Abstract
It has been postulated that the protective effects of lower body subcutaneous adipose tissue (LBSAT) occur via its ability to sequester surplus lipid and thus serve as a “metabolic sink.” However, the mechanisms that mediate this protective function are unknown thus this study addresses this postulate. Ad libitum, chow-fed mice underwent Sham-surgery or LBSAT removal (IngX, inguinal depot removal) and were subsequently provided chow (Chow; typical adipocyte expansion) or high fat diet (HFD; enhanced adipocyte expansion) for 5 weeks. Primary outcome measures included glucose tolerance and subsequent insulin response, muscle insulin sensitivity, liver and muscle triglycerides, adipose tissue gene expression, and circulating lipids and adipokines. In a follow up study the consequences of extended experiment length post-surgery (13 wks) or pre-existing glucose intolerance were examined. At 5 wks post-surgery IngX in HFD-fed mice reduced glucose tolerance and muscle insulin sensitivity and increased circulating insulin compared with HFD Sham. In Chow-fed mice, muscle insulin sensitivity was the only measurement reduced following IngX. At 13 wks circulating insulin concentration of HFD IngX mice continued to be higher than HFD Sham. Surgery did not induce changes in mice with pre-existing glucose intolerance. IngX also increased muscle, but not liver, triglyceride concentration in Chow- and HFD-fed mice 5 wks post-surgery, but chow group only at 13 wks. These data suggest that the presence of LBSAT protects against triglyceride accumulation in the muscle and HFD-induced glucose intolerance and muscle insulin resistance. These data suggest that lower body subcutaneous adipose tissue can function as a “metabolic sink.”
Keywords: adipose tissue distribution, lipectomy, metabolic sink, muscle, subcutaneous adipose issue, triglyceride, visceral adipose tissue
Introduction
A high body mass index (BMI) is a risk factor for diabetes, non-alcoholic fatty liver and cardiovascular disease1 and a strong predictor of mortality.2 However, some obese individuals are characterized by a reduced risk for type-2-diabetes, hypertension and heart disease,3-5 whereas some non-obese individuals develop these co-morbidities.6,7 Results such as these have led to the notion that fat distribution is an important determinant of disease risk. Indeed, intra-abdominal or visceral adipose tissue accumulation is associated with dyslipidemia, cardiovascular disease, insulin resistance and type-2-diabetes.8,9 In contrast, greater lower body adiposity (femoral-gluteal region) appears to exert protective effects against glucose intolerance, dyslipidemia and inflammation.10-14 It has been proposed that lower body subcutaneous adipose tissue (LBSAT) functions as a “metabolic sink,” storing lipids that might otherwise lead to disturbances in non-adipose tissues.15 Although metabolic outcomes associated with LBSAT removal in humans have been investigated,16-20 the “metabolic sink” postulate has not been systematically examined, in part, due to inherent experimental limitations in humans.
Large volume reduction of LBSAT, via liposuction, does not alter insulin sensitivity or circulating markers of inflammation in humans.21 The lack of effect following liposuction, however, may be related to the fact that subjects were morbidly obese prior to and after the procedure. Other studies that have either concluded that liposuction was with19,20 or without effect16-18 were also confounded by wide variations in BMI (24 to 50), age (18–50 + years), duration of study (12 weeks to years) and amount of adipose tissue removed (5–20 Ls).16-20 Although liposuction/lipectomy remains a useful tool to evaluate the role of LBSAT in relation to metabolic abnormalities, human data remains inconsistent due, in part, to variability in experimental approach and study populations.
In 1953 Gordon Kennedy proposed the “lipostatic theory” which predicted that the removal of adipose tissue would result in compensation in other adipose tissue depots.22 There is evidence in humans that liposuction increases body fat in non-excised areas,23,24 such as central/visceral adipose tissue depots.18 This redistribution of lipid to other adipose tissue depots, in particular visceral adipose tissue, may override, supersede and/or contribute to effects induced directly by LBSAT removal. Thus, to investigate the specific role of LBSAT in glucose homeostasis requires a model in which this depot can be removed without significant compensatory accumulation of lipid in other adipose tissue depots.
Studies investigating the effects of LBSAT on metabolic indices in rodents are limited. Whereas in chow or high fat diet-fed mice the removal of LBSAT (inguinal depot) had no effect on glucose regulation,25 its removal in Syrian hamsters resulted in hypertriglyceridemia and increased liver lipid deposition.26 Differences between these 2 rodent studies may be linked to the species used, the amount of tissue removed (15 vs 50%), location of the depots that were removed and/or the duration of the study (∼3 vs. Twelve wks). Additional studies that have demonstrated metabolic deterioration following subcutaneous adipose tissue removal utilized models (chow fed immune deficient mice;27 acute/immediate effects of fat removal via ultrasound in chow fed animals)28 that may not be applicable to obesity-related disease.
We postulate that if LBSAT functions as a “metabolic sink” that its removal will increase the risk of developing diet-induced metabolic dysregulation. To comprehensively examine the effects of LBSAT, both in relation to insulin-mediated regulation of glucose metabolism and non-adipose tissue lipid accumulation, requires a model in which subcutaneous adipose tissue “the metabolic sink” can be removed in the absence of significant compensation in non-excised adipose tissue depots. In addition, this model should also include comparison of dietary conditions associated with standard and enhanced energy storage to delineate if loss of LBSAT exacerbates the risk factors associated with Western diet intake. In the present study, we have used bilateral inguinal fat pad removal to systematically examine the putative protective role of LBSAT. The experimental conditions include 2 terminations time points to address consistency of metabolic alterations (early (5 wks) and late adipose depot compensation (13 wks)) and 2 post-surgery dietary conditions (standard chow vs. Western diet) to delineate if alterations are standard across diets or altered with increased energy intake/storage. In addition, theses surgery conditions will be examined in the context of a pre-existing state of glucose intolerance to determine if a deleterious pre-existing metabolic milieu results in an additive or diminished incremental effect.
Results
Inguinal fat pad removal and subsequent short-term high fat diet feeding
Ad libitum, chow-fed C57BL mice underwent Sham or IngX surgery as previously discussed. Sham and IngX were subsequently provided chow or HFD for 5 weeks.
Body and adipose tissue mass and food intake
Cumulative energy intake was significantly greater in HFD groups compared with Chow (Table 1; P ≤ 0.05). Recovered inguinal adipose mass was significantly reduced in IngX mice (Table 1; P ≤ 0.05). IngX did not result in significant changes in the mass of non-excised adipose depots.
Table 1.
Terminal cumulative food intake and body and adipose tissue mass of Chow or HFD mice 5 weeks post Sham or IngX surgery
| 5 weeks Post-surgery | ||||
|---|---|---|---|---|
| Chow Sham (n = 10) | Chow IngX (n = 10) | HFD Sham (n = 10) | HFD IngX (n = 10) | |
| Food Intake (Total KCAL) | 242.5 ± 5.06a | 259.3 ± 6.72a | 285.1 ± 7.42b | 288.9 ± 5.27b |
| Body Weight (g) | 28.51 ± 0.51 | 28.78 ± 0.49 | 29.99 ± 0.86 | 30.43 ± 0.49 |
| Inguinal (g) | 0.27 ± 0.03a | 0.04 ± 0.01b | 0.58 ± 0.08c | 0.12 ± 0.01d |
| Brown Interscapular (g) | 0.23 ± 0.02a | 0.25 ± 0.03a | 0.40 ± 0.06b | 0.40 ± 0.03b |
| Epididymal (g) | 0.34 ± 0.04a | 0.40 ± 0.04a | 0.82 ± 0.14b | 1.01 ± 0.12b |
| Perirenal (g) | 0.25 ± 0.02a | 0.21 ± 0.02a | 0.43 ± 0.07b | 0.48 ± 0.04b |
| Visceral (g) | 0.23 ± 0.02a | 0.27 ± 0.03a | 0.46 ± 0.05b | 0.49 ± 0.03b |
Cumulative energy intake was significantly greater in HFD groups compared with Chow. Recovered inguinal adipose mass was significantly reduced in IngX mice. IngX did not result in changes in the mass of non-excised adipose depots. (Unlike letters indicate significance; P ≤ 0.05)
Glucose tolerance test and insulin concentration
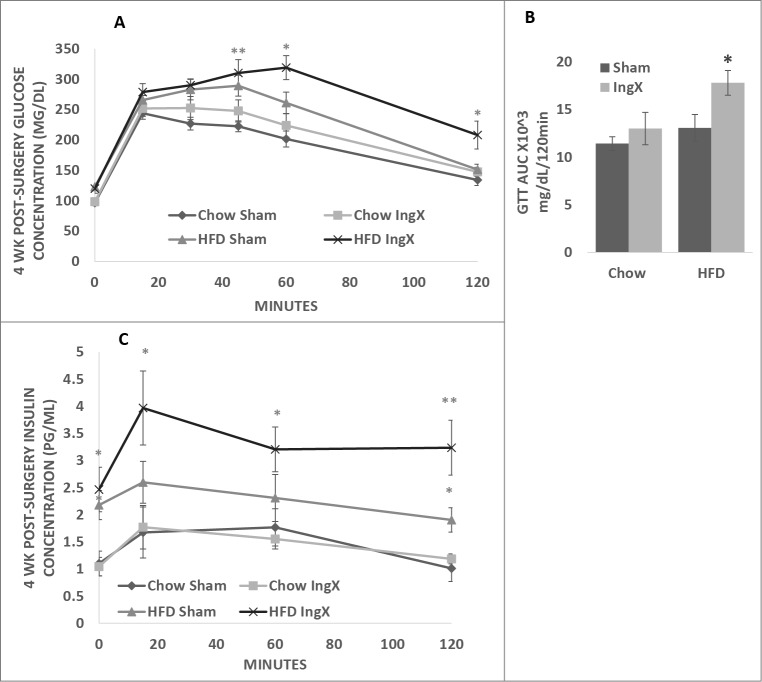
Glucose tolerance tests were performed pre-surgery and one week prior to termination, 4 weeks post-surgery. Pre-surgery glucose concentrations values were not different among groups (data not shown). Four weeks post-surgery glucose concentrations were highest in HFD IngX mice and significantly higher than both Chow groups at 45, 60 and 120 minutes (Fig. 1A; P ≤ 0.05). In addition, glucose concentration of the HFD IngX group was significantly higher than that of the HFD Sham at 60 and 120 minutes. Area under the curve of the HFD IngX group was significantly higher than HFD Sham and both Chow groups (Fig. 1B; P ≤ 0.05).
