Abstract
The purpose of this study was to examine exercise-induced pain modulation in diabetic adults with painful diabetic neuropathy (PDN) compared to diabetic adults without PDN. Eighteen adults diagnosed with Type 2 diabetes with and without PDN (mean age of 49 yrs) completed two sessions. During the familiarization session, participants completed questionnaires, were familiarized with the pain testing protocols, and completed maximal isometric contractions. During the exercise session, experimental pain testing was completed before and following exercise consisting of three minutes of isometric exercise performed at 25% MVC. Ratings of perceived exertion (RPE) and muscle pain (MP) were assessed every 30 seconds during exercise. Results indicated RPE and MP during exercise were significantly higher (p < 0.05) for diabetic adults with PDN vs diabetic adults without PDN. Diabetic adults with PDN did not experience changes in thermal pain ratings following exercise while diabetic adults without PDN reported significantly lower pain ratings following exercise. It is concluded that diabetic adults with PDN experienced high levels of muscle pain during exercise and a lack of exercise-induced hypoalgesia following exercise in comparison to diabetic adults without PDN who experienced lower levels of muscle pain during exercise and a hypoalgesic response following exercise.
Keywords: diabetes, neuropathic pain, exercise-induced analgesia
INTRODUCTION
One of the most common complications of diabetes is neuropathy which may result in pain, loss of mobility and amputation.1 Research indicates that adults with painful diabetic neuropathy have annual health care costs almost three times higher than costs for matched control populations.2 Painful diabetic neuropathy (PDN) has proven difficult to treat and in the majority of patients, existing therapies for neuropathic pain are far from effective.3 It is well-established that lifestyle changes which include exercise can significantly reduce the prevalence of diabetes and associated complications such as neuropathy, however, very little research has focused on the effects of exercise on PDN. Results from several animal studies suggest that an exercise training program reduces behavioral symptoms of neuropathic pain and tactile sensitivity.28, 37, 38, 40 In addition, one human study indicated improvements in neuropathic symptoms and cutaneous nerve fiber branching following an exercise training program.20
Despite the limited number of studies conducted examining the impact of exercise on pain in individuals with PDN, there is research indicating that exercise is associated with an attenuation of pain in healthy individuals,11, 16, 22, 23, 25, 35 and investigators have typically found hypoalgesia (diminished pain in response to a noxious stimulus) occurs during and/or following an exercise session. Various modes of exercise have been used including aerobic exercise (e.g., cycling, running), resistance exercise (e.g., weight lifting), and isometric exercise (e.g., static muscular contraction), and results indicate that exercise-induced hypoalgesia (EIH) occurs during and/or following a single exercise session.5, 16, 22, 23, 24, 25, 35 Limited research is available, however, examining changes in pain after an immediate (i.e., acute) bout of exercise in individuals with PDN. In fact, there are reports that individuals with chronic pain (e.g., fibromyalgia, temporomadibular disorders) may experience a hyperalgesic response during and/or following an exercise session,18, 26, 32, 42 and it has been suggested that this hyperalgesic response may be a reflection of endogenous pain modulatory system dysfunction.32,34,42 Whether these results generalize to individuals with PDN is currently unclear. Further research is needed to expand our understanding of the impact of exercise on PDN. Thus, the purpose of this study was to examine exercise-induced pain modulation in diabetic adults with and without painful diabetic neuropathy.
MATERIALS AND METHODS
Participants
All protocols, methods, and procedures were approved by the Health Sciences Institutional Review Board (IRB) at the University of Wisconsin – Madison. Adults (18 – 60 yrs) clinically diagnosed with type 2 diabetes mellitus with and without PDN by their primary care physician were recruited to participate in this study. Exclusion criteria included: 1) any acute medical or psychiatric illness, 2) uncontrolled chronic medical conditions (e.g., hypertension, heart disease, hypercholestrolemia, hyperglycemia, etc.), 3) upper extremity neuropathy or arthritis that limits exercise, 4) neuropathies from other causes than diabetes, and 5) cognitive or reading impairments which would preclude completing the questionnaires. Participants were recruited from university hospital and clinics, diabetes clinics, and community health care centers. Prior to participating in the study, informed consent was obtained from each participant. Participants who enrolled in this study completed two sessions (i.e., familiarization and exercise sessions) and were paid $25 for completing both sessions.
Procedures
Familiarization Session
During the familiarization session, participants completed an informed consent document and a packet of questionnaires which consisted of: 1) a Demographic, Pain, and Health History, 2) a 24-Hour Health History, 3) the Short Form McGill Pain Questionnaire (SF-MPQ),31 4) the Profile of Mood States (POMS),30 and 5) the International Physical Activity Questionnaire (IPAQ).6, 13, 15 In addition, participants completed three neuropathic pain questionnaires including the Neuropathic Pain Questionnaire (NPQ),27 the painDETECT(PD-Q),14 and the Neuropathic Pain Symptom Inventory (NPSI)8 in order to assess various aspects of neuropathic pain (e.g., symptoms, gradation of pain, the pain course pattern, etc).
