Abstract
Background
The incidence and prevalence of diabetes are increasing all over the world. Complications of diabetes constitute a burden for the individuals and the whole society.
Methods
In the present paper, ordinary differential equations and numerical approximations are used to monitor the size of populations of diabetes with and without complications.
Results
Different scenarios are discussed according to a set of parameters and the dynamical evolution of the population from the stage of diabetes to the stage of diabetes with complications is clearly illustrated.
Conclusions
The model shows how efficient and cost-effective strategies can be obtained by acting on diabetes incidence and/or controlling the evolution to the stage of complications.
Background
It is now commonly admitted that diabetes is sweeping the globe as a silent epidemic largely contributing to the growing burden of non-communicable diseases and mainly encouraged by decreasing levels of activity and increasing prevalence of obesity. The recent reports released by the World Health Organization [1] and the International Diabetes Federation [2] are alarming. In 2003, it was estimated that 194 million people were diabetic, representing a global prevalence exceeding 3% (5.1% for those aged 20 to 79) of the world population. The trend is increasing and the number is expected to reach 333 million (6.3%) by the year 2025. Moreover, for the first time, an estimation of 314 million (8.2%) is given for people in the pre-diabetic stage which constitutes a compartment from which at least one third will evolve to the diabetic stage after 10 years.
Dramatic increase have occurred in both prevalence and incidence of diabetes globally, especially with the new threshold proposed by the Expert Committee on the diagnosis and classification of diabetes mellitus in 1997 [3] and adopted by the World Health Organization. But it is worth noting the growing part of developing countries as stressed by many authors [4-6] and summarized in Table 1 for the ten leading countries [2]. In general, two forms of diabetes are considered: Type 1 diabetes, also known as Insulin Dependent Diabetes Mellitus (IDDM), affecting people under the age of 40 and representing 10 to 15% of the diabetic population, and Type 2 diabetes formerly known as Non Insulin Dependent Diabetes Mellitus(NIDDM), representing the major part (85–90%). However, with the growing epidemic of obesity in all age categories, it is expected that in ten years time, there will be more children with type 2 than with type 1 [7].
Table 1.
Estimated numbers of diabetics (in Million)
| Country | 2003 | 2025 |
| India | 35.5 | 73.5 |
| China | 23.8 | 46.1 |
| United States | 16 | 23.1 |
| Russia | 9.7 | 10.7 |
| Japan | 6.7 | 7.1 |
| Germany | 6.3 | 7.1 |
| Pakistan | 6.2 | 11.6 |
| Brazil | 5.7 | 10.7 |
| Mexico | 4.4 | 9 |
| Egypt | 3.9 | 7.8 |
Indeed, Obesity is another burden challenging the health authorities in almost all countries(high-income and low income alike), although with some variations within and between countries. In the United States, obesity prevalence has increased from 30% in the sixties to more that 60% as indicated by a recent study on obesity and the risk of heart failure which considered hypertension, diabetes and myocardial infarction, stressing that obesity promotes all three, and these promote heart failure [8]. In Prance, the proportion of obese children has grown from 12% in 1990 to 16% in 2000, which lead the health authorities to launch the Programme National Nutrition Santé in 2001 [9]. However, a special attention must be given to the contrasting effect in developing countries where obesity often coexists in the same population with chronic malnutrition and the burden of deaths and disability caused by non-communicable diseases outweighs that imposed by long-standing communicable diseases [1,10]. This global diagnosis being given, it is essential to stress that much of the cost of diabetes treatment is attributable to long term complications, such as blindness, kidney failure, heart disease, amputations and their economic and social consequences (care, hospitalization, absenteeism,...). Indeed, diabetes is:
– the leading cause of end-stage kidney failure necessitating dialysis or transplantation,
– the leading cause of blindness in people of working age,
– the leading cause of amputation,
– the first cause -with other risk factors- of mortality and morbidity by cardiovascular diseases.
The burden of diabetes and its complications
The exact costs of diabetes are not easy to pin down but estimations can be obtained according to three levels:
1. Cost directly related to the diagnosis and management of diabetes without complications. This includes the in-patient and out-patient care, means of treatment by insulin or tablets and the equipment of self control (blood and urine testing).
2. Costs generated by complications of diabetes. These are difficult to quantify because diabetes is linked to micro and macro vascular diseases such as heart disease, kidney failure, eye disease and amputation. Moreover, diabetes may add a cost of care by complicating other unrelated medical situations like infections, accidents and surgery.