Figure 1.
4-week glucose tolerance test (GTT) and subsequent insulin concentrations of Chow and HFD Sham and IngX mice. (A) IngX in Chow mice did not alter glucose tolerance, or (C) insulin concentration. (A) IngX in HFD mice significantly increased glucose concentration at 60 and 120 minutes (P ≤ 0.05; *= compared with HFD Sham) and (B) area under the curve of the GTT (P ≤ 0.05; * = compared with all other groups). (C) Subsequent insulin concentration was increased in HFD IngX groups, with significant increase at 120 minutes (P ≤ 0.05; *= compared with sham controls).
Insulin response to the GTT was also highest in HFD IngX mice. Insulin concentration of the HFD IngX group was significantly higher than both Chow groups at baseline, 15, 60 and 120 minutes and significantly higher than HFD Sham 120 minutes (Fig. 1C; P ≤ 0.05). HFD Sham mice also had significantly higher insulin concentration than both chow groups at baseline and 120 minutes.
Tissue triglyceride and muscle insulin sensitivity
Femoral muscle, but not liver, triglyceride concentration was significantly higher in IngX mice compared with Sham (Fig. 2; P ≤ 0.05). HFD significantly increased femoral muscle triglyceride in Sham mice, however this diet differences did not occur in following IngX (Fig. 2A; P ≤ 0.05). Liver triglyceride was significantly higher in HFD Sham than all other groups (Fig. 2B; P ≤ 0.05). IngX significantly reduced insulin-stimulated pAKT/AKT in both Chow and HFD groups (Fig. 3B; P ≤ 0.05). Overall, insulin-stimulated pAKT/AKT was significantly lower in HFD mice than chow and significantly the lowest in HFD IngX mice (Fig. 3B; P ≤ 0.05).
Figure 2.
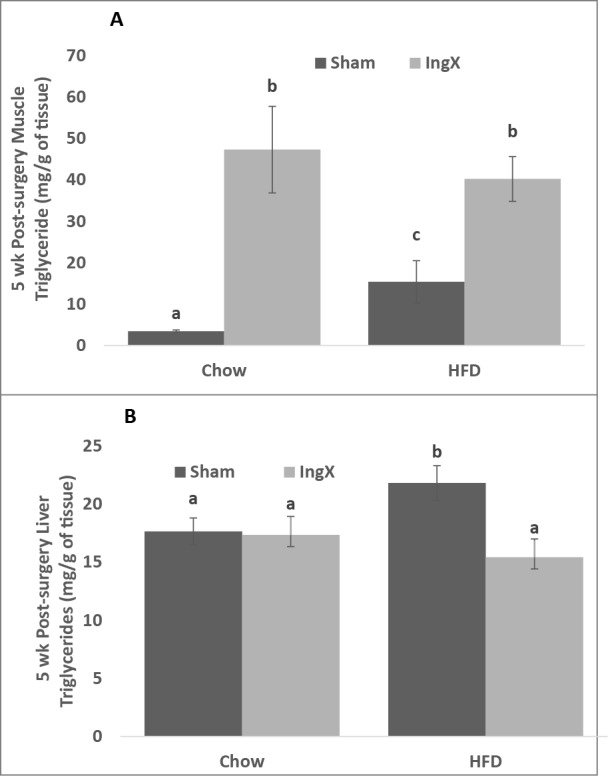
Tissue triglyceride concentration 5 weeks after Sham or IngX surgery in Chow and HFD mice. (A) Muscle triglyceride concentration was significantly increased in Chow and HFD adipose tissue removal groups. (B) Liver triglyceride concentration was significantly decreased in HFD mice with IngX (Unlike letters indicate significance; P ≤ 0.05).
Figure 3.
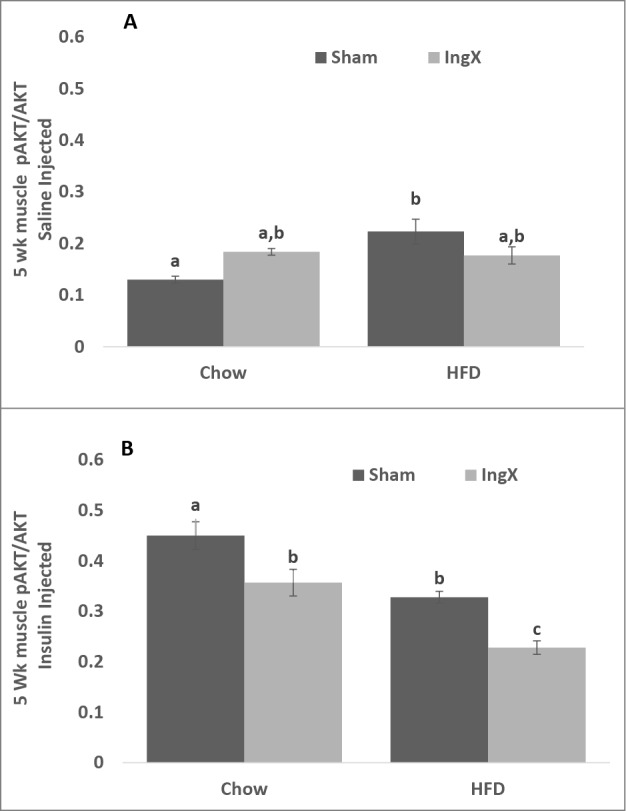
Muscle insulin sensitivity, pAKT/AKT 5 weeks after surgery. (A) Saline injected mice. (B) IngX reduced insulin-stimulated pAKT/AKT in both Chow and HFD groups, the greatest decrease occurred in HFD ingX mice (Unlike letters indicate significance; P ≤ 0.05).
Systemic and portal circulating factors
Blood was sampled from both the systemic and portal circulation to examine effluent differences between peritoneal and systemic organs, such as, but not limited to, intra-abdominal and subcutaneous adipose tissue depots. IngX did not alter systemic or portal free fatty acid, IL-6, MCP-1, TNFα or leptin concentration in Chow or HFD groups (Table 2). However, systemic and portal leptin concentration and systemic free fatty acids were significantly increased in HFD-fed mice compared with Chow groups (Table 2; P ≤ 0.05).
Table 2.
