Summary
Inorganic polyphosphate (polyP), a linear polymer of phosphates, is present in many infectious microorganisms and is secreted by mast cells and platelets. PolyP has recently been shown to accelerate blood clotting and slow fibrinolysis, in a manner that is highly dependent on polymer length. Very long-chain polyP (of the type present in microorganisms) is an especially potent trigger of the contact pathway, enhances the proinflammatory activity of histones, and may participate in host responses to pathogens. PolyP also inhibits complement, providing another link between polyP and inflammation/innate immunity. Platelet-size polyP (which is considerably shorter) accelerates factor V activation, opposes the anticoagulant action of tissue factor pathway inhibitor, modulates fibrin clot structure, and promotes factor XI activation. PolyP may have utility in treating bleeding. It is also a potential target for the development of anti-thrombotic drugs with a novel mechanism of action and potentially fewer bleeding side effects compared to conventional anticoagulants.
Keywords: Blood Coagulation, Coagulation Factor XI, Inflammation, Kallikrein-Kinin System, Platelets, Polyphosphates
Introduction
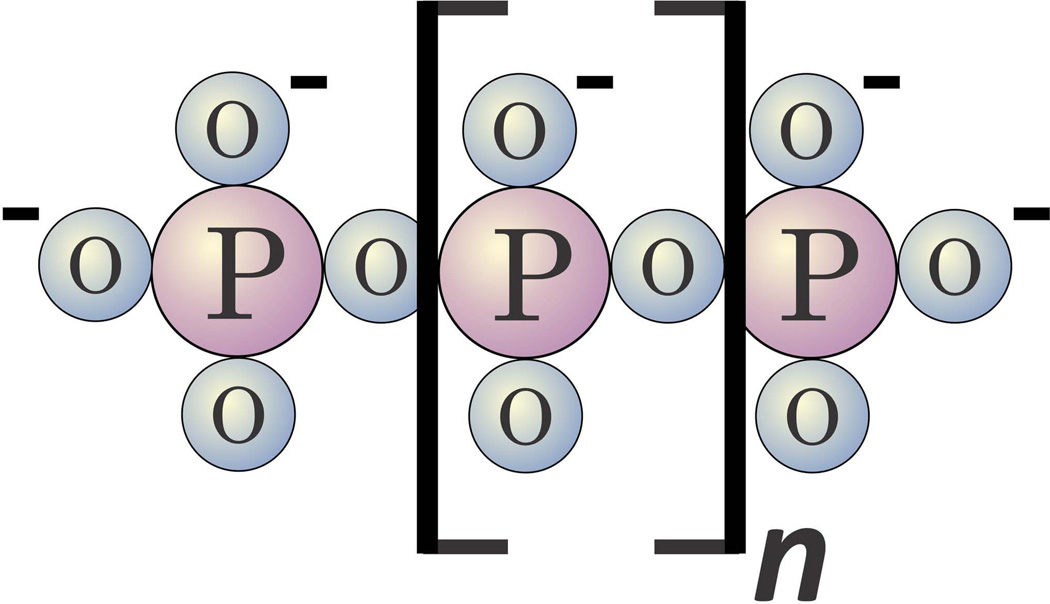
Inorganic polyphosphates (polyP) are linear chains of phosphates held together by the same sort of high-energy phosphoanhydride bonds that occur in ATP (Fig. 1). Widespread throughout biology, polyP can vary greatly in size depending on organism and cell type, ranging from just a few phosphates to thousands of phosphates long [1, 2]. Biosynthesis of polyP has been studied most extensively in microorganisms, and is typically catalysed by polymerases that transfer the γ-phosphate from ATP onto the end of the growing polyP chain [3]. How polyP is synthesised in higher organisms remains unknown, however. A number of phosphatases have been identified that can degrade polyP, including endopolyphosphatases that cleave internal phosphoanhydride bonds, and exopolyphosphatases that sequentially remove the terminal phosphates of polyP [4]. PolyP decays in human serum or plasma with a half-life of about 90 minutes [5].
Figure 1.
Structure of inorganic polyphosphate (polyP). PolyP is a linear, negatively charged polymer of phosphates held together by high-energy phosphoanhydride bonds. Microbial polyP ranges in size from tens of phosphates to thousands of phosphates long [3, 63], while polyP secreted from human platelets and mast cells is shorter and much less heterodisperse — approximately 60 to 100 phosphate units long [7, 8, 15, 34].
