Abstract
Cathepsin B has been demonstrated to be involved in several proteolytic processes that support tumor progression and metastasis and neurodegeneration. To further clarify its role, defined monoclonal antibodies are needed. As the primary structure of human cathepsin B is almost identical to that of the mouse, cathepsin B-deficient mice were used in a novel approach for generating such antibodies, providing the chance of an increased immune response to the antigen, human cathepsin B. Thirty clones were found to produce cathepsin B-specific antibodies. Seven of these antibodies were used to detect cathepsin B in MCF10-DCIS human breast cancer cells by immunocytochemistry and immunoblotting. Five different binding sites were identified by epitope mapping giving the opportunity to combine these antibodies in oligoclonal antibody mixtures for an improved detection of cathepsin B.
Keywords: cathepsin B, cathepsin B-deficient mice, epitope-defined monoclonal anti-cathepsin B antibodies
The lysosomal cysteine proteinases of the papain family – the cathepsins B, C, F, H, K, L, O, S, V, W and X/Z – are involved in a variety of physiological and pathological processes. Most of the cysteine cathepsins are endopeptidases and eight of them were shown to contribute to tumor development and progression (Gocheva et al., 2006 ; Watson and Kreuzaler, 2009 ; Reiser et al., 2010 ; Mullins et al., 2012). Cathepsin B (EC 3.4.22.1), which also has a peptidyl-dipeptidase activity, is constitutively expressed in normal cells and overexpressed in many human malignancies by tumor cells and tumor-associated cells at the mRNA and protein levels (Podgorski and Sloane, 2003 ; Mohamed and Sloane, 2006 ; Vasiljeva et al., 2006 ; Andl et al., 2010). Cathepsin B has been linked to apoptosis, tumor-associated inflammation, angiogenesis and tumor progression and metastasis by contributing to the altered intracellular protein metabolism of cancer cells and to proteolytic cascades in the microenvironment of tumors. In cancer cells, lysosomes are redistributed from the perinuclear area to the cellular periphery, where they can release cathepsins or be secreted into the extracellular space to contribute to matrix degradation and tumor cell invasion. Cathepsin B is a prognostic marker in several types of cancer and its increased expression by tumor cells is correlated with poor outcome, e.g., in breast cancer (Podgorski and Sloane, 2003 ; Joyce et al., 2004 ; Sloane et al., 2005 ; Nagaraj et al., 2006 ; Fehrenbacher et al., 2008 ; Malla et al., 2011 ; Sevenich et al., 2011 ; Gopinathan et al., 2012 ; Rafn and Kallunki, 2012, Rafn et al., 2012).
There is growing evidence that cathepsin B may have the potential to be a therapeutic target for reducing the malignant progression of tumor cells and for treating some kinds of metastatic cancer because ablation or inhibition of cathepsin B in tumor models decreased or delayed metastasis (Mohanam et al., 2001 ; Bervar et al., 2003 ; Fehrenbacher and Jäättelä, 2005 ; Bell-McGuinn et al., 2007 ; Vasiljeva et al., 2008 ; Gopinath et al., 2010 ; Victor et al., 2011 ; Reinheckel et al., 2012 ; Withana et al., 2012 ; Rothberg et al., 2013). The depletion of cathepsins B and L is able to completely reverse the invasive phenotype of MCF7 cells and HER2-expressing SKBR-3 and MDA-MB-453 cells (Rafn et al., 2012). The overexpression of mouse mammary tumor virus-polyoma middle T antigen (PyMT) in mouse mammary gland epithelium results in higher cathepsin B levels and increased metastasis (Vasiljeva et al., 2006 ; Sevenich et al., 2011 ; Bengsch et al., 2013).
Cathepsin B has also been shown to participate in the production of brain pyroglutamate amyloid-beta, thus contributing to the development of Alzheimer’s disease (Hook et al., 2014).
