Abstract
Precise spatiotemporal control of axon guidance factor expression is a prerequisite for formation of functional neuronal connections. Although Netrin/Dcc- and Robo/Slit-mediated attractive and repulsive guidance of commissural axons have been extensively studied, little is known about mechanisms controlling mediolateral positioning of longitudinal axons in vertebrates. Here, we use a genetic approach in zebrafish embryos to study pathfinding mechanisms of dopaminergic and neuroendocrine longitudinal axons projecting from the hypothalamus into hindbrain and spinal cord. The transcription factors Sim1a and Arnt2 contribute to differentiation of a defined population of dopaminergic and neuroendocrine neurons. We show that both factors also control aspects of axon guidance: Sim1a or Arnt2 depletion results in displacement of hypothalamo-spinal longitudinal axons towards the midline. This phenotype is suppressed in robo3 guidance receptor mutant embryos. In the absence of Sim1a and Arnt2, expression of the robo3 splice isoform robo3a.1 is increased in the hypothalamus, indicating negative control of robo3a.1 transcription by these factors. We further provide evidence that increased Robo3a.1 levels interfere with Robo2-mediated repulsive axon guidance. Finally, we show that the N-terminal domain unique to Robo3a.1 mediates the block of Robo2 repulsive activity. Therefore, Sim1a and Arnt2 contribute to control of lateral positioning of longitudinal hypothalamic-spinal axons by negative regulation of robo3a.1 expression, which in turn attenuates the repulsive activity of Robo2.
Keywords: Robo/Slit signalling, Robo receptor isoforms, Dcc/Netrin signalling, Dopaminergic neurons, Neuroendocrine systems, Hypocretinergic neurons, Hypothalamo-spinal projections, Orthopedia, Zebrafish
INTRODUCTION
Understanding how transcription factors genetically encode the specification of connectivity in the nervous system is a challenge in neurobiology. Knowledge of molecular mechanisms that link transcription factor function to regulation of axon guidance in vertebrates is limited to a few well-studied systems, including spinal motoneuron projections and retinotectal trajectories (for a review, see Butler and Tear, 2007; Polleux et al., 2007). However, for most central nervous system projection pathways, it is unknown how connectivity is transcriptionally determined.
Hypothalamic projections into the spinal cord (HTS) are an attractive model for understanding how CNS nuclei establish long-range projections. A well-characterised transcriptional network specifies hypothalamic cell lineages. The homeodomain transcription factor Orthopedia (Otp) is co-expressed with the two PAS domain factors Sim1 and Arnt2, the latter two forming functional heterodimers. Otp/Sim1/Arnt2-dependent cell lineages in mammals (Acampora et al., 1999; Michaud et al., 2000; Michaud et al., 1998; Wang and Lufkin, 2000) and zebrafish (Blechman et al., 2007; Del Giacco et al., 2006; Eaton and Glasgow, 2006; Eaton and Glasgow, 2007; Löhr et al., 2009; Ryu et al., 2007) include neuroendocrine cells characterised by expression of the peptide hormones corticotropin-releasing hormone, thyrotropin-releasing hormone, arginine vasopressin, oxytocin and somatostatin. In addition to neuroendocrine lineages, Otp/Sim1/Arnt2 have been shown to control development of hypothalamic A11-type dopaminergic (DA) neurons in zebrafish and, for Otp, mammals (Borodovsky et al., 2009; Löhr et al., 2009; Ryu et al., 2007). Despite the diverse neurotransmitter/neuropeptide phenotypes, Otp/Sim1/Arnt2-specified cell types share similar descending ipsilateral projections. Neuroendocrine cell lineages have been shown to innervate spinal target fields in mammals (Hancock, 1976; Swanson, 1977) and zebrafish (M. Hammerschmidt, personal communication). Furthermore, A11-type DA neurons project directly to the spinal cord in mammals (Björklund and Skagerberg, 1979) and zebrafish (Tay et al., 2011). The similarity in projection patterns of diverse Otp/Sim1/Arnt2-specified hypothalamic neurons prompted us to investigate whether this transcription factor network might also regulate axon guidance.
Ipsilateral longitudinal axon guidance is regulated in part by the Robo/Slit and Netrin/Dcc receptor/ligand pairs (Farmer et al., 2008; Kastenhuber et al., 2009). Slit and Netrin guidance cues are expressed by ventral midline cells and bind to receptors of the Robo and Dcc families, respectively (Brose et al., 1999; Keino-Masu et al., 1996). In mouse, loss of the two Robo receptors Robo1 or Robo2, as well as loss of Slit1 and Slit2 ligands, disrupts proper establishment of longitudinal pioneer tracts in the midbrain and hindbrain (Farmer et al., 2008), and Robo/Slit signalling also affects ascending nigrostriatal tracts of mesencephalic dopaminergic neurons (Dugan et al., 2011). Similarly, longitudinal axons of A11-related DA neurons in zebrafish integrate midline repulsion via Robo2/Slit and midline attraction via Dcc/Netrin1 to specify lateral positioning (Kastenhuber et al., 2009). A role for Robo3 during ipsilateral longitudinal axon guidance has not been reported. Robo3, with at least four splice variants (Ypsilanti et al., 2010), displays the greatest structural heterogeneity within the Robo receptor family, and controls pre- and post-crossing behaviour of commissural axons (Chen et al., 2008; Sabatier et al., 2004).
Here, we provide evidence that Arnt2 and Sim1a are required to determine the lateral position of neuroendocrine and DA longitudinal axons en route to the spinal cord. Loss of Arnt2 or Sim1a function leads to displacement of HTS axons towards the midline. By performing expression analyses together with gain- and loss-of-function studies, we show that the abnormal projection pattern observed is caused by misexpression of the specific Robo3 isoform robo3a.1. We further show that Robo3a.1 is sufficient to block Robo2-mediated midline repulsion of HTS longitudinal axons. Our data suggest that Sim1a and Arnt2 negatively regulate robo3a.1, which in turn attenuates repulsive activity of Robo2, and thereby controls the lateral positioning of HTS longitudinal axons.
MATERIALS AND METHODS
Fish maintenance and strains
Zebrafish were maintained at 28.5°C (Westerfield, 1995). To inhibit pigmentation, embryos were incubated in 0.2 mM phenylthiourea. For a complete list of used zebrafish lines, see supplementary material Table S1. Compound mutants were identified by PCR. Genotyping of arnt2hi2639c, astti272z, astte284 and twttw204 alleles was previously described (Chalasani et al., 2007; Fricke et al., 2001; Golling et al., 2002; Kastenhuber et al., 2009).
Immunohistochemistry and in situ hybridization
Whole-mount immunohistochemistry (Holzschuh et al., 2003), in situ hybridization and fluorescent in situ hybridization were performed as described previously (Filippi et al., 2007). To enhance signal intensity in fluorescent in situ hybridization, 5% dextrane sulphate was added in the hybridization buffer and 450 μg/ml iodophenol during peroxidase reaction. For confocal microscopy stained embryos were embedded in medium containing 1.2% low melting agarose in 80% glycerol. For details on antibodies and probes used, see supplementary material Table S2.
Plasmid construction and injections
For expression constructs, a multisite Gateway system was used (Kwan et al., 2007). For details on generation of constructs see supplementary material Table S3. For transient overexpression analyses and generation of stable lines, 25 pg plasmid and 50 pg Tol2 transposase RNA (pCS2FA-transposase) (Kwan et al., 2007) were co-injected into one-cell stage embryos.
Morpholino injections and photomorph experiments
Morpholino oligonucleotides (MOs) were obtained from Gene Tools (Philomath, OR). The sim1a (sim1a e2i2), arnt2 (arnt2 e2i2) and dcc (dcc-MO2) morpholinos have been described previously (Löhr et al., 2009; Suli et al., 2006). For controls, standard negative control morpholino from Gene Tools was used. Morpholinos were injected at one-cell stage: 0.25-1.0 ng (sim1a MO), 1-4.5 ng (arnt2 MO) and 4.5 ng (dcc MO) per embryo.
