Abstract
Amyloid-β (Aβ) peptide, which is generated from proteolytic cleavage of amyloid precursor protein (APP), is a key molecule involved in the pathology of Alzheimer disease. Both APP and Aβ peptides are expressed in adipose tissue, however it is currently unclear whether Aβ can affect the key functions of adipose tissue. We aimed to explore whether Aβ affected lipolysis and adipokine secretion in cultured human adipose tissue. We found that Aβ25–35, which contains the main functional domain of the Aβ, stimulated lipolysis via PKA and ERK1/2-dependent pathways and that Aβ25–35 induced leptin and IL-6 secretion. It is concluded that Aβ peptide exerts functional effects on adipose tissue that may lead to increased release of free fatty acids and pro-inflammatory adipokines.
Keywords: adipokine, adipose tissue, amyloid-β peptide, inflammation, lipolysis
Introduction
Amyloid-β (Aβ) peptide plays a critical role in Alzheimer disease (AD) pathology.1 Aβ peptides are generated from amyloid precursor protein (APP) after sequential cleavage by β and γ secretases.2 Various Aβ isoforms can be generated from cleavage in the Aβ N-terminus such as Aβ3–40/42, Aβ11–40/42 and Aβ17–40/42. Alternative peptides can be derived from cleavage at the C-terminus including Aβ1–39, Aβ1–38, Aβ1–33 and Aβ1–16. Aβ25–35 is derived from cleavage at both termini, and is considered the core functional domain of Aβ peptides.3 Although much of the study of APP and Aβ has focused on changes in the central nervous system during AD pathology, as early as in 1989 Joachim et al.4 reported that APP protein and mRNA are expressed in other tissues besides brain including adipose tissue, heart, muscle, kidney, liver, spleen, skin and intestine.4 The widespread expression of APP suggests that Aβ and associated peptides could have diversified roles in both normal and disease physiology in organs other than the brain.
With respect to adipose tissue, evidence from both rodent and human studies indicate that APP expression in adipose tissue is up-regulated under obese conditions and is positively regulated by direct pro-inflammatory stimulation of adipocytes.5-6 For example, APP mRNA expression is increased in subcutaneous adipose tissue of obese human subjects.5 Elevated human adipocyte APP gene expression in vivo is not only positively associated with insulin resistance, hyperinsulinemia, and an increase in the expression profile of the pro-inflammatory genes5 but also correlates with increased plasma Aβ levels.6 Furthermore, full-length APP protein and several Aβ cleavage peptides have also been reported in adipose tissue of obese subjects.5 At the cellular level, treatment of 3T3-L1 adipocytes with tumor necrosis factor (TNF)-α resulted in a significant increases in APP protein levels in a dose-dependent manner.7 This is in consistent with the findings from a study in diet-induced obese mice where TNF-α and APP expression were increased in adipose tissue.8 Studies exploring how Aβ may influence adipose tissue function are limited; however, treatment of isolated murine subcutaneous fat with an APP agonist antibody in vitro had no effect on lipid storage or TNF-α secretion.8 Collectively, it is of interest to further explore how Aβ impacts adipose tissue function.
Lipolysis is a unique function of adipose tissue under energy demanding conditions such as exercise and fasting.9-10 Excessive lipolysis also contributes to increased release of free fatty acids, and subsequent ectopic lipid storage, in conditions of insulin resistance. Two key signaling pathways involved in the lipolytic pathways are the protein kinase A (PKA) and extracellular-signal-regulated kinase 1/2 (ERK1/2) signaling pathways.11-13 The activation of PKA and ERK1/2 results in the phosphorylation of hormone sensitive lipase (HSL) at multiple sites,13-14 leading to the breakdown of stored triglycerides and release of glycerol and free fatty acids from adipose tissue.