To elucidate its role in these processes, the cathepsin B protein must be efficiently and thoroughly detected, e.g., by specific antibodies. In order to get specific and high affinity mouse anti-human cathepsin B monoclonal antibodies we tried a novel approach, i.e., cathepsin B-knockout mice as the basis for generating antibodies to human cathepsin B. As the sequence of human cathepsin B differs from that of the mouse in only a few amino acids, the chance of human cathepsin B being recognized as a foreign protein by the mouse immune system is low. We, therefore, tried to provoke an immune response in cathepsin B-deficient knockout mice as the basis for the generation of anti-cathepsin B monoclonal antibodies. We also used active human cathepsin B for immunization because recombinant cathepsin B had failed in normal mice in several previous efforts to result in high affinity antibodies against native cathepsin B. Cathepsin B was purified from the supernatants of the human non-small cell lung cancer cell line 32M1 according to a novel isolation protocol (Figure 1).
Figure 1.
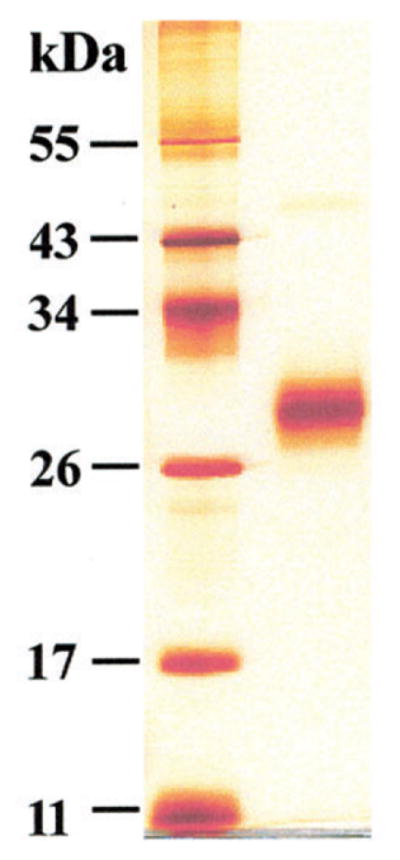
SDS-PAGE (12% gels) under reducing conditions of purified single-chain human cathepsin B.
Silver staining. Left lane: marker proteins (11, 17, 26, 34, 43, 55 kDa). Right lane: single-chain cathepsin B (0.75 μg) of a molecular mass of 31 kDa. Human procathepsin/cathepsin B was purified from the conditioned medium of the human non-small cell lung cancer cell line EPLC-32M1 according to a novel protocol. Cells (1 × 108) were cultured in RPMI-1640 medium supplemented with 10% FCS. After dialyzing the supernatants against 0.1 M glycine-buffer (pH 3.0) to degrade bovine serum albumin of the medium by activating procathepsin D, also secreted by the EPLC-32M1 cells, the supernatants were equilibrated with 50 mM Tris-HCl (pH 7.3) containing KCl (100 mM) and run on a Blue-Sepharose (GE Healthcare Europe) column to remove remnants of albumin. Procathepsin B containing fractions were collected, dialyzed against tri-ethanolamine (20 mM, pH 7.4) and fractionated with a NaCl gradient (0 to 0.15 m) on a DEAE-Sephacel (GE Healthcare Europe) column equilibrated in the same buffer. During this fractionation step procathepsin B was processed to active single-chain cathepsin B (Weber, unpublished data). Its activity was determined with Z-Arg-Arg-NHMec as substrate (10 μM Z-Arg-Arg-NHMec, 50 mM phosphate buffer, 2.5 mM DTT, 2.5 mM EDTA, pH 5.5) and its specificity with the highly selective cathepsin B inhibitor CA-074 (10 μM), which completely blocked the activity (Murata et al., 1991). The catalytic rate constant, kcat, was 172 (S−1) and the Michaelis constant, Km, was 175 μM. 3 mg purified cathepsin B were obtained from 1200 ml conditioned medium.
