The 2015 Genitourinary Cancers Symposium (GU-ASCO 2015) was a forum for the presentation of new research in a number of tumour types, highlighting the most recent basic science and clinical work in the GU cancer field. The following pages summarize some of the most interesting new data and findings presented at this symposium.
New research in early-stage prostate cancer
Risk associated with testicular cancer
Investigators at the University of Maryland retrospectively analyzed the Surveillance, Epidemiology, and End Results (SEER) database to identify individuals with a history of testicular cancer and conduct a comparative analysis to determine whether or not these individuals were at higher risk for subsequent intermediate and high-risk prostate cancer (PCa) (Gleason score 7 or higher) compared to individuals with a history of melanoma.1
The researchers identified 32 435 individuals with testicular cancer history and matched them to 147 044 patients with a history of melanoma. Compared to those individuals with a history of melanoma, those with a history of testicular cancer had a 4.7-fold risk of developing any PCa and a 5.2-fold risk of intermediate-high risk PCa (multivariate analyses, both p < 0.0001). This retrospective data are provocative and hypothesis-generating.
Caution on active surveillance for intermediate-risk PCa
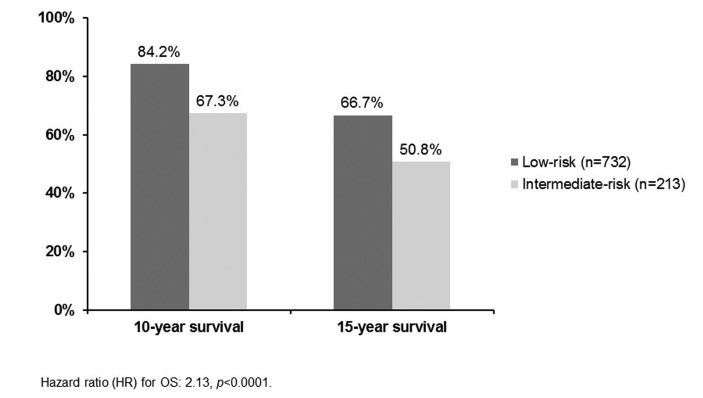
This Canadian study sought to determine the survival outcomes of patients with intermediate-risk PCa (prostate-specific antigen [PSA] >10 ng/ml or Gleason score 7 or clinical stage T2b/2c).2 Study researchers identified 213 intermediate-risk patients managed with active surveillance from the single-centre database at Sunnybrook Health Sciences Centre in Toronto. These patients had a median follow-up of 6.7 years. A total of 732 low-risk patients managed with active surveillance were also included in the analysis as the control group.
Of the 213 intermediate-risk patients, 34.7% received treatment (mainly radiation). Most of these patients were treated due to short PSA doubling times. The 10- and 15-year overall survival (OS) rates are shown in Figure 1. For each analysis, the survival rate was significantly lower for those patients in the intermediate-risk group.
Fig. 1.
10- and 15-year overall survival rates with active surveillance: Low-risk and intermediate-risk patients.
New research in metastatic, hormone-sensitive PCa (mHSPC)
GETUG-AFU: No significant survival advantage for androgen-deprivation therapy (ADT) + docetaxel
At GU-ASCO 2015, researchers presented an updated analysis of data from the Androgen-Deprivation Therapy Alone or with Docetaxel in Non-Castrate Metastatic Prostate Cancer (GETUG-AFU 15) study.3 This study included 385 patients with metastatic non-castrate PCa (mCRPC) and randomized them to treatment with ADT alone (luteinizing-hormone-releasing hormone [LHRH] agonist, maximum androgen blockade, or orchiectomy) or ADT with docetaxel (75 mg/m2 every three weeks for up to nine cycles). The primary endpoint was OS. To help facilitate a comparison with the previously reported ECOG 3805: ChemoHormonal Therapy Versus Androgen Ablation Randomized Trial for Extensive Disease in Prostate Cancer (CHAARTED) study, which stratified patients by high or low tumour volume, the investigators of the GETUG-AFU 15 study retrospectively assessed tumour volume and performed sub-analyses on these separate populations.
The median follow-up for this analysis was 82.9 months. Over this time, the median OS was 46.5 months for ADT alone and 60.9 months for ADT plus docetaxel. This numerical difference was not, however, statistically significant (HR 0.9, 95% confidence interval [CI] 0.7 to 1.2; p = 0.44). There were significant differences noted in secondary endpoints. Median biological progression-free survival (PFS) was 12.9 months with ADT alone and 22.9 months with ADT plus docetaxel (HR 0.7, 95% CI 0.6–0.9, p = 0.0021).
