Abstract
Background/Aims
Intranasal mupirocin and chlorhexidine bathing are candidate strategies to prevent healthcare-associated infections caused by methicillin-resistant Staphylococcus aureus (MRSA). In Korea, intranasal mupirocin is not available, and mupirocin ointment, an over-the-counter drug, has been used indiscriminately. Furthermore, because it is covered by health insurance, mupirocin is easy to prescribe within hospitals.
Methods
We performed a mupirocin drug utilization review (DUR) within Hallym University Sacred Heart Hospital. Annual use of mupirocin was investigated between 2003 and 2013, and monthly consumption of mupirocin was assessed during the final 2-year period. The DUR focused on August 2012, the period of highest use of mupirocin. Also, we investigated trends in mupirocin resistance in MRSA between 2011 and 2013.
Results
Annual consumption of mupirocin increased from 3,529 tubes in 2003 to 6,475 tubes in 2013. During August 2012, 817 tubes were prescribed to 598 patients; of these, 84.9% were prescribed to outpatients, and 77.6% at the dermatology department. The most common indication was prevention of skin infections (84.9%), and the ointment was combined with systemic antibiotics in 62.9% of cases. The average duration of systemic antibiotic administration was about 7.8 days. The rate of low-level mupirocin resistance in MRSA increased from 8.0% to 22.0%, and that of high-level mupirocin resistance increased from about 4.0% to about 7.5%.
Conclusions
Inappropriate use of mupirocin is prevalent. Considering the increase in resistance and the future application of intranasal mupirocin, prophylactic use of mupirocin in dermatology departments should be reconsidered.
Keywords: Drug utilization review; Mupirocin; Drug resistance, bacterial; Methicillin-resistant Staphylococcus aureus
INTRODUCTION
Intranasal mupirocin and bathing in 2% chlorhexidine has been reported to reduce methicillin-resistant Staphylococcus aureus (MRSA) colonization and infection [1,2]. In Korea, however, intranasal mupirocin is not available, and mupirocin ointment, an over-the-counter drug, has been used indiscriminately. Furthermore, because health insurance covers mupirocin ointment, it is relatively easy to prescribe within hospitals. Mupirocin resistance is related to the inappropriate use of mupirocin, and ultimately lessens the effect of intranasal mupirocin on MRSA decolonization. Previous studies have concluded that mupirocin resistance in MRSA parallels mupirocin consumption [3,4]. High-level mupirocin resistance is associated with a failure of MRSA treatment, and combined low-level mupirocin and genotypic chlorhexidine resistance significantly increases the risk of persistent MRSA even after decolonization therapy [5,6]. The number of MRSA isolates with high-level mupirocin resistance declined, followed by the number of low-level mupirocin-resistant isolates, following administrative prescription control of mupirocin [4]. To our knowledge, however, mupirocin ointment consumption or utilization in Korea has not been evaluated.
The prevalence of high-level mupirocin resistance has been reported at about 4% among MRSA isolates in North America, and varies widely between and within countries in Europe [5]. Low-level mupirocin resistance among MRSA isolates from single-hospital studies range in prevalence from 0% to 80% [5]. In Korea, rates of low-level and high-level mupirocin resistance in a tertiary-care hospital were recently reported to be 17% and 2%, respectively [7], although different results were reported in the early 2000s [8,9]. In particular, the rate of low-level mupirocin resistance in a tertiary-care hospital increased from 14% in 2006 to 22% in 2009 [7]. Therefore, we performed a mupirocin drug utilization review (DUR) to evaluate the appropriateness of mupirocin use within Hallym University Sacred Heart Hospital, and investigated trends in mupirocin resistance among MRSA isolates.