The antibodies were generated by a modified version of the Köhler and Milstein (1975) method. Three-month old cathepsin B-deficient mice were immunized with 20 μg of single-chain human cathepsin B emulsified in 300 μl of complete Freund’s adjuvant (CFA). The intraperitoneal immunization was repeated 48 and 117 days after the first injection and 1 day before hybridization on day 121 of the immunization process with 20 μg of the antigen in 300 μl of incomplete Freund’s adjuvant (IFA).
Mouse spleen cells (2 × 107) and cells (5 × 107) of the mouse myeloma cell line P3X63Ag8.653 were thoroughly mixed and cell fusion was mediated by polyethylene glycol 1500 (Boehringer, Mannheim, Germany). Fused cells were cultured in RPMI-based HAT selection medium (Invitrogen, Karlsruhe, Germany; 100 μM hypoxanthine, 4 mM aminopterine, 160 μM thymidine) for 3 weeks and another week in HT-medium (Invitrogen, Karlsruhe, Germany; 100 μM hypoxanthine, 160 μM thymidine). Surviving hybridomas were grown in RPMI 1640 medium supplemented with 10% of an ultra low IgG fetal calf serum (Life Technologies, Germany), HEPES (4.77 g/l), glucose (2.5 g/l), 2-mercaptoethanol (1 mM), L-glutamine (2 mM), insulin (Roche, Mannheim, Germany; 5 mg/l) and gentamycin (Serva, Heidelberg, Germany; 80 mg/l). Hybridomas were screened for the production of cathepsin B-specific antibodies in an indirect ELISA.
Thirty different anti-cathepsin B antibody-producing clones were identified among approximately 650 hybridomas. The specificity of these clones and their functionality were further examined by immunoblotting against purified human cathepsin B. Nine of these antibodies were selected for further characterization by determination of the epitopes (Figures 2 and 3 ; Table 1) they recognize, and seven of them by their performance in detecting cathepsin B in human MCF10-DCIS breast cancer cells by immunoblotting (Figure 4) and immunocytochemistry (Figure 5). These antibodies did not cross-react with cathepsins H, L and S.
Figure 2.
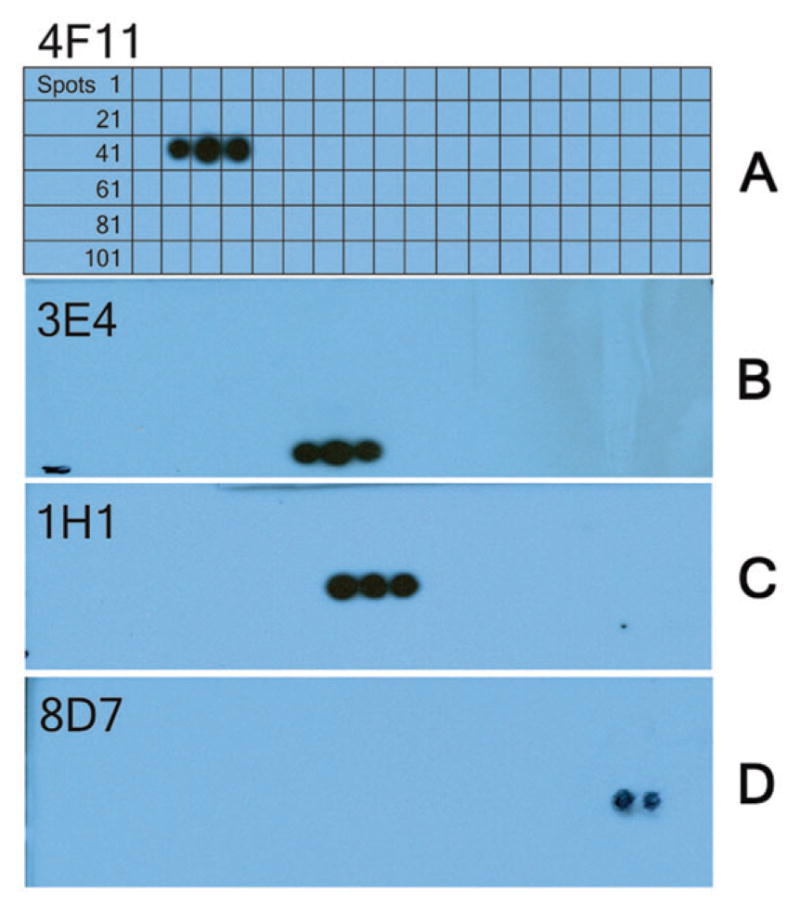
Epitope mapping.