Upon completion of the packet of questionnaires, participants were exposed to the thermal stimuli in order to familiarize them with the pain testing to be completed in the exercise session. A thermal stimulus using a computer controlled Medoc Sensory Analyzer (CHEPS model) was applied to the thenar eminence of the dominant hand and volar suface of the dominant forearm. Two different thermal protocols were used because the manner in which stimuli are applied can influence responses (e.g., Type-III vs Type-IV responses). One protocol that was used was the Ramp and Hold protocol which activates predominantly Type-III (Aδ) nociceptors.51 Stimulus response functions of the volar surface of the forearm were determined by rapidly increasing the thermode temperatures (10 – 20°C/sec) from a baseline of 32 – 35°C to four different peak temperatures ranging between 42 – 49°C and holding the temperature for four seconds. The second protocol involved administration of brief, repetitive suprathreshold heat pulses that evoked Type-IV (C-fiber) pain summation (i.e., temporal summation). A thermode was programmed to deliver pulses that rapidly rise from an adapting temperature to a peak temperature of 51°C at a rate of 30°C/sec, remain at this level for 0.25sec, and then return to baseline at a rate of 30°C/sec. Ten heat pulses were delivered to the thenar eminence of the hand according to the adjusted protocol described by Staud et al. (2006).42 The temperature of the thermode increased during the first four stimuli in this manner: 35°C (baseline) to 45°C (peak temperature) by 30°C/pulse (1st stimulus), 36°C to 47°C (2nd stimulus), 37°C to 48°C (3rd stimulus), 38°C to 49°C (4th stimulus). The baseline and peak temperatures of the 6 remaining stimuli were 38°C and 51°C. During exposure to the thermal stimuli, participants rated late sensations of pain (approx 1 second after each pulse) using a 0 – 100 numerical scale.41, 49 Participants then completed a recovery period (sat quietly for 3 minutes). Following the recovery period, participants completed the two thermal testing protocols again to ensure that the protocols were set and participants were familiar with the testing to be completed in the exercise session.
Finally, participants were asked to squeeze a hand dynamometer with their dominant hand at their maximum strength for five seconds two times, with a two minute interval between the two trials. The average of the two maximum strength values was used to compute the participant’s 25% MVC for the next session. Prior to leaving, participants were given a pedometer to wear for 5 consecutive days (Saturday – Wednesday) to record the number of steps taken per day, as well as a physical activity log to record any exercise performed on those days.
Exercise Session
In the isometric exercise session, participants returned the pedometer and physical activity log, and then completed a 24-Hour Health History and the Short Form McGill Questionnaire (SF-MPQ). Participants then underwent thermal pain testing prior to exercise. Next, participants squeezed the hand dynamometer at their respective 25% MVC for 3 minutes. Participants were asked to rate their muscle pain and perceived exertion every 30 seconds during exercise using validated rating scales. Exercise-induced muscle pain was assessed during the exercise session using a pain intensity scale.10 The scale developed by Cook et al. (1997) consists of 11 vertically aligned numerical responses that range from 0 to 10, with verbal descriptors attached to nine numerical responses. This scale represents a continuum from no pain sensation to unbearable levels of pain, and allows participants to use a number greater than 10. The pain intensity scale has been shown to be a reliable and valid measure of muscle pain during exercise.10 In addition, perceived exertion was assessed using the Ratings of Perceived Exertion (RPE) scale. The RPE scale developed by Borg (1998) consists of 15 vertically aligned numerical responses that range from 6 – 20, with verbal descriptors attached to nine numerical responses. The RPE scale has been shown to be a reliable and valid measure to assess an individual’s perceived exertion during exercise.7 Immediately following the isometric exercise session, the participants underwent experimental pain testing once more and then completed the SF-MPQ.
Statistical Analyses
Descriptive statistics were computed for the neuropathic pain measures and independent samples t-tests were performed to analyze whether there were differences between groups. Muscle pain responses and RPE were assessed every 30 seconds during the 3 minute isometric exercise session and the data were analyzed with a 2 (groups) × 6 (time: every 30 seconds during isometric exercise) ANOVA. For the Ramp and Hold protocol, participants rated pain intensity before and following exercise to four different temperatures, and this data were analyzed with a 2 (groups) × 2 (trials: pre and post) × 4 (temperatures) mixed ANOVA. For the Temporal Summation protocol, participants rated pain intensity before and following exercise to 10 heat pulses, and this data were analyzed with a 2 (groups) × 2 (trials: pre and post) × 3 (ratings 1, 5, and 10) mixed ANOVA. The data for the SF-MPQ (Visual Analog Scale, Present Pain Index, and SF-MPQ Total), were analyzed using a 2 (groups) × 2 (trials) repeated measures ANOVA. The data for the pedometers were analyzed using a 2 (groups) × 5 (days) repeated measures ANOVA. The data for the POMS were analyzed using independent samples t-tests to determine if there were differences between groups. The data for the IPAQ were analyzed using a Mann-Whitney U Test, in order to determine differences between groups. The level of statistical significance was set at α = 0.05 for all analyses. Post-hoc analyses were performed if significant main effects or between group differences were observed and Bonferroni corrections for multiple comparisons were made.