3. Indirect costs correlated to the quality of life and the economic productivity which can be somehow estimated by the degree of disability.
In order to facilitate meaningful comparisons across world regions, costs are often expressed in international dollars (an international dollar has the same purchasing power as one US dollar has in the USA) and cost-effectiveness is measured in terms of years lived with disability (YLD) or disability adjusted life years (DALY) [11,12]. Studies in different countries have shown that diabetes is a costly disease accounting for between 2.5 and 15% of the total healthcare expenditure. For the age category 20–79, the world annual direct cost is estimated to be over 153 billion and expected to double in 2025 [2,13-16]. According to the National Institute of Diabetes and Digestive Kidney Disease (NIDDK) and the American Diabetes Association, diabetes was the sixth leading cause of death in 1999 with a direct cost of $44 billion and an indirect cost of $54 billion annually. In 2002, the direct and indirect cost totaled $132 billion [14]. In France, an estimation of $5.7 billion was given for the direct cost of diabetes [5], whereas, an equivalent cost of £5.2 billion, representing approximately 9% of the annual national health service (NHS) budget, was given for UK in 2000 [15]. The burden affects also developing countries as stressed by the different authors who attended the seventh congress of the Pan-African diabetes study group in 2001 [16] and the Metabolic Syndrome type II Diabetes and Artherosclerosis Congress in 2004 [17]. In these, countries, until recently, it was widely believed that economic development was a necessary prerequisite for improving a population health status and the health was often classified as a non productive sector. Now, politicians and health policy makers are timidly recognizing that investing in people's health is a necessary condition for economic development but energetic decisions are needed for the adoption of urgent and consequent strategies. The need for such strategies is enhanced by the fact that risk factors like cholesterol, tobacco, blood pressure, and obesity are no more a specificity of industrialized countries, they are becoming more prevalent in developing nations, where they double the burden of infectious diseases that have always afflicted poorer countries [10].
The literature dealing with modeling for diabetes is mainly concerned with glucose and insulin dynamics [6,18-20], the epidemiology of the disease [21-23] and economic cost and risk models [24-29]. In previous papers, the authors considered continuous and matrix models for age structured populations of diabetics [30,31] and Dynamics of a disabled population in Morocco [32]. In the present paper, while stressing the growing burden of disease caused by diabetes and its complications, a model is proposed to monitor the size of the diabetic population and to deal with the evolution from the stage of diabetes without complications to the stage of diabetes with complications. Parameters can be handled to illustrate the effect of an increasing or decreasing incidence of diabetes and its complications. Consequently, different strategies can be adopted. The main purpose is to show that investment in primary health care is a necessary and cost-effective strategy that allow to control the incidences of diabetes and its complications and hence, to convince policy makers that bold decisions must be taken for a sustainable development which ensures better quality of life and well-being for the present and future generations of humans.
Methods
The mathematical model
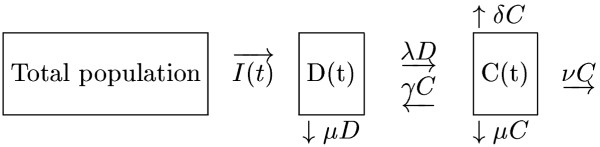
Suppose that C = C(t) and D = D(t) represent the numbers of diabetics with and without complications, respectively, and let N = N(t) = C(t) + D(t) denote the size of the population of diabetics at time t (see Nomenclature). Then, as was noted earlier, N(t) ~ 3% of the world population. Let I = I(t) denote the incidence of diabetes mellitus. The model parameters to be incorporated are μ (the natural mortality rate), λ (the probability of a diabetic person developing a complication), γ (the rate at which complications are cured), ν (the rate at which diabetic patients with complications become severely disabled) and δ (the mortality rate due to complications).
A schematic representation of the model is shown in Figure 1.
Figure 1.
The mathematical model
The diagram shows that I = I(t) cases are diagnosed in a time interval of length t and are assumed to have no complications upon diagnosis. In that same time interval, the number of sufferers without complications, D = D(t), is seen to decrease by the amounts μD (natural mortality) and λD (sufferers who develop complications), and to increase by the amount γC (sufferers whose complications are cured). During this time interval, the number of diabetics with complications is increased by the afore-mentioned amount γC and by the amount μC (natural mortality), νC (patients who become severely disabled and whose disabilities cannot be cured) and δC (those who die from their complications).