Photomorph 2.0 caging strand (SuperNova Life Science) was designed to bind and block the sim1a morpholino. Prior to injection, caging strand and sim1a morpholino were mixed at a molar ratio of 7:1 and hybridised at 70°C for 30 minutes. Subsequently, the mixture was cooled down to 4°C and stored overnight prior to injection. Special care was taken to keep the caged MO solution and injected embryos in the dark until time of uncaging. Uncaging of the morpholino was performed by exposure to UV light (Peqlab, UV superbright light table, 312 nm, 100% intensity) for 30 minutes. Efficiency and function of the Photomorph caging strand was confirmed by PCR on cDNA derived from embryos injected with Photomorph caging strand and sim1a MO (with and without UV light treatment) using primers as described previously (Löhr et al., 2009).
Microscopy, quantification and statistical analyses
Confocal z-stacks were recorded using Zeiss LSM 510. LSM or NIH ImageJ software was used to create z-projections. Adobe Photoshop or Illustrator were used to assemble figures. Distances between axons were determined using Zeiss LSM510 Software. Midline crossing of MA axons was determined using a Leica MZ16F stereomicroscope. Quantification of fluorescence from in situ hybridization analyses: to determine the regions of interest for single neurons, we used the GFP channel and the region of interest manager embedded in ImageJ. Ten neurons in the anterior and six neurons in the posterior hypothalamus (see Fig. 4J) were randomly selected per embryo; on average, three slices per fish were used. To measure the average signal intensity within each selected neuron, as outlined by the regions of interest, we switched to the channel carrying the gene expression information maintaining the region of interest masks from the GFP channel. All samples were analysed at the LSM510 using identical acquisition parameters. Statistical analysis was performed using Student’s t-test. Error bars represent s.d.
Fig. 4.
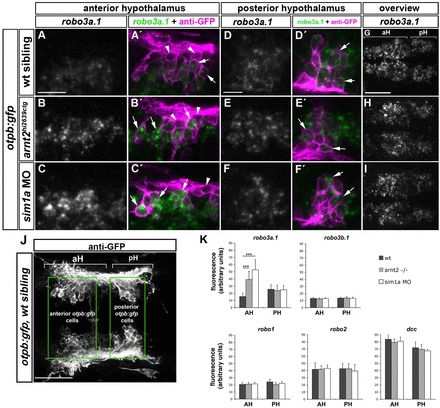
Spatiotemporal expression of robo3a.1 in the hypothalamus is altered in sim1a morphants and arnt2 mutants. Dorsal views of confocal z-projections of 48 hpf embryos. Anterior is towards the left. (A-F′) High magnification of otpb:gfp cells in anterior (aH) or posterior hypothalamus (pH). (A,A′) Expression of robo3a.1 is detected at low levels in a subset of GFP-positive cells (arrows in A′) in otpb:gfp embryos (B-C′). Instead, otpb:gfp; arnt2hi2639c mutant embryos (B,B′) and otpb:gfp embryos injected with sim1a MO (C,C′) show strong robo3a.1 signal in GFP-positive cells (arrows in B′ and C′). (D-F′) Expression levels of robo3a.1 are comparable in GFP-positive cells in pH of otpb:gfp embryos (D,D′), otpb:gfp; arnt2hi2639c mutants (E,E′) and otpb:gfp embryos injected with sim1a MO (F,F′). Arrowheads indicate GFP-positive cells, which do not express robo3a.1. (G-I) Overview of the robo3a.1 expression domain in the hypothalamus of otpb:gfp embryos (G), otpb:gfp; arnt2hi2639c mutant embryos (H) and otpb:gfp embryos injected with sim1a MO (I). (J,K) Quantification (K) of Alexa-488 signal intensity derived from fluorescent in situ hybridization staining for indicated genes in otpb:gfp cells located in aH or pH (J). ***P<0.0001. Scale bars: in A, 20 μm for A-C′; in D, 10 μm for D-F′; in G, 50 μm for G-I.
RESULTS
A novel role for Sim1 and Arnt2 in establishment of HTS axon tracts
Absence of Otp, Sim1 or Arnt2 results in loss of cell type-specific terminal differentiation markers of defined hypothalamic neuroendocrine cell groups and of A11-related DA neurons in zebrafish (Löhr et al., 2009; Ryu et al., 2007). Although not fully differentiated, these cells are maintained at least until 4 dpf, as judged by persistent expression of otp and sim1 transcripts. To study the role of Sim1a and Arnt2 during longitudinal HTS tract formation, we used a transgenic zebrafish line visualizing otpb-expressing neurons in the hypothalamus and their longitudinal projections towards the spinal cord by GFP (supplementary material Fig. S1) (Fujimoto et al., 2011). To analyse a potential effect of Sim1a on HTS tract formation, we injected a sim1a morpholino into otpb:gfp transgenic embryos. otpb:gfp-positive longitudinal axons were significantly displaced towards the midline after depletion of sim1a when compared with controls (Fig. 1A-D,I; supplementary material Table S4). A similar medial displacement of otpb:gfp longitudinal HTS axons was observed upon injection of arnt2 MO (Fig. 1E,F,I; supplementary material Table S4) and in arnt2hi2639c mutant embryos (supplementary material Fig. S2A-E; Table S4). Moreover, co-injection of sub-threshold amounts of sim1a (0.25 ng) and arnt2 (1.0 ng) morpholinos, which do not display a phenotype when injected individually, did result in a medial displacement of otpb:gfp HTS axons (Fig. 1G,H,I; supplementary material Table S4). To determine the specificity of the observed phenotype, we used a transgenic reporter line that labels hypocretinergic (Hcrt) neurons (hcrt:tdtomato). Hcrt neurons reside in the lateral hypothalamus and also project to spinal target regions (Faraco et al., 2006; van den Pol, 1999), but do not depend on Sim1a and Arnt2 transcription factors for differentiation (data not shown). Analysis of otpb:gfp;hcrt:tdtomato transgenic embryos revealed that HTS axons of Hcrt neurons and otpb:gfp-positive neurons run at similar mediolateral positions, yet longitudinal axons of Hcrt neurons did not display any change in lateral positioning upon sim1a knockdown (supplementary material Fig. S3A-G, Table S4). These results demonstrate that sim1a and arnt2 are selectively and synergistically required for lateral positioning of otpb:gfp-positive HTS axons.
Fig. 1.
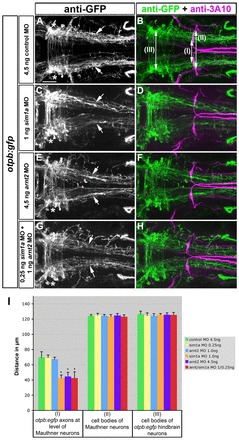
Sim1a and Arnt2 act synergistically during longitudinal HTS axon guidance. Dorsal views of hindbrain confocal z-projections of otpb:gfp transgenic embryos at 72 hpf. Anterior is towards the left. (A-H) Mediolateral positioning of otpb:gfp axons (arrows) after indicated treatments. otpb:gfp hindbrain neurons (asterisks in A,C,E,G) do not contribute to longitudinal projections. (I) Quantification of the distance between otpb:gfp axons (I), of the distance between MA neurons (II) or between otpb:gfp hindbrain neurons (III). *P<0.0001 for sim1a MO 1.0 ng, arnt2 MO 4.5 ng, arnt2/sim1a MO 1.0/4.5 ng versus control MO 4.5 ng. Scale bar: 50 μm.
Temporally controlled knockdown links Sim1 function to axonal pathfinding of dopaminergic neurons
The medial displacement of otpb:gfp HTS axons upon depletion of arnt2 and sim1a could be caused by transdifferentiation of DA (and neuroendocrine) cells into other cell lineages. To ascertain whether Sim1 is instructional for proper HTS pathfinding of DA neurons, we performed a temporally controlled loss-of-function experiment. We decided to knock down sim1a at a time point when DA terminal differentiation marker tyrosine hydroxylase (TH) expression has already been initiated, but HTS development is still ongoing. The sim1a morpholino was kept inactive by hybridization with a complementary caging strand (Tomasini et al., 2009) until uncaging and activation of the sim1a morpholino was achieved by photocleavage (supplementary material Fig. S4). Injection of sim1a morpholino resulted in complete loss of TH expression of A11-related DA cells and their HTS projections (Fig. 2C,D). Conversely, caged sim1a morpholino-injected embryos displayed the full complement of hypothalamic DA neurons and developed HTS tracts similar to controls (Fig. 2A,B,E,F). Next, we injected a caged sim1a morpholino and photo-activated the morpholino at 22 hpf. This time point was chosen, because the earliest A11-related DA neurons differentiate between 18 and 24 hpf. Following this treatment, we detected individual TH-positive DA neurons in 84% of the embryos analysed. These embryos displayed a significant medial displacement of TH-positive HTS axons (Fig. 2G,H,I; supplementary material Table S5). This reveals that Sim1a is essential for lateral positioning of DA HTS axons, and thus has multiple functions during distinct steps of DA cell differentiation and maturation.