RESULTS
Eighteen individuals diagnosed with Type 2 diabetes with and without PDN who participated in this study had a mean age of 49 yrs (sd = 11). Six men and 12 women participated in this study and the racial/ethnic composition of the sample included 11 Caucasian participants, 3 African American participants, 3 Asian participants, and one Latino participant. All of the participants reported taking insulin in order to control Type 2 diabetes, and participants with PDN reported taking a variety of medications to treat painful neuropathy including: gabapentin, amitritylene, acetomenophen, nortryptyline, and amlodipine. Results indicated significant group differences for the Neuropathic Pain Questionnaire, Neuropathic Pain Symptom Inventory, and painDETECT Questionnaire (p < .05). No significant group differences were found for age, duration of diabetes, and BMI (p > 0.05). The baseline data for the participants are summarized in Table 1.
Table 1.
Means and Standard Deviations for the Baseline Data
| Diabetics | PDN | |
|---|---|---|
| M (SD) | M (SD) | |
| Age (yrs) | 46 (13) | 53 (7) |
| Duration of Diabetes (yrs) | 6 (6) | 6 (3) |
| Duration of PDPN (yrs) | 0 (0) | 3 (2) |
| Blood Pressure (SBP/DBP mmHg) | 119/76 (13/3) | 119/75 (11/5) |
| Heart Rate (bpm) | 71 (2) | 72 (2) |
| BMI (0 – 100) | 38 (10) | 34 (9) |
| NPQ (−1.41 – 10.00)* | −1.41 (0) | 0.32 (1.2) |
| NPSI (0 – 100)* | 0 (0) | 18 (10) |
| PD-Q (0 – 35)* | 0 (0) | 13 (5) |
Denotes significance at the p < .05 level.
PDN = Painful Diabetic Neuropathy; SBP = Systolic Blood Pressure; DBP = Diastolic Blood Pressure; BMI = Body Mass Index; NPQ = Neuropathic Pain Questionnaire; NPSI = Neuropathic Pain Symptom Inventory; PD-Q = painDETECT Questionnaire; M = Mean; SD = Standard Deviation; yrs = Years; bpm = Beats Per Minute; mmHg = Millimeters of Mercury
Muscle Pain Ratings During Exercise
Muscle pain was assessed every 30 seconds during exercise. The results indicated that diabetic adults with PDN rated muscle pain significantly higher (p < 0.05) in comparison to diabetic adults without PDN. The results for muscle pain ratings are summarized in Figure 1.
Figure 1.
Means and Standard Errors for Muscle Pain Ratings During Exercise (Muscle Pain Ratings scale = 0 – 10). *Denotes significance at the p < .05 level.
Ratings of Perceived Exertion
Ratings of perceived exertion were measured every 30 seconds during exercise. The results indicated that RPE increased significantly during exercise for both groups (p < .01). In addition, diabetic adults with PDN rated isometric exercise as more effortful at 180 seconds during exercise in comparison to diabetic adults without PDN (p < .05).
Experimental Pain Ratings: Ramp & Hold and Temporal Summation Protocols
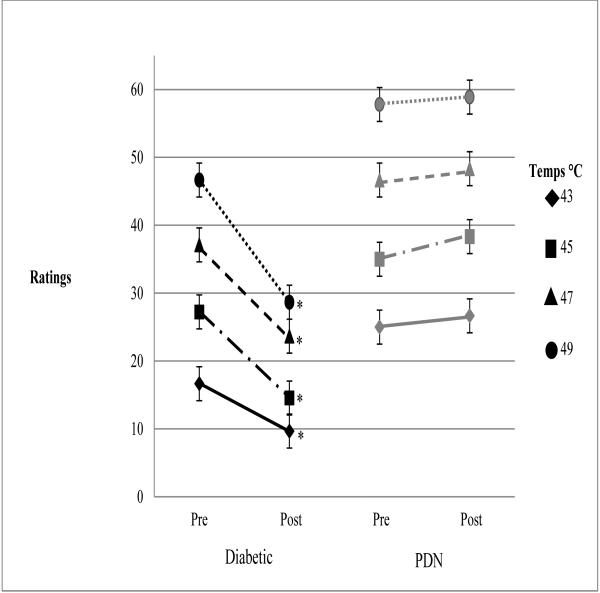
For the ramp and hold protocol, experimental pain ratings were assessed before and following exercise (i.e., trials) using four separate temperatures (43°C, 45°C, 47°C, and 49°C). The results indicated that diabetic adults with PDN rated experimental pain significantly higher (p < 0.05) than diabetic adults without PDN. Further, pain ratings for all four temperatures decreased significantly (p < 0.05) following exercise in diabetic adults without PDN, but there were no changes in pain ratings following exercise in diabetic adults with PDN. The results for the four temperatures are summarized in Figure 3.
Figure 3.
Means and Standard Errors for Ramp and Hold. *Denotes significance at the p < .05 level.
For the temporal summation protocol, experimental pain ratings for the first, fifth and tenth pulses were analyzed before and following exercise. The results indicated that temporal summation pain ratings were significantly lower (p < 0.05) following exercise for the diabetic adults without PDN, however, for diabetics with PDN no significant differences were found after exercise. The results for temporal summation of heat pain are summarized in Figure 4.
