Abstract
Background/Aims
Infections are major causes of both early and late death after lung transplantation (LT). The development of prophylaxis strategies has altered the epidemiology of post-LT infections; however, recent epidemiological data are limited. We evaluated infections after LT at our institution by time of occurrence, site of infections, and microbiologic etiologies.
Methods
All consecutive patients undergoing lung or heart-lung transplantation between October 2008 and August 2014 at our institution were enrolled. Cases of infections after LT were initially identified from the prospective registry database, which was followed by a detailed review of the patients' medical records.
Results
A total of 108 episodes of post-LT infections (56 bacterial, 43 viral, and nine fungal infections) were observed in 34 LT recipients. Within 1 month after LT, the most common bacterial infections were catheter-related bloodstream infections (42%). Pneumonia was the most common site of bacterial infection in the 2- to 6-month period (28%) and after 6 months (47%). Cytomegalovirus was the most common viral infection within 1 month (75%) and in the 2- to 6-month period (80%). Respiratory viruses were the most common viruses after 6 months (48%). Catheter-related candidemia was the most common fungal infection. Invasive pulmonary aspergillosis developed after 6 months. Survival rates at the first and third years were 79% and 73%, respectively.
Conclusions
Although this study was performed in a single center, we provide valuable and recent detailed epidemiology data for post-LT infections. A further multicenter study is required to properly evaluate the epidemiology of post-LT infections in Korea.
Keywords: Lung transplantation, Infection, Epidemiology
INTRODUCTION
Lung transplantation (LT) is an accepted treatment option in patients with end-stage lung disease. After the first LT procedure in 1983, 47,647 adult lung and 3,772 adult heart-lung transplantations had been performed world-wide up to June 2013 [1]. LT recipients have a median survival of 5.7 years, with survival rates of 80% at 1 year, 65% at 3 years, and 53% at 5 years [1]. According to the official report of the Korean Network for Organ Sharing, 230 LTs were performed from the first LT of 1996 through November 2014 in Korea [2]. Survival rates of Korean LT recipients at the first, third, and fifth years are 48%, 39%, and 35%, respectively.
The major causes of post-LT mortality are graft failure and infectious complications [1]. Infectious complications are major causes of both early and late death after LT [3,4]. It is important to know the epidemiology of post-LT infections to prevent and treat infections. Although infections after LT were described in reports from the 1990s [5,6], recent epidemiological data are limited [7]. The development of prophylaxis strategies has altered the epidemiology of post-LT infections and mortality. Therefore, we evaluated infections after LT at our institution by time of occurrence, site of infections, and microbiologic etiologies.
METHODS
Patient population
The study population consisted of all patients who received lung or heart-lung transplantation between October 2008 and August 2014 at our institution. We have prospectively maintained a LT registry containing clinical information on recipients/donors and continue to update it with new clinical information. Cases of infections after LT were initially identified from the LT registry, which was followed by a detailed review of the patients' medical records. The immunosuppressive regimen consisted of induction therapy with basiliximab and triple therapy with tacrolimus, mycophenolate mofetil, and prednisone. Tacrolimus was given to a target trough level of 10 to 15 ng/mL for the first 6 months and 8 to 12 ng/mL thereafter. The study was approved by the Institutional Review Board of the hospital.
Pretransplant screening
Prior to the LT procedure, recipient and donor sputum samples were cultured for bacteria, mycobacteria, and fungi. When Burkholderia cepacia complex was detected, we performed DNA sequencing to confirm the presence of B. cenocepacia. We also performed serologic tests in recipients and donors for herpesviruses (herpes simplex virus, varicella zoster virus, Epstein-Barr virus, and cytomegalovirus), hepatitis viruses (hepatitis A, B, and C viruses), and human immunodeficiency virus. Latent tuberculosis was screened by the tuberculin skin test and QuantiFERON-TB Gold test (Cellestis, Carnegie, Australia) in recipients.
LT candidates without hepatitis A or B antibodies were vaccinated prior to transplantation. Before LT, a 13-valent protein-conjugated pneumococcal vaccine was used for priming, followed 8 weeks later by a 23-valent polysaccharide vaccine. All LT candidates and recipients were recommended to receive annual influenza vaccinations.
Prophylaxis strategies
Antimicrobial prophylaxis was guided by donor and recipient sputum cultures and cefepime was intravenously administered for patients who were sputum culture negative. Regardless of the cytomegalovirus serostatus of recipients and donors, intravenous ganciclovir was given for antiviral prophylaxis at a dosage of 5 mg/kg every 24 hours from 1 to 4 weeks after the LT. Oral valganciclovir was administered at a dosage of 900 mg once daily up to 6 months thereafter. Voriconazole was intravenously administered at a dosage of 4 mg/kg every 12 hours for antifungal prophylaxis and the target trough level was 1.5 to 5.5 mg/dL. If the recipient's creatinine clearance level was less than 50 mL/min, intravenous caspofungin was temporarily administered as a single 70 mg dose followed every 24 hours by a 50 mg dose. We changed to oral voriconazole when the recipient was able to resume a normal diet. If intolerable adverse effects of voriconazole were seen, voriconazole was changed to itraconazole. The total duration of antifungal prophylaxis was 6 months. Serum cytomegalovirus antigenemia (Chemicon, Temecula, CA, USA) and galactomannan (Platelia Aspergillus, Bio-Rad, Hercules, CA, USA) assays were performed regularly at 1, 2, 3, 4, 6, 8, 10, 12, 14, 16, 20, and 24 weeks after LT. Oral trimethoprim/sulfamethoxazole (160/800 mg) was administered every other day for lifetime Pneumocystis jirovecii pneumonia prevention.