In addition to releasing glycerol and fatty acids, adipose tissue is also an active endocrine organ secreting a variety of substances known as adipokines. Accumulating evidence suggests that altered adipokine secretion is involved in promoting chronic low-grade inflammation and insulin resistance in obesity. Leptin was the first adipokine described in 1994 and traditionally acts as a hormone regulating fat storage.15 Human obesity is characterized by high circulating leptin levels16 and higher levels of leptin are associated with a pro-inflammatory response.17 Adiponectin is one of the most abundant adipokines secreted from adipose tissue18 and is reported to have insulin-sensitizing,19 anti-inflammatory and cardioprotective effects.20 IL-6 is classically recognized as a pro-inflammatory cytokine that can induce insulin resistance in adipocytes by reducing adiponectin expression and secretion21 or decreasing insulin-stimulated glucose uptake and lipogenesis.22-23 Collectively, increased secretion of leptin and IL-6 and reduced secretion of adiponectin from adipose tissue in obesity have been linked with systemic inflammation, insulin resistance, and appetite dysregulation.24-25
The aim of this study was to: 1) Determine the effects of Aβ on lipolysis, and associated signaling pathways, in human adipose tissue; and 2) Explore whether Aβ can affect secretion of the key adipokines adiponectin, leptin and IL-6 from human adipose tissue. We hypothesized that 1) Aβ would induce lipolysis in cultured human adipose tissue via PKA- and ERK1/2- dependent signaling pathways and that 2) Aβ would reduce adiponectin secretion while increasing leptin and IL-6 secretion.
Results
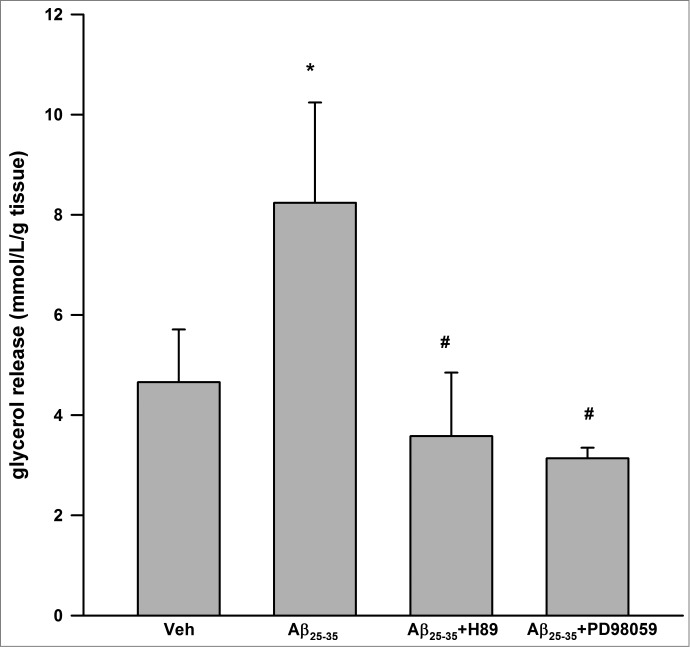
As shown in Figure 1, Aβ25–35 resulted in significant induction of glycerol release at 24 h (4.66 ± 1.05 vs. 8.24 ± 2.00 mmol/L/g tissue, P < 0.05 vs. vehicle control). Aβ25–35 has no effect on glycerol release at 2, 6, 12 h (data not shown). H89, a well-recognized inhibitor of PKA26 partially inhibited Aβ25–35 induced glycerol release (P < 0.05 vs. Aβ25–35 alone). Similarly, PD98059, an inhibitor of ERK1/227 also partially inhibited Aβ25–35 induced glycerol release (P < 0.05 vs. Aβ25–35 alone).
Figure 1.
Aβ25–35 stimulated lipolysis in cultured human visceral adipose tissue via PKA and ERK1/2-dependent signaling pathways. Aβ25–35 (40 μM, 24 h) induced glycerol release. H89 (1 μM) and PD98059 (25 μM) partially inhibited Aβ25–35 induced glycerol release. Data are presented as means + SEM (N = 10). *P < 0.05 versus vehicle control. #P < 0.05 vs. Aβ25–35.