5 week terminal systemic and portal plasma free fatty acid (FFA), cytokine, adipokine and insulin concentrations
| Systemic | Portal | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FFA (mmol/L) | IL6 (pg/ml) | MCP1 (pg/ml) | TNF (pg/ml) | Leptin (pg/ml) | FFA (mmol/L) | IL6 (pg/ml) | MCP1 (pg/ml) | TNF (pg/ml) | Leptin (pg/ml) | |
| Chow | 0.61 ± 0.0a,b | 20.86 ± 4.8 | 69.94 ± 4.8 | 17.41 ± 5.2 | 758.25 ± 156.5a | 0.60 ± 0.1 | 12.23 ± 1.9 | 46.38 ± 14.0 | 11.57 ± 5.0 | 834.5 ± 131.9a |
| Chow IngX | 0.49 ± 0.1a | 17.56 ± 9.4 | 44.38 ± 9.0 | 25.20 ± 8.2 | 958.3 ± 402.1a | 0.57 ± 0.1 | 13.96 ± 1.8 | 31.35 ± 6.6 | 5.0 ± 0.7 | 615.8 ± 89.0a |
| HFD | 0.73 ± 0.1b,c | 40.62 ± 12.6 | 30.46 ± 22.0 | 15.48 ± 2.3 | 2532.5 ± 691.7b | 0.65 ± 0.1 | 27.37 ± 8.9 | 53.48 ± 15.0 | 10.32 ± 1.7 | 2516.5 ± 709.0b |
| HFD IngX | 0.82 ± 0.1c | 28.28 ± 7.8 | 20.86 ± 4.8 | 15.7 ± 2.1 | 4150.25 ± 846.5b | 0.56 ± 0.1 | 18.13 ± 7.2 | 22.82 ± 2.0 | 12.74 ± 3.7 | 4060 ± 884.0b |
Systemic and portal leptin concentration and systemic free fatty acids were significantly increased in HFD-fed mice compared with Chow groups. (Unlike letters indicate significance; P ≤ 0.05)
Adipose tissue and liver gene expression
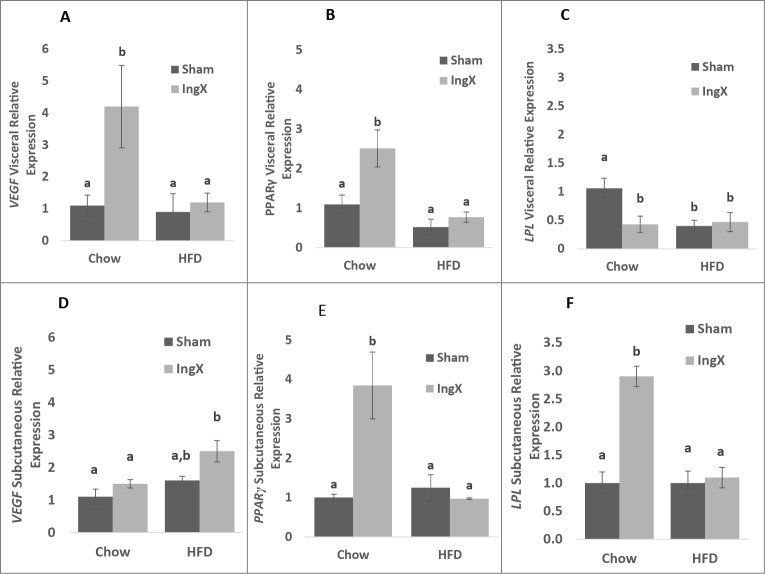
Adipose tissue removal has been demonstrated to induce compensation in non-excised adipose tissue in rodents29-37 and humans.23,24 Therefore, factors demonstrated to play a role in adipose depot expansion such as vasculature expansion (vascular endothelial growth factor: VEGF), cellular differentiation (peroxisome proliferator-activated receptor γ: Pparγ) and lipid partitioning (lipoprotein lipase: LPL) were measured at the gene level in the visceral adipose depot and non-excised remnant of lower body subcutaneous (i.e. inguinal) adipose tissue. IngX significantly increased Vegf and Pparγ and decreased Lpl expression in the visceral depot of Chow mice compared with Chow Sham, but did not cause alterations in HFD mice (Fig. 4A-C; P ≤ 0.05). In LBSAT, Vegf expression was significantly increased in the residual non-excised portion of the inguinal depot of IngX HFD-fed group compared with the intact, non-manipulated inguinal depots of HFD Sham (Fig. 4D; P ≤ 0.05). In Chow animals Pparγ and Lpl expression were significantly increased in residual non-excised inguinal depot of IngX mice compared with Chow Sham (Fig. 4E and F; P ≤ 0.05).
Figure 4.
Visceral and subcutaneous adipose tissue gene expression 5 weeks post-surgery. Vegf (A) and Pparγ (B) expression was significantly increased in the visceral depot of IngX Chow animals, whereas Lpl (C) was decreased. In the residual non-excised portion of the subcutaneous depot Vegf expression (D) was significantly increased in the IngX HFD-fed group, whereas Pparγ (E) and Lpl (F) expression were significantly increased in IngX Chow mice only (Unlike letters indicate significance; P ≤ 0.05).
Gene expression was also used to examine the effects of IngX on liver lipogenesis (Fatty acid synthase (FASN) and sterol regulatory element-binding protein 1c (Srebp-1c)), fibrosis/collagen production (Transforming growth factor β (Tgfb1) and pro-collagen type 1, α 1 (Col1a1)) and markers of ER stress (spliced X box binding protein-1 (XBP1), glucose regulated protein 78 (Grp78), C/EBP homologous protein (Chop) and growth arrest and DNA damage inducible protein 34 (Gadd34)). IngX did not alter selected measurements of lipogenesis or ER stress, but did cause a significant increase in genes selected for fibrosis/collagen production in HFD mice only (Fig. 5B and C; P ≤ 0.05). Genes that were not different among groups were not shown, this includes FASN, XBP1, Grp78, Chop and GADD34.
Figure 5.
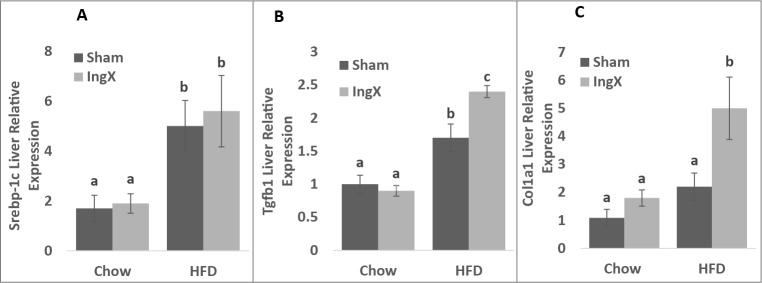
Liver gene expression 5 weeks post-surgery. IngX increased the expression of factors that play a role in fibrosis and collagen production in HFD mice only (B and C; Unlike letters indicate significance; P ≤ 0.05).
Secondary experiments
In a set of follow-up experiments we examined if IngX-induced changes observed at 5 weeks were still present following an extended experiment duration (13 wks) or occurred in mice with existing impaired glucose tolerance.
Inguinal fat pad removal and subsequent long-term high fat diet feeding
Ad libitum, chow-fed C57BL mice underwent IngX or sham-surgery and were subsequently provided chow or HFD until termination at 13 weeks.
Cumulative energy intake, adiposity and body mass of HFD mice were significantly greater than Chow (Table 3; P ≤ 0.05). Recovered inguinal adipose mass was significantly reduced in IngX mice compared to Sham, but IngX did not result in significant changes to the mass of non-excised adipose depots (Table 3; P ≤ 0.05).
Table 3.
Terminal cumulative food intake and body and adipose tissue mass of Chow or HFD mice; (A) 13 weeks following surgery or (B) with preceding glucose intolerance
| 13 weeks HFD Post-surgery | ||||
|---|---|---|---|---|
| Chow Sham (n = 10) | Chow IngX (n = 10) | HFD Sham (n= 10) | HFD IngX (n = 10) | |
| Food Intake (kcal/g) | 886.0 ± 28.6a | 905.9 ± 15.9a | 99.4 ± 22.8b | 984.0 ± 19.40b |
| Body Weight (g) | 31.75 ± 1.08a | 32.50 ± 1.05a | 41.11 ± 1.17b | 41.19 ± 0.69b |
| Inguinal Pad (g) | 0.58 ± 0.05a | 0.12 ± 0.02b | 1.52 ± 0.09c | 0.38 ± 0.03d |
| Brown Interscapular (g) | 0.23 ± 0.02a | 0.23 ± 0.03a | 0.44 ± 0.03b | 0.45 ± 0.02b |
| Epididymal (g) | 0.82 ± 0.10a | 0.90 ± 0.12a | 1.93 ± 0.12b | 2.03 ± 0.08b |
| Perirenal (g) | 0.40 ± 0.05a | 0.38 ± 0.07a | 0.98 ± 0.06b | 1.05 ± 0.05b |
| Visceral (g) | 0.36 ± 0.04a | 0.37 ± 0.05a | 0.82 ± 0.07b | 1.01 ± 0.10b |
| Pre-Glucose Intolerant | ||||
| Chow Sham (n = 10) | HFD Sham (n = 10) | HFD IngX (n = 10) | ||
| Food Intake (Kcal/g) | 274.8 ± 4.24a | 323 ± 11.54b | 324.8 ± 6.53b | |
| Body Weight (g) | 32.91 ± 0.99a | 37.20 ± 1.71b | 37.00 ± 0.66b | |
| Inguinal Pad (g) | 0.52 ± 0.07a | 1.02 ± 0.14b | 0.27 ± 0.05c | |
| Brown Interscapular (g) | 0.23 ± 0.02a | 0.39 ± 0.05b | 0.39 ± 0.03b | |
| Epididymal (g) | 0.78 ± 0.11a | 1.50 ± 0.20b | 1.60 ± 0.15b | |
| Perirenal (g) | 0.41 ± 0.06a | 0.69 ± 0.08b | 0.80 ± 0.09b | |
| Visceral (g) | 0.42 ± 0.07 | 0.59 ± 0.09 | 0.61 ± 0.06 | |
13 week HFS post-surgery—Cumulative energy intake, adiposity, and body mass of HFD mice were significantly greater than Chow. Recovered inguinal adipose mass was significantly reduced in IngX mice compared to Sham, but IngX did not result in significant changes to the mass of non-excised adipose depots. Pre-glucose intolerant—adiposity and body mass and 5 week post-surgery food intake were significantly higher in HFD groups compared with Chow. In HFD mice, IngX significantly decreased inguinal depot mass compared with Sham, but did not result in changes to non-excised depot mass. (Unlike letters indicate significance; P ≤ 0.05)
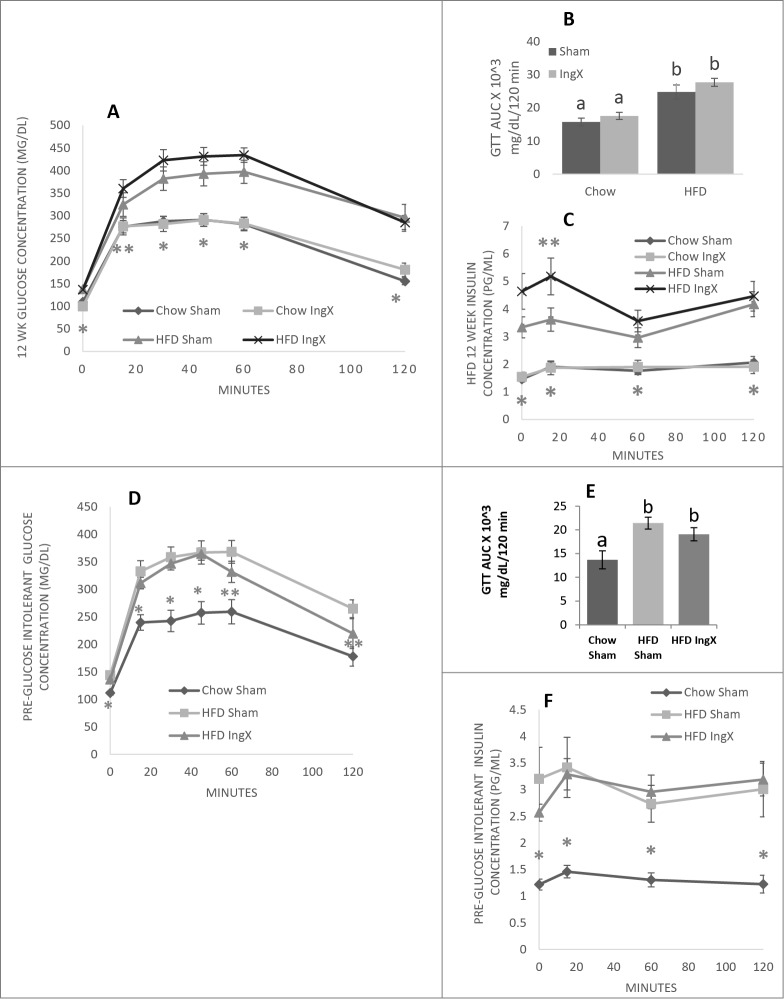
Compared with Chow fed animals 12 weeks of HFD feeding significantly increase glucose concentration curves of the GTT and area under the curves (Fig. 6A and B; P ≤ 0.05). IngX, however, did not further alter glucose response in Chow or HFD mice. Twelve weeks of HFD feeding also significantly increased circulating insulin in both HFD groups, but it was the highest in HFD IngX. IngX in HFD animals exacerbated insulin response to the GTT and was significantly different than Sham IngX at 15 minutes (Fig. 6C; P ≤ 0.05).