METHODS
We performed a mupirocin DUR to assess the pattern of mupirocin use within Hallym University Sacred Heart Hospital. Hallym University Sacred Heart Hospital is a tertiary-care teaching hospital with 829 beds. First, the annual usage rates of antibiotic ointments available at our hospital between 2003 and 2013, mupirocin and fusidic acid, were investigated. In particular, the monthly consumption of mupirocin was assessed during the final 2-year period, during which the use was highest and could be easily extracted using the pharmacy's computer software. We performed a retrospective DUR of prescribed mupirocin for the month of August 2012, the period of highest mupirocin use, by reviewing the electronic medical records of patients who received mupirocin ointment in that month. The following data were collected: number of patients with mupirocin prescriptions, number of tubes of mupirocin ointment prescribed, sites of prescription (inpatient or outpatient), prescribing department, indications, whether bacterial culture and antimicrobial susceptibility testing were requested, whether antibiotic ointment was given alone or with systemic antibiotics, type and duration of co-administered systemic antibiotics, and history or presence of adverse reactions associated with mupirocin.
Mupirocin susceptibility testing of S. aureus isolates was performed using the MicroScan Dried Pos Break-point Combo Panel Type 28 (PBC28, Siemens, West Sacramento, CA, USA), available in our hospital since April 2011. Minimal inhibitory concentration (MIC) breakpoints for mupirocin were defined as: susceptible, below 4 µg/mL; low-level resistant, 8 to 256 µg/mL; and high-level resistant, more than 512 µg/mL. We investigated quarterly trends in low-level and high-level mupirocin resistance rates among MRSA isolates between April 2011 and December 2013. Fusidic acid susceptibility testing of S. aureus isolates was performed using the MicroScan Dried Gram Positive Combo Panel Type 1A (Siemens) before April 2011 and PBC28 after April 2011. MIC breakpoints for fusidic acid were defined as: susceptible, below 2 µg/mL; intermediate resistance, 4 to 16 µg/mL; and resistant, more than 32 µg/mL. We also investigated annual trends in fusidic acid resistance among S. aureus isolates between 2003 and 2013.
RESULTS
Trends in antibiotic ointment consumption
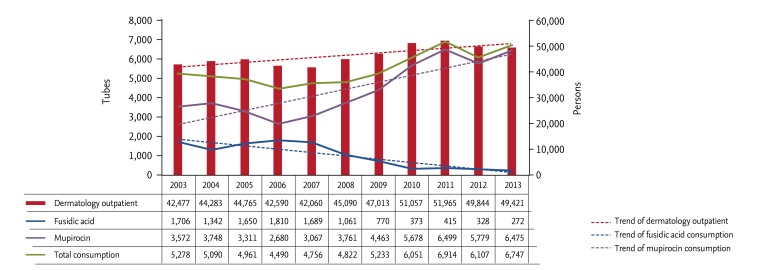
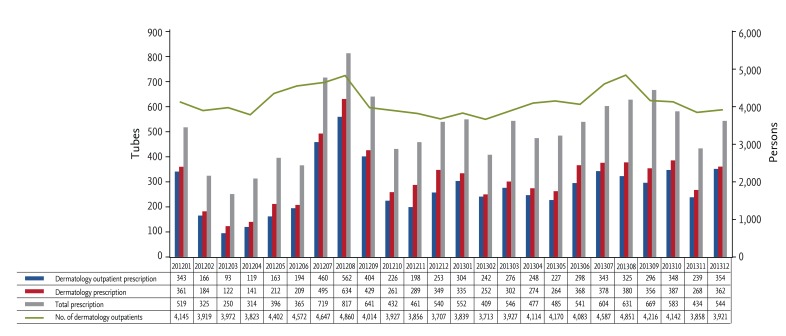
Annual consumption of mupirocin increased from 3,529 tubes in 2003 to 6,475 tubes in 2013, while use of fusidic acid ointment decreased greatly from 1,706 tubes in 2003 to 272 tubes in 2013 (Fig. 1). Monthly use of mupirocin ointment between 2012 and 2013 was highest (817 tubes) in August 2012 (Fig. 2).
Figure 1. Annual consumption of mupirocin and fusidic acid ointment between 2003 and 2013.
Figure 2. Monthly consumption of mupirocin ointment between 2012 and 2013.