Figure 4.
Means and Standard Errors for Temporal Summation of Heat Pain. *Denotes significance at the p < .05 level.
Current Pain
Current sites of pain were assessed with the Demographic, Pain, and Health History questionnaire and included: dental pain (PDN = 5, No PDN = 3), headaches (PDN = 4, No PDN = 4), back pain (PDN = 5, No PDN = 3), knee pain (PDN = 3, No PDN = 3), and shoulder or neck pain (PDN = 2, No PDN = 0). In addition, the SF-MPQ was completed before and following exercise. The SF-MPQ assesses total pain with affective and sensory descriptors, a visual analog scale (VAS), and a present pain index (PPI). The results indicated that ratings were significantly lower following exercise for diabetic adults without PDN for the SF-MPQ total, VAS, and PPI; however, for diabetic adults with PDN only PPI ratings were significantly lower following exercise. The results for the VAS, PPI and SF-MPQ Total are summarized in Table 2.
Table 2.
Means and Standard Deviations for the VAS, PPI, and SF-MPQ Total
| Diabetics | PDN | |||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| M (SD) | M (SD) | M (SD) | M (SD) | |
| VAS (mm 0 – 100) | 13.22 (13.85) | 7.56 (9.66)* | 18.11 (16.14) | 11.89 (13.16) |
| PPI (0 – 5) | 1.00 (0.71) | 0.67 (0.50) * | 1.33 (0.71) | 1.00 (0.50)* |
| SF-MPQ Total (0 – 45) | 2.44 (1.94) | 1.22 (1.30)* | 4.00 (3.96) | 3.33 (2.24) |
Denotes significance at the p < .05 level.
PDN = Painful Diabetic Neuropathy; Pre = Pre-exercise; Post = Post-exercise; M = Mean; SD = Standard Deviation
Physical Activity
Physical activity levels were measured via self-report (i.e., IPAQ) and with a pedometer (i.e., steps per day). Results indicated diabetic adults with PDN reported significantly more Total MET-mins/wk on the IPAQ in comparison to the diabetic adults without PDN (p < 0.01), However, no significant differences were found in the other activity domains of the IPAQ. Results for the IPAQ data are summarized in Table 3.
Table 3.
Medians and Interquartile Ranges for the IPAQ
| IPAQ Category | Diabetics | PDN | ||
|---|---|---|---|---|
| M | (IR) | M | (IR) | |
| IPAQ Job-Related (MET-mins/wk) | 0 | (0 – 800) | 0 | (0 – 311) |
| IPAQ Transportation (MET-mins/wk) | 149 | (0 – 805) | 495 | (165 - 1213) |
| IPAQ Housework (MET-mins/wk) | 90 | (45 – 720) | 610 | (169 – 1860) |
| IPAQ Recreation (MET-mins/wk) | 80 | (0 – 396) | 429 | (8 – 689) |
| IPAQ Walking (MET-mins/wk) | 495 | (149 – 842) | 1336.5 | (396 – 2400) |
| IPAQ Moderate Intensity (MET-mins/wk) | 620 | (45 – 840) | 940 | (327 – 2130) |
| IPAQ Vigorous Intensity (MET-mins/wk) | 0 | (0 – 440) | 0 | (0 – 0) |
| IPAQ Sitting Time (mins/wk) | 2730 | (1260 - 4200) | 3120 | (2070 – 4035) |
| IPAQ Total (MET-mins/wk) | 980 | (540 - 2091)* | 2799 | (2505 – 3228)* |
Denotes significance at the p < .05 level.
IPAQ = International Physical Activity Questionnaire; PDN = Painful Diabetic Neuropathy; MET-mins/wk = Metabolic equivalence minutes per week; M = Mean; IR = Interquartile Ranges (25th and 75th); PDN = Painful Diabetic Neuropathy
Participants wore a pedometer for 5 consecutive days (Saturday – Wednesday). The pedometer data were cleaned prior to analyzing the data, and any individuals who did not meet the following criteria were not included in the analysis: 1) minimum of 500 steps per day, 2) pedometer worn for minimum of 10 hours, and 3) steps per day count less than 20,000. Only one individual was excluded from the analysis due to failing to meet the above criteria. Results indicated no significant differences (p > 0.05) between diabetic adults with PDN (mean = 5765 steps/day, sd = 3044) in comparison to diabetic adults without PDN (mean = 4795 steps/day, sd = 2594).
Profile of Mood States (POMS)
Mood states were assessed at baseline using the POMS (i.e., how participants felt over the past week including the day they came in for testing). Results indicated no significant differences between diabetic adults with or without PDN for tension, depression, anger, vigor, fatigue, confusion, or total mood disturbance (p > .05). The results from the POMS are summarized in Table 4.
Table 4.
