Abstract
A “Holy Grail” sought in medical treatment of obesity is to be able to biologically reprogram their adipose tissues to burn fat rather than store it. White adipose tissue (WAT) stores fuel and its expansion underlines insulin resistance (IR) whereas brown adipose tissue (BAT) burns fuel and stimulates insulin sensitivity. These two types of fats seesaw within our bodies via a regulatory mechanism that involves intricate communication between adipocytes and blood cells, particularly macrophages that migrate into adipose deposits. The coregulator, Receptor Interacting Protein 140 (RIP140), plays a key role in regulating this communication. In mice on a high-fat diet, the level of RIP140 in macrophages is dramatically elevated to activate their inflammatory M1 polarization and enhance their recruitment into WAT, facilitating IR. Conversely, lowering the level of RIP140 in macrophages not only reduces M1 macrophages but also expands alternatively polarized, anti-inflammatory M2 macrophages, triggering white adipose tissue browning, fat burning, and restoration of insulin sensitivity. This suggests a potential therapeutic strategy for reversing IR, obesity, and atherosclerotic or even cosmetic fat deposits: therapeutic browning of white adipose deposits by diminishing RIP140 levels in macrophages.
Keywords: brown adipose tissue, inflammation, insulin resistance, macrophage polarization, metabolism, RIP140, white adipose tissue
Abbreviations: ATM(s), adipose tissue macrophage(s); BAT, brown adipose tissue; HFD, high-fat diet; IR, insulin resistance; ND, normal diet; RIP140, Receptor Interacting Protein 140; (v)WAT, (visceral) white adipose tissue; WT (wild-type mice)
In lean mice and humans, alternatively polarized, or M2 anti-inflammatory, Adipose Tissue Macrophages (ATMs) predominate. Obesity induces the accumulation of classically polarized, or M1 inflammatory, ATMs, leading to a proinflammatory state in adipose tissues and to insulin resistance (IR).1-3 The regulation of M1 vs. M2 macrophage polarization, especially in vivo, is a balancing act that is still not entirely understood. In particular, the question of whether M1 or M2 polarization of macrophages occurs before their recruitment to specific tissues, or the polarization is determined by local environment after their recruitment to adipose tissues, is the subject of intense investigation.
Recent studies have suggested that the M1-M2 switch in adipose tissues is caused by differential recruitment of various monocyte subtypes.4-7 It is also known that most monocytes/macrophages in the adipose tissue are derived from bone marrow.7,8 Related to the development of obesity, it is known that obesity can induce myelopoiesis in bone marrow, which could increase Ly6C+CCR2+ circulating monocytes primed for inflammatory M1 polarization.8,9 In contrast, M2 ATMs come from circulating Ly6C-CCR2- monocytes, which are anti-inflammatory monocytes that usually exist under healthy physiological conditions.10,11 On a molecular level, the CCR2 receptor is a key factor for monocyte egress from bone marrow into the circulation, via interacting with its chemokine ligands CCL2/MCP-1 and CCL7/MCP-3.12 Furthermore, Ly6C+ monocytes are not detected in CCR2 knockout mice, whose adipose tissues have diminished M1 ATM infiltration and are protected from obesity-induced IR.4,6,13 The ensuing hypothesis that M1 macrophages are polarized and recruited to adipose tissue by CCR2-Ly6C is supported by our recent data generated using a new animal model14—a mouse with macrophage-specific knockdown of a regulatory protein important for M1 macrophage activation,15-17 RIP140. We found that in the blood of our mφRIP140KD mice Ly6C+ monocytes are attenuated, and their M1 ATMs are dramatically reduced in number, even under a high fat diet (HFD) that would otherwise induce obesity and adipose inflammation. Also consistent with the CCL2-Ly6C hypothesis, in our mφRIP140KD mice the CCR2 receptor in monocytes and its chemokine ligands (CCL2, CCL7, and CCL8) in adipose tissue are both reduced.18 This result not only supported the CCR2-Ly6C molecular pathway's role in macrophage activation and recruitment, but also suggested a new functional role for RIP140 in promoting monocyte trafficking to inflammatory tissues by regulating the receptor (CCR2) and its ligands (CCL2, CCL7, and CCL8). In this study, we employed PKH26 dye-labeled cells in bone marrow transplantation, and directly monitored in vivo infiltration of labeled monocytes-macrophages into adipose tissue. We found that the process of adipose macrophage recruitment is in fact also drastically decreased in HFD-fed mφRIP140KD mice.14 This established a new functional role for RIP140 in directly regulating monocyte recruitment/infiltration into adipose tissues during the process of inflammation.14
In addition to a decrease in M1 ATMs in the mφRIP140KD adipose tissues, we also found an expansion in M2 ATMs without increasing M2 macrophage proliferation (unpublished data). This revealed that RIP140 not only regulates circulating monocyte activation and trafficking but also contributes to the homeostatic control of M1 vs. M2 ATM polarization within healthy and/or diseased adipose tissues. Supporting this finding, we previously found that the level of RIP140 is dramatically elevated in macrophages of HDF-induced obese mice.14,19 Thus, HFD-associated obesity could involve elevating RIP140 expression, which would induce not only M1 monocyte recruitment but also M1 ATM polarization (or repress M2 ATM polarization). Conversely, reducing RIP140 levels in monocytes would reduce M1 monocyte recruitment and M1 ATM polarization (or derepress M2 ATM polarization). For this model to be established, it will require evidence for a direct role of RIP140 in regulating M2 ATM polarization. This is under investigation.