These rates of change are formalized by the ordinary differential equations (ODEs)
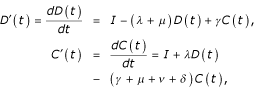
which, since N(t) = D(t) + C(t), give rise to the initial-value problem (IVP)
C'(t) = -(λ + θ)C(t) + λN(t), t > 0; C(0) = C0 (1)
N'(t) = I(t) - (ν + δ)C(t) - μN(t), t > 0; N(0) = N0 (2)
where θ = γ + μ + ν + δ,  and C0, N0 are the initial values of C(t) and N(t), respectively.
and C0, N0 are the initial values of C(t) and N(t), respectively.
In the case when the probability of a diabetic person developing a complication, λ, is constant, the model equations (1), (2) are linear in C(t) and N(t): this linear model will be discussed in the following paragraph. The non-linear model corresponding to a variable λ will be considered by the authors in another paper more devoted to numerical analysis.
The linear case
The critical point and its stability property
The probability of developing a complication, λ, will be estimated to have the constant value
![]()
The initial-value problem (1), (2) may consequently be written in matrix-vector form as
x'(t) = Ax(t) + b(t), t > 0;x(0) = X0 (4)
in which
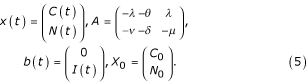
Suppose that I is the steady-state value of the incidence, then the model reaches its critical point when dC/dt and dN/dt given in (1) and (2) vanish simultaneously, that is when
λN - (λ + θ)C = 0, (6)
I - μN - (ν + δ)C = 0. (7)
Solving (6) and (7)gives
![]()
and
![]()
The eigenvalues of the matrix A, χ1, χ2, are the roots of the quadratic equation (the characteristic equation)
χ2 + (λ + θ + μ)χ + μ(λ + θ) + λ(ν + δ) = 0. (9)
The discriminant, Δ, of this equation is given by
Δ = (λ + θ + μ)2 - 4 [μ(λ + θ) + λ(ν + δ)]
and, recalling that θ = ν + μ + δ + γ it follows that
Δ <λ + μ + δ + γ + 2ν.
Solving (9) gives
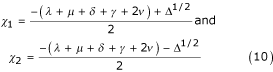
and it is then easy to check that, when the parameters of the model are such that
(a) Δ > 0, χ1, χ2 are both real and negative;
(b) Δ = 0, χ1 = χ2 are real and negative;
(c) Δ < 0, χ1 and χ2 are complex conjugate with negative real parts.
it may be concluded, therefore, that the critical point (C*, N*) of (1), (2), given by (8), is stable.
Numerical solution and stability
It may be shown that the solution x(t) of the IVP (4) satisfies the recurrence relation
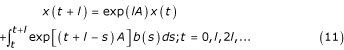
where l > 0 is an increment in t (the time step). This recurrence relation may be used to generate x(tn + 1) in terms of x(tn), thus monitoring C(t) and N(t) at the discrete points t = tn = nl(n = 0, 1, 2, ...).
One very simple way of estimating x(t + l) is to approximate to second order the integral in (11) by the trapezoidal rule, viz.
![]()
and then to replace, also to second order, exp(lA) in (11) and (12) by its (1,1) Padé approximant
exp(lA) = (E - 1/2lA)-1 (E + 1/2lA) (13)
where E is the identity matrix of order two.
Denoting by Xn the numerical approximation to x(tn) calculated using (11), (13), it may be shown, by substituting (12) with (13) in (11) and then by pre-multiplying by (E - 1/2lA), that
(E - 1/2lA)Xn + 1 = (E + 1/2lA)Xn (14)
+
1/2l[(E - 1/2lA)bn + 1 + (E + 1/2lA)bn];n = 0, 1, 2, ...