Fig. 2.
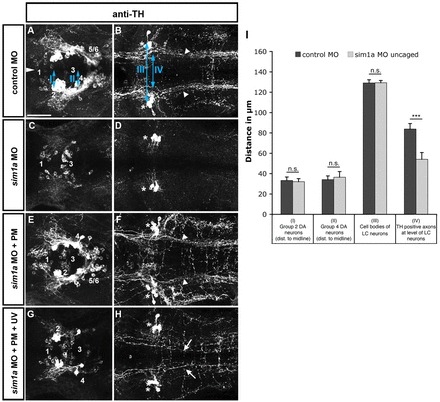
Temporally controlled sim1a knockdown reveals medial displacement of dopaminergic longitudinal projection tracts. Dorsal views of confocal z-projections of 72 hpf embryos at the anterior-posterior levels of forebrain (A,C,E,G) and hindbrain (B,D,F,H). (A,B) Control MO injection. (C,D) sim1a morpholino injection: TH immunoreactivity is lost in cell bodies (C) and axons (D) of the DA HTS systems (DA groups 2,4-6). (E,F) Caging the sim1a morpholino results in wild-type-like formation of DA HTS systems. (G,H) Temporally controlled activation of sim1a MO at 22 hpf permits the formation of individual group 2,4-6 DA neurons (G) but causes a medial shift of TH-positive axons (arrows in H). Arrowheads in B indicate the position of TH-positive HTS tracts. Asterisks in B,D,F,H indicate TH-positive locus coeruleus (LC) neurons. Numbers in A,C,E,G indicate DA groups (Rink and Wullimann, 2002). (I) Quantification of mediolateral positioning of TH-positive HTS axons at 72 hpf at the level of LC neurons (IV in B); of distances of group 2 (I in A) and group 4 cells (II in A) from the midline; and of distances between LC neurons (III in B). ***P<0.0001; n.s., not significant. Scale bar: 50 μm.
Robo2 is required for lateral positioning of HTS axon tracts in Sim1a- and Arnt2-dependent and -independent cell lineages
Proper formation of DA HTS tracts requires Robo2 function (Kastenhuber et al., 2009). As DA neurons are a fraction of otpb:gfp-positive cells, we analysed whether the role of Robo2 applies to otpb:gfp longitudinal axons in general. Owing to similarities in longitudinal projection tract formation, we also included Hcrt neurons in our experiments. robo2 transcripts are present in otpb:gfp-positive cells and in Hcrt neurons (data not shown).
To analyse mediolateral positioning of Hcrt axonal projections in comparison with otpb:gfp-positive projections, we used otpb:gfp;hcrt:tdtomato double transgenic zebrafish that were crossed into Robo2-deficient (astti272z) mutants. In ast homozygous mutant embryos, we observed a significant medial displacement of HTS axons labelled by both transgenic lines when compared with heterozygous siblings (Fig. 3A-G; supplementary material Table S6). Thus, otpb:gfp-positive cells, as well as Hcrt neurons, require Robo2 for lateral positioning of HTS projection tracts.
Fig. 3.
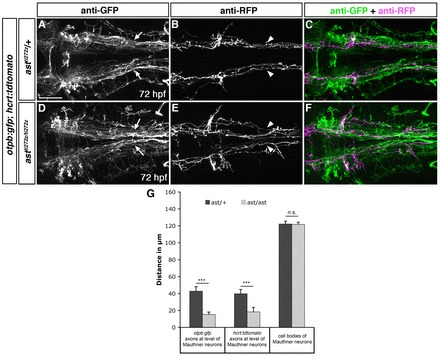
Lateral positioning of longitudinal axons derived from otpb- and hypocretin-positive neurons is altered in ast homozygous mutants. Dorsal views of hindbrain confocal z-projections of otpb:gfp;hcrt:tdtomato double transgenic 72 hpf embryos. (A-F) In ast/ast embryos longitudinal projections of otpb:gfp (D,F; arrows in D) and hcrt:tdtomato (E,F; arrowheads in E) axons are shifted towards the midline compared with ast/+ siblings. (G) Quantification of the distance between otpb:gfp and hcrt:tdtomato HTS axons at the anterior-posterior level of Mauthner neurons, and of the distance between Mauthner neurons. ***P<0.0001; n.s., not significant. Scale bar in A for A-F: 50 μm.
Expression of robo3a.1 is increased in otpb:gfp-positive neurons in the hypothalamus after sim1a or arnt2 depletion
We next analysed the molecular mechanisms underlying the medial displacement of otpb:gfp HTS tracts in sim1a morphants and arnt2hi2639 mutants. A previous study in mouse demonstrated that Robo3 transcripts were upregulated in mammillary body neurons of Sim1–/–/Sim2–/– double mutants (Marion et al., 2005). In zebrafish, two robo3 isoforms (robo3v1 and robo3v2) have been described, which only differ in a short stretch of amino acids at the N terminus (Challa et al., 2005). In accordance with the nomenclature of Robo3 isoforms in mammals (Camurri et al., 2005; Chen et al., 2008), robo3v2 will here be referred to as robo3a.1, and robo3v1 as robo3b.1. Based on its specific and spatially restricted expression in the diencephalon, we focused on robo3a.1 (Challa et al., 2005). To analyse expression of robo3a.1 in relation to otpb:gfp neurons, we performed fluorescent in situ hybridization to robo3a.1 in combination with anti-GFP immunohistochemistry. At 48 hpf, this analysis revealed a bilateral robo3a.1 expression domain in the hypothalamus, which extends medially towards the midline. Based on robo3a.1 and otpb:gfp expression profiles, the robo3a.1 domain could be separated into an anterior (aH) and a posterior (pH) part (Fig. 4G,J). In aH, robo3a.1 is expressed only in a subset of GFP-positive cells at very low levels, whereas in pH, robo3a.1 is co-expressed in most GFP-positive neurons (Fig. 4A-A′,D-D′). This expression profile coincides with the dynamic regulation of robo3a.1 transcription during DA cell development (supplementary material Fig. S5).
We next evaluated robo3a.1 expression in otpb:gfp transgenic arnt2hi2639 mutant embryos and in otpb:gfp embryos after sim1a MO knockdown (Fig. 4B-B′,C-C,E-E′,F-F,H,I). Our analysis reveals that, in response to arnt2 or sim1a loss of function, robo3a.1 expression levels are significantly increased in a subset of GFP neurons in the aH, whereas expression in pH was unchanged (Fig. 4K). By contrast, a change in expression levels of other Robo receptors or of Dcc was not detected (Fig. 4K and data not shown).
Taken together, these results provide evidence that robo3a.1 transcription is dynamically regulated during development of otpb:gfp and DA neurons, and that Sim1a and Arnt2 transcription factors are required for downregulation of robo3a.1 in aH as development proceeds.
Robo3 is required to define lateral positioning of otpb:gfp-positive longitudinal axons
We next tested a potential role of Robo3 during pathfinding of otpb:gfp-positive HTS longitudinal axons using Robo3 twitch twice (twttw204) mutants (Burgess et al., 2009). In twt mutant otpb:gfp embryos, the distance between longitudinal HTS axons in the hindbrain was significantly increased when compared with heterozygous siblings (Fig. 5A-E; supplementary material Table S7). In addition, we analysed pathfinding of Hcrt HTS axons, which do not express robo3a.1 (supplementary material Fig. S6A-C). Formation of hcrt:tdtomato-positive longitudinal axons did not reveal a significant difference between twt mutant and heterozygous sibling embryos (supplementary material Fig. S6D-H, Table S7). Therefore, Robo3 function specifically contributes to mediolateral positioning of otpb:gfp-positive longitudinal axons but not of Hcrt axons.
Fig. 5.