Means and Standard Deviations for the POMS data
| Diabetics | PDN | |||
| M | (SD) | M | (SD) | |
| Tension | 11.56 | (6.41) | 7.11 | (4.14) |
| Depression | 12.78 | (10.53) | 5.67 | (5.20) |
| Anger | 9.00 | (8.25) | 4.56 | (3.21) |
| Vigor | 13.11 | (5.49) | 16.78 | (3.87) |
| Fatigue | 7.67 | (6.06) | 7.78 | (4.14) |
| Confusion | 9.00 | (5.66) | 4.78 | (2.49) |
| Total Mood Disturbance | 136.89 | (33.08) | 113.11 | (11.78) |
POMS = Profile of Mood States; PDN = Painful Diabetic Neuropathy; M = Mean; SD = Standard Deviation
DISCUSSION
The purpose of this study was to examine the influence of exercise on endogenous pain modulation in adults with PDN compared to diabetic adults without PDN. The results from this study indicated that experimental pain responses following exercise differed between the two groups of diabetic adults who participated in this study. Experimental pain ratings were found to decrease significantly following exercise for the diabetic adults without PDN indicating that EIH occurred following exercise.
Very little research has been conducted examining EIH in diabetic adults. The results from this study indicated that pain ratings for the ramp & hold protocol (predominantly Type III fiber responses) were found to be significantly lower following exercise. In addition, pain ratings for the temporal summation protocol (predominantly Type IV fiber responses) were also found to be lower following exercise. In fact, it appears that temporal summation did not occur following exercise since the temporal summation protocol was not rated as painful following exercise (ratings < 20). Further, general levels of pain on the day of testing as measured by the SF-MPQ were also found to be lower following exercise in the diabetic adults without PDN. The results from this study are in agreement with results from research conducted with healthy adults. For example, there are reports that healthy adults experience EIH following a similar exercise paradigm.21, 46
In contrast, the diabetic adults with PDN did not experience a decrease in experimental pain ratings following exercise indicating that EIH did not occur. Also, general levels of pain on the day of testing did not change significantly (with the exception of the PPI). These results are in agreement with results from research conducted with other chronic pain conditions. For example, some investigators have reported that women with fibromyalgia (FM) do not experience EIH following an exercise session.26, 29 In addition, lack of an EIH response has also been reported for adults with temporomandibular jaw disorder (TMD),32 adults with chronic whiplash associated disorders,47 and Gulf War veterans with chronic musculoskeletal pain.12 As for temporal summation, it appears there is only one other study conducted examining the influence of exercise on temporal summation of second pain. Vierck et al. (2001) found that temporal summation ratings increased following exercise for women with FM in comparison to a decrease in temporal summation ratings following exercise for healthy women.49 It has been hypothesized that that this lack of EIH may reflect endogenous pain modulatory system dysfunction.34,43 Investigators have suggested that this dysfunction may be the result of an active deficiency of inhibitory mechanisms or enhanced facilitation that overrides inhibition.29, 34,42
In addition, results indicated that muscle pain during exercise was significantly higher for diabetic adults with PDN. By the end of the three minute exercise bout, diabetic adults with PDN reported very strong pain while the diabetic adults without PDN rated their muscle pain as moderate. These results are in agreement with studies conducted with chronic pain patients in which participants with a chronic pain condition experienced hyperalgesia during exercise compared to healthy controls. For example, Cook et al. (2010) reported that Gulf War veterans with chronic musculoskeletal pain rated muscle pain intensity significantly higher during aerobic exercise in comparison to healthy Gulf War veterans.12 Also, there have been several studies conducted with fibromyalgia patients using an isometric exercise protocol, and results indicate that FM patients rated muscle pain significantly higher than healthy controls.19, 26, 29, 42 The results from these studies suggest dysfunction in regulation of nociceptive processes, thus leading to increases in naturally occurring muscle pain during exercise.12
The mechanisms underlying dysfunction of the endogenous pain modulatory system in chronic pain conditions are poorly understood. While the pain in patients with PDN is believed to originate in the peripheral nervous system, central nervous system mechanisms also contribute to the maintenance of neuropathic pain possibly through central sensitization.4, 9, 48 Experimental and clinical studies indicate that neuronal and afferent fiber hyperexcitability is a key factor in the development of neuropathic pain.17, 36 Central neuronal hyperexcitability underlying neuropathic pain is usually explained by increased input from the periphery.50 Contributing to the hyperexcitability may be decreased activity in endogenous pain inhibitory systems, and research with other chronic pain conditions (e.g., FM, TMD) provides evidence for endogenous pain modulatory system dysfunction.32, 42 It has been suggested that this dysfunction may be due to a deficiency in the pain inhibitory system or there may be enhanced facilitation that overrides inhibition.32, 42 Therefore, more research on the mechanisms underlying dysfunction of the endogenous pain modulatory system in adults with chronic pain warrants further investigation.