For patients with high-volume disease (n=183), median OS was 35.1 months for ADT alone and 39 months for ADT plus docetaxel (HR 0.8, 95% CI 0.6–1.2; p = 0.35). For low-volume disease, median OS was not reached for ADT alone and was 83.1 months for ADT plus docetaxel (HR 1.0, 95% CI 0.6–15, p = 0.87).
The results of this trial stand in contrast to those of the similarly designed E3805 trial, which did report a survival benefit for ADT plus docetaxel in high-volume disease (HVD) patients.4 At GU-ASCO 2015, Dr. Eric Small led a discussion session that included an assessment of the different outcomes of these two studies.5 He postulated that the difference between the two studies is likely explained by a few important differences in the trial designs and populations (Table 1).
Table 1.
Key differences between GETUG-AFU 15 and ECOG 3805 studies
| GETUG | ECOG | |
|---|---|---|
| Total sample size | 385 | 790 |
| High-volume disease | 183 (47.5%) | 514 (65%) |
| Median follow-up | 83 months | 29 months |
| Received post-protocol docetaxel | ADT-only arm: 80% ADT-docetaxel arm: 45% |
ADT-only arm: 33% ADT-docetaxel arm: 12% |
| Received post-protocol abiraterone or enzalutamide | ADT-only arm: No response ADT-docetaxel arm: No response |
ADT-only arm: 20% DT-docetaxel arm: 23% |
GETUG-AFU: Groupe d’Etude des Tumeurs Uro-Genital-Association Française d’Urologie; ECOG: Eastern Cooperative Oncology Group; ADT: androgen deprivation therapy.
The GETUG-AFU 15 trial was underpowered to detect differences in the high-volume group (N = 183). Of potential importance was the median time of follow-up, namely 83 months for GETUG-AFU 15 and 29 months for ECOG 3805. The non-protocol treatments received differed as well. In GETUG-AFU 15, 80% of those in the ADT-only arm and 45% of the ADT plus docetaxel arm received post-protocol docetaxel; in the ECOG study, 33% of the ADT-only arm and 12% of the ADT plus docetaxel arm had received post-protocol docetaxel.
Finally, the use of oral agents (abiraterone or enzalutamide) was not reported in the GETUG-AFU 15 trial, but is thought to be low, whereas these agents were used post-protocol in 20% of the ECOG 3805 study’s ADT-only arm and 23% of the ADT plus docetaxel arm.
Abiraterone in mHSPC
The Southwest Oncology Group (SWOG) S10104 study was a Phase 2, single-arm analysis of abiraterone acetate plus prednisone among 41 men with poor prognosis metastatic disease (PSA >4 ng/ml after ADT).6 The primary endpoint was a PSA of ≤0.2 ng/mL within one year. Of the 41 patients, five (13%) achieved this goal, thereby falling short of the protocol-specified level of six responses; however, the authors were nonetheless encouraged. The median OS noted in this population was 23.9 months, which is substantially longer than that observed in a similar population of patients (SWOG 9346 study, 13 months7).
New Research in mCRPC
No OS benefit of cabozantinib among men with prior docetaxel and abiraterone or enzalutamide
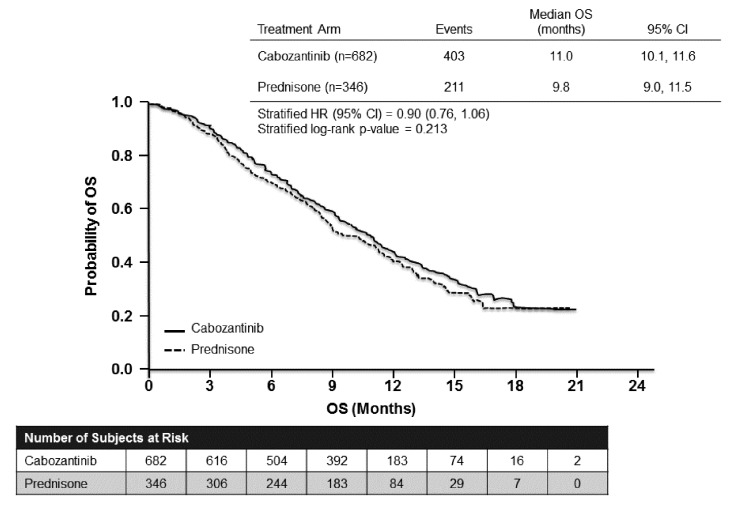
The Study of Cabozantinib (XL184) Versus Prednisone in Men With Metastatic Castration-resistant Prostate Cancer Previously Treated With Docetaxel and Abiraterone or MDV3100 (COMET-1) study was an international, Phase 3 trial involving 960 patients post-progression with docetaxel and post-progression with abiraterone and/or enzalutamide.8 All patients had bone metastases, with or without severe pain. Patients were randomized to receive oral cabozantinib 60 mg daily (n=682) or prednisone 5 mg twice daily (n = 346). The primary study endpoint was OS.