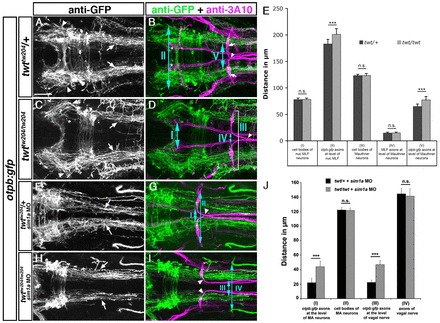
Robo3 is required for lateral positioning of otpb:gfp-positive longitudinal axons. Dorsal views of confocal hindbrain z-projections (72 hpf) are shown. Anterior is towards the left. (A,B) In twt/+ embryos, otpb:gfp longitudinal axons in the midbrain (A, arrowheads) and hindbrain (A, arrows) grow by a defined distance towards the midline. Arrows in B indicate Mauthner neuron somata. (C,D) In twt/twt embryos, otpb:gfp longitudinal axons project more laterally in the midbrain (C, arrowheads) and hindbrain (C, arrows). (E) Quantification of the separation between otpb:gfp longitudinal axons in mid- and hindbrain (double-headed arrows, II and V in B) and between nucMLF somata (I, see asterisks in B), MLF axons (IV) or MA neurons (III) in twt/+ or twt/twt embryos. (F,G) In sim1a MO-injected twt/+ embryos, otpb:gfp longitudinal axons are medially displaced (F, arrows). (H,I) In sim1a MO-injected twt/twt embryos, otpb:gfp longitudinal axons project more laterally (H, arrows). (J) Quantification of the distance between MA neurons (II, marked in G) and vagal axons (IV in H), or of the distance between otpb:gfp longitudinal axons (I in H and III in G) in sim1a MO-injected twt/+ and twt/twt embryos. Arrowheads in B,D,G,I indicate pathfinding of Mauthner neurons in twt/+ and twt/twt embryos. *P<0.001. Scale bar: 50 μm.
Medial displacement of otpb:gfp- and TH-positive longitudinal axons after sim1a or arnt2 depletion is suppressed in robo3 mutants
To examine whether an increase of robo3a.1 expression might be the cause for medial displacement of otpb:gfp-positive longitudinal axons, we assayed longitudinal tract formation after sim1a or arnt2 knockdown in Robo3-deficient embryos. In twt heterozygous otpb:gfp embryos injected with sim1a MO, we observed a medial displacement of longitudinal axons. By contrast, this displacement upon sim1a knockdown was strongly attenuated in twt mutant otpb:gfp embryos (Fig. 5F-J; supplementary material Table S8). Knockdown of arnt2 function resulted in similar findings (supplementary material Fig. S7A-E; Table S8). In order to demonstrate that negative regulation of robo3 by Sim1a is required for the positioning of longitudinal DA tracts, we again used stage-specific knockdown of sim1a by photoactivation of morpholinos (supplementary material Fig. S8A-D). This analysis demonstrated a significant increase in the average distance of TH-positive longitudinal axons for sim1a photomorphants of twt mutant genotype when compared with twt heterozygous embryos (supplementary material Fig. S8E, Table S9). These two sets of experiments indicate that negative control of robo3a.1 by Sim1a and Arnt2 is required for proper lateral positioning of otpb:gfp and DA longitudinal axons.
Overexpression of robo3a.1 induces medial displacement of Robo2-dependent longitudinal axons
We next analysed whether mis-expression of robo3a.1 was sufficient to induce medial displacement of Robo2-dependent longitudinal axons. In addition to robo3a.1, we also decided to analyse a potential role of robo3b.1 in HTS formation. We analysed Otpb and Hcrt neurons, which require Robo2 function for longitudinal tract formation. To temporally control expression of robo3a.1 or robo3b.1, we generated hsp70l:robo3a.1 and hsp70l:robo3b.1 transgenic lines, which enable comparable levels of ubiquitous robo3a.1 or robo3b.1 expression upon heat-shock treatment (data not shown). The heat-shock lines were crossed to the otpb:gfp and hcrt:tdtomato transgenic lines. Transgenic embryos were heat-induced (39°C for 50 minutes) at 24 and 28 hpf to maintain robo3 expression during longitudinal HTS pathfinding. Heat-shock treatment did not affect formation of longitudinal axons in control or hsp70l:robo3b.1 embryos. By contrast, mis-expression of robo3a.1 resulted in medial displacement of longitudinal axons (Fig. 6). Quantification revealed a significant medial displacement phenotype after overexpression of robo3a.1 when compared with controls or with overexpression of robo3b.1 (Fig. 6G,N; supplementary material Tables S10, S11). In addition to the results described above, we observed different phenotypes upon mis-expression of robo3a.1 (enables midline crossing) and robo3b.1 (prevents midline crossing) in commissural Mauthner neuron axons (J.S., unpublished).
Fig. 6.
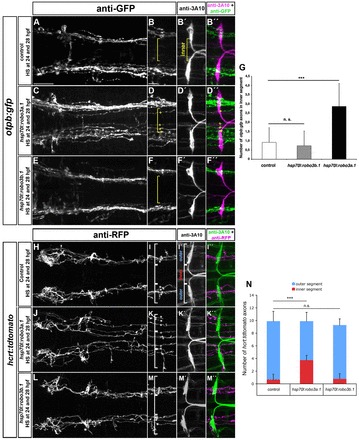
Ectopic expression of robo3a.1 is sufficient to induce medial displacement of HTS axons. (A-F″,H-M″) Dorsal views of confocal z-projections of brain regions of 48 hpf (A-F″) and 72 hpf (H-M″) embryos. Anterior is towards the to left. (A-B″,H-I″) Heat-shock treatment does not affect pathfinding of otpb:gfp or hcrt:tdtomato longitudinal axon in control embryos. (C-D″,J-K″) Longitudinal otpb:gfp or hcrt:tdtomato axons are medially displaced after heat shock-induced overexpression of robo3a.1. (E-F″,L-M″) Pathfinding of otpb:gfp or hcrt:tdtomato longitudinal axons is normal after overexpression of robo3b.1. (G,N) Quantification of the number of otpb:gfp or hcrt:tdtomato longitudinal axons in defined outer or inner sections. Brackets in B,B′,D,F, I,I′,K,M, indicate outer and inner sections as defined by Mauthner neuron somata (outer section) and the area in between (inner section) used for quantification. Asterisks and plus signs indicate longitudinal HTS axons in outer and inner sections, respectively. Scale bars: in A, 50 μm for A,C,E,H,J,L; in B, 25 μm for B-B″,D-D″,F-F″,I-I″,K-K″,M-M″.
Our findings demonstrate that mis-expression of robo3a.1, but not of robo3b.1, is sufficient to induce medial displacement of longitudinal HTS axons. Thus, the Robo3a.1 isoform is important for proper mediolateral positioning of HTS tracts.
Ectopic Robo3a.1 blocks Robo2-dependent repulsion
To analyse how ectopic robo3a.1 expression may affect HTS tract formation, we first tested for a potential role of Robo3a.1 as a chemoattractive receptor. The medial displacement of DA longitudinal axons towards the midline in Robo2-deficient ast embryos can be rescued upon knockdown of attractive Dcc/Netrin signalling (Kastenhuber et al., 2009). If Robo3a.1 serves as an attractive receptor, we would predict that a combined knockdown of sim1a (thereby increasing Robo3a.1) and dcc (thereby removing Dcc-mediated attraction) should still result in medial displacement phenotype of otpb:gfp HTS axons. However, we found that medial displacement of longitudinal axons was significantly reduced following co-injection of sim1a MO and dcc MO when compared with controls (Fig. 7A-E; supplementary material Table S12). This indicates that attraction mediated by Dcc causes medial displacement of longitudinal axons after sim1a knockdown, and argues against a role of Robo3a.1 as an attractive receptor.
Fig. 7.
