Abstract
We recently exploited a transgenic approach to coerce macrophage anti-inflammatory M2 polarization in vivo by lowering Receptor Interacting Protein 140 (RIP140) level in macrophages (mφRIP140KD), which induced browning of white adipose tissue (WAT). In vitro, conditioned medium from cultured adipose tissue macrophages (ATMs) of mφRIP140KD mice could trigger preadipocytes' differentiation into beige cells. Here we describe a cell therapy for treating high fat diet (HFD)-induced insulin resistance (IR). Injecting M2 ATMs retrieved from the WAT of mφRIP140KD mice into HFD-fed obese adult wild-type mice effectively triggers their WAT browning, reduces their pro-inflammatory responses, and improves their insulin sensitivity. These data provide a proof-of-concept that delivering engineered anti-inflammatory macrophages can trigger white fat browning, stimulate whole-body thermogenesis, and reduce obesity-associated IR.
Keywords: browning, beige cells, HFD, insulin resistance, M2 ATM, macrophages, preadipocyte differentiation, RIP140, obesity
Abbreviations: ATM(s), adipose tissue macrophage(s); BAT, brown adipose tissue; FFA, free fatty acid; GTT, glucose tolerance test; HFD, high-fat diet; IL4, Interleukin 4; IR, insulin resistance; ITT, insulin tolerance test; KD, knockdown mice; mφRIP140KD, macrophage-specific RIP140 knockdown mutation; ND, normal diet; PBS, phosphate-buffered saline; RIP140, Receptor Interacting Protein 140; SVF, stromal vascular fraction; TG, triglyceride; (v)WAT, (visceral) white adipose tissue; WT, wild-type mice
Introduction
Adipose tissue in mammals consists of 2 major types—white adipose tissue (WAT) and brown adipose tissue (BAT), each having a unique physiological role.1-3 WAT is the main energy storage site, whereas BAT provides the major energy source via non-shivering thermogenesis. BAT is located in the interscapular region, and detected mainly in infants and small mammals. It has a high mitochondria content and expresses mitochondrial UCP1, which dissipates energy to generate heat. In contrast, WAT typically expands under an obese condition and becomes inflammatory, which triggers insulin resistance (IR).4 Recently, a third type of fat has been identified within the WAT depots of mouse and human, called “beige fat.” 5-8 Because beige fat can also engage in thermogenesis, increasing beige fat is generally considered beneficial. The activity of beige cells can be increased in response to cold exposure or certain stimuli such as IL4 treatment, which enhances thermogenesis. This leads to an interesting phenomenon called “browning” of WAT.9-11 Despite beige cells seeming similar to BAT cells in terms of thermogenesis, evidence indicates that brown vs. beige adipocytes have distinct gene expression profiles and are likely to have tissue-specific actions.12 In light of the potential benefit of turning WAT into beige fat, there is increasing interest in understanding how BAT is activated and how WAT cells may be turned into beige cells. This could provide a new therapeutic strategy for treating obesity and its related metabolic disorders.8,12
Two groups have recently provided evidence that enhancing the anti-inflammatory M2 ATM population can positively affect WAT browning under cold exposure and IL4 treatment.10,11 We previously reported a positive regulatory molecule for inflammatory M1 macrophages, Receptor Interacting Protein 140 (RIP140),13,14 which is a coactivator of NF-κB in the process of M1 activation.15 Based on the notion that RIP140 activates M1 ATMs,15 we engineered a mouse model where M1 activation is reduced by specific knockdown of RIP140 only in the monocyte-macrophage lineage, called mφRIP140KD. As expected, the mφRIP140KD mice indeed are protected against acute inflammation such as sepsis,16 but more interestingly these mice are also protected against diet-induced metabolic diseases including insulin resistance (IR).17 Upon careful dissection of their WAT depots, especially following a long-term high fat diet (HFD) that in wild-type animals induces adipose inflammation and systemic IR, we found significant “browning” in the WAT depots of mφRIP140KD, consistent with their increased thermogenesis and energy expenditure as well as improved insulin sensitivity.17 In addition, we found that mφRIP140KD mice not only have reduced M1 ATM numbers, but in fact they have increased M2 ATMs numbers when compared to the wild-type animals, especially after HFD induction.17
The browning phenotype of these mφRIP140KD animals is associated with reduced RIP140 level in macrophages (including ATMs), which lowers WAT inflammation under HFD feeding. However, the chronic dampening of monocyte-macrophage inflammatory potential in these mice is system-wide; all their macrophages’ RIP140 level is lowered from birth, which could compromise immunity. We therefore conjectured that it might be possible to reduce inflammatory potential only in adults, and only in restricted locales, just sufficient to trigger local WAT browning while preventing unwanted chronic systemic effects. Indeed several studies have shown that injection of macrophages in animal models as therapeutic methods, such as for wound healing,18,19 tumor therapy,20 sepsis and colitis.21 Specifically, we asked whether one could locally inhibit adult-stage adipose inflammation via local delivery of engineered anti-inflammatory macrophages, to trigger “browning” of WAT—and whether it would work even under HFD feeding.