Clinical definitions
The definitions of bacterial, viral, and fungal infections are based on the recommendations of the American Society of Transplantation [8]. Bloodstream infections were defined as primary when the focus could not be defined. Catheter-related bloodstream infections were defined as follows: positive simultaneous blood cultures from the central venous catheter and peripheral vein yielding the same organism and (1) the presence of significant catheter-tip colonization with 15 colony-forming units or more of the same organism isolated from the blood culture or (2) when the blood culture drawn through the central venous catheter became positive at least 120 minutes earlier than a positive culture drawn simultaneously from a peripheral vein [9].
Rejection was diagnosed via biopsy and classified by the International Society for Heart and Lung Transplantation guidelines [10]. Simple spirometry tests were performed at every outpatient visit (every 1 to 3 months) for diagnosis and functional grading of chronic airway rejection (bronchiolitis obliterans syndrome).
Mortality and survival rate
Early mortality was defined as death within 1 month after LT. Late mortality was defined as death after 1 month. Survival rates using the Kaplan-Meier method were evaluated at 1 and 3 years after LT.
RESULTS
A total of 34 consecutive patients were enrolled in this study (24 bilateral lung and 10 heart-lung transplant recipients from deceased donors). The median length of the first hospital stay after LT was 55 days (interquartile range [IQR], 31 to 195). The median follow-up period was 16 months (IQR, 7 to 36) after LT. Table 1 shows the demographic and clinical characteristics of these patients. The median age of the patients was 46 years (IQR, 31 to 56), and the study series included three pediatric patients (5, 11, and 16 years old). The most common reason for LT was interstitial lung disease (44%). All recipients were seropositive for cytomegalovirus prior to the LT. Antiviral prophylaxis was prematurely terminated in 13 patients (38%; median duration of prophylaxis, 17 weeks; range, 4 to 23), antifungal prophylaxis in four patients (12%; median duration, 10 weeks; range, 6 to 21), and antipneumocystis prophylaxis in three patients (9%; median duration, 10 weeks; range, 3 to 36). Rejection was diagnosed in three recipients (9%) during the study period. A total of 108 episodes of post-LT infections (56 bacterial, 43 viral, and nine fungal infections) were observed in the 34 patients during the study period.
Table 1. Demographic and clinical characteristics of the 34 lung transplant recipients analyzed in this study.
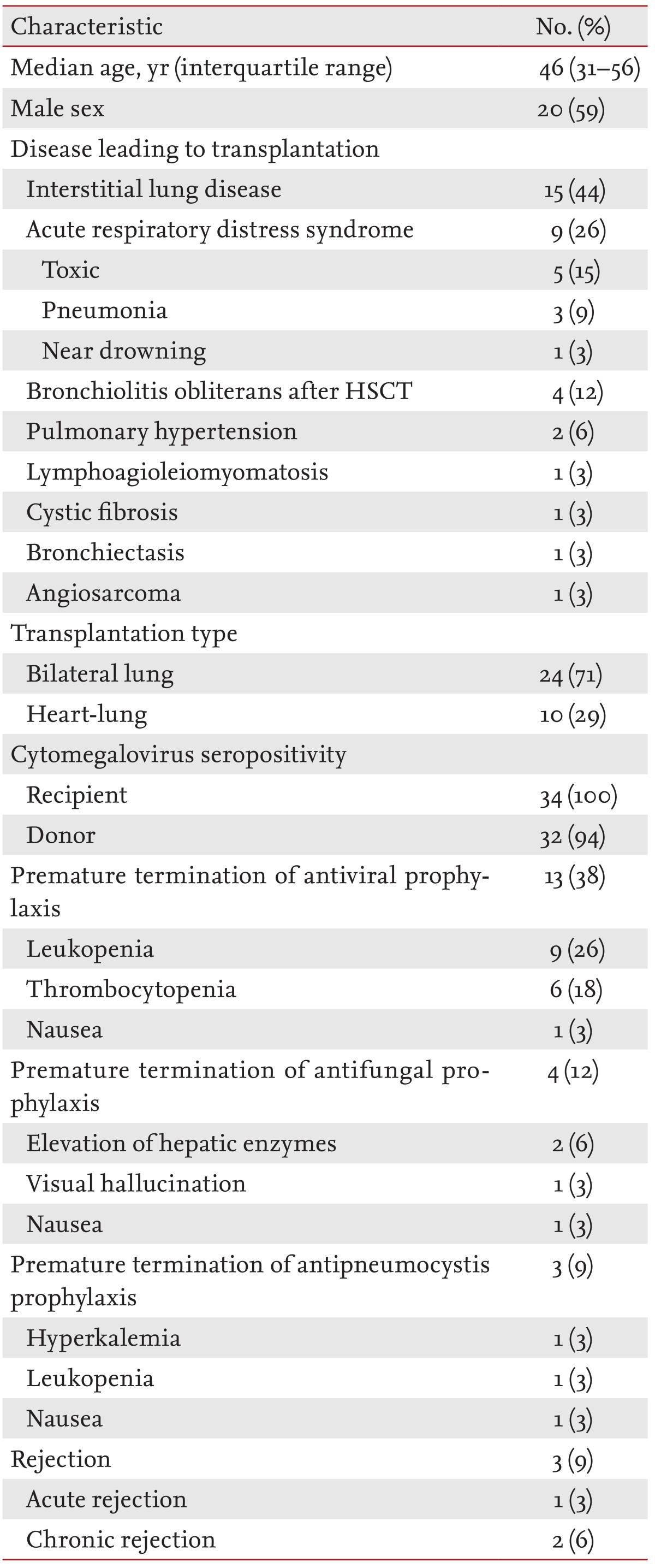
HSCT, hematopoietic stem cell transplantation.