Physical activity levels were assessed both subjectively (i.e., IPAQ) and objectively (i.e., pedometer). Results from the IPAQ indicated that the only group difference was the IPAQ Total MET-mins/wk; however, groups were not significantly different on the individual domains of the IPAQ. Diabetics with PDN reported higher levels of physical activity in comparison to diabetics without PDN. This finding was somewhat unexpected; however, this result may be due to clinicians prescribing exercise to prevent further complications and potential amputations as a result of PDN. In contrast, the results from the pedometer data indicated the two groups did not differ in physical activity levels. The mean number of steps per day indicated that the Type 2 diabetic adults without PDN could be categorized as “sedentary” and the Type 2 diabetic adults with PDN could be categorized as “low active” according to guidelines published by Tudor-Locke & Bassett (2004).45 This is in agreement with previous reports indicating that Type 2 diabetic adults are not regularly active.33, 39, 44 Further research is needed examining strategies to increase physical activity levels in diabetic adults with and without PDN.
Several limitations of the present study should be acknowledged. First, the sample size in this study was small and diabetes disease severity was not assessed. Thus, further research is needed with larger sample sizes and groups matched on disease severity. Also, in the absence of a control condition, the possibility of experimental artifacts (i.e., regression to the mean, effect of multiple testing, etc) contributing to the observed hypoalgesic responses cannot be entirely discounted. Future research examining changes in pain perception following exercise in adults with and without neuropathic pain should incorporate random assignment of participants into both exercise and control conditions to control for these potential experimental artifacts.
In summary, the results from this study can only be generalized to men and women with Type 2 diabetes with and without PDN. The results are in agreement with other investigators who found that individuals with other chronic painful conditions (e.g., fibromyalgia, temporomandibular disorders, chronicwhiplash associated disorders) did not experience an attenuation of pain during or following an exercise session.26, 32, 42, 47 However, the mechanisms underlying dysfunction of the endogenous pain modulatory system remain unclear. Thus, additional research is needed in this area. It is concluded that diabetic adults with PDN experienced high levels of muscle pain during exercise and a lack of EIH following exercise in comparison to diabetic adults without PDN who experienced lower levels of muscle pain during exercise and a hypoalgesic response following exercise.
PERSPECTIVE.
Very little research has been conducted examining the impact of exercise on pain modulation in diabetic adults with PDN. This study provides support that adults with PDN exhibit exercise-induced endogenous pain modulatory system dysfunction.
Figure 2.
Means and Standard Errors for Ratings of Perceived Exertion During Exercise (RPE = 6 – 20). *Denotes significance at the p < .05 level.
Acknowledgements
We would like to thank Dr. Miroslav Backonja for his helpful comments and insight.
This study was funded by the UW-Madison Virginia Horne Henry Fund. All authors participated in the conduct of this study.
Footnotes
Discolsures:There are no conflicts of interest with this study, none of the authors have anything to disclose.
References
- 1.Apelqvist J, Bakker K, van Houtum WH, Schaper NC. Practical guidelines on the management and prevention of the diabetic foot. Diabetes Metab Res and Rev. 2008;24(Suppl 1):181–187. doi: 10.1002/dmrr.848. [DOI] [PubMed] [Google Scholar]
- 2.Argoff CE, Cole BE, Fishbain DA, Irving GA. Diabetic peripheral neuropathic pain: Clinical and quality-of-life issues. Mayo Clinic Proceedings. 2006;81(4):S3–S11. doi: 10.1016/s0025-6196(11)61474-2. [DOI] [PubMed] [Google Scholar]
- 3.Backonja M, Woolf CJ. Future directions in neuropathic pain therapy: closing the translational loop. Oncologist. 2010;15(Suppl 2):24–29. doi: 10.1634/theoncologist.2009-S502. [DOI] [PubMed] [Google Scholar]
- 4.Baron R. Peripheral neuropathic pain: from mechanisms to symptoms. Clin J Pain. 2000;16(Suppl 2):12–20. doi: 10.1097/00002508-200006001-00004. [DOI] [PubMed] [Google Scholar]
- 5.Bartholomew JB, Lewis BP, Linder DE, Cook DB. Post-exercise analgesia: replication and extension. J Sports Sci. 1996;14:329–334. doi: 10.1080/02640419608727718. [DOI] [PubMed] [Google Scholar]
- 6.Booth ML. Assessment of Physical Activity: An International Perspective. Res Q Exerci Sport. 2000;71(2):s114–s120. [PubMed] [Google Scholar]
- 7.Borg G. The Borg RPE Scale. In: Borg G, editor. Borg’s Perceived Exertion and Pain Scales. Human Kinetics; Champaign, IL: 1998. pp. 29–38. [Google Scholar]