This report describes cell therapy experiments in animals to test this concept, and provides a proof-of-concept that direct injection of RIP140KD-ATM into adult animals under HFD feeding indeed can cause visceral WAT (vWAT) browning and therapeutically protect against the development of diet-induced IR in normal mice receiving this macrophage cell therapy.
Results
To test the therapeutic potential of M2 ATMs, we first examined ATMs collected from WT and those from mφRIP140KD (mostly polarized into M2) using in vitro conditioned medium culture experiments where preadipocytes are differentiated into mature adipocytes, provided with conditioned medium of various ATM preparations. In this experiment, we monitored the efficiency of differentiating preadipocytes into beige cells (markers Ucp-1, Pgc-1α, Tmem26 and Cd137). The result (Fig. 1) shows that beige markers in those cultures using conditioned medium from WT-ATM (gray bar) and RIP140KD-ATM (black bar) are significantly elevated as compared to the control (Ctrl, white bar). More importantly, condition medium from RIP140KD ATM is much more effective, as compared to the wild type ATM, in differentiating preadipocytes into beige cells according to the expression of these markers. This result shows that ATM from mφRIP140KD mice can be much more efficient as a therapeutic ATM. We then moved on to test a cell therapy strategy using in vivo injection of ATM collected from mφRIP140KD mice as described in the following.
Figure 1.
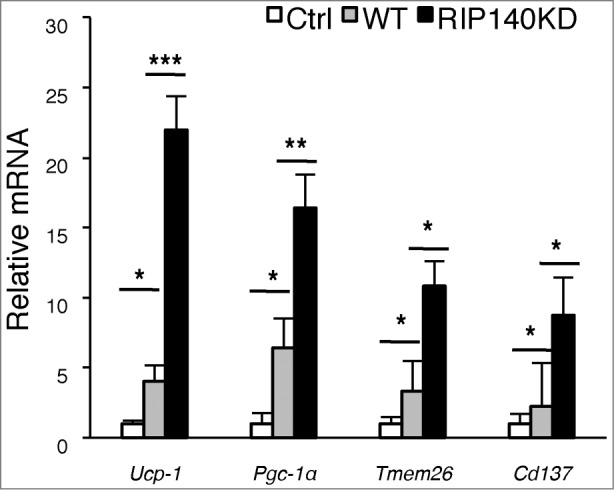
Conditioned medium from RIP140KD macrophage more effectively induces pre-adipocyte differentiation into beige cells. qPCR detection of mRNA levels of beige markers in cells differentiated from primary preadipocytes cultured with conditioned medium of ATMs isolated from WT or mφRIP140KD mice. Statistical significance was determined by Student's t-test, with data presented as mean ± SD, *P < 0.05; **P < 0.01; ***P < 0.001.