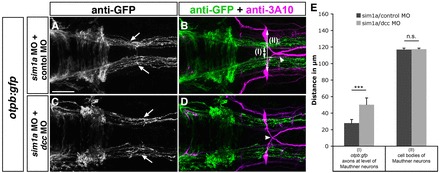
Dcc-mediated attraction causes medial displacement of otpb:gfp longitudinal axons after sim1a knockdown. Dorsal views of hindbrain confocal z-projections of 48 hpf otpb:gfp embryos. Anterior is towards the left. (A-D) GFP-positive longitudinal axons are medially displaced towards the midline (A, arrows) after co-injection of sim1a MO and control MO in otpb:gfp embryos. Medial displacement of longitudinal otpb:gfp axons (C, arrows) is reduced after combined injection of sim1a MO and dcc MO. (E) Quantification of the distance between otpb:gfp-positive longitudinal axons (I) or of the distance between Mauthner neurons (II) of indicated treatments. Arrowheads in B,D indicate normal midline crossing of MA neurons. *P<0.001; n.s., not significant. Scale bar: 50 μm.
We next analysed whether Robo3a.1 interferes with repulsion by Robo2 as suggested previously (Chen et al., 2008). If Robo3a.1 compromised Robo2 repulsion, then increased levels of robo3a.1 in the ast mutant background should not lead to a stronger medial displacement phenotype. Quantification of medial displacement of longitudinal axons after depletion of sim1a in ast/ast;twt/+ embryos (increased robo3a.1 expression), and in ast; twt double mutant embryos (no robo3a.1 expression) compared with ast/ast; twt/twt double mutants injected with control MO revealed similar phenotypic strength (Fig. 8A-G; supplementary material Table S13).
Fig. 8.
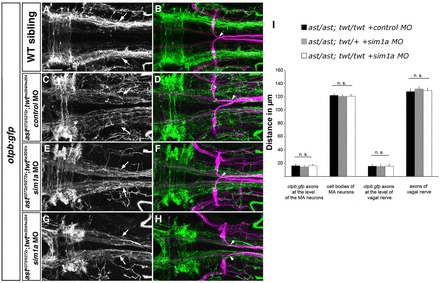
Medial displacement phenotype of otpb:gfp longitudinal HTS axons is not affected by depletion of sim1a in ast/ast; twt/+ and ast/ast; twt/twt embryos. Dorsal views of hindbrain confocal z-projections of 72 hpf otpb:gfp embryos. Anterior is towards the left. (A,B) otpb:gfp HTS axons (arrows) in a wild-type sibling. (C-H) Phenotypic strength of medial displacement of otpb:gfp-positive longitudinal axons is similar in ast/ast; twt/twt embryos injected with control MO (C, arrows), and in ast/ast; twt/+ (E, arrows) or ast/ast; twt/twt (G, arrows) sim1a morphant embryos. Arrowheads in B,D,F,H indicate pathfinding of Mauthner neurons. (I) Quantification of the distance between of otpb:gfp longitudinal axons and vagal axons, and of the distance between Mauthner neurons in ast/ast; twt/twt embryos injected with control MO, and in ast/ast; twt/+ or ast/ast; twt/twt embryos injected with sim1a MO. n.s., not significant. Scale bar: 50 μm.
To further support these findings, we made use of two different ast/robo2 alleles, the strong astti272z and the weaker astte284 allele (Chalasani et al., 2007; Fricke et al., 2001). Consistently, the medial displacement phenotype of HTS axons is less pronounced in the weak ast mutants (Fig. 9A,J,I,R). We hypothesised that residual Robo2 signalling in weak ast embryos should be rendered less effective after robo3a.1 mis-expression. If so, the resulting reduced effectiveness of Robo2 signalling would be predicted to increase HTS medial displacement in weak, but not strong, ast mutants. The experiment showed that the medial displacement phenotype was not increased upon overexpression of robo3a.1 in the strong ast mutants. By contrast, a significant increase in medial displacement phenotype was observed after overexpression of robo3a.1 in the weak ast mutant background (Fig. 9; supplementary material Table S14). Taken together, these results are consistent with Robo3a.1 functioning to attenuate repulsion by Robo2, and that attraction by Dcc then directs longitudinal axons towards the midline.
Fig. 9.
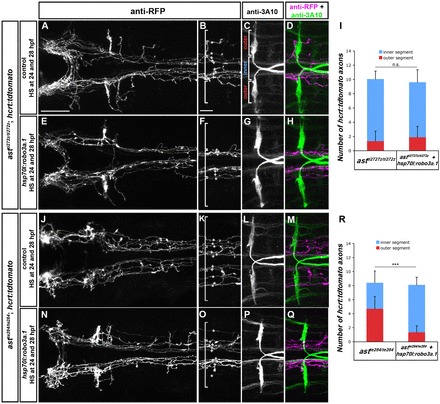
Overexpression of robo3a.1 leads to increased medial displacement of longitudinal HTS axons in astte284 but not in astti272z embryos. Dorsal views of confocal z-projections of the brain and hindbrain of 72 hpf hcrt:tdtomato embryos. Anterior is towards the left. (A-H) Medial displacement phenotype of hcrt:tdtomato HTS axons is similar in astti272z/ti272z and astti272z/ti272z; hsp70l:robo3a.1 embryos after heat-shock treatment. (J-Q) Medial displacement phenotype of hcrt:tdtomato HTS axons is increased in astte284/te284; hsp70l:robo3a.1 embryos when compared with astte284/te284 embryos. (I,R) Quantification of the number of hcrt:tdtomato longitudinal axons in outer or inner sections in astti272z (I) or astte284 (R). Brackets in B,C,F,K,O indicate outer and inner sections used for quantification, defined by Mauthner neuron somata (outer section) and the area in between (inner section). Asterisks and plus signs indicate longitudinal hcrt:tdtomato axons in outer and inner sections, respectively. Scale bars: in A, 50 μm for A,E,J,N; in B, 25 μm for B-D,F-H,K-M,O-Q.
The extracellular N-terminal domain present in the Robo3a.1 isoform is important for interfering with Robo2-dependent repulsion
To identify structural characteristics of Robo3 isoforms required for interference with Robo2-dependent repulsion, we used the Gal4/UAS system for Hcrt-specific expression of different robo3 isoforms together with tdtomato. A hcrt:gal4 driver was co-injected with UAS:robo3-P2A-tdtomatoCAAX responders harbouring different Robo3 isoforms, and embryos showing transient expression of the tdTomato reporter were analysed. Whole-mount in situ hybridization for tdtomato revealed similar expression levels for the different robo3 isoforms (supplementary material Fig. S9). The medial displacement phenotype was quantified when tdTomato-positive longitudinal axons were in close contact with 3A10-positive MLF axons (Fig. 10C,D). All midline crossing events caudal to the level of the nucMLF were considered to be ectopic (Fig. 10C). Expression of tdTomato alone or of robo3b.1 in Hcrt neurons did not affect longitudinal tract formation. By contrast, mis-expression of robo3a.1 resulted in medial displacement of longitudinal axons and ectopic midline crossing events (Fig. 10A-F,K; supplementary material Table S15). To analyse requirement of Robo3A cytoplasmatic domains, we mis-expressed mouse Robo3A.1 and Robo3A.2 in Hcrt neurons. The two mouse Robo3A isoforms encode a similar N-terminus to zebrafish robo3a.1, but distinct C termini and contribute to different aspects of commissural axon guidance (Chen et al., 2008). Similar to our findings upon mis-expression of zebrafish robo3a.1, mis-expression of mouse Robo3A.1 or Robo3A.2 resulted in medial displacement of longitudinal axons and ectopic midline crossing (Fig. 10G-J,K; supplementary material Table S15).
Fig. 10.
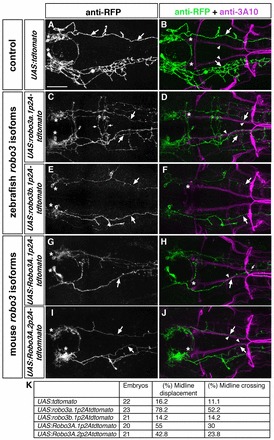
The N-terminal domain present in Robo3A isoforms is important for interfering with Robo2-dependent repulsion. Dorsal views of hindbrain confocal z-projections of 72 hpf embryos. Anterior is towards the left. Embryos were co-injected with hcrt:gal4 and UAS constructs indicated on the left. (A,B) Hcrt neurons form tdTomato-positive longitudinal axons (A, arrows), which project laterally (B, arrows) to the MLF (B, arrowheads). (C,D) After mis-expression of robo3a.1 in Hcrt neurons, tdTomato-positive longitudinal axons are medially displaced towards the midline (C,D, arrows) and project together with the medial longitudinal fascicle (D, arrowheads). Small arrows indicate ectopic midline crossing events (C). (E,F) Upon mis-expression of robo3b.1 in Hcrt neurons, tdTomato-positive longitudinal axons project normally (E,F, arrows). (G-J) After mis-expression of mouse Robo3A.1 (G,H) or Robo3A.2 (I,J) in Hcrt neurons, tdTomato-positive longitudinal axons are displaced towards the midline (G-J, arrows) and project together with the medial longitudinal fascicle (H,J, arrowheads). Small arrow in H indicates midline crossing. (K) Quantification. Asterisks in A,C,E,G,I indicate Hcrt neurons, asterisks in B,D,F,H,J indicate nucMLF neurons. Scale bar: 50 μm.