Drug utilization review of mupirocin ointment
As August 2012 was the month with the highest consumption of mupirocin, we focused our DUR of mupirocin ointment on that period. Physicians prescribed 817 tubes to 598 patients in August 2012. Of those, 694 tubes (84.9%) were administered to outpatients, and 562 tubes (81.0%) were prescribed at the dermatology outpatient clinic. The department responsible for most prescriptions was dermatology (634 tubes, 77.6%), while 20 (2.4%) were prescribed by pediatrics. The dermatology department was responsible for about 60% of all mupirocin prescriptions between 2012 and 2013 (Fig. 2). Most prescriptions (84.9%) were for prophylactic purposes after procedures, such as biopsy (60.0%) or for non-infectious skin lesions (25.0%). Only 1.8% were prescribed after a request for bacterial culture with antimicrobial susceptibility testing. Mupirocin ointment was combined with systemic antibiotics in 62.9% of cases; systemic antibiotics were also usually prophylactic (84.3%). Systemic antibiotics included cefaclor (49%), amoxicillin/clavulanate (38%), and cefditoren (3%). The mean duration of co-administration of systemic antibiotics was 7.8 days, with cefaclor given for an average of 6.6 days, amoxicillin/clavulanate 7.9 days, and cefditoren 10 days. We did not identify any current or past drug adverse reactions associated with mupirocin through electronic chart review.
Trends of mupirocin and fusidic acid resistance among S. aureus isolates
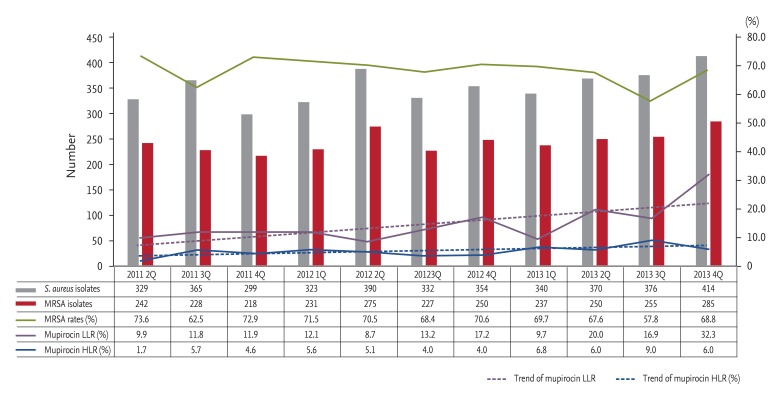
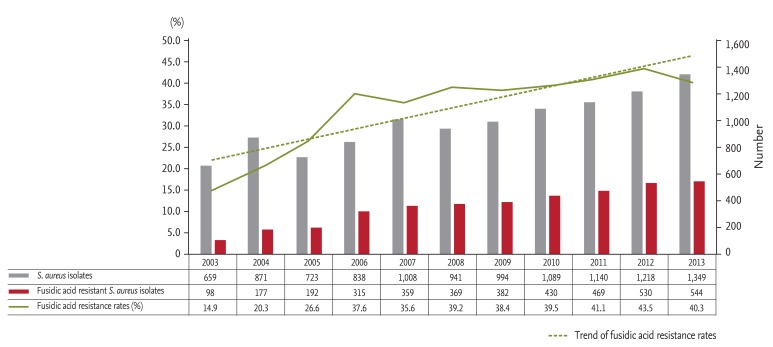
We investigated quarterly trends in low-level and high-level mupirocin resistance among all inpatient and outpatient MRSA isolates between April 2011 and December 2013. The rate of low-level mupirocin resistance among MRSA isolates increased greatly, from 8.0% to 22.0%, while that of high-level mupirocin resistance increased from 4.0% to 7.5% (Fig. 3). The rate of fusidic acid resistance among all inpatient and outpatient S. aureus isolates increased greatly, from 14.9% to 40.3%, between 2003 and 2013 (Fig. 4).
Figure 3. Quarterly trends in low-level and high-level mupirocin resistance among methicillin-resistant Staphylococcus aureus (MRSA) isolates. LLR, low-level resistance; HLR, high-level resistance.
Figure 4. Annual trends in fusidic acid resistance among Staphylococcus aureus isolates.