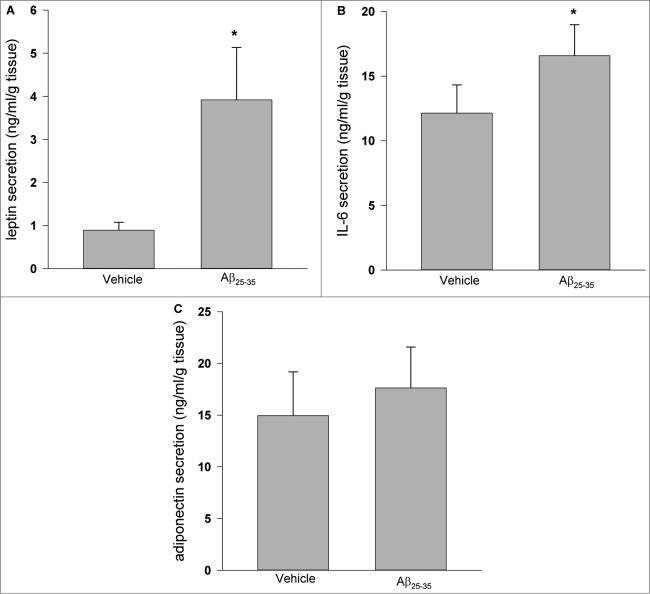
As shown in Figure 2, treatment of cultured human adipose tissue with Aβ25–35 for 24 h resulted in significant induction of leptin and IL-6 secretion (P < 0.05 vs. vehicle control), while there was no detectable effect on adiponectin secretion.
Figure 2.
Effects of Aβ25–35 on leptin, IL-6 and adiponectin secretion. Aβ25–35 (40 μM, 24 h) induced leptin (A) and IL-6 (B) secretion, while there were no observed effects on adiponectin secretion (C). Data are presented as means + SEM (N = 10 ). *P < 0.05 versus vehicle control.
Discussion
While traditionally viewed as a molecule involved in AD pathology, an accumulating body of evidence suggests that Aβ, and its precursor protein APP, could also be involved in physiological and pathological processes in adipose tissue.5-6 However, currently there is no research examining whether Aβ can affect the key functions of human adipose tissue.
In this study, we aimed to explore how Aβ affects lipolysis and secretion of major adipokines from human adipose tissue. Aβ25–35 is the main functional domain of the Aβ molecule that is required for neurotoxic effects.28 We found that Aβ25–35 can induce lipolysis in cultured adipose tissue in a manner that appeared partially dependent on PKA and ERK1/2-dependent signaling pathways. Lipolysis for the provisions of fatty acids as a fuel for other tissues is a unique function of adipose.10 However, chronically elevated circulating levels of fatty acids, commonly observed in obesity, are associated with negative metabolic consequences such as ectoptic lipid deposition and insulin resistance.29 Our results may suggest that over-accumulation of Aβ in adipose tissues might promote lipolysis, which could increase availability of fatty acids and glycerol and contribute to lipotoxicity in other organs. However, we were unable to measure mRNA expression of key lipolytic molecules so we cannot ascertain if changes in gene expression are required for the functional responses seen in human adipose after 24 h treatment with Aβ. Future research is also required to determine whether and how Aβ may directly activate ERK1/2 or PKA signaling pathways in adipose.
We also found that Aβ25–35 can induce leptin and IL-6 secretion in cultured adipose tissue while there was no detectable influence on adiponectin secretion. Serum leptin concentration and adipose leptin mRNA level are positively associated with BMI.30 Similarly, chronic low-grade elevations of IL-6 in adipose tissue are posited to directly contribute to insulin resistance.31-32 Given the previous associations between adipose tissue APP and Aβ in rodent models of obesity, the induction of leptin and IL-6 secretion by Aβ25–35 may indicate that Aβ might contribute to adipose tissue inflammation and promote insulin resistance. It should be noted that in the present study we assessed the impact of aggregated Aβ on cultured human adipose tissue. Although overproduction of Aβ leads to aggregation formation in vivo, future research is needed to determine whether Aβ peptides or other forms of this diverse molecule also affect adipose tissue function.