- Spot 43: AHVSVE VSAEDLL
- Spot 44: SVE VSAEDLL TCC
- Spot 45: VSAEDLL TCCGSM
Detected sequence: V133 SAEDLL139(B) mAb 3E4; (C) mAb 1H1; (D) mAb 8D7.
Figure 3.
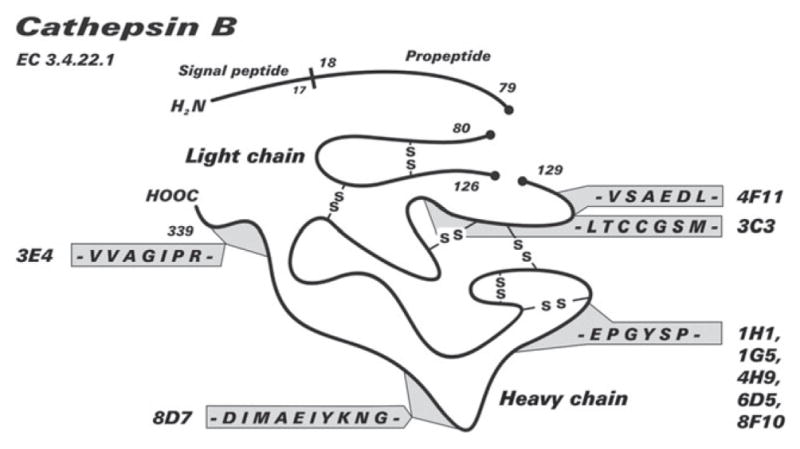
Schematic representation of the epitopes of 9 of the anti-cat B monoclonal antibodies in cathepsin B.
The sequence C211 EPGYSP is located on the surface of cathepsin B, based on the crystal structure of human cathepsin B (PDB file 1CSB-2dcd).
Table 1.
List of monoclonal antibodies and the cathepsin B epitopes they are detecting as determined by the PepSpot technique.
| 4F11 | V133 SAEDLL |
| 3C3 | E136 DLLTCCGSM |
| 1H1, 1G5, 6D5, 4H9, 8F10 | C211 EPGYSP |
| 8D7 | D238 IMAEIYKNG |
| 3E4 | V325 VAGIPR |
All antibodies are IgG1-type antibodies.
Figure 4.
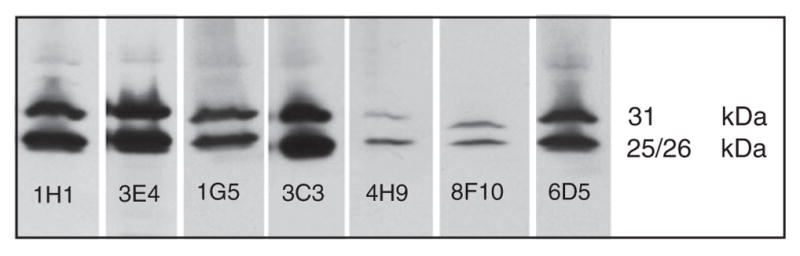
Immunoblot analysis demonstrating that the selected mAbs detect cathepsin B in lysates (0.68 μg DNA/sample) of MCF10-DCIS human breast cancer cells.
Lysates were run under reducing conditions on 12% SDS-PAGE gels at 50 mA and the separated proteins were transferred to nitrocellulose membranes. The membranes were incubated with the mAbs (1:500) for 2 h at room temperature and a secondary anti-mouse antibody (1:10 000). Detected cathepsin B was visualized through Western Lightening Plus ECL – Western Chemiluminescence Substrate. The 25/26 kDa band represents the heavy chain of the active two-chain form of cathepsin B and the 31 kDa band the active single-chain form of cathepsin B. Second step controls were negative and are not shown.