DISCUSSION
The use of mupirocin ointment has increased greatly in the last decade, likely related to the increase in fusidic acid-resistant S. aureus, the number of individuals visiting dermatology outpatient clinics, and the cost to patients of fusidic acid ointment since 2000. Rates of fusidic acid resistance among S. aureus isolates were reported to be ~20% between the late 1990s and the early 2000s [10,11]. Our hospital's resistance rate during 2003 and 2005 was also about 20%, but continued to increase to 35.6% in 2006 and > 40% in 2011. The number of individuals visiting the dermatology outpatient clinic in our hospital increased from about 42,000 persons per year in 2003 to more than 49,000 persons per year in 2013.
The number of mupirocin prescriptions from the dermatology department in the DUR period was representative of the monthly prescriptions between 2012 and 2013. Specifically, the monthly mupirocin prescriptions correlated with the number of individuals visiting the dermatology outpatient department, increasing during the summer vacation, when more dermatological procedures were performed. Therefore, we decided to perform a mupirocin DUR during August 2012, although the study was limited by evaluating the data from only a single university-affiliated tertiary-care hospital during a specific period [12]. Most prescriptions for mupirocin ointment were inappropriately given for prophylactic purposes in the department of dermatology, especially at the dermatology outpatient clinic. Furthermore, systemic antibiotics were used in combination with about two-thirds of the mupirocin prescriptions, though no evidence supports this regimen.
Mupirocin resistance, especially low-level mupirocin resistance, among all inpatient and outpatient MRSA isolates increased with use of mupirocin; however, mupirocin susceptibility was not determined according to Clinical Laboratory and Standards Institute recommendations [7]. Excessive use of mupirocin can lead to mupirocin resistance [13,14]; mupirocin use for more than 5 to 10 days in MRSA nasal carriers with other MRSA colonization sites, such as chronic wounds, and repetitive use of mupirocin resulting from a decolonization failure are associated with development of resistance [3]. The use of mupirocin in our hospital, especially in outpatient departments, increased greatly after 2003 (data not shown), and annual consumption of mupirocin remained high after 2010.
Mupirocin susceptibility testing of MRSA isolates has been available in our hospital since April 2011, but only 1.8% of prescriptions during August 2012 followed a request for bacterial culture. Thus, we could not directly correlate use of mupirocin with resistance among MRSA isolates between 2003 and 2013. However, we assume that use of mupirocin ointment in our hospital would be similar to that in other community hospitals or clinics in the vicinity. Moreover, mupirocin ointment has been used indiscriminately as an over-the-counter drug in the community, although we could not estimate the quantity consumed. Therefore, excessive mupirocin use both as a prescription and over the counter could affect mupirocin resistance at our hospital, and this resistance is likely to reflect that in the community.
The rate of resistance to fusidic acid increased greatly despite the decreasing use of fusidic acid ointment in our hospital. We assume that this increase was related to widespread over-the-counter use of fusidic acid ointment or to the use of fusidic acid as an oral antibiotic. Therefore, an antimicrobial stewardship program to control prescriptions of mupirocin and combined systemic antibiotics, especially at dermatology outpatient clinics, should be established. Further nationwide DURs for antibiotic ointment using large datasets, such as those from the Health Insurance Review and Assessment Service or nationwide multicenter studies evaluating the use of, and monitoring of resistance to, mupirocin in Korea should be conducted. As a first step, as mupirocin ointment is used indiscriminately as an over-the-counter drug, it should be classified as a prescription drug.
In conclusion, the inappropriate use of mupirocin is prevalent. Considering the increase in mupirocin resistance and the future application of intranasal mupirocin, prophylactic use of mupirocin ointment in dermatology departments must be reconsidered. A nationwide DUR using large datasets or further multicenter studies of mupirocin ointment use and resistance in Korea are needed.
KEY MESSAGE
1. Inappropriate use of mupirocin is prevalent.
2. Mupirocin ointment is inappropriately prescribed mainly as a prophylactic in the dermatology department, especially the outpatient clinic.
3. The rate of mupirocin resistance, especially low-level resistance, among methicillin-resistant Staphylococcus aureus isolates tends to increase with increasing use of mupirocin.