where Xn = (Cn, Nn)T, T denoting transpose, and bn = (0, In)T with In = I(tn). It may then be shown that Cn + 1 and Nn + 1 (n = 0, 1, 2,...) may be determined by solving the algebraic equations given by
(Method 1) (1 + 1/2l(λ + θ))Cn + 1 - 1/2lλNn + 1 =
[1 - 1/2l(λ + θ)]Cn + 1/2lλNn - 1/4l2λ(In + 1 - In) (15)
and
1/2l(ν + δ)Cn + 1 + (1 + 1/2lμ)Nn + 1 =
-1/2l(ν + δ)Cn + (1 - 1/2lμ)Nn
+1/2l(1 + lμ)In + 1 + 1/2l(1 - lμ)In (16)
assuming convergence, Cn + 1 = Cn = C, Nn + 1 = Nn = N and In + 1 = In = I, say, then equations (15) and (16) become
(λ + θ)C - λN = 0, (17)
(ν + δ)C + μN = I, (18)
respectively. Obviously (17) and (18) are the same as (6) and (7) and so the fixed point (C+, N+) of the numerical solution sequence (Cn, Nn), n = 0, 1, 2,... is the same as the critical point (C*, N*) of the linear initial value-problem.
For comparison purpose, the IVP (1), (2) was also solved using the well-known Euler method (a first-order method) given by
(Method 2) Cn + 1 = [1 - l(λ + θ)]Cn + lλNn (19)
Nn + 1 = -l(ν + δ)Cn + (1 - lμ) + lINn (20)
The method1 is unconditionally stable whereas the Euler method is conditionally stable provided ([33])
![]()
Numerical experiments
Taking I(t) = I to be constant equations (15) and (16) simplify to
(1 + 1/2l(λ + θ))Cn + 1 - 1/2lλNn + 1 =
[1 - 1/2l(λ + θ)]Cn + 1/2lλNn (22)
1/2l(ν + δ)Cn + 1 + (1 + 1/2lμ)Nn + 1 =
-1/2l(ν + δ)Cn + (1 - 1/2lμ)Nn + lI, (23)
respectively, for n = 0, 1, 2, .... In the numerical experiments, I was given the value 6.106yr-1 and the parameters ν, δ, μ, γ, were given the value shown in Table 2. the critical values of C and N were then calculated from (8) and were found to be
Table 2.
Parameter vlaues used in numerical experiments
| Parameter | Value yr-1 |
| ν | 0.05 |
| δ | 0.05 |
| μ | 0.02 |
| γ | 0.08 or 0 |
C* = 47000000 and N* = 61100000. (24)
Using Matlab, four numerical experiments were carried out taking as initial conditions
C0 = C* ± 500 and N0 = N* ± 500. (25)
A time step of l = 0.01yr-1 was used and the solution to the IVP (1), (2) was computed by solving (22), (23) for n = 0, 1, 2, .... Using all combinations of initial conditions, the computed solution converged to the values of C* and N* given in (24). By way of example, the fixed points, C+ and N+, to which the numerical solution converged are shown in Table 3.
Table 3.
Fixed point values (× 1O7) for the linear model using different values of l, I = 6 × 107, λ = 0.66
| N+ | N+ | C+ | C+ | |
| l yr | Euler | Method1 | Euler | Method1 |
| 0.01 | 6.08 | 6.08 | 4.75 | 4.75 |
| 0.02 | 6.11 | 6.11 | 4.77 | 4.77 |
| 0.05 | 6.11 | 6.11 | 4.7 | 4.7 |
| 0.1 | 6.11 | 6.11 | 4.7 | 4.7 |
| 0.2 | 6.11 | 6.11 | 4.7 | 4.7 |
| 0.5 | 6.11 | 6.11 | 4.7 | 4.7 |
| 1 | 6.11 | 6.11 | 4.7 | 4.7 |
| 2 | 6.11 | 6.11 | 4.7 | 4.7 |
| 2.5 | div | 6.11 | div | 4.7 |
| 3 | div | 6.11 | div | 4.7 |
| 3.5 | div | 6.11 | div | 4.7 |
| 4 | div | 6.11 | div | 4.7 |
div = divergence of numerical methods.
The initial conditions in (25) are close to the steady-state solutions C* and N*. Other initial conditions, further from C* and N*, will converge to the same values of C+ and N+ for the same value of l, though these values will be reached at different times.
Retaining the parameters values shown in Table 2 the effect of the choice of time step was monitored in a series of 11 further experiments. The fixed-point values, C+ and N+ to which convergence occurred are shown in Table 3, where it may be seen that, for the larger values of l (l ≥ 0.5yr), there is very close agreement with the critical-point values given in (24).