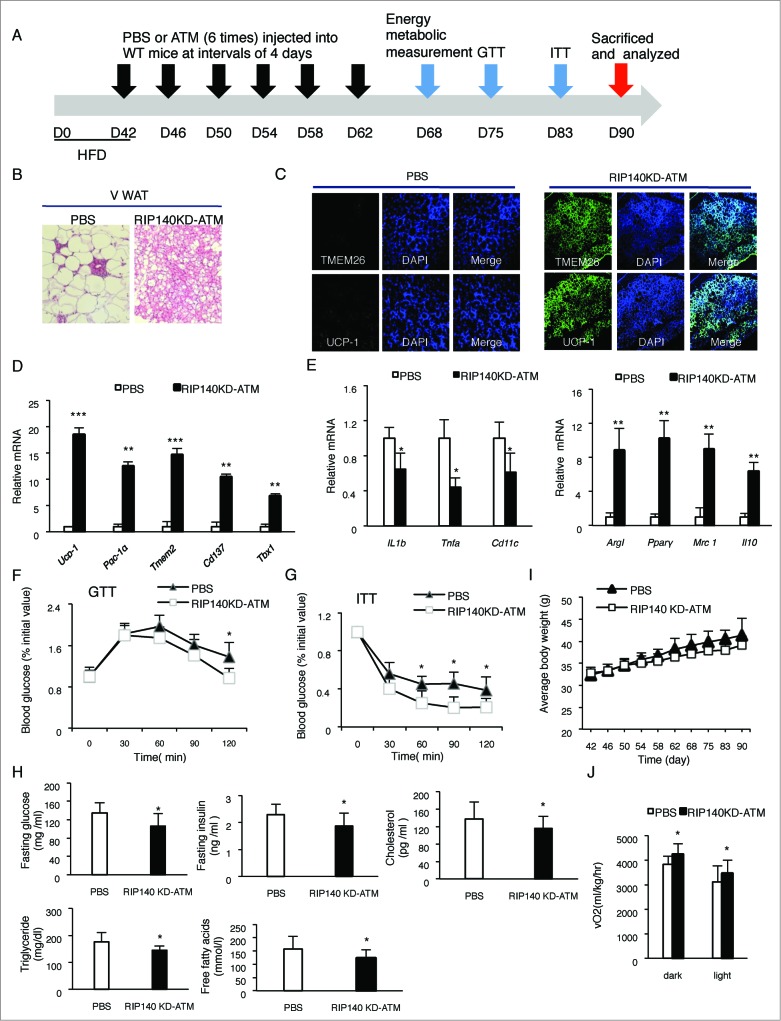
Our experimental cell therapy design is shown in Figure 2A. Wild type (WT) C57/BL6 male mice were fed with a HFD for 42 d to cause obesity. To these obese WT mice, PBS (phosphate-buffered saline, as a negative control) or RIP140KD-ATM isolated from the WAT depots of MφRIP140KD mice was injected into the peritoneum, every 4 d injections were conducted for a total of 6 times. Six days after the final injection of engineered macrophages, multiple tests were conducted to profile the recipient mice's metabolic phenotype. Figure 2B-J shows the collected data including: B) Histological examination; C) Immunological staining; D) Adipocyte gene markers; E) M1 vs. M2 ATM markers; F) GTT; G) ITT; H) Serum insulin, glucose, cholesterol, TG and FFA; I) Body weight; and J) Energy expenditure.
Figure 2 (See previous page).
Experimental design and results of ATM cell therapy. (A) Control PBS or experimental ATMs isolated from minced WAT of mφRIP140KD mice and labeled with live-dye PKH26 were intraperitoneally (i.p.) injected into HFD-fed WT mice 6 times, at 4 day intervals. After the 6 injections, these mice were assayed for functional GTT, ITT and energy metabolism measurements, then were sacrificed and samples were analyzed. (B) Histological staining of vWAT. (C) Sections of vWAT were analyzed by immunological staining of UCP-1 (green), TMEM26 (green), and co-stained with DAPI (blue). (D) qPCR results of mRNA levels in brown markers in vWAT. (E) qPCR determined mRNA levels of M1 and M2 markers in the SVF of vWAT. (F)-(G) Glucose tolerance test (GTT) and Insulin tolerance test (ITT). (H) Serum insulin, glucose, cholesterol, triglyceride and free fatty acid levels in PBS- or mφRIP140KD-ATM- (RIP140KD-ATM-) injected wild type (WT) mice. (I) Average body weight of WT mice injected with PBS or RIP140KD-ATM (J) Analyses of energy expenditure of PBS- or RIP140KD-ATM- injected mice, with vO2 consumption measured in both dark and light circadian phases. Statistical significance was determined by Student's t-test, with data presented as mean ± SD, *P < 0.05; **P < 0.01; ***P < 0.001.