Figure 6.
Glucose tolerance test (GTT) and subsequent insulin concentrations (A-C) Following long-term HFD feeding or (D-F) in mice with preceding glucose intolerance. (C) 12 weeks post-surgery insulin concentration was significantly increased at 15 minutes in HFD IngX mice (P ≤ 0.05; compared with HFD Sham). IngX did not alter GTT (D) or insulin measurements (F) in mice with preceding glucose intolerance. With AUC unlike letters indicate significance; P ≤ 0.05.
Femoral muscle triglyceride was significantly increased in HFD groups compared with Chow, however IngX-induced increases in triglyceride concentration occurred in Chow mice only (Fig. 7A; P ≤ 0.05).
Figure 7.
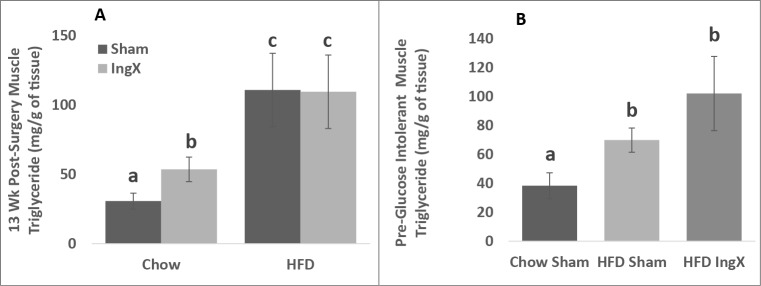
Femoral muscle triglyceride (A and B) was significantly increased in HFD groups compared with Chow. In 13 week experiment IngX-induced increases in femoral muscle triglyceride concentration only occurred in Chow mice (Unlike letters indicate significance; P ≤ 0.05).
Inguinal fat pad removal in glucose intolerant mice
Mice were fed Chow or HFD ad libitum for 6 weeks before surgery, hence HFD-fed animals had significantly higher glucose concentration curves (data not shown) and area under the curve than Chow (AUC; CHOW = 11548 ± 879.0 and HFD = 16551 ± 1571.3). HFD mice received IngX or Sham surgery (HFD Sham and HFD IngX) whereas Chow only received Sham surgery (Chow Sham) because the Chow IngX model was investigated in the primary experiment. Mice were terminated 5 weeks post-surgery and tissues were analyzed as described above.
Adiposity and body mass and 5 week post-surgery food intake (Table 3; P ≤ 0.05), GTT/insulin response (Fig. 6D-F; P ≤ 0.05) and femoral muscle triglyceride concentration (Fig. 7B; P ≤ 0.05) were significantly higher in HFD groups compared with Chow. In HFD mice, IngX significantly decreased inguinal depot mass compared with Sham, but did not result in changes to non-excised depot mass, glucose/insulin response to GTT (Fig. 6D-F) or muscle triglyceride concentration (Fig. 7B).
Discussion
Excessive adipose tissue accumulation is associated with insulin resistance, glucose intolerance, non-alcoholic fatty liver disease and cardiovascular disease risk. Furthermore, adipose tissue distribution appears to play an important role in the advancement of these obesity-induced pathophysiologies. Increased visceral adipose tissue in particular, is strongly associated with adverse metabolic risk factor profiles, whereas increases in LBSAT are not associated with the prevalence of these risk factors.13 For example, increased LBSAT mass is associated with decreased risk of impaired glucose metabolism and dyslipidemia, thus supporting its proposed protective role.38 We postulate that if LBSAT functions as a “metabolic sink” that its removal will increase the risk of developing diet-induced metabolic dysregulation. We used bilateral inguinal fat pad removal to examine the putative protective role of LBSAT on glucose tolerance, insulin sensitivity and lipids. These results demonstrate that removal of LBSAT (IngX) results in glucose intolerance and decreased muscle insulin sensitivity, which, in turn, may be due to adipose tissue-muscle interactions.
The protective effects of LBSAT are proposed to occur via its ability to sequester surplus lipid and thus serve as a “metabolic sink.”15 The primary goal of the present study was to addresses this postulate using surgical reduction of LBSAT without significant redistribution/compensation in non-excised adipose tissue depots. Results from the present study support and extend the “metabolic sink” concept. First, IngX resulted in glucose intolerance and muscle insulin resistance in HFD mice. Our data are consistent with a previous study in which subcutaneous adipose tissue removal (∼50%) in Syrian hamsters resulted in insulin resistance.26 Second, IngX resulted in lipid accumulation in muscle proximal to the excision site in Chow and HFD mice. Ectopic triglyceride deposition in non-adipose tissue can result from increased import of fatty acids, increased de novo synthesis and/or decreased fatty acid oxidation.39 Therefore, peripheral adipose tissue removal may upregulate the capacity for fatty acid transport/uptake and/or reduce fatty acid oxidation in skeletal muscle, in particular muscle proximal to the adipose depot excision site, this however remains to be tested. Subcutaneous adipose tissue removal has also been demonstrated to increase systemic circulating lipids in Syrian hamsters26 and humans.40 In the present study, however, IngX did not result in significant changes to portal or systemic circulating lipids. This indicates lipid spillover, in the present model, may be localized to the region of adipose depot removal. Consistent with this postulate, IngX did not increase liver triglycerides in the present experiment.
IngX-induced changes in muscle insulin sensitivity correlated to the accumulation of triglyceride in muscle. In both Chow and HFD mice IngX increased femoral muscle triglyceride concentration and decreased muscle insulin sensitivity. These data are consistent with the well-established relation between intramyocellular lipid accumulation and insulin resistance in humans and rodents.41,42 However, glucose intolerance and increased circulating insulin occurred exclusively in HFD-fed IngX mice. This evidence suggests that adipose tissue removal-induced increases in muscle lipid storage, per se, are not sufficient to explain impairments in glucose tolerance. Studies demonstrate lipid accumulation, itself, is not deleterious whereas excessive lipid intermediates linked to HFD, such as but not limited to diaclyglycerol, ceramides and acyl-CoA, are.43-46 Therefore we postulate that removal of “the protective” subcutaneous adipose depot in HFD-fed mice promotes greater accumulation of tissue lipid intermediates than both Chow and HFD Sham. Consequently these alterations would account for the enhanced tissue insulin resistance and whole body glucose intolerance demonstrated in HFD IngX.