Bacterial infections
Among 56 episodes of bacterial infections, 12 (21%) occurred within 1 month of the LT, 25 (45%) in the 2- to 6-month period, and 19 (34%) after 6 months (Table 2). Within 1 month of the LT, the most common site of infection was catheter-related bloodstream infections (5/12 episodes, 42%). Pneumonia was the most common bacterial infection in the 2- to 6-month period (7/25, 28%) and after 6 months (9/19, 47%). Mycoplasma pneumonia was only observed at 6 months after LT. Polymicrobial pneumonia was present in four episodes of bacterial pneumonia; three developed in the 2- to 6-month period and one developed after 6 months. In one episode, Acinetobacter baumannii, Pseudomonas aeruginosa, and influenza A virus were isolated from bronchoalveolar lavage fluid culture and multiplex reverse-transcription polymerase chain reaction (Seeplex RV 15 ACE Detection kit, Seegene, Seoul, Korea). No patient was co-infected with bacterial and fungal pneumonia.
Table 2. Bacterial infections after lung transplantation in the study cohort.
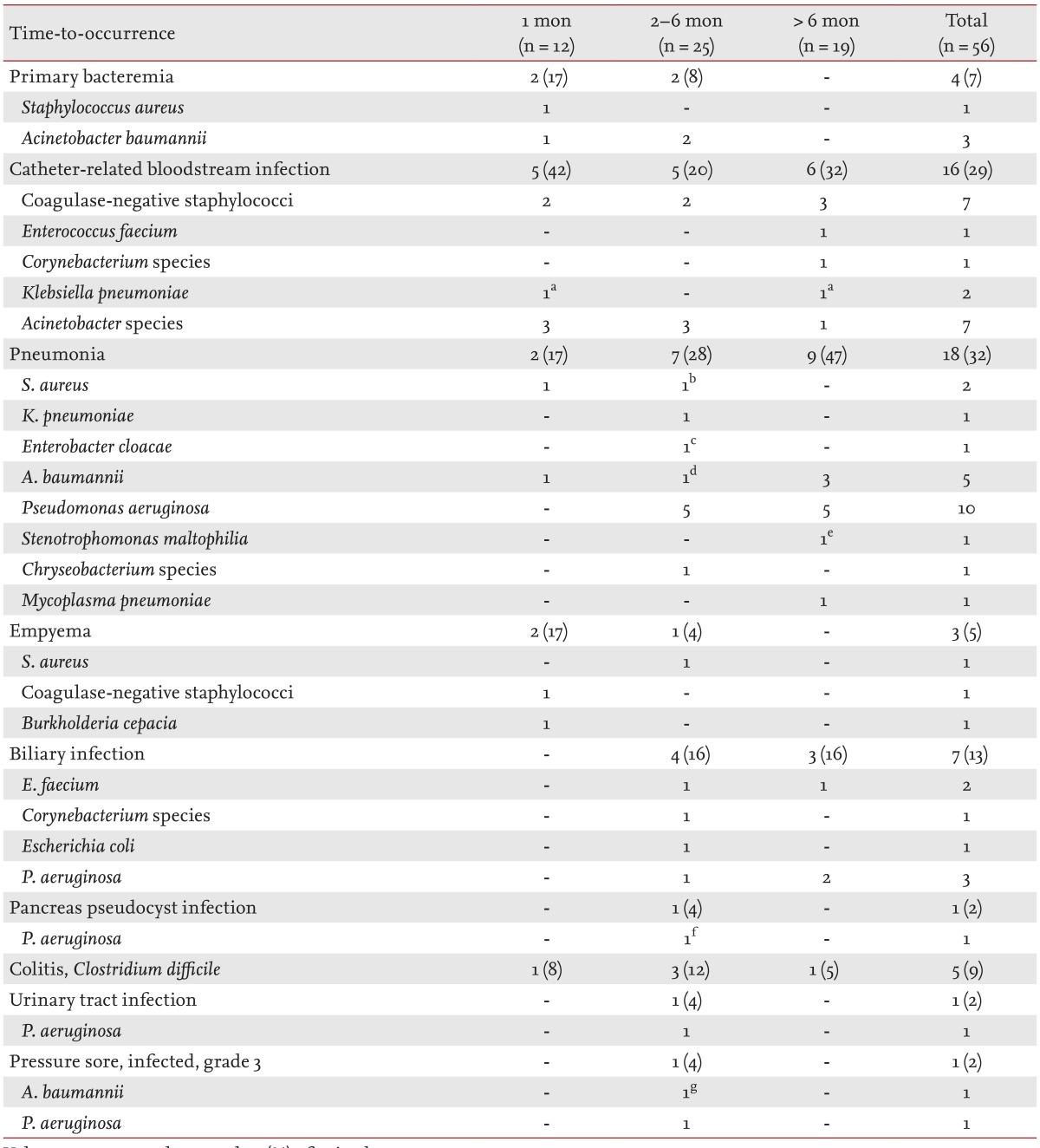
Values are presented as number (%) of episodes.