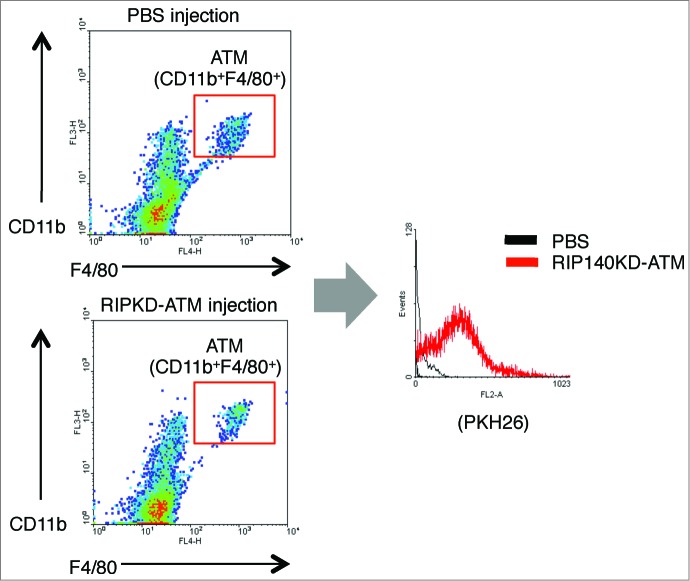
As shown in Figure 2B, recipient obese mice's vWAT histology clearly demonstrated indicators of browning, such as multilocular morphology, in response to RIP140KD-ATM cell injections (right). We have confirmed that PKH26-labeled ATM indeed incorporates into the recipient animals’ WAT as shown in Figure 3. Figure 2C confirms that browning markers (such as UCP1 and TMEM26) are present in the vWAT sections of RIP140KD-ATM injected mice (right panel). Consistently, mRNA levels of such browning markers (Ucp-1, Pgc-1α Tmem26 and Cd137 and Tbx1) are prominently elevated in the vWAT of RIP140KD-ATM injected mice (Fig. 2D). We have also observed that the injected macrophages do not seem to incorporate into other organs such as liver, spleen or kidney, at least based upon our results using PKH-26 detection, and that injecting engineered macrophages has little effect on interscapular BAT and subcutaneous WAT (Data not shown). Figure 2E shows that gene expression associated with inflammatory M1 ATM polarization (Il1β, Tnfα and Cd11c) indeed is decreased, whereas that associated with anti-inflammatory M2 ATM polarization (Arg1, Pparγ, Mrc1 and Il10) is increased in the stromal vascular fraction (SVF) of vWAT in RIP140KD-ATM injected mice. Significant improvement in glucose tolerance (Fig. 2F) and insulin sensitivity (Fig. 2G) was seen in RIP140KD-ATM injected mice. Other metabolic profiles, such as glucose level, insulin level, cholesterol level, triglyceride and free fatty acid level all show improvement in the RIP140KD-ATM injected mice as compared to PBS-injected mice (Fig. 2H). A time-dependent trend toward mean body weight reduction was also observed in the RIP140KD-ATM injected obese mice (Fig. 2I), but the trend did not achieve significance within the duration of our study. However, as shown in Figure 2J, RIP140KD-ATM injected mice indeed have a significantly higher level of energy expenditure (O2 consumption) in both the dark and light phases. Accordingly, we conclude that local injection of anti-inflammatory RIP140KD-ATM cells into adult mice even under a HFD feeding can trigger vWAT browning and improve metabolic parameters. As a result, HFD-induced vWAT inflammation is reduced, whole body energy expenditure is supercharged, and metabolic syndrome is alleviated.