For the primary endpoint analysis, there was no significant difference between the groups. The median was 11.0 months for cabozantinib and 9.8 months with prednisone (p = 0.213; Fig. 2). In subgroup analyses, the only group who showed a significant difference in favor of cabozantinib were those who had previously been treated with cabazitaxel (n = 393).
Fig. 2.
Cabozantinib after progression on docetaxel and abiraterone or enzalutamide (COMET-1): Overall survival.
With respect to secondary endpoints, there were some benefits observed with cabozantinib. The proportion with a bone scan response at week 12 (≥30% reduction in bone scan lesion area by central review) was 42% with cabozantinib and 3% with prednisone (p < 0.001). Bone biomarkers were also improved relative from baseline. Median PFS was also longer with cabozantinib (5.6 months) versus with prednisone (2.8 months) (p < 0.001).
In the analysis of safety, 33% of those in the cabozantinib group discontinued the study due to adverse events, compared to 12% of the prednisone group.
Enzalutamide safe and effective in older men (sub-analysis)
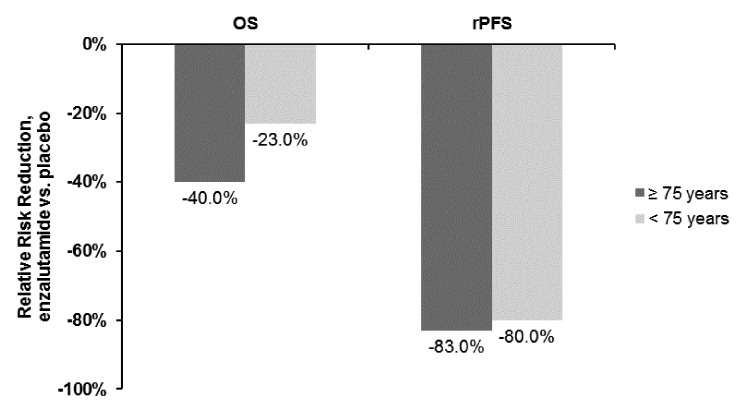
Researchers from the Safety and Efficacy Study of Oral MDV3100 in Chemotherapy-Naive Patients With Progressive Metastatic Prostate Cancer (PREVAIL) study group presented an analysis of efficacy and safety results from this study for the subgroups of patients younger than 75 years (n = 1108) or 75 years and older (n = 609).9 The PREVAIL study was a randomized, controlled clinical trial comparing oral enzalutamide to placebo among 1,717 chemotherapy-naïve men with mCRPC. The study showed that enzalutamide treatment was associated with significant improvements in OS and radiographic PFS compared to placebo in these patients. Those patients over 75 years had a slightly higher incidence of decreased appetite, asthenia and falls.
In this sub-analysis, the investigators reported that patients in the older subgroup had worse prognostic features at baseline relative to the younger patients (e.g., higher proportion with ECOG performance status grade 1, higher median PSA, higher proportion with >20 bone metastases and higher prevalence of cardiovascular disease). Enzalutamide treatment was associated with significant risk reductions for death of 40% in the older subgroup and 23% in the younger group (both statistically significant vs. placebo). Radiographic PFS was reduced by 83% with enzalutamide in the older subgroup and 80% among the younger patients (Fig. 3).
Fig. 3.
Overall and radiographic progression-free survival in the PREVAIL study (enzalutamide vs. placebo) stratified by age.
Some new insight on sequencing of therapies
A post-hoc analysis of data from the Abiraterone Acetate in Asymptomatic or Mildly Symptomatic Patients With Metastatic Castration-Resistant Prostate Cancer (COU-AA-302) trial showed that taxane treatment after progression on abiraterone acetate plus prednisone is a viable strategy.10 The median time to PSA progression on subsequent taxane therapy was 7.59 months in this analysis.