Co-infected episodes: aK. pneumoniae/A. baumannii, bS. aureus/Chryseobacterium species, cE. cloacae/P. aeruginosa, dA. baumannii/P. aeruginosa/influenza A virus, eA. baumannii/S. maltophilia, fP. aeruginosa/Candida krusei, gA. baumannii/P. aeruginosa.
Multidrug-resistant bacteria, such as methicillin-resistant staphylococci, vancomycin-resistant enterococci, and carbapenem-resistant or extended-spectrum β-lactamase producing gram-negative bacilli, were frequently involved (37/56 episodes, 66%) in these infections. The Staphylococcus aureus and coagulase-negative staphylococci in our patients were all methicillin-resistant. All three episodes of Klebsiella pneumoniae infections in our study cohort were associated with multidrug-resistant organisms (two carbapenem-resistant and one extended-spectrum β-lactamase producing). Most P. aeruginosa (14/16 episodes, 88%) and Acinetobacter species (14/16, 88%) infections were associated with carbapenem-resistant organisms.
Among the 34 LT recipients, 18 already had respiratory colonization with carbapenem-resistant P. aeruginosa, A. baumannii, or K. pneumoniae. Although bacterial infections were found to be significantly more common in those colonized than those not-colonized (14/18 [78%] vs. 5/16 [31%], p = 0.006 by the chi-square test), mortality was not significantly different between the two groups (6/18 [33%] vs. 2/16 [13%], p = 0.23 by the Fisher exact test).
Viral infections
Of the 43 episodes of viral infections in our patient series, 12 (28%) occurred within 1 month after LT, 10 (23%) in the 2- to 6-month period, and 21 (49%) after 6 months (Table 3). Cytomegalovirus was the most common viral infection within 1 month (9/12 episodes, 75%) and in the 2- to 6-month period (8/10, 80%). All seven episodes of asymptomatic cytomegalovirus infections of the first period developed within 10 days (median, 7 days [range, 1 to 10]). Two episodes of gastrointestinal diseases (oral/perianal ulcers and colitis), without prior cytomegalovirus antigenemia, developed at 14 and 17 days after LT during the antiviral prophylaxis. Five of seven episodes (71%) of asymptomatic cytomegalovirus infections of the second period (2 to 6 months) developed in patients with premature termination of antiviral prophylaxis. Two episodes of cytomegalovirus diseases developed after 6 months. Pathologically proven cytomegalovirus pneumonia developed 8 months after LT in the one patient with acute rejection. An episode of cytomegalovirus retinitis developed 13 months after LT. Epstein-Barr virus-related posttransplant lymphoproliferative disorders developed in two of our pediatric recipients (at 5.5 and 18 months after LT).
Table 3. Viral infections after lung transplantation in the study cohort.