Visceral adipose tissue accumulation has a greater association to the pathogenesis of obesity-related disease, however it only constitutes ∼10% of total body adiposity whereas subcutaneous is approximately 85%.47 Visceral adipose tissue is postulated to play a fundamental role in obesity-induced health consequence because of its anatomical location. Specifically, it is proposed that metabolites and secretory products released from visceral adipocytes enter the portal vein which subsequently drains to liver insulin-sensitive hepatocytes,48-50 however this postulate remains controversial. Some estimate subcutaneous adipose tissue supplies the majority of effluent, such as but not limited to free fatty acids, to the portal vein51 and systemic circulation,51,52 whereas visceral adipose tissue is estimated to minimally contribute. Therefore obesity-induced pathophysiology of the liver may primarily be due to the limited ability of subcutaneous adipose tissue to store excess energy and subsequently visceral adipose accumulation occurs. Hence we examined early effects of subcutaneous adipose tissue removal in relation to liver perturbations. Non-alcoholic fatty liver disease (NAFLD) is a co-morbidity of obesity and is associated with increased hepatic fibrosis/collagen, steatosis, inflammation and endoplasmic reticulum (ER) stress.53 Evidence suggests ER stress, induced by increased steatosis, is a pro-fibrotic stimulus,54 thus indicating markers of lipogenesis, ER stress and fibrosis/collagen production should be positively correlated. However, markers of liver dysfunction in the current study did not correlate as previously demonstrated. Ingx further stimulated HFD-induced increases in fibrosis/collagen markers, but did not increase liver triglyceride concentration or markers of lipogenesis or ER stress. Overall, subcutaneous adipose tissue removal does induce markers of liver perturbations, but gene expression changes at the protein level may better indicate how subcutaneous adipose tissue deposition protects liver function during obesity development.
Fat removal-induced metabolic alterations were examined before and after significant adipose tissue compensation to examine the direct role of LBSAT in relation to glucose tolerance and insulin sensitivity. Adipose tissue removal in humans18,23,24 and rodents30,35 induces compensation in remaining non-excised adipose tissue depots. Rodent studies demonstrate that compensatory adipose depot mass differences are not detectable 5 weeks post-surgery,55 but significant compensatory redistribution of lipids to non-excised adipose depots occurs ∼12 weeks post-surgery. The extent of compensation differs according to the depot removed, hence intra-abdominal adipose tissue depot removal (i.e., epididymal) significantly increases mass of non-excised adipose depots, whereas inguinal does not.35 Consistent with previous studies, IngX (inguinal removal) did not induce adipose depot compensation at 5 or 12 weeks post-surgery. Depot mass is a principal measurement of compensation, but it may not account for compensatory changes in adipocyte proliferation (increased smaller cell size) or adipose tissue gene expression. Proliferation markers would precede increases in adipose depot growth, hence factors involved in adipogenesis (peroxisome proliferator-activated receptor gamma; Pparγ), vasculature growth (vascular endothelial growth factor; Vegf) and lipogenesis (lipoprotein lipase; Lpl) where measured 5 weeks post-surgery in the visceral depot and non-excised subcutaneous adipose remnant. In general, early adipocyte hyperplasia associated HFD feeding is demonstrated to be preceded by an increase in proliferation markers in the stromal vascular cells of adipose tissue.56-58 However, other studies demonstrate HFD-induced proliferation potential is dependent on the magnitude of adiposity,59 which can vary according to diet composition or experiment length, and/or depot location.60 In the present experiment 5 week HFD feeding did not alter adipose depot markers of proliferation in Sham mice, but adipose depot compensatory changes at the molecular level were initiated in response to IngX in Chow fed mice only. This suggests that IngX-induced compensatory modifications are inhibited in HFD mice, but whether this outcome is the cause or consequence of HFD IngX-induced glucose intolerance and high circulating insulin remains unclear.
Although the effects of subcutaneous adipose tissue removal have been investigated in humans and rodents, results from these studies were equivocal. Some of this inconsistency may be due to variability in experimental approach. We, therefore, performed 2 secondary studies to determine whether IngX-induced metabolic impairments were sustained following an extended experimental duration, 13 weeks, or was exacerbated in mice with existing glucose intolerance. While pronounced short-term metabolic improvements have been demonstrated with LBSAT removal,61 many have demonstrated that large volume removal of LBSAT in obese humans results in minimal metabolic improvement, with short term decreases in circulating lipids and fasting insulin,17,19-21,62 but no long-term differences.16 In the present study IngX-induced metabolic effects were sustained, yet to some extent attenuated, in both Chow and HFD mice after a longer experiment duration. Mice characterized by impaired glucose tolerance prior to surgery were not affected by IngX. Consistent with this other have demonstrated LBSAT removal in obese/glucose intolerant mice is without metabolic effect.25 The present study supports the notion that IngX-mediated perturbations to glucose homeostasis are sustained at longer durations, but are influenced by the pre-existing metabolic milieu. HFD consequences of IngX are greatest early post-surgery, attenuated following a longer post-surgical duration and don't occur if obesity/glucose intolerance is present at surgery. Therefore IngX effects diminish as HFD-induced glucose intolerance and other obesity associated co-morbidities reach upper limits.
In conclusion, it is postulated that obesity-induced pathophysiology may be due to the limited ability of lower body subcutaneous adipose tissue to store excess energy/ surplus lipid that otherwise ectopically deposit in tissues such as liver, muscle and pancreas, however a direct causal role had not been demonstrated. Results from the present systematic study suggest that lower body subcutaneous adipose tissue serves to protect muscle from excessive triglyceride deposition and ultimately helps to preserve whole body glucose homeostasis. The relative contribution of visceral and/or subcutaneous adipose tissue deposition to the development of insulin resistance remains to be clarified. Studies suggest, however, that the subcutaneous and visceral adipose tissue depots work concomitantly in lipid partitioning and both regions influence insulin sensitivity and glucose homeostasis. The chronological order of regional adipose tissue depot dysregulation in obesity likely varies among individuals, but some propose that dysregulation of the visceral depot is a result of inadequate subcutaneous lipid deposition.63 Future studies investigating adipose tissue distribution and its relation to metabolic disease should no longer independently investigate distinct depots, but rather examine alterations in mechanisms that balance lipid deposition between different adipose regions and consequent adaptations of muscle or organs proximal to adipose depots.
Materials and Methods
Animals
Adult male C57BL/6 mice (Jackson Laboratory, Bar Harbor, Maine) (∼24 g) were individually housed under controlled conditions (12:12 light-dark cycle, 50–60% humidity, and 25°C) and were allowed to acclimate for one week following arrival. During experiments mice had free access to standard chow (Harlan Teklad LM485, Madison, WI) or a high-fat Western diet containing 21% milk fat and 34% sucrose (HFD; Harlan Laboratories, Madison, WI; TD.08811; 45% kcal from fat; 4.7 kcal/g). Post-surgery body mass and food intake were recorded weekly. Procedures were reviewed and approved by the Colorado State University Institutional Animal Care and Use Committee.
Fat removal surgeries
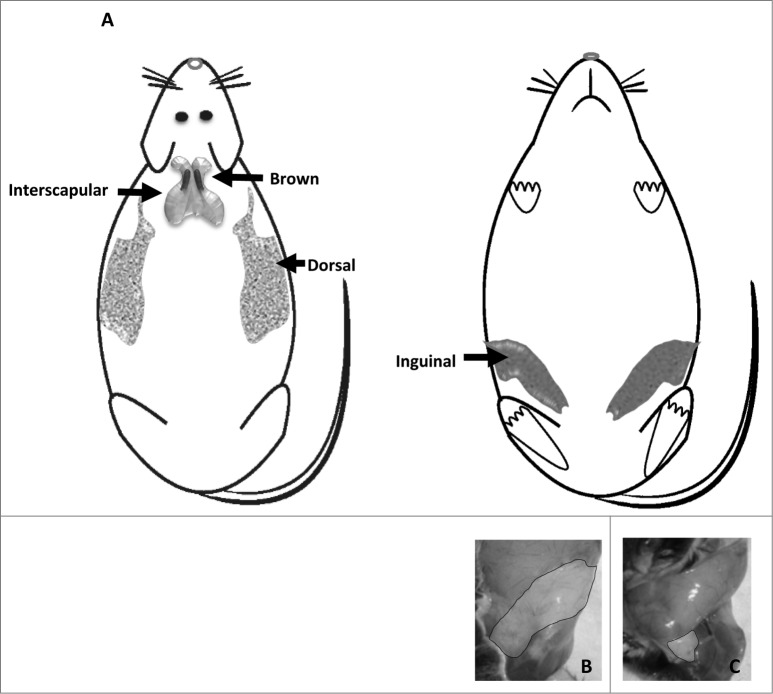
Subcutaneous adipose tissue is located between the muscle and the skin. In rodents there are 3 distinct regions inguinal, dorsal and interscapular (Fig. 8A), the latter contains brown adipose tissue. Surgeries in the present experiment include removal of the inguinal adipose depot.
Figure 8.
(A) Subcutaneous adipose tissue depot location, dorsal and ventral. (B) Inguinal adipose tissue depot before removal, and (C) after ∼300mg removal.