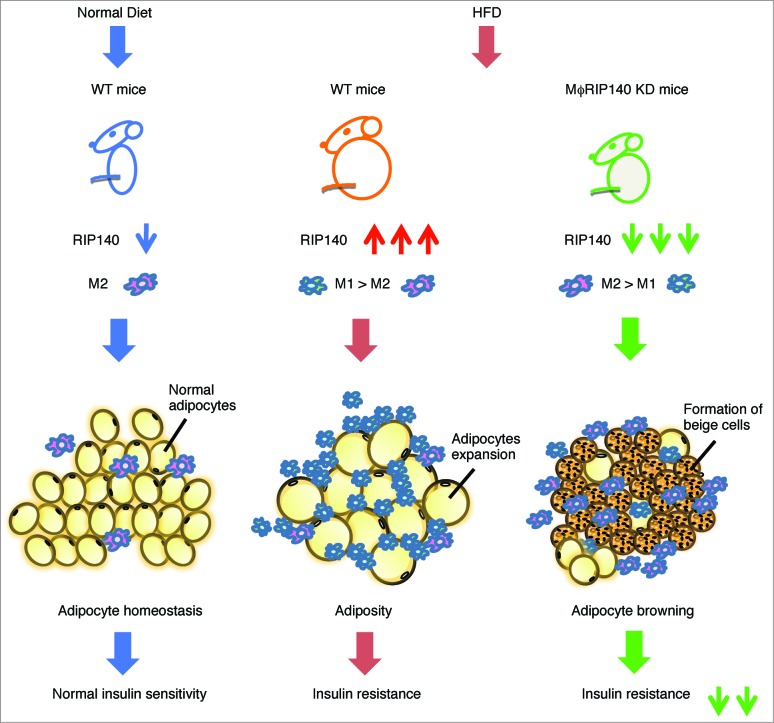
In conclusion (Fig. 1), our recent study showed that RIP140 is involved in regulating monocyte recruitment to adipose tissues.14 More recent unpublished results indicate its additional function in regulating M2 expansion by a means other than proliferation. As such, we propose that RIP140 also has a direct role in suppressing M2 macrophage polarization. Regarding M1 or M2 polarization, most studies show effects via environmental stimuli. Based upon our studies, we propose that RIP140 is a principal intrinsic factor that tips the balance of M1 versus M2 polarization of the macrophage population. Manipulating this important regulator will gear the direction of macrophage. For instance, dampening RIP140, as shown in our mφRIP140KD mice, not only reduces their M1 monocyte infiltration into visceral white adipose tissues (vWATs) but also enhances their M2 macrophage polarization within those adipose tissues, which facilitates a dramatic switch in M1/M2 homeostasis of these mice even under HFD feeding. The switch in macrophage polarization results in a superior physiological condition in animals, in spite of HFD feeding, that triggers WAT browning and increases thermogenesis and energy expenditure, ultimately improving insulin sensitivity.
Figure 1.
The function of ATM recruitment and contribution to adipose tissue. Left panel: In lean mice fed a normal diet (or ND), the function of ATMs as M2 type maintains adipose tissue homeostasis and normal insulin sensitivity. Middle panel: In HFD-induced obese mice, RIP140 is overexpressed in monocytes/macrophages compared with ND-fed WT mice, resulting in M1 over M2 ATM infiltration and in situ polarization, triggering WAT adiposity and insulin resistance. Right panel: Attenuation of RIP140 in the monocytes/macrophages could result in M2 over M1 ATM polarization, triggering WAT browning and alleviating HFD–induced insulin resistance.
Given these encouraging results, it is tempting to speculate several potential applications of targeting RIP140, or engineering cells that express RIP140 such as macrophages, to develop new therapeutics. For instance, it may be possible to use tailored macrophage with a certain level of RIP140 as a therapeutic agent, such as in cell therapy. Depending upon disease conditions, which can benefit from an inflammatory response, or an anti-inflammatory status, specific macrophages may be used to combat these diseases. It may also be possible to develop compounds targeting the expression, or protein stability, of RIP140, in treating those diseases where RIP140 is overtly elevated. Recently, we have obtained a preliminary result, testing the benefit of injecting tailored macrophages, such as those derived from the mφRIP140KD mice, in preventing HFD induced I.R. This provides a proof-of-concept that engineered macrophages with altered RIP140 expression may be used in treating Type II diabetes.