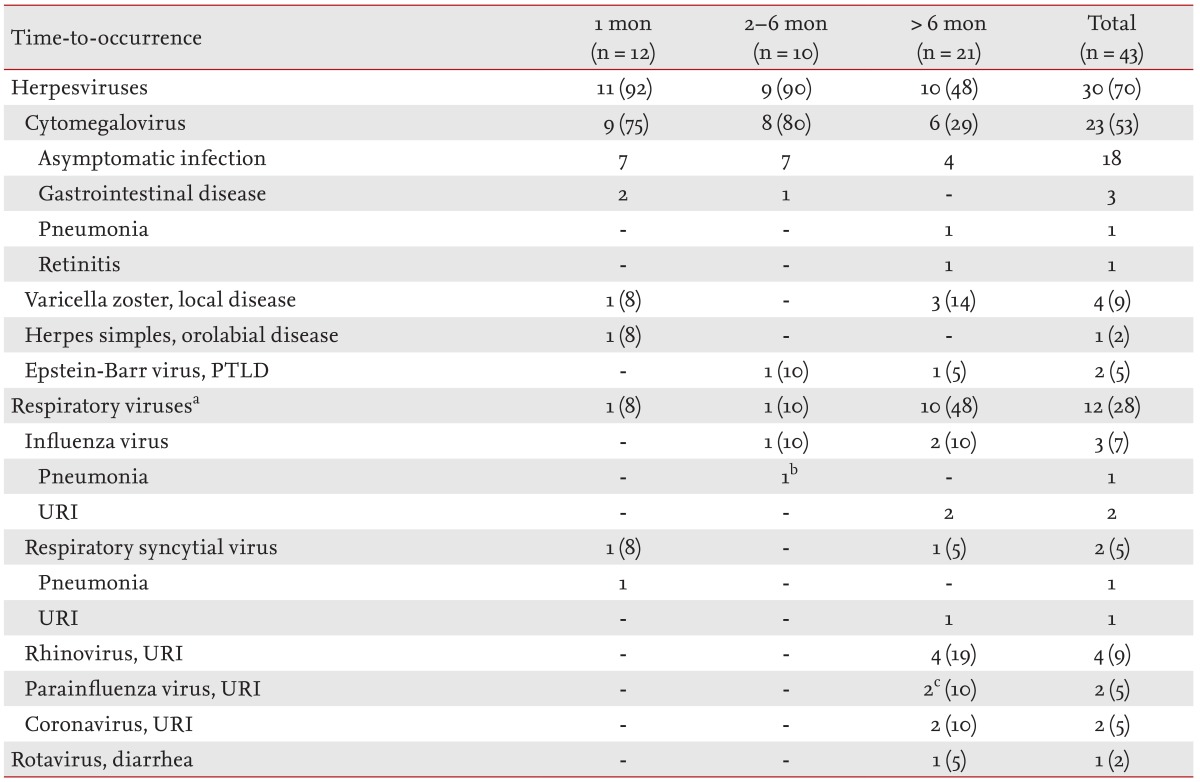
Values are presented as number (%) of episodes.
PTLD, post-transplant lymphoproliferative disorder; URI, upper respiratory tract infection.
aRespiratory viruses were detected from nasopharyngeal aspirates or bronchoalveolar lavage f luid using multiplex reverse-transcription polymerase chain reaction.
Co-infected episodes: bAcinetobacter baumannii/Pseudomonas aeruginosa/influenza A virus from bronchoalveolar lavage fluid, cRhinovirus and parainfluenza virus were co-infected in one episode.
Respiratory viruses were the most common viruses detected after 6 months (10/21, 48%). All 10 episodes of that period among our patients were upper respiratory tract infections, whereas two episodes within 6 months (one within 1 month and one in the 2- to 6-month period) were pneumonia due to respiratory syncytial and influenza viruses, respectively. Only one patient developed diarrhea due to rotavirus 2 years after LT. Rotavirus was transmitted to the patient from his two sons.
Fungal infections
Of the nine episodes of fungal infections in our study patients, one (11%) occurred within 1 month after LT, two (22%) in the 2- to 6-month period, and six (67%) after 6 months (Table 4). One episode of catheter-related candidemia (Candida parapsilosis) of the first period developed on the day after the LT, before an adequate voriconazole concentration was achieved. A pancreas pseudocyst infection (co-infection of Candida krusei and P. aeruginosa) developed 2 months after LT, despite the adequate voriconazole prophylaxis. All three episodes of catheter-related candidemia of the last period (after 6 months) were due to Candida albicans. No episodes of Aspergillus tracheobronchitis were observed in our study cohort. Three episodes of probable invasive pulmonary aspergillosis developed at 7, 21, and 33 months after LT. None of the three patients had any episode of rejection, augmented immunosuppression, or Aspergillus colonization before the development of invasive pulmonary aspergillosis.
Table 4. Fungal infections after lung transplantation in the study cohort.
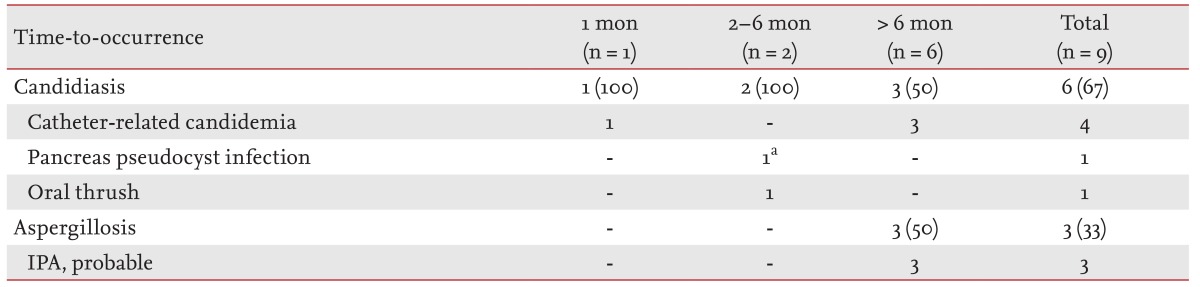
Values are presented as number (%) of episodes.