Taken together, our results show that ectopic expression of zebrafish robo3a.1 and mouse Robo3A.1 or Robo3A.2 isoforms is sufficient to induce medial displacement of Robo2-dependent longitudinal axons. This suggests that blocking of Robo2 activity primarily occurs via the specific N-terminal domain present in the Robo3A isoforms.
DISCUSSION
Our data reveal a novel function for Arnt2 and Sim1a transcription factors in establishment of HTS longitudinal axon tracts. Arnt2 and Sim1 are key regulators for differentiation of at least five different neuroendocrine cell lineages and of A11-related DA neurons in the hypothalamus (Löhr et al., 2009; Michaud et al., 2000; Michaud et al., 1998). As these diverse neuronal types all develop descending projections to the spinal cord, we analysed whether Sim1a and Arnt2 would contribute to control of axonal pathfinding. We found that loss of Arnt2 or Sim1a function results in a medial shift of HTS trajectories while passing the hindbrain. Our data reveal that Sim1a and Arnt2 negatively regulate expression of robo3a.1, a specific robo3 isoform, which in turn inhibits Robo2 signalling activity.
In mouse embryos that lack Sim1 and/or Sim2 function, projection behaviour of hypothalamic neurons of the mammillary body is altered, resulting in aberrant midline crossing (Marion et al., 2005). These findings indicate that Sim transcription factor function may contribute to axon pathfinding for diverse neural populations. By contrast, Arnt2 function has been reported to be dispensable in this system. Our data indicate that sim1a and arnt2, similar to their role during neuronal differentiation (Michaud et al., 2000), act synergistically during HTS axon guidance. Robo3 transcripts have been reported to be upregulated in the mammillary body of Sim1–/–/Sim2–/– double mutants, suggesting that Robo3 might contribute to the reported axon guidance defects (Marion et al., 2005). Our analysis of HTS tract formation reveals similar mechanisms for Sim1a and Arnt2, and elucidates for both factors downstream molecular mechanisms affecting axon guidance. However, mediolateral positioning of HTS axons is not completely restored upon depletion of sim1a or arnt2 in robo3 mutant embryos, suggesting additional HTS axon guidance control mechanisms by Sim1a and Arnt2. Downstream targets of Sim1 and Arnt2 include transcriptional regulators, as well as cell migration-related genes (Liu et al., 2003), which may contribute to HTS axon guidance independent of Robo3. In Drosophila, abrogation of sim leads to an axonal fasciculation phenotype (Freer et al., 2011), which differs from the mouse Sim1 or zebrafish sim1a loss-of-function defects. Thus, Sim1 may control different aspects of axonal pathfinding by regulating different axon guidance factors.
How does upregulated robo3a.1 misguide pathfinding of HTS longitudinal axons? Previous work suggested that Robo3a.1 may inactivate Robo1 and Robo2 receptors and thereby render pre-crossing commissural axons unresponsive to Slit repulsion (Chen et al., 2008). Several observations argue that a similar mechanism between Robo3A.1 and Robo2 may lead to the medial displacement phenotype of HTS longitudinal axons. The sim1a or arnt2 loss-of-function axon tract phenotypes share many features with Robo2 mutants. These similarities suggest that Robo3A.1 interferes with Robo2 activity. This notion is further strengthened by our findings that increased activity of Robo3a.1 (by either sim1a depletion or robo3a.1 overexpression) enhances the medial displacement phenotype of HTS axons in weak, but not strong, ast mutants. These genetic experiments reveal that Robo3 acts only through Robo2 in this developmental context, but has no Slit-dependent signalling activity independent of Robo2. This indicates that Robo3A.1 does not serve as an attractive receptor. Furthermore, our observations after combined knockdown of sim1a and dcc suggest that attraction mediated by Dcc is the driving force that directs otpb:gfp longitudinal axons towards the midline. This finding relates to our previous results demonstrating that knockdown of dcc in ast mutants restores mediolateral positioning of DA longitudinal axons (Kastenhuber et al., 2009). Our data indicate that Robo3a.1 inhibits Robo2-mediated repulsion and that attraction by Dcc then directs HTS longitudinal axons towards the midline.
How does Robo3A.1 interfere with Robo2 activity? Our results on the mis-expression of different combinations of N- and C-terminal zebrafish and mouse Robo3a and Robo3b isoforms in Hcrt neurons suggest that the N-terminal domain present in Robo3A is important for inhibiting Robo2 activity during HTS axon guidance. A recent study demonstrated that different C-terminal domains present in mouse Robo3A.1 and Robo3A.2 contribute to distinct aspects of commissural axon guidance (Chen et al., 2008). Robo3A.1 allows midline crossing by potentially blocking activity of Robo1 and Robo2, whereas Robo3A.2 may prevent re-crossing by collaboration with Robo1 and Robo2. How can the observed differences be explained? Commissural and ipsilateral axon guidance mechanisms use similar sets of guidance cues, but respond with different projections (for a review, see Evans and Bashaw, 2010a). Thus, it is conceivable that DA and otpb:gfp-positive longitudinal growth cones, in contrast to commissural neurons, use a distinct pathway involving solely the N-terminal domain found in Robo3A isoforms to attenuate Robo2 activity. In Drosophila, different functions of Robo2 and Robo3 are specified by their ectodomains, and do not reflect differences in cytoplasmic signalling (Evans and Bashaw, 2010b; Spitzweck et al., 2010). Alternatively, as we failed to identify different C-terminal robo3 isoforms in zebrafish (data not shown), it is also possible that zebrafish solely use the N-terminal Robo3a.1 and Robo3b.1 isoforms to control different aspects of axon guidance. In addition to the results presented here, a role for Robo3a.1 and Robo3b.1 during axon guidance, has also been suggested by others (Challa et al., 2005; Devine and Key, 2008). Our findings indicate that inhibition of Robo2 activity via the N-terminal domain present in Robo3A isoforms occurs extracellularly during pathfinding of HTS axons. However, the precise molecular interactions remain elusive. The Robo3A specific domain could interfere with Robo2 by direct physical interaction, and thereby mask the Slit-binding domain of Robo2 mapped within the immunoglobulin-like domains 1 and 2 (Hohenester, 2008). Alternatively, repulsion by Robo2 may be inhibited by ectodomain-dependent heterodimerisation of Robo3A.1 and Robo2 in a Slit-independent fashion, similar to findings in Drosophila (Evans and Bashaw, 2010b).
What is the role of Robo3A.1 during longitudinal pathfinding? Lateral positioning of longitudinal axons is primarily governed by repulsion through midline derived Slit proteins (Farmer et al., 2008; Kastenhuber et al., 2009). In zebrafish, the four known Slit genes display strong expression at the midline of the midbrain and hindbrain, and lower expression within the spinal cord, suggesting different Slit levels (supplementary material Fig. S10) (Hutson et al., 2003; Yeo et al., 2001). Therefore, in order to maintain constant mediolateral positioning in regions with different Slit levels, the growth cones of DA and otpb:gfp-positive longitudinal axons may have adopted a mechanism to modulate Slit-dependent repulsion from the midline (Fig. 11). Robo3A.1 may contribute to this mechanism by reducing the activity of Robo2 and thus attenuating repulsion by Slit proteins. The Robo3A.1 dependent ‘attenuation’ mechanism may then enable the navigation of longitudinal otpb:gfp or DA growth cones to keep lateral positioning in the mid- and hindbrain despite high expression levels of slits (Fig. 11A,A′). Sim1a and Arnt2 contribute to this mechanism by controlling negative regulation of robo3a.1 in a stage-dependent manner synchronized with progress of axonogenesis. Complete downregulation of robo3a.1 then leads to full activation of Robo2, which allows for proper lateral positioning within the lower slit levels expressed in the spinal cord (Fig. 11B,11B′). In arnt2 or sim1a loss-of-function embryos, stage-dependent negative control of robo3a.1 does not occur. Therefore, Robo3a.1 is still present and inhibits Robo2 activity when longitudinal axons approach the spinal cord. When Robo2 activity is severely attenuated by Robo3a.1 overexpression, repulsion and attraction are imbalanced and longitudinal growth cones are directed towards the midline by Dcc/Netrin-mediated attraction (Fig. 11C,11C′).