- 8.Bouhassira D, Attal N, Fermanian J, Alchaar H, Gautron M, Masquelier E, Rostaing S, Lanteri-Minet M, Collin E, Grisart J, Boureau F. Development and validation of the Neuropathic Pain Screening Inventory. Pain. 2004;108(3):248–257. doi: 10.1016/j.pain.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 9.Chen X, Levine JD. Altered temporal pattern of mechanically evoked c-fiber activity in a model of diabetic neuropathy in the rat. Neuroscience. 2003;121:1007–1015. doi: 10.1016/s0306-4522(03)00486-x. [DOI] [PubMed] [Google Scholar]
- 10.Cook DB, O’Connor PJ, Eubanks SA, Smith JC, Lee M. Naturally occurring muscle pain during exercise: assessment and experimental evidence. Med Sci Sports Exerc. 1997;29:999–1012. doi: 10.1097/00005768-199708000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Cook DB, Koltyn KF. Pain and exercise. Int J Sport Psychol. 2000;31:256–277. [Google Scholar]
- 12.Cook DB, Stegner AJ, Ellingson LD. Exercise alters pain sensitivity in gulf war veterans with chronic musculoskeletal pain. J Pain. 2010;11:764–772. doi: 10.1016/j.jpain.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P. International Physical Activity Questionnaire: 12-Country Reliability and Validity. Med Sci Sports Exerc. 2003;35(8):1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 14.Freynhagen R, Baron R, Gockel U, Tölle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006;22(10):1911–1920. doi: 10.1185/030079906X132488. [DOI] [PubMed] [Google Scholar]
- 15.Hagströmer M, Oja P, Sjöström M. The International Physical Activity Questionnaire: a study of concurrent and construct validity. Public Health Nutr. 2006;9(6):755–762. doi: 10.1079/phn2005898. [DOI] [PubMed] [Google Scholar]
- 16.Janal MN. Pain sensitivity, exercise and stoicism. J R Soc Med. 1996;89:376–381. doi: 10.1177/014107689608900706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen TS, Gottrup H, Sindrup SH, Bach FW. The clinical picture of neuropathic pain. Eur J Pharmacol. 2001;429:1–11. doi: 10.1016/s0014-2999(01)01302-4. [DOI] [PubMed] [Google Scholar]
- 18.Kadetoff D, Kosek E. The effects of static muscular contraction on blood pressure, heart rate, pain ratings, and pressure pain thresholds in healthy individuals and patients with fibromyalgia. European Journal of Pain. 2007;11:39–47. doi: 10.1016/j.ejpain.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 19.Kadoglou NP, Iliadis F, Angelopoulou N, Perrea D, Ampatzidis G, Liapis CD, Alevizos M. The anti-inflammatory effects of exercise training in patients with type 2 diabetes mellitus. Eur J Cardiovasc Prev Rehabil. 2007;14:837–843. doi: 10.1097/HJR.0b013e3282efaf50. [DOI] [PubMed] [Google Scholar]
- 20.Kluding PM, Pasnoor M, Singh R, Jernigan S, Farmer K, Rucker J, Sharma NK, Wright DE. The effect of exercise on neuropathic symptoms, nerve fiber function, and cutaneous innervations in people with diabetic peripheral neuropathy. J Diabetes Complications. 2012;26(5):424–429. doi: 10.1016/j.jdiacomp.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knauf MT, Koltyn KF. Exercise-induced modulation of pain. Med Sci Sports Exerc. 2010;42(Suppl 1):660. [Google Scholar]
- 22.Koltyn KF, Arbogast RW. Perception of pain after resistance exercise. Br J Sports Med. 1998;32:20–24. doi: 10.1136/bjsm.32.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koltyn KF. Analgesia Following Exercise: A review. Sports Med. 2000;29:85–98. doi: 10.2165/00007256-200029020-00002. [DOI] [PubMed] [Google Scholar]
- 24.Koltyn KF. Exercise-Induced Hypoalgesia and Intensity of Exercise. Sports Med. 2002;32:477–487. doi: 10.2165/00007256-200232080-00001. [DOI] [PubMed] [Google Scholar]
- 25.Koltyn KF, Umeda M. Exercise, hypoalgesia, and blood pressure. Sports Med. 2006;36:207–214. doi: 10.2165/00007256-200636030-00003. [DOI] [PubMed] [Google Scholar]
- 26.Kosek E, Ekholm J, Hansson P. Modulation of pressure pain thresholds during and following isometric contraction in patients with fibromyalgia and in healthy controls. Pain. 1996;64:415–423. doi: 10.1016/0304-3959(95)00112-3. [DOI] [PubMed] [Google Scholar]
- 27.Krause SJ, Backonja MM. Development of a neuropathic pain questionnaire. Clin J Pain. 2003;19(5):306–314. doi: 10.1097/00002508-200309000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Kuphal KE, Fibuch EE, Taylor BK. Extended swimming exercise reduces inflammatory and peripheral neuropathic pain in rodents. J Pain. 2007;8:989–997. doi: 10.1016/j.jpain.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Lannersten L, Kosek E. Dysfunction of endogenous pain inhibition during exercise with painful muscles in patients with shoulder myalgia and fibromyalgia. Pain. 2010;151:77–86. doi: 10.1016/j.pain.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 30.McNair DM, Lorr M, Droppleman LF. Profile of Mood States. Educational and Industrial Testing Service; San Diego, CA: 1971. [Google Scholar]