Figure 3.
FACS analyses of PKH-26-labeled ATMs incorporation into WAT of injected mice. Left 2 panels show FACS analyses of ATMs collected from animals injected with either control (injection with PBS) or RIP140KD ATMs labeled with PKH-26 label, by gating macrophage specific markers CD11b and F4/80. Right panel shows PKH-26 label in the gated CD11b+F4/80+ ATMs.
Discussion
Inducing BAT activation and/or the differentiation of WAT into beige cells could provide new strategies for treating obesity, IR and diseases associated with impaired glucose/lipid homeostasis.6,8 Here, we report that local, visceral injections of engineered (from MφRIP140KD mice) anti-inflammatory ATMs into obese adult mice under chronic HFD feeding can trigger browning and heightened metabolism in their vWAT. This phenomena is consistent with our previous in vitro study that treatment of primary preadipocyte cell cultures with conditioned medium from macrophage cultures derived from MφRIP140KD mice could induce cultured preadipocytes to differentiate into beige cells.17 It is possible that RIP140KD ATMs release factors promoting preadipocyte differentiation into beige cells, mimicking “browning.” It is known that cold exposure and IL4 treatment trigger M2 ATM activation and cause WAT browning in animals, and that production of catecholamines by M2 ATMs may play a role,9,11 but how or whether catecholamines act on beige cell progenitors or mature white adipocytes remains to be determined. Our current report provides in vivo support for the medical potential of cell-therapy based induction of beige fat in preventing/treating diet-induced IR,17 although what factors may be secreted from the anti-inflammatory, engineered ATMs that trigger WAT browning awaits further study. Nevertheless, based upon our animal data, a protective and/or therapeutic strategy to reduce local tissue inflammation based upon “injection of engineered therapeutic macrophages” in adults seems feasible. While in this report we only demonstrated beneficial “browning” of inflammatory WAT as a proof-of-concept, it may also be possible to counteract inflammation in other tissues by delivering therapeutic macrophages to different target areas.
RIP140 is a master coregulator for a variety of transcription factors, including the inflammatory master regulator NF-κB. Our previous study found HFD to be an important trigger that elevates macrophages’ RIP140 level.16 Apparently, the level of RIP140 is crucial to the homeostasis of macrophage polarization and controls inflammation vs. anti-inflammation. While lowering the level of RIP140 might seem generally beneficial to animals’ wellbeing, it nevertheless could compromise their immune system by tipping the balance toward anti-inflammation, which could cause other diseases such as cancer progression. Therefore, transgenic approaches to systemically inhibit RIP140 in vivo would not seem to be a viable solution for inflammatory diseases. However, the non-systemic ex vivo cellular strategy described in this report – locally injecting engineered therapeutic macrophages into adult subjects – might provide a more feasible alternative.
In summary, our report describes a new strategy that exploits engineered ATMs to temporarily inhibit diet-induced local WAT inflammatory status and induce adult-stage vWAT browning, delivering a protective/therapeutic effect against diet-induced metabolic diseases. This strategy could also be adapted to alleviate other inflammatory diseases in various tissues/organs.
Materials and Methods
Animals
All studies were carried out using male C57Bl/6J mice as wild type (WT) mice, purchased from The Jackson Laboratory and maintained in the animal facility of University of Minnesota. The University of Minnesota Institutional Animal Care and Use Committee approved all animal studies. MφRIP140KD transgenic mice were generated as previously described.15 Eight-week-old male C56BL/6J mice were fed a HFD containing 60% calories from fat and 345 mg cholesterol/kg (F3282; Bio-Serv, West Chester, PA, USA).