For l ≤ 2yr (approximately), the two methods give similar results but for l > 2.5yr (approximately (21)) the Euler method diverged. The values of C+ and N+ using Euler method are given in Table 3.
It may be concluded from these results that the Euler method may be used with confidence if the diabetic population is to be monitored at time intervals up to approximately two years using the linear model (1), (2). However, to monitor the population less frequently the numerical method (Method 1) should be used.
Results and discussion
Nine scenarios were considered (Low-Low, Low-Medium, Low-High, Medium-Low, Medium-Medium, Medium-High, High-Low, High-Medium, and High-High) by combining the levels of incidence of diabetes and its complications. For instance, High-High refers to a high incidence of diabetes and high incidence of complications. These combinations are given by different values of the parameters I for incidence of diabetes and A for incidence of complications. The model shows clearly the differences in the global number of diabetics (N) and the number of diabetics with complications(C) as illustrated by Tables 4. The number of diabetics resulting from a strategy with high incidence may be reduced by half if a strategy with medium incidence is applied during a dozen of years. A further reduction by three can be obtained by a strategy with low incidence. But, more importantly, the rate of complications reaches 78% in a high strategy of complications, it can be reduced to 63% (respectively 53%) with a medium (respectively a low) strategy of complications. Now, are we really able to act on these incidences and how? Precise answers have been given at different levels.
Table 4.
Output of the nine scenarios according to each level of incidence I and complications λ (× 107)
| I λ | L | M | H |
| L | C = 0.72 | C = 2.18 | C = 4.36 |
| N = 1.36 | N = 4.09 | N = 8.18 | |
| M | C = 0.75 | C = 0.22 | C = 4.55 |
| N = 1.20 | N = 3.6 | N = 7.21 | |
| H | C = 0.79 | C = 2.38 | C = 4.77 |
| N = 1.0 | N = 3.05 | N = 6.11 |
For the incidence of diabetes, worldwide, it is now commonly admitted that efforts must be conjugated to reverse or at least to attenuate its growing trend, otherwise, health authorities will be unable to provide care and treatments for millions of people who will be affected by diabetes in the future [1,2,7,16,34]. Propositions are mainly directed towards risk factors like obesity, tobacco inactivity, alcohol, blood pressure, cholesterol, inheritance and diet habits in general [4,6,35]. Many authors have dealt with complications [1,2,36,37]. The strategy can be summarized by the recommendations of The Saint Vincent Declaration [38] which fixed in 1989 the following objectives:
– Reduce new blindness due to diabetes by one third or more
– Reduce numbers of people entering end-stage diabetic renal failure by at least one third
– Reduce by one half the rate of limb amputations for diabetic gangrene
– Cut morbidity and mortality from coronary disease in the diabetic by vigorous programmes of risk factor reduction
– Achieve pregnancy outcome in the diabetic woman that approximates that of the non-diabetic woman.
However, although most of developed countries have reacted by pragmatic measures, the trend remain globally passive mainly because developing countries have been, so far, satisfied with adopting national conventions and adhering to international recommendations instead of working in the field. As stressed earlier, this behaviour can be partly explained by lack of means and poor budget affected to health care but, in general, bad management and absence of goodwill assume a large part of responsibility. The illustration yielded by our mathematical model confirms the diagnosis and the recommendations given by specialists and experts in the field of diabetes and health management in general. Moreover, it gives to health decision makers guide lines of comparison between the social and economic costs of uncontrolled diabetes, and the benefit gained by a productive investment in primary healthcare.
Conclusion
In this paper, a mathematical model was proposed to deal with the dynamics of a population of diabetes. The model was formalized by a system of ordinary differential equations, then numerical approximations were used to obtain numerical results. Although linear and non-linear cases were considered, for sake of clarity and simplicity, only numerical results of the linear model were given.
The model showed clearly the results given according to different scenarios. The main purpose was to stress the importance to control the incidence of diabetes and its complications and hence to convince decision makers that investment in healthcare is a cost-effective strategy.
Authors contributions
AB participated to the proposition and discussion of the model.
EHT elaborated numerical analysis and English writing.
KA elaborated numerical experiments.
AC elaborated numerical procedures and TEX writing.