More specifically, mice anesthetized with isoflurane received a mid-ventral abdominal incision through which sham surgery (Sham, skin was separated from muscle, but adipose tissue was not removed) or lower body subcutaneous adipose tissue, inguinal, removal (IngX, bilateral removal totaling ∼300 mg of inguinal adipose tissue) occurred (Fig. 8). Roughly 80% of inguinal adipose tissue can be visualized and removed through abdominal incision in IngX (Fig. 8B and C), which is ∼30% of total subcutaneous and ∼10% of whole body adiposity. Following surgery, muscle and skin were closed with suture and meloxicam analgesic (0.025mg/10 g body weight) was injected subcutaneously.
Glucose tolerance test (GTT)
Glucose tolerance tests (GTTs) were conducted pre-surgery and one week before experiment termination. Following a 6 hour fast, blood glucose was determined from tail vein (Freestyle Lite Glucometer; Abbott, Abbott Park, IL) and ∼75 ul of blood was collected for later measurement of plasma insulin. Mice then received a 1.5 g/kg dextrose injection (ip) and blood glucose was again assessed from tail vein blood samples 15, 30, 45, 60 and 120 min post-injection. An additional, ∼75 ul of blood was collected at 15, 60 and 120 minutes for later measurement of plasma insulin.
Terminal procedures (blood collection and tissue harvesting)
All mice were fasted 4 hours before being anesthetized with isoflurane for termination where blood, liver, adipose tissue and muscle were collected. Blood was collected (∼150 ul) from the hepatic portal vein before systemic blood was collected via cardiac puncture. Subsequently plasma was separated from blood samples and stored at −80°C. The right lobe of the liver was snap-frozen in liquid nitrogen and stored at −80°C. Inguinal (IWAT), epididymal (EWAT), perirenal (PWAT), inter-scapular brown adipose (BAT) and visceral white adipose tissue (VWAT; mesenteric and omentum) were dissected and weighed. The inguinal (from Sham and non-excised remnants IngX) and visceral adipose depots were snap frozen and stored at −80°C. The femoral muscle was also collected and frozen due to its close proximity to the adipose tissue removed.
Tissue triglycerides and free fatty acids
Skeletal muscle and liver lipids were extracted using the procedure of Bligh and Dyer.64 Muscle and liver triglyceride concentration (Sigma Chemical Co, St. Louis, MO) and plasma non-esterified fatty acids (Wako, Richmond, VA) were determined enzymatically using commercially available kits.
Muscle insulin sensitivity
Four hour fasted mice were injected (ip) with saline or insulin (1 mU/g; SAFC Biosciences, Inc.., Lenexa, KS, USA) ∼15 mins before being anesthetized with isoflurane for terminal. Insulin sensitivity was estimated in the femoral muscle using the ratio of phospho-Akt1 (Ser473) to total Akt (EMD Millipore Corporation, Billerica, MA).
Mouse plasma adipokine assay
Systemic and portal plasma insulin, leptin, tumor necrosis factor α (TNFα), monocyte chemotactic protein-1 (MCP-1) and interleukin 6 (IL-6) concentrations were determined using commercial kits (EMD Millipore Corporation, Billerica, MA) and analyzed on a Luminex instrument (LX200; Millipore, Austin, TX).
Quantitative RT PCR
RNA isolation and cDNA synthesis
Lipid-specific RNAeasy mini-kit columns (QIAGEN, Valencia, CA) were used to isolate RNA from adipose tissue and Trizol (Life Technologies, Grand Island, NY) was used to isolate liver RNA according to the manufacturer's instructions. iScript (Bio-Rad, Hercules, CA) was used to synthesize cDNA from 0.25 μg total RNA.
Quantitative real-time PCR
Sequences of primers for liver and adipose tissue are shown in Table 4. Primers were optimized as previously described.65 Samples were run in triplicate using an iCycler (Bio-Rad) and iQ SYBR Green Supermix (Bio-Rad). Expression patterns of genes of interest were normalized to constitutively expressed β2 microglobulin (B2M) and relative expression was quantified as previously described.65
Table 4.
Sequences of primers for adipose or liver tissue. Beta-2 microglobulin (B2M), C/EBP homologous protein (CHOP), collagen-α1 (Col1a1) fatty acid synthase (FASN), growth arrest and DNA damage inducible protein 34 (GADD34), glucose regulated protein 78 (GRP78), lipoprotein lipase (LPL), peroxisome proliferator-activated receptor γ (PPARγ), sterol regulatory element-binding protein-1c (SREBP-1c), transforming growth factor-β (Tgfb1), vascular endothelial growth factor (VEGF) and spliced X box binding protein-1 (XBP-1)
| Target Gene | Sequence |
|---|---|
| B2M | F-CGGTCGCTTCAGTCGTCAG |
| R-ATGTTCGGCTTCCCATTCTCC | |
| CHOP | F-CGCTCTCCAGATTCCAGTCAG |
| R-GTTCTCCTGCTCCTTCTCCTT | |
| Col1a1 | F-ATGTATCACCAGACGCAGAAG |
| R-TCTTGAGGTTGCCAGTCTGC | |
| FASN | F-AGACTACAGACGACAGCAACC |
| R-CTCTCAGACAGGCACTCAGC | |
| GADD34 | F-CAGAAGATGACACAGAAGAGG |
| R-TCTCTCCTGGTAGACAACGC | |
| GRP78 | F-GAGGCGTATTTGGGAAAGAAGG |
| R-GCTGCTGTAGGCTCATTGATG | |
| LPL | F-TTCCAGCCAGGATGCAACA |
| R-GGTCCACGTCTCCGAGTCC | |
| PPARγ | F-GCG GTG AAC CAC TGA TAT TCA GGA CA |
| R-TCCGAAGTTGGTGGGCCAGA | |
| Srebp-1c | F-TGGTGGGCACTGAAGCAAAG |
| R-CACTTCGTAGGGTCAGGTTCTC | |
| Tgfb1 | F-TGGACACACAGTACAGCAAGG |
| R-GTAGTAGACGATGGGCAGTGG | |
| Vegf | F-TGTGCAGGCTGCTGTAACGATGAA |
| R-ATGTGCTGGCTTTGGTGAGGTTTG | |
| XBP-1 | F-GGCATTCTGGACAAGTTGG |
| R-GAAAGGGAGGCTGGTAAGG |
Statistical analysis
Data are expressed as mean ± standard error of the mean (SEM). Comparisons among multiple groups were performed using 2-way ANOVA between-subjects analysis of variance (ANOVA) (IBM SPSS for Windows, release 21; SPSS, Chicago, IL) with diet (Chow and HFD) and surgery (Sham and IngX) as the factors. This was performed on most dependent variables including body weight, tissue mass, AUC, lipid measurements, gene expression, obesity markers and insulin sensitivity. Glucose and insulin concentrations from the GTT were analyzed using 2-way repeated measures ANOVA with time as a within-subject variables. Post-hoc tests of individual groups were made using Tukey's tests. For all experiments, differences among groups were considered statistically significant if P ≤ 0.05. Exact probabilities and test values were omitted for simplicity and clarity of presentation of the results.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Haffner SM. Relationship of metabolic risk factors and development of cardiovascular disease and diabetes. Obesity (Silver Spring) 2006; 14 Suppl 3:121S-7S; PMID:16931493; http://dx.doi.org/ 10.1038/oby.2006.291 [DOI] [PubMed] [Google Scholar]
- 2. Prospective Studies C, Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, Qizilbash N, Collins R, Peto R. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 2009; 373:1083-96; PMID:19299006; http://dx.doi.org/ 10.1016/S0140-6736(09)60318-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sims EA. Are there persons who are obese, but metabolically healthy? Metabolism 2001; 50:1499-504; PMID:11735101; http://dx.doi.org/ 10.1053/meta.2001.27213 [DOI] [PubMed] [Google Scholar]
- 4. Reaven GM. Importance of identifying the overweight patient who will benefit the most by losing weight. Ann Intern Med 2003; 138:420-3; PMID:12614095; http://dx.doi.org/ 10.7326/0003-4819-138-5-200303040-00012 [DOI] [PubMed] [Google Scholar]
- 5. Ferrannini E, Natali A, Bell P, Cavallo-Perin P, Lalic N, Mingrone G. Insulin resistance and hypersecretion in obesity. European Group for the Study of Insulin Resistance (EGIR). J Clin Invest 1997; 100:1166-73; PMID:9303923; http://dx.doi.org/ 10.1172/JCI119628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Voulgari C, Tentolouris N, Dilaveris P, Tousoulis D, Katsilambros N, Stefanadis C. Increased heart failure risk in normal-weight people with metabolic syndrome compared with metabolically healthy obese individuals. J Am Coll Cardiol 2011; 58:1343-50; PMID:21920263; http://dx.doi.org/ 10.1016/j.jacc.2011.04.047 [DOI] [PubMed] [Google Scholar]