In conclusion, we revealed that Aβ25–35 stimulated lipolysis and induced leptin and IL-6 secretion in cultured human adipose tissue. The effects of Aβ25–35 on lipolysis appeared to be partially dependent on PKA and ERK1/2-dependent signaling pathways but future research is warranted to fully characterize how Aβ influences adipose tissue function. Our current findings implicate Aβ in the regulation of lipolysis and adipokine secretion in human adipose tissue and suggest that the previously observed increase in adipose tissue Aβ in obesity might have functional effects in this tissue.
Materials and Methods
Aβ25–35 (cat#A-1060–1) was purchased from R-Peptide (GA, USA). Human enzyme-linked immunosorbent assay (ELISA) Duoset kits for adiponectin (cat#DY1065), leptin (cat#DY398) and IL-6 (cat#DY206) were from R&D systems (NE, USA). Specific chemical inhibitors PD98059 (cat# 10006726) and H89 (cat#10010556) were obtained from Cayman Chemicals (KS, USA). Medium 199 was from Life Technologies (NY, USA). Glycerol assay kit (cat# F6428) was from Sigma (MO, USA). Fatty acid-free bovine serum albumin (FA-free BSA) (cat#152401) was from MP Biomedical (OH, USA). All other chemicals were purchased from Sigma (MO, USA).
Aβ25–35 was dissolved in endotoxin-free deionized distilled water at a concentration of 2 mM and incubated at 37°C for 7 d to induce aggregation as described by Xian et al.33 After aggregation, the solution was stored at −20°C until experimentation.
Preperitoneal adipose tissue samples were obtained from 10 non-obese male subjects; age = 56 ± 4 yr, body mass index (BMI) = 25.2 ± 1.0 kg/m2] undergoing abdominal surgeries. The samples of adipose tissue were put in sterile PBS and immediately transported to the laboratory for experiments. Patients were free of acute or chronic disease and were not currently taking any medications known to affect lipid metabolism or inflammation, with the exception of one participant who had previous history of colon cancer. The present study was conducted according to the guideline laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by University of British Columbia Clinical Research Ethics Board (H12–02330). Written informed consent was obtained from all subjects. Adipose tissue was sectioned into ∼100 mg pieces and weighed on an analytical balance (Mettler Toledo MS204S) before being minced into ∼5–10 mg pieces and placed into culture dishes containing 3 ml of M199 supplemented with 1% antibiotic/antimycotic, 50 μU insulin and 2.5 nM dexamethasone, as described previously.9 The cultures were placed in a humidified 5% CO2 incubator at 37°C to equilibrate for 24 h. On the morning of the experiment, media was replaced with fresh M199 supplemented with 2.5% fatty Acid-free BSA and treated with Aβ25–35 (40 μM), Aβ25-35 +H89 (1 μM, an inhibitor of PKA), Aβ25-35 +PD98059 (25 μM, an inhibitor of ERK1/2). Media were collected at 24 h for further analysis of glycerol, adiponectin, IL-6 and leptin levels. We chose these concentrations of Aβ25–35 and signal inhibitors based on published literature33-34 and pilot experiments from our laboratory.
Culture media was analyzed for glycerol concentrations using colorimetric assays according to the manufacturer's instructions. Glycerol concentrations were corrected for tissue weight and reported as mmol/L released per g tissue. The coefficient of variation for these assays in our laboratory based on duplicate measurements is <10%.