IPA, invasive pulmonary aspergillosis.
aPseudomonas aeruginosa/Candida krusei.
Outcomes of LT recipients
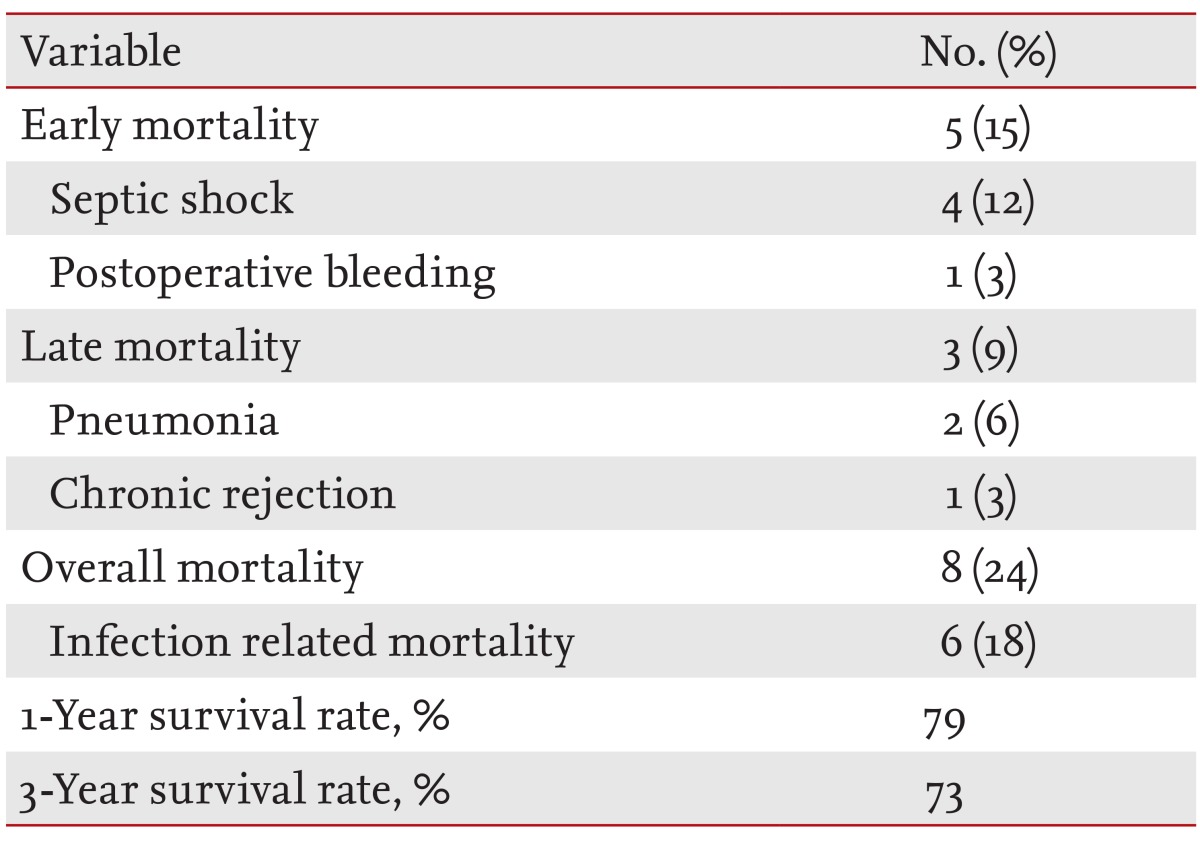
Of the 34 LT recipients in our series, eight patients (24%) died and six patients (18%) had infection-related mortality (Table 5). Five patients (15%) died within 1 month after LT (septic shock in four patients and postoperative bleeding in one patient). Carbapenem-resistant Acinetobacter species were identified in blood cultures of all septic shock patients (three catheter-related bloodstream infections and one primary bacteremia). Prior to septic shock, the four patients had received combination antimicrobial treatment, including at least one susceptible agent, because of respiratory colonization with multidrug-resistant Acinetobacter species. Three patients (9%) died after 1 month (pneumonia in two patients and chronic rejection in one patient). Survival rates at the first and third years were 79% and 73%, respectively.
Table 5. Clinical outcomes of the 34 lung transplant recipients analyzed in this study.
DISCUSSION
Infection is one of the most significant complications in LT recipients and is a significant determinant of transplantation outcomes. Various prophylaxis strategies have thus evolved gradually for LT recipients. The epidemiology of post-LT infections was reported in the 1990s [5,6]; however, data from the modern era are limited [7]. Although our current study was performed in a single center, we have detailed the very recent epidemiology of post-LT infections in our present analysis.