- 7. Succurro E, Marini MA, Frontoni S, Hribal ML, Andreozzi F, Lauro R, Perticone F, Sesti G. Insulin secretion in metabolically obese, but normal weight, and in metabolically healthy but obese individuals. Obesity (Silver Spring) 2008; 16:1881-6; PMID:18551117; http://dx.doi.org/ 10.1038/oby.2008.308 [DOI] [PubMed] [Google Scholar]
- 8. Bjorntorp P. Metabolic implications of body fat distribution. Diabetes Care 1991; 14:1132-43; PMID:1773700; http://dx.doi.org/ 10.2337/diacare.14.12.1132 [DOI] [PubMed] [Google Scholar]
- 9. Kissebah AH, Krakower GR. Regional adiposity and morbidity. Physiol Rev 1994; 74:761-811; PMID:7938225 [DOI] [PubMed] [Google Scholar]
- 10. Snijder MB, Dekker JM, Visser M, Yudkin JS, Stehouwer CD, Bouter LM, Heine RJ, Nijpels G, Seidell JC. Larger thigh and hip circumferences are associated with better glucose tolerance: the Hoorn study. Obes Res 2003; 11:104-11; PMID:12529492; http://dx.doi.org/ 10.1038/oby.2003.18 [DOI] [PubMed] [Google Scholar]
- 11. Snijder MB, Dekker JM, Visser M, Bouter LM, Stehouwer CD, Yudkin JS, Heine RJ, Nijpels G, Seidell JC. Trunk fat and leg fat have independent and opposite associations with fasting and postload glucose levels: the Hoorn study. Diabetes Care 2004; 27:372-7 [DOI] [PubMed] [Google Scholar]
- 12. Snijder MB, Dekker JM, Visser M, Bouter LM, Stehouwer CD, Kostense PJ, Yudkin JS, Heine RJ, Nijpels G, Seidell JC. Associations of hip and thigh circumferences independent of waist circumference with the incidence of type 2 diabetes: the Hoorn Study. Am J Clin Nutr 2003; 77:1192-7; PMID:12716671 [DOI] [PubMed] [Google Scholar]
- 13. Porter SA, Massaro JM, Hoffmann U, Vasan RS, O'Donnel CJ, Fox CS. Abdominal subcutaneous adipose tissue: a protective fat depot? Diabetes Care 2009; 32:1068-75; PMID:19244087; http://dx.doi.org/ 10.2337/dc08-2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Manolopoulos KN, Karpe F, Frayn KN. Gluteofemoral body fat as a determinant of metabolic health. Int J Obes (Lond) 2010; 34:949-59; PMID:20065965; http://dx.doi.org/ 10.1038/ijo.2009.286 [DOI] [PubMed] [Google Scholar]
- 15. Kral JG. Surgical treatment of regional adiposity. Lipectomy versus surgically induced weight loss. Acta med Scand Suppl 1988; 723:225-31; PMID:3164970 [PubMed] [Google Scholar]
- 16. Mohammed BS, Cohen S, Reeds D, Young VL, Klein S. Long-term effects of large-volume liposuction on metabolic risk factors for coronary heart disease. Obesity (Silver Spring) 2008; 16:2648-51; PMID:18820648; http://dx.doi.org/ 10.1038/oby.2008.418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hong YG, Kim HT, Seo SW, Chang CH, Rhee EJ, Lee WY. Impact of large-volume liposuction on serum lipids in orientals: a pilot study. Aesthetic Plast Surg 2006; 30:327-32; PMID:16733777; http://dx.doi.org/ 10.1007/s00266-005-0010-7 [DOI] [PubMed] [Google Scholar]
- 18. Hernandez TL, Kittelson JM, Law CK, Ketch LL, Stob NR, Lindstrom RC, Scherzinger A, Stamm ER, Eckel RH. Fat redistribution following suction lipectomy: defense of body fat and patterns of restoration. Obesity (Silver Spring) 2011; 19:1388-95; PMID:21475140; http://dx.doi.org/ 10.1038/oby.2011.64 [DOI] [PubMed] [Google Scholar]
- 19. Gonzalez-Ortiz M, Robles-Cervantes JA, Cardenas-Camarena L, Bustos-Saldana R, Martinez-Abundis E. The effects of surgically removing subcutaneous fat on the metabolic profile and insulin sensitivity in obese women after large-volume liposuction treatment. Horm Metab Res 2002; 34:446-9; PMID:12198600; http://dx.doi.org/ 10.1055/s-2002-33603 [DOI] [PubMed] [Google Scholar]
- 20. Giese SY, Bulan EJ, Commons GW, Spear SL, Yanovski JA. Improvements in cardiovascular risk profile with large-volume liposuction: a pilot study. Plast Reconstr Surg 2001; 108:510-9; discussion 20-1; PMID:11496197; http://dx.doi.org/ 10.1097/00006534-200108000-00035 [DOI] [PubMed] [Google Scholar]
- 21. Klein S, Fontana L, Young VL, Coggan AR, Kilo C, Patterson BW, Mohammed BS. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. N Engl J Med 2004; 350:2549-57; PMID:15201411; http://dx.doi.org/ 10.1056/NEJMoa033179 [DOI] [PubMed] [Google Scholar]
- 22. Kennedy GC. The role of depot fat in the hypothalamic control of food intake in the rat. Proc R Soc Lond B Biol Sci 1953; 140:578-96; PMID:13027283 [DOI] [PubMed] [Google Scholar]
- 23. Yost TJ, Rodgers CM, Eckel RH. Suction lipectomy: outcome relates to region-specific lipoprotein lipase activity and interval weight change. Plast Reconstr Surg 1993; 92:1101-8; discussion 9-11; PMID:8234508; http://dx.doi.org/ 10.1097/00006534-199311000-00016 [DOI] [PubMed] [Google Scholar]
- 24. Lambert EV, Hudson DA, Bloch CE, Koeslag JH. Metabolic response to localized surgical fat removal in nonobese women. Aesthetic Plast Surg 1991; 15:105-10; PMID:2035358; http://dx.doi.org/ 10.1007/BF02273842 [DOI] [PubMed] [Google Scholar]
- 25. Shi H, Strader AD, Woods SC, Seeley RJ. The effect of fat removal on glucose tolerance is depot specific in male and female mice. Am J Physiol Endocrinol Metab 2007; 293:E1012-20; PMID:17652151; http://dx.doi.org/ 10.1152/ajpendo.00649.2006 [DOI] [PubMed] [Google Scholar]
- 26. Weber RV, Buckley MC, Fried SK, Kral JG. Subcutaneous lipectomy causes a metabolic syndrome in hamsters. Am J Physiol Regul Integr Comp Physiol 2000; 279:R936-43; PMID:10956251 [DOI] [PubMed] [Google Scholar]
- 27. Ishikawa K, Takahashi K, Bujo H, Hashimoto N, Yagui K, Saito Y. Subcutaneous fat modulates insulin sensitivity in mice by regulating TNF-α expression in visceral fat. Horm Metab Res 2006; 38:631-8; PMID:17075771; http://dx.doi.org/ 10.1055/s-2006-954580 [DOI] [PubMed] [Google Scholar]
- 28. Goncalves WL, Graceli JB, Santos RL, Cicilini MA, Bissoli NS, Abreu GR, Moysés MR. Ultrasound lipoclasia on subcutaneous adipose tissue to produce acute hyperglycemia and enhance acute inflammatory response in healthy female rats. Dermatol Surg 2009; 35:1741-5; PMID:19737292; http://dx.doi.org/ 10.1111/j.1524-4725.2009.01286.x [DOI] [PubMed] [Google Scholar]
- 29. Mauer MM, Harris RB, Bartness TJ. The regulation of total body fat: lessons learned from lipectomy studies. Neurosci Biobehav Rev 2001; 25:15-28; PMID:11166075; http://dx.doi.org/ 10.1016/S0149-7634(00)00047-6 [DOI] [PubMed] [Google Scholar]
- 30. Mauer MM, Bartness TJ. Fat pad-specific compensatory mass increases after varying degrees of lipectomy in Siberian hamsters. Am J Physiol 1997; 273:R2117-23; PMID:9435669 [DOI] [PubMed] [Google Scholar]
- 31. Mauer MM, Bartness TJ. Temporal changes in fat pad mass and cellularity after lipectomy in Siberian hamsters. Physiol Behav 1997; 62:1029-36; PMID:9333196; http://dx.doi.org/ 10.1016/S0031-9384(97)00233-3 [DOI] [PubMed] [Google Scholar]
- 32. Mauer MM, Bartness TJ. Short-day-like body weight changes do not prevent fat pad compensation after lipectomy in Siberian hamsters. Am J Physiol 1997; 272:R68-77; PMID:9038992 [DOI] [PubMed] [Google Scholar]
- 33. Mauer MM, Bartness TJ. Photoperiod-dependent fat pad mass and cellularity changes after partial lipectomy in Siberian hamsters. Am J Physiol 1996; 270:R383-92; PMID:8779869 [DOI] [PubMed] [Google Scholar]
- 34. Mauer MM, Bartness TJ. A role for testosterone in the maintenance of seasonally appropriate body mass but not in lipectomy-induced body fat compensation in Siberian hamsters. Obes Res 1995; 3:31-41; PMID:7712357; http://dx.doi.org/ 10.1002/j.1550-8528.1995.tb00118.x [DOI] [PubMed] [Google Scholar]
- 35. Mauer MM, Bartness TJ. Body fat regulation after partial lipectomy in Siberian hamsters is photoperiod dependent and fat pad specific. Am J Physiol 1994; 266:R870-8; PMID:8160883 [DOI] [PubMed] [Google Scholar]