The concentrations of adiponectin, IL-6 and leptin were measured by ELISA according to the manufacturer's instruction. Adiponectin, IL-6 and leptin concentrations were corrected for tissue weight and reported as ng/mL per g tissue. The coefficient of variation for these assays in our laboratory based on duplicates is <10%.
All values are expressed as the means ± standard error of the mean (SEM). Effects of Aβ25–35 and inhibitors on Aβ25–35 induced glycerol release versus vehicle control were compared by one-way ANOVA with LSD post-hoc comparisons. Effects of Aβ25–35 vs. vehicle control on adiponectin, IL-6 and leptin secretion were compared by Student's t-test. All statistics were performed using GraphPad Prism v6.0. Statistical significance was set at P < 0.05.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This study is supported by a Natural Sciences and Engineering Research Council (NSERC) Discovery Grant to JPL. ZW is supported by an Alzheimer Society Research Program (ASRP) postdoctoral fellowship from the Alzheimer Society of Canada.
References
- 1. Landau SM, Lu M, Joshi AD, Pontecorvo M, Mintun MA, Trojanowski JQ, Shaw LM, Jagust WJ. Comparing positron emission tomography imaging and cerebrospinal fluid measurements of beta-amyloid. Ann Neurol 2013; 74:826-36; PMID:23536396; http://dx.doi.org/ 10.1002/ana.23908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. De Strooper B, Vassar R, Golde T. The secretases: enzymes with therapeutic potential in Alzheimer disease. Nat Rev Neurol 2010; 6:99-107; PMID:20139999; http://dx.doi.org/ 10.1038/nrneurol.2009.218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Millucci L, Ghezzi L, Bernardini G, Santucci A. Conformations and biological activities of amyloid beta peptide 25-35. Curr Protein Pept Sci 2010; 11:54-67; PMID:20201807 [DOI] [PubMed] [Google Scholar]
- 4. Joachim CL, Mori H, Selkoe DJ. Amyloid beta-protein deposition in tissues other than brain in Alzheimer's disease. Nature 1989; 341:226-30; PMID:2528696; http://dx.doi.org/ 10.1038/341226a0 [DOI] [PubMed] [Google Scholar]
- 5. Lee YH, Tharp WG, Maple RL, Nair S, Permana PA, Pratley RE. Amyloid precursor protein expression is upregulated in adipocytes in obesity. Obesity (Silver Spring) 2008; 16:1493-500; PMID:18483477; http://dx.doi.org/ 10.1038/oby.2008.267 [DOI] [PubMed] [Google Scholar]
- 6. Lee YH, Martin JM, Maple RL, Tharp WG, Pratley RE. Plasma amyloid-beta peptide levels correlate with adipocyte amyloid precursor protein gene expression in obese individuals. Neuroendocrinology 2009; 90:383-90; PMID:19672057; http://dx.doi.org/ 10.1159/000235555 [DOI] [PubMed] [Google Scholar]
- 7. Sommer G, Kralisch S, Lipfert J, Weise S, Krause K, Jessnitzer B, Lossner U, Bluher M, Stumvoll M, Fasshauer M. Amyloid precursor protein expression is induced by tumor necrosis factor alpha in 3T3-L1 adipocytes. J Cell Biochem 2009; 108:1418-22; PMID:19862700 [DOI] [PubMed] [Google Scholar]
- 8. Puig KL, Floden AM, Adhikari R, Golovko MY, Combs CK. Amyloid precursor protein and proinflammatory changes are regulated in brain and adipose tissue in a murine model of high fat diet-induced obesity. PLoS One 2012; 7:e30378; PMID:22276186; http://dx.doi.org/ 10.1371/journal.pone.0030378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wan Z, Thrush AB, Legare M, Frier BC, Sutherland LN, Williams DB, Wright DC. Epinephrine-mediated regulation of PDK4 mRNA in rat adipose tissue. Am J Physiol Cell Physiol 2010; 299:C1162-70; PMID:20739620; http://dx.doi.org/ 10.1152/ajpcell.00188.2010 [DOI] [PubMed] [Google Scholar]