Isolation and injection of RIP140KD-ATM
WAT from MφRIP140KD mice was minced and digested for 30 min at 37°C with type II collagenase (Sigma-Aldrich-C0130) in PBS + 2% BSA (pH 7.4). The cell suspension was filtered through a 100 mM nylon sieve and centrifuged at 500g for 15 min, separating the floating adipocyte fraction from the stromal vascular fraction (SVF) pellet. The SVF was collected, treated with an RBC lysis buffer (Sigma-R7757) and washed with PBS with 2% FBS. F4/80+Cd11b+ cells as ATM were collected by using FACSAria II (BSL2). Isolated RIP140KD-ATMs (3 × 106 cells) were labeled with PKH26 (Sigma-PKH26PCL) for 12 min and then intraperitoneally (i.p.) injected into HFD-fed WT mice.
ITT and GTT
Insulin tolerance test (ITT) or glucose tolerance test (GTT) was performed after overnight fasting. After baseline blood collection, D-glucose (2 g/kg; sigma-G8270) or insulin (0.75 units/kg; sigma-91077) were i.p. injected into animals. A standard glucometer was used to measure blood glucose levels at indicated time points.
Metabolic measurement
Indirect calorimetric assessment was performed on mice to measure volumetric oxygen consumption (vO2). Before measurement, animals were habituated to metabolic cages for 3 d Animals were maintained in a 12 hr light/dark cycle in individual chambers, with free access to chow and water. vO2 was recorded every 4 minutes for 2 d and normalized to body mass using Oxymax (Columbus Instrument, Columbus OH.)
RNA isolation and gene expression analyses
Total RNA was isolated using TRIzol (Invitrogen; 15596–026). Reverse transcription (RT) of RNA was performed with a High-Capacity cDNA Reverse Transcription Kit containing RNase Inhibitor (Applied Biosystems; N8080119). Quantitative real-time PCR (qPCR) was performed as described previously.15 Each gene expression experiment was performed in triplicate. The primer sequence information for qPCR is available upon request. Expression levels were normalized to β-actin level, with the wild-type HFD treatment set as value 1.
Immunohistochemistry
Tissues samples were fixed with 10% formalin, embedded in paraffin and sectioned. A morphometric study of adipocyte size was performed in vWAT sections with hematoxylin-eosin staining. Rabbit anti-TMEM26 (IMGENEX; IMG6633A) and rabbit anti-UCP-1 (Abcam; ab10983) were used for immunofluorescence.
Funding
This work was supported by NIH grants DK54733, DK60521, DK54733–11S, NIH 3 R01 DK60521–12S1, the Dean's Commitment and the Distinguished McKnight Professorship of University of Minnesota (LNW).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol 2011; 12:722-34; PMID:21952300; http://dx.doi.org/ 10.1038/nrm3198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tchkonia T, Thomou T, Zhu Y, Karagiannides I, Pothoulakis C, Jensen MD, Jensen MD, Kirkland JL. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab 2013; 17:644-56; PMID:23583168; http://dx.doi.org/ 10.1016/j.cmet.2013.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sanchez-Gurmaches J, Guertin DA. Adipocyte lineages: tracing back the origins of fat. Biochim Biophys Acta 2014; 1842:340-51; PMID:23747579; http://dx.doi.org/ 10.1016/j.bbadis.2013.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Attie AD, Scherer PE. Adipocyte metabolism and obesity. J Lipid Res 2009; 50 Suppl:S395-9; PMID:19017614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Enerback S. Human brown adipose tissue. Cell Metab 2010; 11:248-52; PMID:20374955; http://dx.doi.org/ 10.1016/j.cmet.2010.03.008 [DOI] [PubMed] [Google Scholar]
- 6. Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med 2013; 19:1252-63; PMID:24100998; http://dx.doi.org/ 10.1038/nm.3361 [DOI] [PubMed] [Google Scholar]