Figure 5.
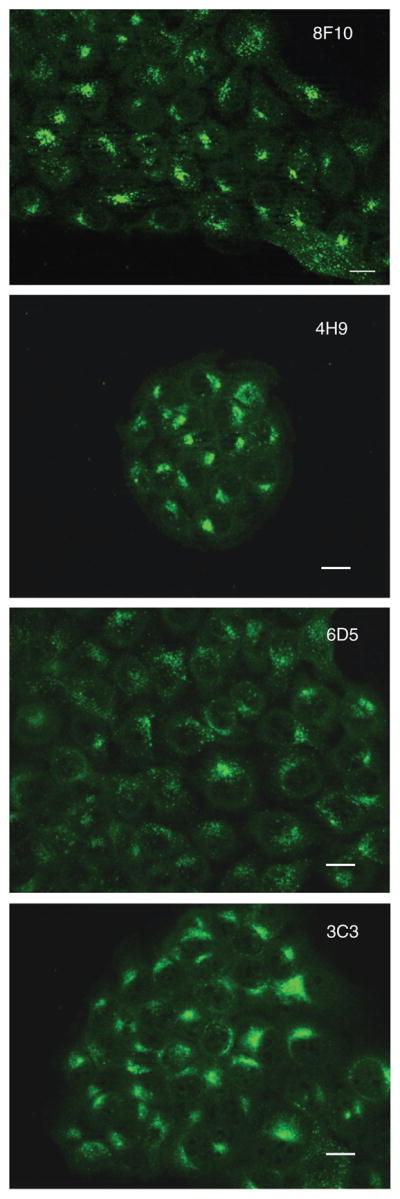
Immunofluorescence staining with the antibodies 8F10, 4H9, 6D5 and 3C3 for intracellular cathepsin B (green) in methanol fixed (−20° C) MCF10-DCIS cells cultured on glass coverslips for 48 h. When cell cultures reached 80% confluency, cells were fixed using cold methanol (−20° C) for 5 min. For blocking, non-specific binding sites cells were incubated with 0.2% BSA/PBS at room temperature for 45 min and then stained with the primary antibodies diluted 1:40 in 0.1% saponin/PBS overnight at 4° C. Excess primary antibodies were removed by washing with 0.1% saponin/PBS and the secondary antibody (1:1000) was then added for 1 h at room temperature under dark conditions. Finally, cells were washed with aqua dest. and left to dry. Coverslips were mounted on microscope slides. A 2 μl aliquot of an Antifade solution was added and clear nail polish was used to seal the edges. The cells were observed with a Zeiss LSM 310 microscope – epitome setting with optical sectioning and DIC phase contrast under oil immersion at an original magnification of 40x. Bar, 10 μM.
The PepSpot technique was used for identifying the epitopes detected by the antibodies. The sequence of procathepsin B was synthesized as a library of 110 spots of 13mer peptides onto a cellulose membrane (Jerini, Germany). The sequences of the peptides of neighboring spots were overlapping to ensure the continuous presentation of the complete procathepsin B sequence. After washing the membrane with a casein-based blocking buffer, it was incubated with the primary antibody (1 μg/ml) and after washing with Tween-TBS buffer with HRP-conjugated secondary antibody. The chemiluminescent light detection of the recognized spots was performed with a FujiFilm LAS4000 luminescent image analyzer directly from the blots.
A direct comparison of the performance of the obtained antibodies in immunoblotting and immunocytochemistry revealed only minor differences in their effectiveness in detecting cathepsin B. They all recognize cathepsin B. Based on immunoblotting, mAb 3E4 clearly is the most effective, followed by 1H1, 1G5, 6D5 and 3C3. MAb 8F10 and 4H9 gave the weakest signal and had the lowest efficacy in recognizing cathepsin B in immunoblots, whereas in immunofluorescence mAB 4H9 and 8F10 are the most effective in detecting the protein. Their signals in the images were bright, and punctuate staining was well-defined in the periphery of all cells. All other mAbs, i.e., 3C3, 6D5, 1H1, 1G5 and 3E4, also detected cathepsin B.