- 10. Ahmadian M, Duncan RE, Jaworski K, Sarkadi-Nagy E, Sul HS. Triacylglycerol metabolism in adipose tissue. Future Lipidol 2007; 2:229-237; PMID:19194515; http://dx.doi.org/ 10.2217/17460875.2.2.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tansey JT, Huml AM, Vogt R, Davis KE, Jones JM, Fraser KA, Brasaemle DL, Kimmel AR, Londos C. Functional studies on native and mutated forms of perilipins. A role in protein kinase A-mediated lipolysis of triacylglycerols. J Biol Chem 2003; 278:8401-6; PMID:12477720; http://dx.doi.org/ 10.1074/jbc.M211005200 [DOI] [PubMed] [Google Scholar]
- 12. Egan JJ, Greenberg AS, Chang MK, Wek SA, Moos MC, Jr, Londos C. Mechanism of hormone-stimulated lipolysis in adipocytes: translocation of hormone-sensitive lipase to the lipid storage droplet. Proc Natl Acad Sci U S A 1992; 89:8537-41; PMID:1528859; http://dx.doi.org/ 10.1073/pnas.89.18.8537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Greenberg AS, Shen WJ, Muliro K, Patel S, Souza SC, Roth RA, Kraemer FB. Stimulation of lipolysis and hormone-sensitive lipase via the extracellular signal-regulated kinase pathway. J Biol Chem 2001; 276:45456-61; PMID:11581251; http://dx.doi.org/ 10.1074/jbc.M104436200 [DOI] [PubMed] [Google Scholar]
- 14. Watt MJ, Holmes AG, Pinnamaneni SK, Garnham AP, Steinberg GR, Kemp BE, Febbraio MA. Regulation of HSL serine phosphorylation in skeletal muscle and adipose tissue. Am J Physiol Endocrinol Metab 2006; 290:E500-8; PMID:16188906; http://dx.doi.org/ 10.1152/ajpendo.00361.2005 [DOI] [PubMed] [Google Scholar]
- 15. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 1994; 372:425-32; PMID:7984236; http://dx.doi.org/ 10.1038/372425a0 [DOI] [PubMed] [Google Scholar]
- 16. Fried SK, Ricci MR, Russell CD, Laferrere B. Regulation of leptin production in humans. J Nutr 2000; 130:3127S-3131S; PMID:11110887 [DOI] [PubMed] [Google Scholar]
- 17. Lee SM, Choi HJ, Oh CH, Oh JW, Han JS. Leptin increases TNF-alpha expression and production through phospholipase D1 in raw 264.7 cells. PLoS One 2014; 9:e102373; PMID:25047119; http://dx.doi.org/ 10.1038/10.1371/journal.pone.0102373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem 1995; 270:26746-9; PMID:7592907; http://dx.doi.org/ 10.1074/jbc.270.45.26746 [DOI] [PubMed] [Google Scholar]
- 19. Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 2001; 7:941-6; PMID:11479627; http://dx.doi.org/ 10.1038/90984 [DOI] [PubMed] [Google Scholar]
- 20. Ohashi K, Parker JL, Ouchi N, Higuchi A, Vita JA, Gokce N, Pedersen AA, Kalthoff C, Tullin S, Sams A, et al. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J Biol Chem 2010; 285:6153-60; PMID:20028977; http://dx.doi.org/ 10.1074/jbc.M109.088708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fasshauer M, Kralisch S, Klier M, Lossner U, Bluher M, Klein J, Paschke R. Adiponectin gene expression and secretion is inhibited by interleukin-6 in 3T3-L1 adipocytes. Biochem Biophys Res Commun 2003; 301:1045-50; PMID:12589818 [DOI] [PubMed] [Google Scholar]