- 7. Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012; 150:366-76; PMID:22796012; http://dx.doi.org/ 10.1016/j.cell.2012.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pfeifer A, Hoffmann LS. Brown, beige, and white: the new color code of fat and its pharmacological implications. Annu Rev Pharmacol Toxicol 2014; PMID:25149919; http://dx.doi.org/ 10.1146/annurev-pharmtox-010814-124346 [DOI] [PubMed] [Google Scholar]
- 9. Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature 2011; 480:104-8; PMID:22101429; http://dx.doi.org/ 10.1038/nature10653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rao RR, Long JZ, White JP, Svensson KJ, Lou J, Lokurkar I, Jedrychowski MP, Ruas JL, Wrann CD, Lo JC,et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 2014; 157:1279-91; PMID:24906147; http://dx.doi.org/ 10.1016/j.cell.2014.03.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Qiu Y, Nguyen KD, Odegaard JI, Cui X, Tian X, Locksley RM, Palmiter RD, Chawla A. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell 2014; 157:1292-308; PMID:24906148; http://dx.doi.org/ 10.1016/j.cell.2014.03.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Keipert S, Jastroch M. Brite/beige fat and UCP1 - is it thermogenesis? Biochim Biophys Acta 2014; 1837:1075-82; PMID:24530356; http://dx.doi.org/ 10.1016/j.bbabio.2014.02.008 [DOI] [PubMed] [Google Scholar]
- 13. Mostaqul Huq MD, Gupta P, Wei LN. Post-translational modifications of nuclear co-repressor RIP140: a therapeutic target for metabolic diseases. Curr Med Chem 2008; 15:386-92; PMID:18288993; http://dx.doi.org/ 10.2174/092986708783497382 [DOI] [PubMed] [Google Scholar]
- 14. Ho PC, Wei LN. Negative regulation of adiponectin secretion by receptor interacting protein 140 (RIP140). Cell Signal 2012; 24:71-6; PMID:21872658; http://dx.doi.org/ 10.1016/j.cellsig.2011.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ho PC, Tsui YC, Feng X, Greaves DR, Wei LN. NF-kappaB-mediated degradation of the coactivator RIP140 regulates inflammatory responses and contributes to endotoxin tolerance. Nature immunology 2012; 13:379-86; PMID:22388040; http://dx.doi.org/ 10.1038/ni.2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ho PC, Chang KC, Chuang YS, Wei LN. Cholesterol regulation of receptor-interacting protein 140 via microRNA-33 in inflammatory cytokine production. FASEB J 2011; 25:1758-66; PMID:21285396; http://dx.doi.org/ 10.1096/fj.10-179267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu PS, Lin YW, Lee B, McCrady-Spitzer SK, Levine JA, Wei LN. Reducing RIP140 expression in macrophage alters ATM infiltration, facilitates white adipose tissue browning and prevents high fat diet-induced insulin resistance. Diabetes 2014; 63:4021-31; PMID:24969109; http://dx.doi.org/ 10.2337/db14-0619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Danon D, Kowatch MA, Roth GS. Promotion of wound repair in old mice by local injection of macrophages. Proc Natl Acad Sci U S A 1989; 86:2018-20; PMID:2928316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Danon D, Madjar J, Edinov E, Knyszynski A, Brill S, Diamantshtein L, Diamantshtein L,Shinar E. Treatment of human ulcers by application of macrophages prepared from a blood unit. Exp Gerontol 1997; 32:633-41; PMID:9785089; http://dx.doi.org/ 10.1016/S0531-5565(97)00094-6 [DOI] [PubMed] [Google Scholar]
- 20. Fidler IJ. Inhibition of pulmonary metastasis by intravenous injection of specifically activated macrophages. Cancer Res 1974; 34:1074-8; PMID:4842910 [PubMed] [Google Scholar]
- 21. Anderson P, Souza-Moreira L, Morell M, Caro M, O'Valle F, Gonzalez-Rey E, Delgado M. Adipose-derived mesenchymal stromal cells induce immunomodulatory macrophages which protect from experimental colitis and sepsis. Gut 2013; 62:1131-41; PMID:22637701; http://dx.doi.org/ 10.1136/gutjnl-2012-302152 [DOI] [PubMed] [Google Scholar]