Appendix
t : time,
l : increment in t (the time step),
C(t) : number of diabetics with complications,
D(t) : number of diabetics without complications,
N(t) : number of diabetics (N = C + D),
I(t) : incidence of diabetes mellitus,
J : jacobien,
μ : natural mortality rate,
λ : probability of developing a complication,
γ : rate at which complications are cured,
ν: rate at which patients with complications become severely disabled,
δ : mortality rate due to complications,
θ = μ + δ + γ + ν,
χ1, χ2 : eigenvalues,
C0, N0 : initial values of C and N,
C*, N* : critical-point values of C and N,
C+, N+ : fixed-point values of C and N,
x: x = [C, N]T, T denoting transpose,
X0 : X0 = [C0, N0]T,
A : constant square matrix of order two (linear model),
b(t): b(t) = [0, I(t)]T,
Cn, Nn : approximations to C(nl), N(nl),
Xn : Xn = [Cn, Nn]T,
In : In = I(nl).
Acknowledgments
Acknowledgements
One of the authors (A.B) is grateful to the British Council (Morocco) for financial support during the period of research. This paper is dedicated to Wiam Boutayeb and David M. Barlett.
Contributor Information
A Boutayeb, Email: masraab@brunel.ac.uk.
EH Twizell, Email: E.H.Twizell@brunel.ac.uk.
K Achouayb, Email: aassil@hotmail.com.
A Chetouani, Email: a-chetouani@sciences.univ-oujda.ac.ma.
References
- The world health report Today's challenges. Geneva, World Health Organization. http://www.who.int/whr/2003/en
- International Diabetes Federation IFD report. 2003. http://www.idf.org/home/index.cfm
- The Expert Committee on the diagnosis and classification of diabetes mellitus Report. Diabetes care. 1997;20:1183–96. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- Diet, Nutrition and the prevention of Chronic Diseases Report of joint WHO/FAO Expert Consultation. Geneva, World Health Organization, WHO Technical report Series 916. 2003. [PubMed]
- Mayo M. Le diabète: Une épidémie silencieuse. Science et vie. 2004;1037:100–113. [Google Scholar]
- Derouich M, Boutayeb A. The effect of physical exercise on the dynamics of glucose and insulin. Journal of Biomechanics. 2002;35:911–917. doi: 10.1016/S0021-9290(02)00055-6. [DOI] [PubMed] [Google Scholar]
- Borys JM. Diabète et prédiabète: repenser la prévention http://www.diabsurf.com
- Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- Beme D. Intervenir en amont de 1'obésité http://www.doctissimo.fr/html/sante/obesite
- The world health report 2002 Reducing Risk: Promoting Health Life. Geneva, World Health Organization. 2002. http://www.who.int/whr/2002/en
- Hutubessy R, Chisholm D, Edjer TT. For WHO-CHOICE Generalized cost-effectiveness analysis for national-level priority setting in health sector. Cost Effectiveness and Resources Allocation. 2003;1:8. doi: 10.1186/1478-7547-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers CD, Bernard C, Iburg KM, Inoue M, Fat DM, Shibuya K, Stein C, Tomijima N, Xu H. Global Burden of Disease in 2002 data sources, methods and results. Paper 54 WHO.
- Currie JC, Krans D, Morgan CL, Gill L, Stott NC, Peters JR. NHS acute sector expenditure for diabetes, and excess in-patient cost of care. Diabetic Medecine. 1997;14:686–692. doi: 10.1002/(SICI)1096-9136(199708)14:8<686::AID-DIA434>3.3.CO;2-4. [DOI] [PubMed] [Google Scholar]
- OHSU Health General diabetes statistics http://www.ohsuhealth.com/diabetes/stats.asp
- Fact sheet N03 Diabetes: cost and complications http://www.diabete.org.uk/infocentre/
- Belkhadir J. Le diabète en milieu marocain en. 2001. http://www.marocsante.com/html/diabetes
- MSDAC 2004 Metabolic syndrome type II diabetes, and atherosclerosis congress http://www.msdac.com
- Lehmann ED, Deutsch T. Application of computers in diabetes care a review. Med Inform. 1995;20:281–302. doi: 10.3109/14639239509024284. [DOI] [PubMed] [Google Scholar]