- 36. Hamilton JM, Wade GN. Lipectomy does not impair fattening induced by short photoperiods or high-fat diets in female Syrian hamsters. Physiol Behav 1988; 43:85-92; PMID:3413255; http://dx.doi.org/ 10.1016/0031-9384(88)90102-3 [DOI] [PubMed] [Google Scholar]
- 37. Dark J, Forger NG, Stern JS, Zucker I. Recovery of lipid mass after removal of adipose tissue in ground squirrels. Am J Physiol 1985; 249:R73-8; PMID:4014499 [DOI] [PubMed] [Google Scholar]
- 38. Snijder MB, Visser M, Dekker JM, Goodpaster BH, Harris TB, Kritchevsky SB, De Rekeneire N, Kanaya AM, Newman AB, Tylavsky FA, et al. Low subcutaneous thigh fat is a risk factor for unfavourable glucose and lipid levels, independently of high abdominal fat. The Health ABC Study. Diabetologia 2005; 48:301-8; PMID:15660262; http://dx.doi.org/ 10.1007/s00125-004-1637-7 [DOI] [PubMed] [Google Scholar]
- 39. Schaffer JE. Lipotoxicity: when tissues overeat. Curr Opin Lipidol 2003; 14:281-7; PMID:12840659; http://dx.doi.org/ 10.1097/00041433-200306000-00008 [DOI] [PubMed] [Google Scholar]
- 40. Ybarra J, Blanco-Vaca F, Fernandez S, Castellvi A, Bonet R, Palomer X, Ordóñez-Llanos J, Trius A, Vila-Rovira R, Pérez A. The effects of liposuction removal of subcutaneous abdominal fat on lipid metabolism are independent of insulin sensitivity in normal-overweight individuals. Obes Surg 2008; 18:408-14; PMID:18264836; http://dx.doi.org/ 10.1007/s11695-007-9261-5 [DOI] [PubMed] [Google Scholar]
- 41. Machann J, Haring H, Schick F, Stumvoll M. Intramyocellular lipids and insulin resistance. Diabetes Obes Metab 2004; 6:239-48; PMID:15171747; http://dx.doi.org/ 10.1111/j.1462-8902.2004.00339.x [DOI] [PubMed] [Google Scholar]
- 42. Larson-Meyer DE, Newcomer BR, Ravussin E, Volaufova J, Bennett B, Chalew S, Cefalu WT, Sothern M. Intrahepatic and intramyocellular lipids are determinants of insulin resistance in prepubertal children. Diabetologia 2011; 54:869-75; PMID:21181394; http://dx.doi.org/ 10.1007/s00125-010-2022-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Badin PM, Langin D, Moro C. Dynamics of skeletal muscle lipid pools. Trends Endocrinol Metab: TEM 2013; 24:607-15; PMID:23988586; http://dx.doi.org/ 10.1016/j.tem.2013.08.001 [DOI] [PubMed] [Google Scholar]
- 44. Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest 2000; 106:171-6; PMID:10903330; http://dx.doi.org/ 10.1172/JCI10583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schmitz-Peiffer C. Protein kinase C and lipid-induced insulin resistance in skeletal muscle. Ann N Y Acad Sci 2002; 967:146-57; PMID:12079844 [DOI] [PubMed] [Google Scholar]
- 46. Timmers S, Schrauwen P, de Vogel J. Muscular diacylglycerol metabolism and insulin resistance. Physiol Behav 2008; 94:242-51; PMID:18207474; http://dx.doi.org/ 10.1016/j.physbeh.2007.12.002 [DOI] [PubMed] [Google Scholar]
- 47. Klein S, Allison DB, Heymsfield SB, Kelley DE, Leibel RL, Nonas C, Kahn R, Association for Weight Management and Obesity Prevention , NAASO , Obesity Society, et al. Waist circumference and cardiometabolic risk: a consensus statement from shaping America's health: Association for Weight Management and Obesity Prevention; NAASO, the Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Diabetes Care 2007; 30:1647-52; PMID:17360974; http://dx.doi.org/ 10.2337/dc07-9921 [DOI] [PubMed] [Google Scholar]
- 48. Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science 1996; 271:665-8; PMID:8571133; http://dx.doi.org/ 10.1126/science.271.5249.665 [DOI] [PubMed] [Google Scholar]
- 49. Bergman RN. Non-esterified fatty acids and the liver: why is insulin secreted into the portal vein? Diabetologia 2000; 43:946-52; PMID:10952470; http://dx.doi.org/ 10.1007/s001250051474 [DOI] [PubMed] [Google Scholar]
- 50. Williamson JR, Kreisberg RA, Felts PW. Mechanism for the stimulation of gluconeogenesis by fatty acids in perfused rat liver. Proc Natl Acad Sci U S A 1966; 56:247-54; PMID:4381783; http://dx.doi.org/ 10.1073/pnas.56.1.247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. J Clin Invest 2004; 113:1582-8; PMID:15173884; http://dx.doi.org/ 10.1172/JCI21047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Guo Z, Hensrud DD, Johnson CM, Jensen MD. Regional postprandial fatty acid metabolism in different obesity phenotypes. Diabetes 1999; 48:1586-92; PMID:10426377; http://dx.doi.org/ 10.2337/diabetes.48.8.1586 [DOI] [PubMed] [Google Scholar]
- 53. Pagliassotti MJ. Endoplasmic reticulum stress in nonalcoholic fatty liver disease. Annu Rev Nutr 2012; 32:17-33; PMID:22809102; http://dx.doi.org/ 10.1146/annurev-nutr-071811-150644 [DOI] [PubMed] [Google Scholar]
- 54. Tanjore H, Lawson WE, Blackwell TS. Endoplasmic reticulum stress as a pro-fibrotic stimulus. Biochim Biophys Acta 2013; 1832:940-7; PMID:23201247; http://dx.doi.org/ 10.1016/j.bbadis.2012.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Foster MT, Shi H, Seeley RJ, Woods SC. Transplantation or removal of intra-abdominal adipose tissue prevents age-induced glucose insensitivity. Physiol Behav 2010; 101:282-8; PMID:20570685; http://dx.doi.org/ 10.1016/j.physbeh.2010.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dugail I, Quignard-Boulange A, Dupuy F. Role of adipocyte precursors in the onset of obesity induced by overfeeding in suckling rats. J Nutr 1986; 116:524-35; PMID:3958800 [DOI] [PubMed] [Google Scholar]
- 57. Klyde BJ, Hirsch J. Increased cellular proliferation in adipose tissue of adult rats fed a high-fat diet. J Lipid Res 1979; 20:705-15; PMID:490049 [PubMed] [Google Scholar]
- 58. Ellis JR, McDonald RB, Stern JS. A diet high in fat stimulates adipocyte proliferation in older (22 month) rats. Exp Gerontol 1990; 25:141-8; PMID:2369929; http://dx.doi.org/ 10.1016/0531-5565(90)90045-4 [DOI] [PubMed] [Google Scholar]
- 59. Li J, Yu X, Pan W, Unger RH. Gene expression profile of rat adipose tissue at the onset of high-fat-diet obesity. Am J Physiol Endocrinol Metab 2002; 282:E1334-41; PMID:12006364 [DOI] [PubMed] [Google Scholar]
- 60. Joe AW, Yi L, Even Y, Vogl AW, Rossi FM. Depot-specific differences in adipogenic progenitor abundance and proliferative response to high-fat diet. Stem Cells 2009; 27:2563-70; PMID:19658193; http://dx.doi.org/ 10.1002/stem.190 [DOI] [PubMed] [Google Scholar]
- 61. D'Andrea F, Grella R, Rizzo MR, Grella E, Nicoletti G, Barbieri M, Paolisso G. Changing the metabolic profile by large-volume liposuction: a clinical study conducted with 123 obese women. Aesthetic Plast Surg 2005; 29:472-8; discussion 9-80, 81; PMID:16328631; http://dx.doi.org/ 10.1007/s00266-005-0089-x [DOI] [PubMed] [Google Scholar]
- 62. Ramos-Gallardo G, Perez Verdin A, Fuentes M, Godinez Gutierrez S, Ambriz-Plascencia AR, Gonzalez-Garcia I, Gómez-Fonseca SM, Madrigal R, González-Reynoso LI, Figueroa S. et al. Effect of abdominoplasty in the lipid profile of patients with dyslipidemia. Plast Surg Int 2013; 2013:861348; PMID:23956856; http://dx.doi.org/ 10.1155/2013/861348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Taksali SE, Caprio S, Dziura J, Dufour S, Cali AM, Goodman TR, Papademetris X, Burgert TS, Pierpont BM, Savoye M. et al. High visceral and low abdominal subcutaneous fat stores in the obese adolescent: a determinant of an adverse metabolic phenotype. Diabetes 2008; 57:367-71; PMID:17977954; http://dx.doi.org/ 10.2337/db07-0932 [DOI] [PubMed] [Google Scholar]
- 64. Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 1959; 37:911-7; PMID:13671378; http://dx.doi.org/ 10.1139/o59-099 [DOI] [PubMed] [Google Scholar]
- 65. Kim DH, Sandoval D, Reed JA, Matter EK, Tolod EG, Woods SC, Seeley RJ. The role of GM-CSF in adipose tissue inflammation. Am J Physiol Endocrinol Metab 2008; 295:E1038-46; PMID:18765677; http://dx.doi.org/ 10.1152/ajpendo.00061.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]