With this novel approach we were able to generate a panel of monoclonal anti-human cathepsin B antibodies that proved to be able to detect cathepsin B in standard techniques like immunoblotting and immunofluorescence. The determination of the binding sites in the cathepsin B molecule revealed that these selected antibodies recognized five different epitopes (Figure 3). This allows the combination of single monoclonal antibodies in oligoclonal mixtures in order to enhance the intensity of the analytical signals in immunoblotting or immunocytochemistry or both. We saw that the signals were more intense when several combinations of these antibodies were used, clearly improving the detection of cathepsin B.
Contributor Information
Ekkehard Weber, Institute of Physiological Chemistry Medical Faculty, Martin Luther University Halle-Wittenberg, Hollystrasse 1, D-06097 Halle, Germany.
Elena Barbulescu, Department of Pharmacology, Wayne State University School of Medicine, Detroit, MI 48201, USA.
Rita Medek, Institute of Physiological Chemistry, Medical Faculty, Martin Luther University Halle-Wittenberg, D-06097 Halle, Germany.
Thomas Reinheckel, Institute of Molecular Medicine and Cell Research, Albert Ludwigs University Freiburg, D-79104 Freiburg, Germany.
Mansoureh Sameni, Department of Pharmacology, Wayne State University School of Medicine, Detroit, MI 48201, USA.
Arulselvi Anbalagan, Department of Pharmacology, Wayne State University School of Medicine, Detroit, MI 48201, USA.
Kamiar Moin, Department of Pharmacology, Wayne State University School of Medicine, Detroit, MI 48201, USA.
Bonnie F. Sloane, Department of Pharmacology, Wayne State University School of Medicine, Detroit, MI 48201, USA
References
- Andl CD, McCowan KM, Allison GL, Rustgi AK. Cathepsin B is the driving force of esophageal cell invasion in a fibroblast-dependent manner. Neoplasia. 2010;12:485–498. doi: 10.1593/neo.10216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell-McGuinn KM, Garfall AL, Bogyo M, Hanahan D, Joyce JA. Inhibition of cysteine cathepsin protease activity enhances chemotherapy regimens by decreasing tumor growth and invasiveness in a mouse model of multistage cancer. Cancer Res. 2007;67:7378–7385. doi: 10.1158/0008-5472.CAN-07-0602. [DOI] [PubMed] [Google Scholar]
- Bengsch F, Buck A, Günther SC, Seiz JR, Tacke M, Pfeifer D, von Elverfeldt D, Sevenich L, Hillebrand LE, Kern U, et al. Cell type-dependent pathogenic functions of overexpressed human cathepsin B in murine breast cancer progression. Oncogene. 2013 doi: 10.1038/onc.2013.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bervar A, Zajc I, Sever N, Katunuma N, Sloane BF, Lah TT. Invasiveness of transformed human breast epithelial cell lines is related to cathepsin B and inhibited by cysteine proteinase inhibitors. Biol Chem. 2003;384:447–455. doi: 10.1515/BC.2003.050. [DOI] [PubMed] [Google Scholar]
- Fehrenbacher N, Jäättelä M. Lysosomes as targets for cancer therapy. Cancer Res. 2005;65:2993–2995. doi: 10.1158/0008-5472.CAN-05-0476. [DOI] [PubMed] [Google Scholar]
- Fehrenbacher N, Bastholm L, Kirkegaard-Soerensen T, Weber E, Shirasawa S, Kalulunki T, Jäättelä M. Sensitization to lysosomal cell death by oncogene-induced down-regulation of LAMP-1 and -2. Cancer Res. 2008;68:6623–6633. doi: 10.1158/0008-5472.CAN-08-0463. [DOI] [PubMed] [Google Scholar]