- 31.Melzack R. The short-form McGill pain questionnaire. Pain. 1987;30(2):191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 32.Mohn C, Vassend O, Knardahl S. Experimental pain sensitivity in women with temporomandibular disorders and pain-free controls: the relationship to orofacial muscular contraction and cardiovascular responses. Clin J Pain. 2008;24:343–352. doi: 10.1097/AJP.0b013e318162eaf4. [DOI] [PubMed] [Google Scholar]
- 33.Morrato EH, Hill JO, Wyatt HR, Ghushchyan V, Sullivan P. Physical activity in U.S. adults with diabetes and at risk for developing diabetes, 2003. Diabetes Care. 2007;30:203–209. doi: 10.2337/dc06-1128. [DOI] [PubMed] [Google Scholar]
- 34.Nijs J, Kosek E, Van Oosterwijck J, Meeus M. Dysfunctional endogenous analgesia during exercise in patients with chronic pain: exercise or not to exercise? Pain Physician. 2012;15:ES205–ES213. [PubMed] [Google Scholar]
- 35.O’Connor PJ, Cook DB. Exercise and pain: the neurobiology, measurement, and laboratory study of pain in relation to exercise in humans. Exerc Sport Sci Rev. 1999;27:119–166. [PubMed] [Google Scholar]
- 36.Ørstavik K, Namer B, Schmidt R, Schmelz M, Hilliges M, Weidner C, Carr RW, Handwerker H, Jørum E, Torebjörk HE. Abnormal function of C-fibers in patients with diabetic neuropathy. J Neurosci. 2006;26:11287–11294. doi: 10.1523/JNEUROSCI.2659-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rossi DM, Valenti VE, Navega MT. Exercise training attenuates acute hyperalgesia streptozotocin-induced diabetic female rats. Clinics (Sao Paulo) 2011;66:1615–1619. doi: 10.1590/S1807-59322011000900019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shankarappa SA, Piedras-Rentería ES, Stubbs EB., Jr Forced-exercise delays neuropathic pain in experimental diabetes effects on voltage-activated calcium channels. J Neurochem. 2011;118:224–236. doi: 10.1111/j.1471-4159.2011.07302.x. [DOI] [PubMed] [Google Scholar]
- 39.Shultz JA, Sprague MA, Branen LJ, Lambeth S. A comparison of views of individuals with type 2 diabetes mellitus and diabetes educators about barriers to diet and exercise. J Health Comm. 2001;6:99–115. doi: 10.1080/108107301750254457. [DOI] [PubMed] [Google Scholar]
- 40.Stagg NJ, Mata HP, Ibrahim MM, Henriksen EJ, Porreca F, Vanderah TW, Philip Malan T., Jr Regular exercise reverses sensory hypersensitivity in a rat neuropathic pain model: role of endogenous opioids. Anesthesiology. 2011;114:940–948. doi: 10.1097/ALN.0b013e318210f880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Staud R, Robinson ME, Vierck CJ, Cannon RC, Mauderli AP, Price DD. Ratings of experimental pain and pain-related negative affect predict clinical pain in patients with fibromyalgia syndrome. Pain. 2003;105:215–222. doi: 10.1016/s0304-3959(03)00208-2. [DOI] [PubMed] [Google Scholar]
- 42.Staud R, Robinson ME, Price DD. Isometric exercise has opposite effects on central pain mechanisms in fibromyalgia patients compared to normal controls. Pain. 2005;118:176–184. doi: 10.1016/j.pain.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 43.Staud R, Price DD, Fillingham RB. Advanced continuous-contact heat pulse design for efficient temporal summation of second pain (windup) J Pain. 2006;7:575–582. doi: 10.1016/j.jpain.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 44.Thomas N, Alder E, Leese GP. Barriers to physical activity in patients with diabetes. Postgrad Med J. 2004;80:287–291. doi: 10.1136/pgmj.2003.010553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tudor-Locke C, Bassett DR., Jr How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med. 2004;34:1–8. doi: 10.2165/00007256-200434010-00001. [DOI] [PubMed] [Google Scholar]
- 46.Umeda M, Newcomb LW, Ellingson LD, Koltyn KF. Examination of the dose-response relationship between pain perception and blood pressure following isometric exercise. Med Sci Sports Exerc. 2008;40(Suppl 1):117. [Google Scholar]
- 47.Van Oosterwijck J, Nijs J, Meeus M, Paul L. Evidence for central sensitization in chronic whiplash: a systematic literature review. Eur J Pain. 2012 Sep 25; doi: 10.1002/j.1532-2149.2012.00193.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 48.Veves A, Backonja M, Malik RA. Painful diabetic neuropathy: epidemiology, natural history, early diagnosis, and treatment options. Pain Med. 2008;9:660–674. doi: 10.1111/j.1526-4637.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 49.Vierck CJ, Staud R, Price DD, Connon RL, Mauderli AP, Martin AD. The effect of maximal exercise on temporal summation of second pain (windup) in patients with fibromyalgia syndrome. J Pain. 2001;2:334–344. doi: 10.1054/jpai.2001.25533. [DOI] [PubMed] [Google Scholar]
- 50.Witting N, Svensson P, Jensen T. Differential recruitment of endogenous pain inhibitory systems in neuropathic pain patients. Pain. 2003;103:75–81. doi: 10.1016/s0304-3959(02)00421-9. [DOI] [PubMed] [Google Scholar]
- 51.Yeomans DC, Cooper BY, Vierck CJ. Effects of systematic morphine on responses of primates to first or second pain sensations. Pain. 1996;66:253–263. doi: 10.1016/0304-3959(96)03082-5. [DOI] [PubMed] [Google Scholar]