- 22. Rotter V, Nagaev I, Smith U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J Biol Chem 2003; 278:45777-84; PMID:12952969 [DOI] [PubMed] [Google Scholar]
- 23. Lagathu C, Bastard JP, Auclair M, Maachi M, Capeau J, Caron M. Chronic interleukin-6 (IL-6) treatment increased IL-6 secretion and induced insulin resistance in adipocyte: prevention by rosiglitazone. Biochem Biophys Res Commun 2003; 311:372-9; PMID:14592424; http://dx.doi.org/ 10.1016/j.bbrc.2003.10.013 [DOI] [PubMed] [Google Scholar]
- 24. Montecucco F, Mach F, Pende A. Inflammation is a key pathophysiological feature of metabolic syndrome. Mediators Inflamm 2013; 2013:135984; PMID:23710114; http://dx.doi.org/ 10.1155/2013/135984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harwood HJ, Jr. The adipocyte as an endocrine organ in the regulation of metabolic homeostasis. Neuropharmacology 2012; 63:57-75; PMID:22200617; http://dx.doi.org/ 10.1016/j.neuropharm.2011.12.010 [DOI] [PubMed] [Google Scholar]
- 26. Engh RA, Girod A, Kinzel V, Huber R, Bossemeyer D. Crystal structures of catalytic subunit of cAMP-dependent protein kinase in complex with isoquinolinesulfonyl protein kinase inhibitors H7, H8, and H89. Structural implications for selectivity. J Biol Chem 1996; 271:26157-64; PMID:8824261; http://dx.doi.org/ 10.1074/jbc.271.42.26157 [DOI] [PubMed] [Google Scholar]
- 27. Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem 1995; 270:27489-94; PMID:7499206; http://dx.doi.org/ 10.1074/jbc.270.46.27489 [DOI] [PubMed] [Google Scholar]
- 28. Ashenafi S, Fuente A, Criado JM, Riolobos AS, Heredia M, Yajeya J. Beta-Amyloid peptide25-35 depresses excitatory synaptic transmission in the rat basolateral amygdala "in vitro". Neurobiol Aging 2005; 26:419-28; PMID:15653170 [DOI] [PubMed] [Google Scholar]
- 29. Kok BP, Brindley DN. Myocardial fatty acid metabolism and lipotoxicity in the setting of insulin resistance. Heart Fail Clin 2012; 8:643-61; PMID:22999246; http://dx.doi.org/ 10.1016/j.hfc.2012.06.008 [DOI] [PubMed] [Google Scholar]
- 30. Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 1996; 334:292-5; PMID:8532024; http://dx.doi.org/ 10.1056/NEJM199602013340503 [DOI] [PubMed] [Google Scholar]
- 31. Bastard JP, Maachi M, Van Nhieu JT, Jardel C, Bruckert E, Grimaldi A, Robert JJ, Capeau J, Hainque B. Adipose tissue IL-6 content correlates with resistance to insulin activation of glucose uptake both in vivo and in vitro. J Clin Endocrinol Metab 2002; 87:2084-9; PMID:11994345; http://dx.doi.org/ 10.1210/jcem.87.5.8450 [DOI] [PubMed] [Google Scholar]
- 32. Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab 2001; 280:E745-51; PMID:11287357 [DOI] [PubMed] [Google Scholar]
- 33. Xian YF, Lin ZX, Mao QQ, Ip SP, Su ZR, Lai XP. Protective effect of isorhynchophylline against beta-amyloid-induced neurotoxicity in PC12 cells. Cell Mol Neurobiol 2012; 32:353-60; PMID:22042506; http://dx.doi.org/ 10.1007/s10571-011-9763-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ronsisvalle N, Di Benedetto G, Parenti C, Amoroso S, Bernardini R, Cantarella G. CHF5074 protects SH-SY5Y Human neuronal-like cells from amyloidbeta 25-35 and tumor necrosis factor related apoptosis inducing ligand toxicity in vitro. Curr Alzheimer Res 2014; 11:714-24; PMID:24938499 [DOI] [PubMed] [Google Scholar]