Funding
This work was supported by NIH grants DK54733, DK60521, DK54733-11S, NIH 3 R01 DK60521-12S1, the Dean's Commitment and the Distinguished McKnight Professorship of University of Minnesota to LNW, and by The University of Minnesota Foundation to FHB.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol 2010; 72:219-46; PMID:20148674; http://dx.doi.org/ 10.1146/annurev-physiol-021909-135846 [DOI] [PubMed] [Google Scholar]
- 2. Biswas SK, Mantovani A. Orchestration of metabolism by macrophages. Cell metabolism 2012; 15:432-7; PMID:22482726; http://dx.doi.org/ 10.1016/j.cmet.2011.11.013 [DOI] [PubMed] [Google Scholar]
- 3. Odegaard JI, Chawla A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science 2013; 339:172-7; PMID:23307735; http://dx.doi.org/ 10.1126/science.1230721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW, Jr. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest 2006; 116:115-24; PMID:16341265; http://dx.doi.org/ 10.1172/JCI24335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nishimura S, Manabe I, Nagasaki M, Seo K, Yamashita H, Hosoya Y, Ohsugi M, Tobe K, Kadowaki T, Nagai R, et al. . In vivo imaging in mice reveals local cell dynamics and inflammation in obese adipose tissue. J Clin Invest 2008; 118:710-21; PMID:18202748; http://dx.doi.org/ 10.1172/JCI33328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes 2008; 57:3239-46; PMID:18829989; http://dx.doi.org/ 10.2337/db08-0872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol 2005; 5:953-64; PMID:16322748; http://dx.doi.org/ 10.1038/nri1733 [DOI] [PubMed] [Google Scholar]
- 8. Gordon S. Macrophage heterogeneity and tissue lipids. J Clin Invest 2007; 117:89-93; PMID:17200712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tacke F, Randolph GJ. Migratory fate and differentiation of blood monocyte subsets. Immunobiology 2006; 211:609-18; PMID:16920499; http://dx.doi.org/ 10.1016/j.imbio.2006.05.025 [DOI] [PubMed] [Google Scholar]
- 10. Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 2003; 19:71-82; PMID:12871640; http://dx.doi.org/ 10.1016/S1074-7613(03)00174-2 [DOI] [PubMed] [Google Scholar]
- 11. Sunderkotter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, Leenen PJ. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol 2004; 172:4410-7; PMID:15034056; http://dx.doi.org/ 10.4049/jimmunol.172.7.4410 [DOI] [PubMed] [Google Scholar]
- 12. Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, Charo IF. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest 2007; 117:902-9; PMID:17364026; http://dx.doi.org/ 10.1172/JCI29919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 2007; 117:175-84; PMID:17200717; http://dx.doi.org/ 10.1172/JCI29881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu PS, Lin YW, Lee B, McCrady-Spitzer SK, Levine JA, Wei LN. Reducing RIP140 expression in macrophage alters ATM infiltration, facilitates white adipose tissue browning and prevents high fat diet-induced insulin resistance. Diabetes 2014; PMID:24969109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ho PC, Tsui YC, Feng X, Greaves DR, Wei LN. NF-kappaB-mediated degradation of the coactivator RIP140 regulates inflammatory responses and contributes to endotoxin tolerance. Nat Immunol 2012; 13:379-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mostaqul Huq MD, Gupta P, Wei LN. Post-translational modifications of nuclear co-repressor RIP140: a therapeutic target for metabolic diseases. Curr Med Chem 2008; 15:386-92; PMID:18288993; http://dx.doi.org/ 10.2174/092986708783497382 [DOI] [PubMed] [Google Scholar]
- 17. Ho PC, Wei LN. Negative regulation of adiponectin secretion by receptor interacting protein 140 (RIP140). Cell Signal 2012; 24:71-6; PMID:21872658; http://dx.doi.org/ 10.1016/j.cellsig.2011.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 2004; 25:677-86; PMID:15530839; http://dx.doi.org/ 10.1016/j.it.2004.09.015 [DOI] [PubMed] [Google Scholar]
- 19. Ho PC, Chang KC, Chuang YS, Wei LN. Cholesterol regulation of receptor-interacting protein 140 via microRNA-33 in inflammatory cytokine production. FASEB J 2011; 25:1758-66; PMID:21285396; http://dx.doi.org/ 10.1096/fj.10-179267 [DOI] [PMC free article] [PubMed] [Google Scholar]