Footnotes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Ridenour G, Lampen R, Federspiel J, Kritchevsky S, Wong E, Climo M. Selective use of intranasal mupirocin and chlorhexidine bathing and the incidence of methicillin-resistant Staphylococcus aureus colonization and infection among intensive care unit patients. Infect Control Hosp Epidemiol. 2007;28:1155–1161. doi: 10.1086/520102. [DOI] [PubMed] [Google Scholar]
- 2.Huang SS, Septimus E, Kleinman K, et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med. 2013;368:2255–2265. doi: 10.1056/NEJMoa1207290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Talon D, Marion C, Thouverez M, Bertrand X. Mupirocin resistance is not an inevitable consequence of mupirocin use. J Hosp Infect. 2011;79:366–367. doi: 10.1016/j.jhin.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Walker ES, Levy F, Shorman M, David G, Abdalla J, Sarubbi FA. A decline in mupirocin resistance in methicillin-resistant Staphylococcus aureus accompanied administrative control of prescriptions. J Clin Microbiol. 2004;42:2792–2795. doi: 10.1128/JCM.42.6.2792-2795.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hetem DJ, Bonten MJ. Clinical relevance of mupirocin resistance in Staphylococcus aureus. J Hosp Infect. 2013;85:249–256. doi: 10.1016/j.jhin.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Lee AS, Macedo-Vinas M, Francois P, et al. Impact of combined low-level mupirocin and genotypic chlorhexidine resistance on persistent methicillin-resistant Staphylococcus aureus carriage after decolonization therapy: a case-control study. Clin Infect Dis. 2011;52:1422–1430. doi: 10.1093/cid/cir233. [DOI] [PubMed] [Google Scholar]
- 7.Lee H, Lim H, Bae IK, et al. Coexistence of mupirocin and antiseptic resistance in methicillin-resistant Staphylococcus aureus isolates from Korea. Diagn Microbiol Infect Dis. 2013;75:308–312. doi: 10.1016/j.diagmicrobio.2012.11.025. [DOI] [PubMed] [Google Scholar]
- 8.Yun HJ, Lee SW, Yoon GM, et al. Prevalence and mechanisms of low- and high-level mupirocin resistance in staphylococci isolated from a Korean hospital. J Antimicrob Chemother. 2003;51:619–623. doi: 10.1093/jac/dkg140. [DOI] [PubMed] [Google Scholar]
- 9.Yang JA, Park DW, Sohn JW, Yang IS, Kim KH, Kim MJ. Molecular analysis of isoleucyl-tRNA synthetase mutations in clinical isolates of methicillin-resistant Staphylococcus aureus with low-level mupirocin resistance. J Korean Med Sci. 2006;21:827–832. doi: 10.3346/jkms.2006.21.5.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee K, Lee HS, Jang SJ, et al. Antimicrobial resistance surveillance of bacteria in 1999 in Korea with a special reference to resistance of enterococci to vancomycin and gram-negative bacilli to third generation cephalosporin, imipenem, and fluoroquinolone. J Korean Med Sci. 2001;16:262–270. doi: 10.3346/jkms.2001.16.3.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim HB, Jang HC, Nam HJ, et al. In vitro activities of 28 antimicrobial agents against Staphylococcus aureus isolates from tertiary-care hospitals in Korea: a nationwide survey. Antimicrob Agents Chemother. 2004;48:1124–1127. doi: 10.1128/AAC.48.4.1124-1127.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee H, Jung D, Yeom JS, et al. Evaluation of ceftriaxone utilization at multicenter study. Korean J Intern Med. 2009;24:374–380. doi: 10.3904/kjim.2009.24.4.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caffrey AR, Quilliam BJ, LaPlante KL. Risk factors associated with mupirocin resistance in meticillin-resistant Staphylococcus aureus. J Hosp Infect. 2010;76:206–210. doi: 10.1016/j.jhin.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 14.Lee AS, Macedo-Vinas M, Francois P, et al. Trends in mupirocin resistance in meticillin-resistant Staphylococcus aureus and mupirocin consumption at a tertiary care hospital. J Hosp Infect. 2011;77:360–362. doi: 10.1016/j.jhin.2010.11.002. [DOI] [PubMed] [Google Scholar]