Fig. 11.
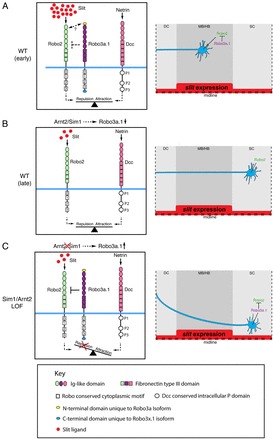
Model for control of Robo2 activity during longitudinal HTS pathfinding. (A) During early stages, Robo3a.1 reduces repulsive activity of Robo2, while HTS axons navigate through areas of high slit expression levels in mid- and hindbrain. Double-headed arrow indicates potential Robo2/Robo3a.1 interaction domains. (B) Sim1a and Arnt2 negatively regulate robo3a.1 at later stages when HTS axons have passed regions of high slit expression. Lateral positioning in the spinal cord is then controlled by full activity of Robo2/Slit signalling. (C) Model of abnormal HTS axon trajectories in Arnt2 and Sim1a loss-of-function embryos: ‘attenuation’ of Robo/Slit signalling activity persists when axons travel through hindbrain because Robo3a.1 downregulation fails to occur after Arnt2/Sim1a depletion. As a result, HTS axons receive less Robo/Slit signalling activity and are medially displaced. For conserved Robo3 domains, see key. DC, diencephalon; MB/HB, midbrain-hindbrain; sc, spinal cord.
In summary, we have shown that Arnt2 and Sim1a transcription factors contribute to proper positioning of HTS longitudinal axon tracts. Sim1a and Arnt2 perform this function by negative regulation of robo3a.1 transcription at a specific stage of neuroendocrine and DA cell differentiation. Precise control of robo3a.1 expression is essential for proper longitudinal tract formation, as Robo3A.1 attenuates Robo2 activity to adjust lateral positioning within different Slit levels. Thus, our findings reveal a novel mechanism to ensure correct lateral positioning of longitudinal projections navigating through changing signalling environments.
Supplementary Material
Acknowledgments
We thank C. Beattie and M. Tessier-Lavigne for robo3 cDNAs, P. Mourrain for the hcrt promoter and M. Hammerschmidt for sharing unpublished results. We thank the zebrafish community for sharing reagents and S. Götter for expert zebrafish care. We thank Virginie Lecaudey, Erin Beddows, Marcus Frank and Jochen Holzschuh for comments on the manuscript.
Footnotes
Funding
This work was supported by the German Research Foundation [DFG-SFB780-B6 to W.D. and DFG-SCHW1404/1-1 to J.S.) and by the European Commission (FP7, mdDANEURODEV, to W.D.).
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.087825/-/DC1
These authors contributed equally to this work
Present address: Institute for Developmental Biology, University of Cologne, Zuelpicher Strasse 47b, 50674 Cologne, Germany
References
- Acampora D., Postiglione M. P., Avantaggiato V., Di Bonito M., Vaccarino F. M., Michaud J., Simeone A. (1999). Progressive impairment of developing neuroendocrine cell lineages in the hypothalamus of mice lacking the Orthopedia gene. Genes Dev. 13, 2787–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björklund A., Skagerberg G. (1979). Evidence for a major spinal cord projection from the diencephalic A11 dopamine cell group in the rat using transmitter-specific fluorescent retrograde tracing. Brain Res. 177, 170–175. [DOI] [PubMed] [Google Scholar]
- Blechman J., Borodovsky N., Eisenberg M., Nabel-Rosen H., Grimm J., Levkowitz G. (2007). Specification of hypothalamic neurons by dual regulation of the homeodomain protein Orthopedia. Development 134, 4417–4426. [DOI] [PubMed] [Google Scholar]
- Borodovsky N., Ponomaryov T., Frenkel S., Levkowitz G. (2009). Neural protein Olig2 acts upstream of the transcriptional regulator Sim1 to specify diencephalic dopaminergic neurons. Dev. Dyn. 238, 826–834. [DOI] [PubMed] [Google Scholar]
- Brose K., Bland K. S., Wang K. H., Arnott D., Henzel W., Goodman C. S., Tessier-Lavigne M., Kidd T. (1999). Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell 96, 795–806. [DOI] [PubMed] [Google Scholar]
- Burgess H. A., Johnson S. L., Granato M. (2009). Unidirectional startle responses and disrupted left-right co-ordination of motor behaviors in robo3 mutant zebrafish. Genes Brain Behav. 8, 500–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler S. J., Tear G. (2007). Getting axons onto the right path: the role of transcription factors in axon guidance. Development 134, 439–448. [DOI] [PubMed] [Google Scholar]
- Camurri L., Mambetisaeva E., Davies D., Parnavelas J., Sundaresan V., Andrews W. (2005). Evidence for the existence of two Robo3 isoforms with divergent biochemical properties. Mol. Cell. Neurosci. 30, 485–493. [DOI] [PubMed] [Google Scholar]
- Chalasani S. H., Sabol A., Xu H., Gyda M. A., Rasband K., Granato M., Chien C. B., Raper J. A. (2007). Stromal cell-derived factor-1 antagonizes slit/robo signaling in vivo. J. Neurosci. 27, 973–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challa A. K., McWhorter M. L., Wang C., Seeger M. A., Beattie C. E. (2005). Robo3 isoforms have distinct roles during zebrafish development. Mech. Dev. 122, 1073–1086. [DOI] [PubMed] [Google Scholar]
- Chen Z., Gore B. B., Long H., Ma L., Tessier-Lavigne M. (2008). Alternative splicing of the Robo3 axon guidance receptor governs the midline switch from attraction to repulsion. Neuron 58, 325–332. [DOI] [PubMed] [Google Scholar]
- Del Giacco L., Sordino P., Pistocchi A., Andreakis N., Tarallo R., Di Benedetto B., Cotelli F. (2006). Differential regulation of the zebrafish orthopedia 1 gene during fate determination of diencephalic neurons. BMC Dev. Biol. 6, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine C. A., Key B. (2008). Robo-Slit interactions regulate longitudinal axon pathfinding in the embryonic vertebrate brain. Dev. Biol. 313, 371–383. [DOI] [PubMed] [Google Scholar]
- Dugan J. P., Stratton A., Riley H. P., Farmer W. T., Mastick G. S. (2011). Midbrain dopaminergic axons are guided longitudinally through the diencephalon by Slit/Robo signals. Mol. Cell. Neurosci. 46, 347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton J. L., Glasgow E. (2006). The zebrafish bHLH PAS transcriptional regulator, single-minded 1 (sim1), is required for isotocin cell development. Dev. Dyn. 235, 2071–2082. [DOI] [PubMed] [Google Scholar]
- Eaton J. L., Glasgow E. (2007). Zebrafish orthopedia (otp) is required for isotocin cell development. Dev. Genes Evol. 217, 149–158. [DOI] [PubMed] [Google Scholar]
- Evans T. A., Bashaw G. J. (2010a). Axon guidance at the midline: of mice and flies. Curr. Opin. Neurobiol. 20, 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T. A., Bashaw G. J. (2010b). Functional diversity of Robo receptor immunoglobulin domains promotes distinct axon guidance decisions. Curr. Biol. 20, 567–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraco J. H., Appelbaum L., Marin W., Gaus S. E., Mourrain P., Mignot E. (2006). Regulation of hypocretin (orexin) expression in embryonic zebrafish. J. Biol. Chem. 281, 29753–29761. [DOI] [PubMed] [Google Scholar]