- Gocheva V, Zeng W, Ke D, Klimstra D, Reinheckel T, Peters C, Hanahan D, Joyce JA. Distinct roles for cysteine cathepsin genes in multistage tumorigenesis. Genes Dev. 2006;20:543–556. doi: 10.1101/gad.1407406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath S, Malla RR, Gondi CS, Alapati K, Fassett D, Klopfenstein JD, Dinh DH, Gujrati M, Rao JS. Co-depletion of cathepsin B and uPAR induces G0/G1 arrest in glioma via FOXO3a mediated p27 upregulation. PLoS One. 2010;5:e11668. doi: 10.1371/journal.pone.0011668. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gopinathan A, Denicola GM, Frese KK, Cook N, Karreth FA, Mayerle J, Lerch MM, Reinheckel T, Tuveson DA. Cathepsin B promotes the progression of pancreatic ductal adenocarcinoma in mice. Gut. 2012;61:877–884. doi: 10.1136/gutjnl-2011-300850. [DOI] [PubMed] [Google Scholar]
- Hook G, Yu J, Toneff T, Kindy M, Hook V. Brain pyroglutamate amyloid-beta is produced by cathepsin B and is reduced by the cysteine protease inhibitor E64d, representing a potential Alzheimer’s Disease therapeutic. J Alzheimer’s Dis. 2014;41:129–149. doi: 10.3233/JAD-131370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce JA, Baruch A, Chehade K, Meyer-Morse N, Giraudo E, Tsai FY, Greenbaum DC, Hager JH, Bogyo M, Hanahan D. Cathepsin cysteine proteases are effectors of invasive growth and angiogenesis during multistage tumorigenesis. Cancer Cell. 2004;5:443–453. doi: 10.1016/s1535-6108(04)00111-4. [DOI] [PubMed] [Google Scholar]
- Köhler G, Milstein C. Continuous culture of fused cells secreting antibodies of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Malla RR, Gopinath S, Gondi CS, Alapati K, Dinh DH, Gujrati M, Rao JS. Cathepsin B and uPAR knockdown inhibits tumor-induced angiogenesis by modulating VEGF expression in glioma. Cancer Gene Ther. 2011;18:419–434. doi: 10.1038/cgt.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed MM, Sloane BF. Cysteine cathepsins: multifunctional enzymes in cancer. Nat Rev Cancer. 2006;6:764–775. doi: 10.1038/nrc1949. [DOI] [PubMed] [Google Scholar]
- Mohanam S, Jasti SL, Kondraganti SR, Chandrasekar N, Lakka SS, Kin Y, Fuller GN, Yung AW, Kyritsis AP, Dinh DH, et al. Down-regulation of cathepsin B expression impairs the invasive and tumorigenic potential of human glioblastoma cells. Oncogene. 2001;20:3665–3673. doi: 10.1038/sj.onc.1204480. [DOI] [PubMed] [Google Scholar]
- Mullins SR, Sameni M, Blum G, Bogyo M, Sloane BF, Moin K. Three-dimensional cultures modeling premalignant progression of human breast epithelial cells: role of cysteine cathepsins. Biol Chem. 2012;393:1405–1416. doi: 10.1515/hsz-2012-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata M, Miyashita S, Yokoo C, Tamai M, Hanada K, Hatayama K, Towatari T, Nikawa T, Katanuma N. Novel epoxysuccinyl peptides. Selective inhibitors of cathepsin B in vitro. FEBS Lett. 1991;280:307–310. doi: 10.1016/0014-5793(91)80318-w. [DOI] [PubMed] [Google Scholar]
- Nagaraj NS, Vigneswaran N, Zacharias W. Cathepsin B mediates TRAIL-induced apoptosis in oral cancer cells. J Cancer Res Clin Oncol. 2006;132:171–183. doi: 10.1007/s00432-005-0053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podgorski I, Sloane BF. Cathepsin B and its role(s) in cancer progression. Biochem Soc Symp. 2003;70:263–276. doi: 10.1042/bss0700263. [DOI] [PubMed] [Google Scholar]