- Bellazzi R, Nucci G, Cobelli C. The subcutaneous route to insulin-dependent diabetes theory. IEEE Eng Med Biol Mag. 2001;20:56–64. doi: 10.1109/51.897828. [DOI] [PubMed] [Google Scholar]
- Parker RS, Doyle FJ, Peppas NA. The intravenous route to blood glucose control. IEEE Eng Med Biol Mag. 2001;24:65–73. doi: 10.1109/51.897829. [DOI] [PubMed] [Google Scholar]
- Staines A, Bodanshy HJ, Lilley HEB, Stephenson C, McNally RJQ, Cartwright RA. The epidemiolgy of diabetes mellitus in the United Kingdom. Diabetologia. 1993;36:1282–1287. doi: 10.1007/BF00400806. [DOI] [PubMed] [Google Scholar]
- Patterson C, Thorogood M, Smith PG, Heasman MA, Clarke MA, Mam JI. Epidemiology of type 1 diabetes in Scotland : evidence of an increasing incidence. Diabetologia. 1983;24:238–243. doi: 10.1007/BF00282706. [DOI] [PubMed] [Google Scholar]
- Boutayeb A, Kerfati A. Mathematical models in diabetology Modelling. Measurement and Control, C, AMSE. 1994;44:53–63. [Google Scholar]
- Bagust A, Hopkinson PK, Maslove L, Currie CJ. The projected health care burden of type diabetes in the UK from 2000 to 2006. Diabet Med. 2002;19:1–5. doi: 10.1046/j.1464-5491.19.s4.2.x. [DOI] [PubMed] [Google Scholar]
- Richard JS, Khothari V, Amanda IA, Stratton IM, Holman RR. The UKPDS risk engine : a model for the risk of coronary heart disease in type II diabetes (UKPDS 56) Clinical Science. 2001;101:671–679. doi: 10.1042/CS20000335. [DOI] [PubMed] [Google Scholar]
- Bagust A, Hopkinson PK, Maier W, Currie CJ. An economic model of the long-term health care burden of type II diabetes. Diabetologia. 2001;12:2140–55. doi: 10.1007/s001250100023. [DOI] [PubMed] [Google Scholar]
- O'brien JA, Patrick AR, Caro J. Estimates of direct medical costs for microvascular and macrovascular complications resulting from type 2 diabetes mellitus in the United States in 2000. Clin Ther. 2003;3:1017–38. doi: 10.1016/S0149-2918(03)80122-4. [DOI] [PubMed] [Google Scholar]
- Huse DM, Oster G, Killen AR, Lacey MJ, Colditz GA. The economic costs of non-insulin-dependent diabetes mellitus. JAMA. 1989;262:2708–13. doi: 10.1001/jama.262.19.2708. [DOI] [PubMed] [Google Scholar]
- Gozzoli V, Palmer AJ, Brandt A, Spinas GA. Economic and clinical impact of alternative disease management strategies for secondary prevention in type 2 diabetes in the Swiss setting. Swiss Med Wkly. 2001;131:303–10. doi: 10.4414/smw.2001.09716. [DOI] [PubMed] [Google Scholar]
- Boutayeb A, Derouich M. Age structured models for diabetes in East Morocco. Mathematics and Computers in Simulation. 2002;58:215–229. doi: 10.1016/S0378-4754(01)00368-8. [DOI] [Google Scholar]
- Boutayeb A, Twizell EH. An age structure model for complications of diabetes mellitus in Morocco. Simulation Modelling Practice and Theory. 2004;12:77–87. doi: 10.1016/j.simpat.2003.11.003. [DOI] [Google Scholar]
- Boutayeb A, Chetouani A. Dynamics of a disabled population in Morocco. BioMedical Engineering Online. 2003;2:2. doi: 10.1186/1475-925X-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achouyab K. Ph D thesis. Oujda University, Morocco; 1996. Modélisation mathématique en diabétologie: Conception et étude numérique. [Google Scholar]
- Le Jeune S. Le diabète, une catastrophe pire que le SIDA http://www.diabsurf.com
- Recommandations de 1'Alfediam Diabète et Métabolisme. Paris. 1995;21:59–62. [PubMed] [Google Scholar]
- DCCT. New Engl J Med. 1996;329:977–986. [Google Scholar]
- UKPPDS Tight blood pressure and risk of macrovascular and microvascular complications in type 2 diabetes. Brit Med J. 1998;317:703–713. [PMC free article] [PubMed] [Google Scholar]
- Krans HMJ, Porta M, Keen H. Diabetes Care and Research in Europe: The Saint Vincent Declaration Action Program. WHO, regional office for Europe. 1992.