- Farmer W. T., Altick A. L., Nural H. F., Dugan J. P., Kidd T., Charron F., Mastick G. S. (2008). Pioneer longitudinal axons navigate using floor plate and Slit/Robo signals. Development 135, 3643–3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi A., Dürr K., Ryu S., Willaredt M., Holzschuh J., Driever W. (2007). Expression and function of nr4a2, lmx1b, and pitx3 in zebrafish dopaminergic and noradrenergic neuronal development. BMC Dev. Biol. 7, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freer S. M., Lau D. C., Pearson J. C., Talsky K. B., Crews S. T. (2011). Molecular and functional analysis of Drosophila single-minded larval central brain expression. Gene Expr. Patterns 11, 533–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricke C., Lee J. S., Geiger-Rudolph S., Bonhoeffer F., Chien C. B. (2001). astray, a zebrafish roundabout homolog required for retinal axon guidance. Science 292, 507–510. [DOI] [PubMed] [Google Scholar]
- Fujimoto E., Stevenson T. J., Chien C. B., Bonkowsky J. L. (2011). Identification of a dopaminergic enhancer indicates complexity in vertebrate dopamine neuron phenotype specification. Dev. Biol. 352, 393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furley A. J., Morton S. B., Manalo D., Karagogeos D., Dodd J., Jessell T. M. (1990). The axonal glycoprotein TAG-1 is an immunoglobulin superfamily member with neurite outgrowth-promoting activity. Cell 61, 157–170. [DOI] [PubMed] [Google Scholar]
- Golling G., Amsterdam A., Sun Z., Antonelli M., Maldonado E., Chen W., Burgess S., Haldi M., Artzt K., Farrington S., et al. (2002). Insertional mutagenesis in zebrafish rapidly identifies genes essential for early vertebrate development. Nat. Genet. 31, 135–140. [DOI] [PubMed] [Google Scholar]
- Hancock M. B. (1976). Cells of origin of hypothalamo-spinal projections in the rat. Neurosci. Lett. 3, 179–184. [DOI] [PubMed] [Google Scholar]
- Hohenester E. (2008). Structural insight into Slit-Robo signalling. Biochem. Soc. Trans. 36, 251–256. [DOI] [PubMed] [Google Scholar]
- Holst J., Vignali K. M., Burton A. R., Vignali D. A. (2006). Rapid analysis of T-cell selection in vivo using T cell-receptor retrogenic mice. Nat. Methods 3, 191–197. [DOI] [PubMed] [Google Scholar]
- Holzschuh J., Barrallo-Gimeno A., Ettl A. K., Durr K., Knapik E. W., Driever W. (2003). Noradrenergic neurons in the zebrafish hindbrain are induced by retinoic acid and require tfap2a for expression of the neurotransmitter phenotype. Development 130, 5741–5754. [DOI] [PubMed] [Google Scholar]
- Hutson L. D., Jurynec M. J., Yeo S. Y., Okamoto H., Chien C. B. (2003). Two divergent slit1 genes in zebrafish. Dev. Dyn. 228, 358–369. [DOI] [PubMed] [Google Scholar]
- Kastenhuber E., Kern U., Bonkowsky J. L., Chien C. B., Driever W., Schweitzer J. (2009). Netrin-DCC, Robo-Slit, and heparan sulfate proteoglycans coordinate lateral positioning of longitudinal dopaminergic diencephalospinal axons. J. Neurosci. 29, 8914–8926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keino-Masu K., Masu M., Hinck L., Leonardo E. D., Chan S. S., Culotti J. G., Tessier-Lavigne M. (1996). Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell 87, 175–185. [DOI] [PubMed] [Google Scholar]
- Kwan K. M., Fujimoto E., Grabher C., Mangum B. D., Hardy M. E., Campbell D. S., Parant J. M., Yost H. J., Kanki J. P., Chien C. B. (2007). The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev. Dyn. 236, 3088–3099. [DOI] [PubMed] [Google Scholar]
- Liu C., Goshu E., Wells A., Fan C. M. (2003). Identification of the downstream targets of SIM1 and ARNT2, a pair of transcription factors essential for neuroendocrine cell differentiation. J. Biol. Chem. 278, 44857–44867. [DOI] [PubMed] [Google Scholar]
- Löhr H., Ryu S., Driever W. (2009). Zebrafish diencephalic A11-related dopaminergic neurons share a conserved transcriptional network with neuroendocrine cell lineages. Development 136, 1007–1017. [DOI] [PubMed] [Google Scholar]
- Marion J. F., Yang C., Caqueret A., Boucher F., Michaud J. L. (2005). Sim1 and Sim2 are required for the correct targeting of mammillary body axons. Development 132, 5527–5537. [DOI] [PubMed] [Google Scholar]
- Michaud J. L., Rosenquist T., May N. R., Fan C. M. (1998). Development of neuroendocrine lineages requires the bHLH-PAS transcription factor SIM1. Genes Dev. 12, 3264–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud J. L., DeRossi C., May N. R., Holdener B. C., Fan C. M. (2000). ARNT2 acts as the dimerization partner of SIM1 for the development of the hypothalamus. Mech. Dev. 90, 253–261. [DOI] [PubMed] [Google Scholar]
- Polleux F., Ince-Dunn G., Ghosh A. (2007). Transcriptional regulation of vertebrate axon guidance and synapse formation. Nat. Rev. Neurosci. 8, 331–340. [DOI] [PubMed] [Google Scholar]
- Rink E., Wullimann M. F. (2002). Development of the catecholaminergic system in the early zebrafish brain: an immunohistochemical study. Brain Res. Dev. Brain Res. 137, 89–100. [DOI] [PubMed] [Google Scholar]
- Ryu S., Mahler J., Acampora D., Holzschuh J., Erhardt S., Omodei D., Simeone A., Driever W. (2007). Orthopedia homeodomain protein is essential for diencephalic dopaminergic neuron development. Curr. Biol. 17, 873–880. [DOI] [PubMed] [Google Scholar]
- Sabatier C., Plump A. S., Ma L., Brose K., Tamada A., Murakami F., Lee E. Y., Tessier-Lavigne M. (2004). The divergent Robo family protein rig-1/Robo3 is a negative regulator of slit responsiveness required for midline crossing by commissural axons. Cell 117, 157–169. [DOI] [PubMed] [Google Scholar]
- Spitzweck B., Brankatschk M., Dickson B. J. (2010). Distinct protein domains and expression patterns confer divergent axon guidance functions for Drosophila Robo receptors. Cell 140, 409–420. [DOI] [PubMed] [Google Scholar]
- Suli A., Mortimer N., Shepherd I., Chien C. B. (2006). Netrin/DCC signaling controls contralateral dendrites of octavolateralis efferent neurons. J. Neurosci. 26, 13328–13337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson L. W. (1977). Immunohistochemical evidence for a neurophysin-containing autonomic pathway arising in the paraventricular nucleus of the hypothalamus. Brain Res. 128, 346–353. [DOI] [PubMed] [Google Scholar]
- Tay T. L., Ronneberger O., Ryu S., Nitschke R., Driever W. (2011). Comprehensive catecholaminergic projectome analysis reveals single-neuron integration of zebrafish ascending and descending dopaminergic systems. Nat. Commun. 25, 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasini A. J., Schuler A. D., Zebala J. A., Mayer A. N. (2009). PhotoMorphs: a novel light-activated reagent for controlling gene expression in zebrafish. Genesis 47, 736–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Pol A. N. (1999). Hypothalamic hypocretin (orexin): robust innervation of the spinal cord. J. Neurosci. 19, 3171–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Lufkin T. (2000). The murine Otp homeobox gene plays an essential role in the specification of neuronal cell lineages in the developing hypothalamus. Dev. Biol. 227, 432–449. [DOI] [PubMed] [Google Scholar]
- Westerfield M. (1995). The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio), 3rd edn. Eugene, OR: University of Oregon Press. [Google Scholar]
- Yeo S. Y., Little M. H., Yamada T., Miyashita T., Halloran M. C., Kuwada J. Y., Huh T. L., Okamoto H. (2001). Overexpression of a slit homologue impairs convergent extension of the mesoderm and causes cyclopia in embryonic zebrafish. Dev. Biol. 230, 1–17. [DOI] [PubMed] [Google Scholar]
- Ypsilanti A. R., Zagar Y., Chédotal A. (2010). Moving away from the midline: new developments for Slit and Robo. Development 137, 1939–1952. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