- Rafn B, Kallunki T. A way to invade: a story of ErbB2 and lysosomes. Cell Cycle. 2012;11:2415–2416. doi: 10.4161/cc.20860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafn B, Nielsen CF, Andersen SH, Szyniarowski P, Corcelle-Termeau E, Valo E, Fehrenbacher N, Olsen CJ, Daugaard M, Egebjerg C, et al. ErbB2-driven breast cancer cell invasion depends on a complex signaling network activating myeloid zinc finger-1-dependent cathepsin B expression. Mol Cell. 2012;45:764–776. doi: 10.1016/j.molcel.2012.01.029. [DOI] [PubMed] [Google Scholar]
- Reinheckel T, Peters C, Krüger A, Turk B, Vasiljeva O. Differential impact of cysteine cathepsins on genetic mouse models of de novo carcinogenesis: cathepsin B as emerging therapeutic target. Front Pharmacol. 2012;3:133. doi: 10.3389/fphar.2012.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser J, Adair B, Reinheckel T. Specialized roles for cysteine cathepsins in health and disease. J Clin Invest. 2010;120:3421–3431. doi: 10.1172/JCI42918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg JM, Bailey KM, Wojtkowiak JW, Ben-Nun Y, Bogyo M, Weber E, Moin K, Blum G, Mattingly RR, Gillies RJ, et al. Acid-mediated tumor proteolysis: contribution of cysteine cathepsins. Neoplasia. 2013;15:1125–1137. doi: 10.1593/neo.13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevenich L, Werner F, Gajda M, Schurigt U, Sieber C, Müller S, Follo M, Peters C, Reinheckel T. Transgenic expression of human cathepsin B promotes progression and metastasis of polyoma-middle-T-induced breast cancer in mice. Oncogene. 2011;30:54–64. doi: 10.1038/onc.2010.387. [DOI] [PubMed] [Google Scholar]
- Sloane BF, Yan S, Podgorski I, Linebaugh BE, Cher M-L, Mai J, Cavallo-Medved D, Sameni M, Dosescu J, Moin K. Cathepsin B and tumor proteolysis: contribution of the tumor microenvironment. Semin Cancer Biol. 2005;15:149–157. doi: 10.1016/j.semcancer.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Vasiljeva O, Papazoglou A, Kruger A, Brodoefel H, Korovin M, Deussing J, Augustin N, Nielsen BS, Almholt K, Bogyo M, et al. Tumor cell-derived and macrophage-derived cathepsin B promotes progression and lung metastasis of mammary cancer. Cancer Res. 2006;66:5242–5250. doi: 10.1158/0008-5472.CAN-05-4463. [DOI] [PubMed] [Google Scholar]
- Vasiljeva O, Korovin M, Gajda M, Brodoefel H, Bojic L, Kruger A, Schurigt U, Sevenich L, Turk B, Peters C, et al. Reduced tumour cell proliferation and delayed development of high-grade mammary carcinomas in cathepsin B-deficient mice. Oncogene. 2008;27:4191–4199. doi: 10.1038/onc.2008.59. [DOI] [PubMed] [Google Scholar]
- Victor BC, Anbalagan A, Mohamed MM, Sloane BF, Cavallo-Medved D. Inhibition of cathepsin B activity attenuates extracellular matrix degradation and inflammatory breast cancer invasion. Breast Cancer Res. 2011;13:R115. doi: 10.1186/bcr3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CJ, Kreuzaler PA. The role of cathepsins in involution and breast cancer. J Mammary Gland Biol Neoplasia. 2009;14:171–179. doi: 10.1007/s10911-009-9126-8. [DOI] [PubMed] [Google Scholar]
- Withana NP, Blum G, Sameni M, Slaney C, Anbalagan A, Olive MB, Bidwell BN, Edgington L, Wang L, Moin K, et al. Cathepsin B inhibition limits bone metastasis in breast cancer. Cancer Res. 2012;72:1199–1209. doi: 10.1158/0008-5472.CAN-11-2759. [DOI] [PMC free article] [PubMed] [Google Scholar]


