update of the evidence on mass media, print, telephone and website delivery of interventions. J Sci Med Sport. 2004;7((1)(suppl)):74–80. doi: 10.1016/s1440-2440(04)80281-0. [DOI] [PubMed] [Google Scholar]
- 11.Brandon TH, Collins BN, Juliano LM, Lazev AB. Preventing relapse among former smokers: a comparison of minimal interventions through telephone and mail. J Consult Clin Psychol. 2000;68(1):103–113. doi: 10.1037//0022-006x.68.1.103. [DOI] [PubMed] [Google Scholar]
- 12.Backinger CL, Fagan P, Matthews E, Grana R. Adolescent and young adult tobacco prevention and cessation: current status and future directions. Tob Control. 2003;12(suppl 4):IV46–IV53. doi: 10.1136/tc.12.suppl_4.iv46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piantadosi S. Clinical Trials: A Methodologic Perspective. New York, NY: John Wiley & Sons; 1997. [Google Scholar]
- 14.DiClemente RJ, Wingood GM, Rose ES, et al. Efficacy of sexually transmitted disease/human immunodeficiency virus sexual risk-reduction intervention for African American adolescent females seeking sexual health services: a randomized controlled trial. Arch Pediatr Adolesc Med. 2009;163(12):1112–1121. doi: 10.1001/archpediatrics.2009.205. [DOI] [PubMed] [Google Scholar]
- 15.Schulz KF, Grimes DA. Blinding in randomised trials: hiding who got what. Lancet. 2002;359(9307):696–700. doi: 10.1016/S0140-6736(02)07816-9. [DOI] [PubMed] [Google Scholar]
- 16.Locke EA, Latham GP. Building a practically useful theory of goal setting and task motivation: a 35-year odyssey. Am Psychol. 2002;57(9):705–717. doi: 10.1037//0003-066x.57.9.705. [DOI] [PubMed] [Google Scholar]
- 17.Smith K, Harrington K, Wingood G, Oh MK, Hook EW, III, DiClemente RJ. Self-obtained vaginal swabs for diagnosis of treatable sexually transmitted diseases in adolescent girls. Arch Pediatr Adolesc Med. 2001;155(6):676–679. doi: 10.1001/archpedi.155.6.676. [DOI] [PubMed] [Google Scholar]
- 18.Van Der Pol B, Ferrero DV, Buck-Barrington L, et al. Multicenter evaluation of the BDProbeTec ET System for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in urine specimens, female endocervical swabs, and male urethral swabs. J Clin Microbiol. 2001;39(3):1008–1016. doi: 10.1128/JCM.39.3.1008-1016.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turner CF, Ku L, Rogers SM, Lindberg LD, Pleck JH, Sonenstein FL. Adolescent sexual behavior, drug use, and violence: increased reporting with computer survey technology. Science. 1998;280(5365):867–873. doi: 10.1126/science.280.5365.867. [DOI] [PubMed] [Google Scholar]
- 20.Carey MP, Carey KB, Maisto SA, Gordon CM, Weinhardt LS. Assessing sexual risk behaviour with the Timeline Followback (TLFB) approach: continued development and psychometric evaluation with psychiatric outpatients. Int J STD AIDS. 2001;12(6):365–375. doi: 10.1258/0956462011923309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fishbein M, Pequegnat W. Evaluating AIDS prevention interventions using behavioral and biological outcome measures. Sex Transm Dis. 2000;27(2):101–110. doi: 10.1097/00007435-200002000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Rochon J. Application of GEE procedures for sample size calculations in repeated measures experiments. Stat Med. 1998;17(14):1643–1658. doi: 10.1002/(sici)1097-0258(19980730)17:14<1643::aid-sim869>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 23.Pocock SJ. Clinical Trials. New York, NY: John Wiley & Sons; 1993. [Google Scholar]
- 24.Hardin JW, Hilbe JM. Generalized Estimating Equations. New York, NY: Chapman & Hall/CRC; 2003. [Google Scholar]
- 25.Efron B. Nonparametric estimates of standard error: the jackknife, the bootstrap, and other methods. Biometrika. 1981;68(3):589–599. [Google Scholar]
- 26.Weinstock H, Berman S, Cates W., Jr Sexually transmitted diseases among American youth: incidence and prevalence estimates, 2000. Perspect Sex Reprod Health. 2004;36(1):6–10. doi: 10.1363/psrh.36.6.04. [DOI] [PubMed] [Google Scholar]
- 27.Wasserheit JN. Epidemiological synergy: interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex Transm Dis. 1992;19(2):61–77. [PubMed] [Google Scholar]
- 28.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999;75(1):3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bozzette SA, Joyce G, McCaffrey DF, et al. HIV Cost and Services Utilization Study Consortium. Expenditures for the care of HIV-infected patients in the era of highly active antiretroviral therapy. N Engl J Med. 2001;344(11):817–823. doi: 10.1056/NEJM200103153441107. [DOI] [PubMed] [Google Scholar]
- 30.Chesson HW, Blandford JM, Gift TL, Tao G, Irwin KL. The estimated direct medical cost of sexually transmitted diseases among American youth, 2000. Perspect Sex Reprod Health. 2004;36(1):11–19. doi: 10.1363/psrh.36.11.04. [DOI] [PubMed] [Google Scholar]
- 31.Pinkerton SD, Layde PM. NIMH Multisite HIV Prevention Trial Group. Using sexually transmitted disease incidence as a surrogate marker for HIV incidence in prevention trials: a modeling study. Sex Transm Dis. 2002;29(5):298–307. doi: 10.1097/00007435-200205000-00009. [DOI] [PubMed] [Google Scholar]
- 32.McBride CM, Rimer BK. Using the telephone to improve health behavior and health service delivery. Patient Educ Couns. 1999;37(1):3–18. doi: 10.1016/s0738-3991(98)00098-6. [DOI] [PubMed] [Google Scholar]
- 33.Rimer BK, Conaway M, Lyna P, et al. The impact of tailored interventions on a community health center population. Patient Educ Couns. 1999;37(2):125–140. doi: 10.1016/s0738-3991(98)00122-0. [DOI] [PubMed] [Google Scholar]
- 34.Cowan FM. Adolescent reproductive health interventions. Sex Transm Infect. 2002;78(5):315–318. doi: 10.1136/sti.78.5.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collins C, Harshbarger C, Sawyer R, Hamdallah M. The diffusion of effective behavioral interventions project: development, implementation, and lessons learned. AIDS Educ Prev. 2006;18((4)(suppl A)):5–20. doi: 10.1521/aeap.2006.18.supp.5. [DOI] [PubMed] [Google Scholar]
- 36.Bull A. Technology-Based Health Promotion. Thousand Oaks, CA: Sage; 2011. [Google Scholar]
- 37.Kaiser NC, Owen JE, Winzelberg AJ. Technological advances in modifying adolescent health risk behaviors. In: DiClemente RJ, Santelli JS, Crosby RA, editors. Adolescent Health: Understanding and Preventing Risk Behaviors. San Francisco, CA: Jossey-Bass; 2009. pp. 447–472. [Google Scholar]
- 38.Lenhart A, Ling R, Campbell S, Purcell K. Teens and Mobile Phones. Washington, DC: Pew Internet & American Life Project; 2010. [Google Scholar]
- 39.Ybarra ML, Bull SS. Current trends in Internetand cell phone-based HIV prevention and intervention programs. Curr HIV/AIDS Rep. 2007;4(4):201–207. doi: 10.1007/s11904-007-0029-2. [DOI] [PubMed] [Google Scholar]