Similarly, in a post-hoc analysis of data from a subset of patients from the PREVAIL study showed that taxanes retain their efficacy among patients pre-treated with enzalutamide.11
Impact of AR-V7 splice variant on treatment efficacy
Previous research had shown that the AR-V7 splice variant was associated with primary resistance to both abiraterone acetate and enzalutamide. At GU-ASCO 2015, researchers presented data on 37 men who initiated therapy with a taxane; 17 of these patient had the AR-V7 splice variant confirmed.12 The study showed that the presence of AR-V7 was not associated with resistance to taxane therapy. The authors suggested that this could be used as a biomarker to help guide the selection of therapy among patient with mCRPC.
Also of interest in the AR-V7 discussion is the upcoming Androgen Receptor Modulation Optimized for Response in Splice Variant (ARMOR3-SV) trial comparing enzalutamide and the small molecule galeterone among patients with the AR-V7 variant.13 This randomized, open-label, Phase 2 trial is expected to begin recruiting in 2015.
New evidence with radium-223
At GU-ASCO 2015, the evidence continued to accumulate for radium-223 in the mCRPC setting. This included two real-world analyses from the expanded access program (EAP) in the United States.14–16 The EAP includes 184 patients with symptomatic bone metastases, two or more skeletal metastases on imaging, and no lung, liver, or brain metastases, treated with radium-223 50 kBq/kg every four weeks for six injections (in addition to best standard of care). The primary endpoint of the study is safety.
Analysis of these patients did not reveal any new safety concerns, and radium-223 was well tolerated overall14 and among those patients concurrently receiving abiraterone acetate or enzalutamide.15 Median OS, one of the key exploratory efficacy analyses, was 17 months.14 The impact of radium-223 on pain was significant; 43% of patients reported pain relief on the Brief Pain Inventory (Short Form), while 19% reported no change and 28% reported worsening of pain.16
Also presented at GU-ASCO 2015 was a study of 46 patients with mCRPC showing that the addition of radium-223 to docetaxel therapy was associated with a greater proportion of patients achieving normalization of bone alkaline phosphatase (bALP), a bone turnover marker, compared to docetaxel alone.17
ODM-201, a promising novel therapy for mCRPC
With respect to novel therapies in development for mCRPC, one of the more promising compounds appears to be ODM-201, an oral AR-inhibitor. In an open-label Phase 1 study, this compound was well tolerated and showed substantial anti-tumour activity.18 The Phase 3 Efficacy and Safety Study of ODM-201 in Men With High-risk Non-metastatic Castration-resistant Prostate Cancer (ARAMIS) is ongoing.
New research in radiation therapy
No OS benefit from dose escalation in intermediate-risk patients
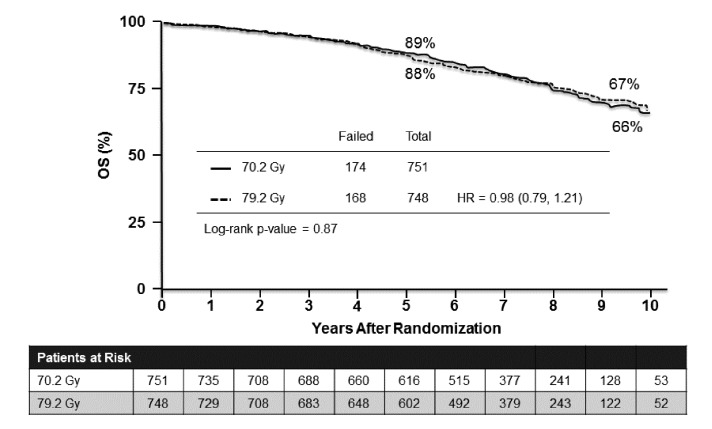
Results of the Radiation Therapy in Treating Patients With Stage II Prostate Cancer (RTOG 0126) trial were presented at GU-ASCO 2015. This was a Phase 3, randomized study of high-dose three-dimensional conformal radiation therapy (3DCRT)/intensity-modulated radiation therapy (IMRT) versus standard dose in 1,532 men with localized PCa, stage cT1b-T2b with Gleason Score 2 to 6 and PSA ≥10 and <20.19 The study treatments were 3DCRT or IMRT to 79.2 Gy in 44 fractions (high-dose) or 70.2 Gy in 39 fractions (standard dose). The primary endpoint was OS.
There was no significant difference in OS (HR 0.98, 95% CI 0.79–1.21, p = 0.87) (Fig. 4); however, there were significant improvements noted in favour of the high-dose group for local control, distant-metastasis-free survival, and biochemical disease failure rates, as well as less use of salvage therapies. These benefits were offset by a significant increase in gastrointestinal and GU toxicity than the standard dose.