Multidrug-resistant bacteria were frequently present in our current study cohort. LT candidate patients with chronic pulmonary diseases usually have undergone frequent admissions, intensive care, and administrations of broad-spectrum antimicrobial agents. Therefore, care needs to be taken with regard to multidrug-resistant bacterial infections in the immediate post-LT care period, especially in intensive care units. In a previous report from Korea [11], multidrug-resistant gram-negative bacteria were also the respiratory pathogens most commonly identified in the late post-LT period (> 1 month after LT). In general, infections during the first month after solid organ transplantation are often donor- or recipient-derived or are associated with surgical and nosocomial complications. However, a period of 1 month may be too short to capture all bacterial infections, especially multidrug-resistant infections related to postoperative complications. LT recipients usually need more prolonged hospital care than other organ transplant recipients. Therefore, multidrug-resistant bacterial infections can often occur during the 2- to 6-month period in LT patients.
In our current study subjects, multidrug-resistant bacterial infections contributed to morbidity and early mortality after LT. Burkholderia cenocepacia infections could be considered relative contraindication to LT in patients with cystic fibrosis because these patients are at increased risk of poor post-LT outcomes [12]. However, in general, colonization or infection of multidrug-resistant P. aeruginosa or A. baumannii is not considered a contraindication for LT [13]. Although bacterial infections were found to be significantly more common in those colonized with multidrug-resistant gram-negative bacilli than those not-colonized, mortality was not significantly different between the two groups.
Most cytomegalovirus infections of the first period (within 1 month after LT) developed in the very early period after LT, possibly due to our antiviral prophylaxis protocol. In our institution, intravenous ganciclovir had started from 1 week in LT recipients, given the risk of neutropenia due to simultaneous administration of potent immunosuppressive and antiviral agents. After we completed our current study; however, we modified this protocol and antiviral prophylaxis now begins immediately after LT in our hospital. Antiviral prophylaxis is usually targeted at tissue-invasive cytomegalovirus diseases. Although two episodes of gastrointestinal diseases developed in the very early period after LT in our present study cohort, the prognosis of gastrointestinal diseases is usually good. More serious cytomegalovirus diseases are pneumonia and retinitis in LT recipients. Indeed, cytomegalovirus pneumonia and retinitis developed after the discontinuation of antiviral prophylaxis in this study. Therefore, the duration of antiviral prophylaxis is an important issue in LT recipients. In a randomized controlled trial [14], extending the duration of antiviral prophylaxis in LT recipients from 3 to 12 months further reduced the incidence of cytomegalovirus infection and disease. After that study, a recent guideline recommended 12-month antiviral prophylaxis in LT recipients [15]. However, in Korea, we need to carefully decide whether to follow the recommendation because most Korean patients are cytomegalovirus seropositive and the prophylactic use of valganciclovir is very expensive because the national health insurance scheme in Korea does not cover this drug.
Two adult cases of posttransplant lymphoproliferative disorders that developed 6 months and 2 years after LT were previously reported in Korea [16,17]. In our current study, Epstein-Barr virus-related posttransplant lymphoproliferative disorders developed in two pediatric recipients. Although some centers employ chemoprophylaxis and/or preemptive strategies using Epstein-Barr virus load as a surveillance tool, for prevention of posttransplant lymphoproliferative disorders, published data in support of these protocols are currently limited [18].
Antifungal prophylaxis is usually targeted at invasive aspergillosis in LT recipients. Among solid organ transplantations, the lung and heart-lung have the highest incidence of invasive aspergillosis [19]. Aspergillus infection in LT recipients manifests in several ways, including airway colonization, various forms of tracheobronchitis, and invasive pulmonary aspergillosis. In our present study series, there was no observed episode of tracheobronchitis or invasive pulmonary aspergillosis during the period of voriconazole prophylaxis. The serum concentrations of voriconazole were adequate in all patients who received the drug. Although three episodes of probable invasive pulmonary aspergillosis developed after the end of prophylaxis among our patient subjects, all fully improved with standard treatments. Infection due to azole-resistant Candida species (C. krusei) developed in one of our patients during voriconazole prophylaxis. All episodes of candidiasis that developed after the end of voriconazole prophylaxis were due to an azole-susceptible species, C. albicans.
The main limitation of our study was that only 34 patients from a single center were analyzed. Because of this limitation, it is difficult to state that our study data are completely representative of the Korean LT recipients. However, despite this potential drawback, we do provide valuable and recent detailed epidemiology data for post-LT infections in a Korean cohort. Continuous monitoring of infections after transplantation and a multicenter study are required in the future to further evaluate the proper epidemiology of infections after LT in Korea.
KEY MESSAGE
1. Multidrug-resistant bacterial infections increase morbidity and early mortality after lung transplantation.
2. Antifungal prophylaxis prevented any episodes of invasive aspergillosis.
3. Most respiratory virus infections manifest as upper respiratory infections and develop 6 months after lung transplantation.
Acknowledgments
We thank the lung transplantation team at Asan Medical Center, particularly In-Ok Kim, RN, for their invaluable assistance with this study.