Fig. 4.
High-dose versus standard dose radiotherapy for localized prostate cancer: Overall survival.
Additional benefit with short-term ADT with radiotherapy in intermediate-risk patients
A Canadian study presented at GU-ASCO evaluated the impact of short-term ADT (STADT) among patients with intermediate-risk PCa treated with radiotherapy.20 In the Study on the Role of Hormonal Treatment for Two Dosage Levels of Prostate Radiation Therapy Versus Prostate Radiation Therapy Alone (PCS III), patients were randomized to receive STADT in combination with either a 70 Gy or 76 Gy dose of radiotherapy, or with radiation alone (76 Gy). The co-primary endpoints were biochemical failure and disease-free survival.
After a median follow-up of 75.4 months, there was a significant benefit for both co-primary endpoints in favour of the inclusion of STADT compared to radiotherapy alone. There was not significant between-group difference, however, with respect to OS (secondary endpoint).
Low-dose-rate brachytherapy is superior to dose-escalated external beam radiation in unfavorable-risk PCa
The Androgen Suppression Combined With Elective Nodal and Dose Escalated Radiation Therapy (ASCENDE-RT) study included 122 patients with intermediate- and high-risk PCa.21 All patients received one year of ADT with an LHRH agonist, plus a non-steroidal anti-androgen for at least one month. After eight months, all patients received external-beam, whole-pelvis radiation therapy (EBRT; 46 Gy in 23 fractions). Subjects were randomized to treatment with dose-escalated EBRT (EBRT-B; boost of 32 Gy in 16 fractions) or low-dose-rate brachytherapy (LDR-B; Iodine-125 LDR boost prescribed to a minimum peripheral dose of 115Gy). The primary study endpoint was relapse-free survival, defined by biochemical criteria.
Over a median follow-up of 6.5 years, LDR-PB was associated with a 50% reduction in biochemical relapse compared to EBRT-B; however, there was no significant between-group difference in OS (or in PCa-specific survival or metastasis-free survival).
Insights in imaging for prostate cancer
One of the recognized shortcomings of the PCa field is the inadequacy of current imaging modalities as means of monitoring for emerging metastases. At GU-ASCO 2015, Dr. Peter Pinto presented an overview of recent research with novel imaging techniques.22
Perhaps the most promising modality he discussed was fluoride positron emission tomography–computed tomography (PET/CT) scanning, which has been shown to be superior to technetium (TC) bone scan. Ferumoxytol (an iron-based compound)-enhanced magnetic resonance imaging (MRI) may also be useful for lymph node imaging. F-FDHT is an androgen analog with high affinity for the androgen receptor. This has promise as a non-invasive way to monitor treatment response.
Modalities that have not proven to be useful include choline PET or acetate/PET for low PSA metastases. Prostate-specific membrane antigen (PSMA), a transmembrane protein found in the prostate, has been shown to be more specific than either choline or acetate PET.
References
- 1.Riggin J, Siddiqui MM. Development of intermediate and high-risk prostate cancer after testicular cancer. J Clin Oncol. 2015;33(suppl 7) abstr 177. [Google Scholar]
- 2.Musunuru HB, et al. Cautionary tale of active surveillance in intermediate-risk patients: Overall and cause-specific survival in the Sunnybrook experience. J Clin Oncol. 2015;33(suppl 7) abstr 163. [Google Scholar]
- 3.Gravis G, et al. Androgen deprivation therapy (ADT) plus docetaxel (D) versus ADT alone for hormone-naïve metastatic prostate cancer (PCa): Long-term analysis of the GETUG-AFU 15 phase III trial. J Clin Oncol. 2015;33(suppl 7) abstr 140. [Google Scholar]
- 4.Sweeney C, et al. Impact on overall survival (OS) with chemohormonal therapy versus hormonal therapy for hormone-sensitive newly metastatic prostate cancer (mPrCa): An ECOG-led phase III randomized trial. J Clin Oncol. 2014;32:5s. (suppl; abstr LBA2). [Google Scholar]
- 5.Small EJ. Looking in the crystal ball or could the negative outcomes of these phase 3 trials been predicted?. Discussion presented at GU-ASCO 2015; Abstract Discussion. [Google Scholar]
- 6.Flaig TW, et al. Phase II trial of abiraterone acetate (AA) treatment for metastatic prostate cancer (PC) patients with a PSA of more than four following initial androgen deprivation therapy: SWOG S1014. J Clin Oncol. 2015;33(suppl 7) abstr 152. [Google Scholar]
- 7.Hussain M, et al. Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: data from Southwest Oncology Group Trial 9346 (INT-0162) J Clin Oncol. 2006;24(24):3984–90. doi: 10.1200/JCO.2006.06.4246. [DOI] [PubMed] [Google Scholar]
- 8.Smith MR, et al. Final analysis of COMET-1: Cabozantinib (Cabo) versus prednisone (Pred) in metastatic castration-resistant prostate cancer (mCRPC) patients (pts) previously treated with docetaxel (D) and abiraterone (A) and/or enzalutamide (E) J Clin Oncol. 2015;33(suppl 7) abstr 139. [Google Scholar]
- 9.Graff JN, et al. Clinical outcomes and safety in men ≥75 and <75 years with metastatic castration-resistant prostate cancer (mCRPC) treated with enzalutamide in the phase 3 PREVAIL trial. J Clin Oncol. 2015;33(suppl 7) abstr 200. [Google Scholar]
- 10.De Bono JS, et al. Response to taxane chemotherapy as first subsequent therapy after abiraterone acetate (AA) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC): Post-hoc analysis of COU-AA-302. J Clin Oncol. 2015;33(suppl 7) abstr 184. [Google Scholar]
- 11.Gizzi M, et al. Previous enzalutamide therapy and response to subsequent taxane therapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2015;33(suppl 7) abstr 227. [Google Scholar]
- 12.Antonarakis ES, et al. AR splice variant 7 (AR-V7) and response to taxanes in men with metastatic castration-resistant prostate cancer (mCRPC) J Clin Oncol. 2015;33(suppl 7) abstr 138. [Google Scholar]
- 13.Roberts J, et al. Androgen receptor modulation optimized for response in splice variant (ARMOR3-SV): Randomized, open-label, multicenter, controlled study of galeterone versus enzalutamide (enz) in men expressing AR-V7 splice variant (SV), metastatic castrate resistant prostate cancer (mCRPC) J Clin Oncol. 2015;33(suppl 7) abstr 259. [Google Scholar]
- 14.Vogelzang NJ, et al. Radium-223 dichloride (Ra-223) in U.S. expanded access program (EAP) J Clin Oncol. 2015;33(suppl 7) abstr 247. [Google Scholar]
- 15.Sartor AO, et al. Prior and concurrent use of abiraterone and enzalutamide with Ra-223 in an expanded access setting. J Clin Oncol. 2015;33(suppl 7) abstr 253. [Google Scholar]
- 16.Morris MJ, et al. Effect of radium-223 dichloride (Ra-223) on pain from US EAP. J Clin Oncol. 2015;33(suppl 7) abstr 160. [Google Scholar]
- 17.Morris MJ, et al. Effects of radium-223 dichloride (Ra-223) with docetaxel (D) versus D on prostate-specific antigen (PSA) and bone alkaline phosphatase (bALP) in patients (pts) with castration-resistant prostate cancer (CRPC) and bone metastases (mets): A phase 1/2a clinical trial. J Clin Oncol. 2015;33(suppl 7) abstr 202. [Google Scholar]
- 18.Massard C, et al. Pharmacokinetics, activity, and safety of ODM-201 in chemotherapy-naïve patients with metastatic castration-resistant prostate cancer: An open-label phase I trial with long-term extension. J Clin Oncol. 2015;33(suppl 7) abstr 230. [Google Scholar]
- 19.Michalski JM, et al. A randomized trial of 79.2Gy versus 70.2Gy radiation therapy (RT) for localized prostate cancer. J Clin Oncol. 2015;33(suppl 7) abstr 4. [Google Scholar]
- 20.Nabid A, et al. Place of short-term androgen deprivation therapy in intermediate-risk prostate cancer treated with radiotherapy: a phase III trial. J Clin Oncol. 2015;33(suppl 7) abstr 5. [Google Scholar]
- 21.Morris WJ, et al. ASCENDE-RT: A multicenter, randomized trial of dose-escalated external beam radiation therapy (EBRT-B) versus low-dose-rate brachytherapy (LDR-B) for men with unfavorable-risk localized prostate cancer. J Clin Oncol. 2015;33(suppl 7) abstr 3. [Google Scholar]
- 22.Pinto PA. New imaging modalities to identify early metastatic disease. Presentation at the at GU-ASCO 2015; General Session 2. [Google Scholar]