Footnotes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Yusen RD, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-first adult lung and heart-lung transplant report: 2014; focus theme: retransplantation. J Heart Lung Transplant. 2014;33:1009–1024. doi: 10.1016/j.healun.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Korean Network for Organ Sharing. National data for organ transplantation [Internet] Seoul (KR): Korean Network for Organ Sharing; c2014. [cited 2014 Dec 1]. Available from: http://www.konos.go.kr/konosis/sub4/sub04_03_01_pop.jsp. [Google Scholar]
- 3.de Perrot M, Chaparro C, McRae K, et al. Twenty-year experience of lung transplantation at a single center: influence of recipient diagnosis on long-term survival. J Thorac Cardiovasc Surg. 2004;127:1493–1501. doi: 10.1016/j.jtcvs.2003.11.047. [DOI] [PubMed] [Google Scholar]
- 4.Zander DS, Baz MA, Visner GA, et al. Analysis of early deaths after isolated lung transplantation. Chest. 2001;120:225–232. doi: 10.1378/chest.120.1.225. [DOI] [PubMed] [Google Scholar]
- 5.Kanj SS, Tapson V, Davis RD, Madden J, Browning I. Infections in patients with cystic fibrosis following lung transplantation. Chest. 1997;112:924–930. doi: 10.1378/chest.112.4.924. [DOI] [PubMed] [Google Scholar]
- 6.Maurer JR, Tullis DE, Grossman RF, Vellend H, Winton TL, Patterson GA. Infectious complications following isolated lung transplantation. Chest. 1992;101:1056–1059. doi: 10.1378/chest.101.4.1056. [DOI] [PubMed] [Google Scholar]
- 7.Parada MT, Alba A, Sepulveda C. Early and late infections in lung transplantation patients. Transplant Proc. 2010;42:333–335. doi: 10.1016/j.transproceed.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Humar A, Michaels M AST ID Working Group on Infectious Disease Monitoring. American Society of Transplantation recommendations for screening, monitoring and reporting of infectious complications in immunosuppression trials in recipients of organ transplantation. Am J Transplant. 2006;6:262–274. doi: 10.1111/j.1600-6143.2005.01207.x. [DOI] [PubMed] [Google Scholar]
- 9.Raad I, Hanna HA, Alakech B, Chatzinikolaou I, Johnson MM, Tarrand J. Differential time to positivity: a useful method for diagnosing catheter-related bloodstream infections. Ann Intern Med. 2004;140:18–25. doi: 10.7326/0003-4819-140-1-200401060-00007. [DOI] [PubMed] [Google Scholar]
- 10.Stewart S, Fishbein MC, Snell GI, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26:1229–1242. doi: 10.1016/j.healun.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 11.Kim SY, Shin JA, Cho EN, et al. Late respiratory infection after lung transplantation. Tuberc Respir Dis (Seoul) 2013;74:63–69. doi: 10.4046/trd.2013.74.2.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murray S, Charbeneau J, Marshall BC, LiPuma JJ. Impact of burkholderia infection on lung transplantation in cystic fibrosis. Am J Respir Crit Care Med. 2008;178:363–371. doi: 10.1164/rccm.200712-1834OC. [DOI] [PubMed] [Google Scholar]
- 13.Avery RK. Infections after lung transplantation. Semin Respir Crit Care Med. 2006;27:544–551. doi: 10.1055/s-2006-954612. [DOI] [PubMed] [Google Scholar]
- 14.Palmer SM, Limaye AP, Banks M, et al. Extended valganciclovir prophylaxis to prevent cytomegalovirus after lung transplantation: a randomized, controlled trial. Ann Intern Med. 2010;152:761–769. doi: 10.7326/0003-4819-152-12-201006150-00003. [DOI] [PubMed] [Google Scholar]
- 15.Razonable RR, Humar A AST Infectious Diseases Community of Practice. Cytomegalovirus in solid organ transplantation. Am J Transplant. 2013;13(Suppl 4):93–106. doi: 10.1111/ajt.12103. [DOI] [PubMed] [Google Scholar]
- 16.Chang YS, Kim Y, Kim DY, et al. Two cases of post transplant lymphoproliferative disorder in lung transplant recipients. Korean J Intern Med. 2004;19:276–281. doi: 10.3904/kjim.2004.19.4.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paik HC, Hwang JJ, Kim DH, Joung EK, Kim HK, Lee DY. The 10 years experience of lung transplantation. Korean J Thorac Cardiovasc Surg. 2006;39:822–827. [Google Scholar]
- 18.Allen UD, Preiksaitis JK AST Infectious Diseases Community of Practice. Epstein-Barr virus and posttransplant lymphoproliferative disorder in solid organ transplantation. Am J Transplant. 2013;13(Suppl 4):107–120. doi: 10.1111/ajt.12104. [DOI] [PubMed] [Google Scholar]
- 19.Minari A, Husni R, Avery RK, et al. The incidence of invasive aspergillosis among solid organ transplant recipients and implications for prophylaxis in lung transplants. Transpl Infect Dis. 2002;4:195–200. doi: 10.1034/j.1399-3062.2002.t01-2-02002.x. [DOI] [PubMed] [Google Scholar]