PolyP is typically stored in intracellular organelles that have been given a variety of names depending on the organism. Docampo and colleagues have proposed naming these organelles “acidocalcisomes” [6], since they are acidic and, in addition to their polyP content, they also generally contain substantial stores of calcium ions. In mammalian cells, polyP was found to be a major constituent of the dense granules of human platelets [7] and to be abundant in a subset of the secretory granules of mast cells [8]. Both platelet dense granules and the polyP-containing secretory granules of mast cells have properties in common with acidocalcisomes of lower organisms, as they are acidic compartments in which polyP is stored in a highly condensed state along with metal ions such as Ca2+. Additionally, polyP has been detected in lysosomes [9], mitochondria and nuclei of mammalian cells [10],
Recent studies have begun to elucidate the roles that polyP plays in regulatory and metabolic functions in mammalian cells (reviewed by Azevedo and Saiardi [11]). In this review, we will focus on recent findings from our laboratory and others that polyP is a potent modulator of the blood clotting system in haemostasis, thrombosis and inflammation.
PolyP in blood
PolyP is secreted by activated human platelets
The dense granules of human platelets (also called delta granules) are spherical organelles that are acidic [12], electron-dense [13], and contain metal ions such as Ca2+ and Mg2+ along with inorganic monophosphate and pyrophosphate [14]. In 2004, Ruiz et al. [7] first reported that platelet dense granules share many properties with acidocalcisomes, including an abundant store of polyP. PolyP in platelet dense granules is both shorter and far less heterodisperse than microbial polyP, ranging in length from about 60 to 100 phosphate units [7, 15]. Upon platelet activation, polyP is secreted from dense granules along with the other granule contents [7, 15], such that polyP can reach concentrations of about 1 to 2 μM when all platelets in whole blood are activated. (PolyP concentrations are typically given in terms of the concentration of phosphate monomer.) We subsequently showed that platelet polyP is a potent modulator of blood coagulation and fibrinolysis [5], findings that are reviewed in detail below.
Human patients with defects in platelet dense granules are known, and the deficiency is associated with a bleeding tendency [16]. Indeed, patients with platelet dense granule defects were recently reported to have approximately tenfold reduced levels of polyP in their platelets [17]. In an interesting recent study, Ghosh et al. [18] studied mice in which the gene encoding the enzyme, inositol hexakisphosphate kinase 1 (Ip6k1), was deleted. Yeast lacking inositol hexakisphosphate kinase are known to be devoid of polyP, so these authors hypothesised that knocking out the homologous gene in mice may also affect polyP accumulation in these animals. Mammals have three isoforms of this enzyme, but knocking out the gene for one of them (Ip6k1) resulted in mice whose platelets had approximately tenfold lower polyP levels than normal. These mice had compromised haemostasis and were protected against experimentally-induced thrombosis [18]. The relationship between the activity of inositol hexakisphosphate kinase and accumulation of polyP is not understood, underscoring our lack of knowledge of how polyP is synthesised in higher eukaryotes.
PolyP in other blood cell types
Mast cells and basophils contain numerous secretory granules, a subset of which were shown recently to share features with acidocalcisomes, including being spherical, electron-dense, and containing phosphate and divalent metal ions [8]. Furthermore, these acidocalcisome-like secretory granules in mast cells and basophils were found to contain abundant polyP, with polymer lengths very similar to those found in platelets (about 60–100 phosphates long). Mast cell polyP is localised in serotonin- but not histamine-containing granules, and is released when these cells secrete their granule contents [8]. Notably, platelet dense granules also contain serotonin.
PolyP has been reported to trigger apoptosis in plasma cells and myeloma cells, although not normal lymphocytes [19]. Myeloma cells are reported to contain higher levels of polyP in their nuclei compared with normal plasma cells [20]. And finally, polyP has also been reported in human red blood cells as a component of the Ca2+-ATPase pump [21, 22].
PolyP modulates the blood clotting cascade
Triggering clotting via the contact pathway
The contact pathway of blood clotting (sometimes call the “intrinsic” pathway) is triggered when two protease zymogens, prekallikrein and factor XII, assemble together with the protein cofactor, high molecular weight kininogen, on artificial surfaces such as clay or glass, or on certain anionic polymers. This results in both autoactivation of factor XII and the reciprocal activation of prekallikrein by factor XIIa and of factor XII by kallikrein. The resulting factor XIIa then converts factor XI to XIa, which then activates factor IX, leading to the final common pathway of blood clotting. This culminates in a burst of thrombin, which in turn converts fibrinogen to fibrin and robustly activates platelets (Fig. 2). The contact pathway is totally dispensable for normal haemostasis, since humans or mice with complete factor XII deficiency have no bleeding problems [23].
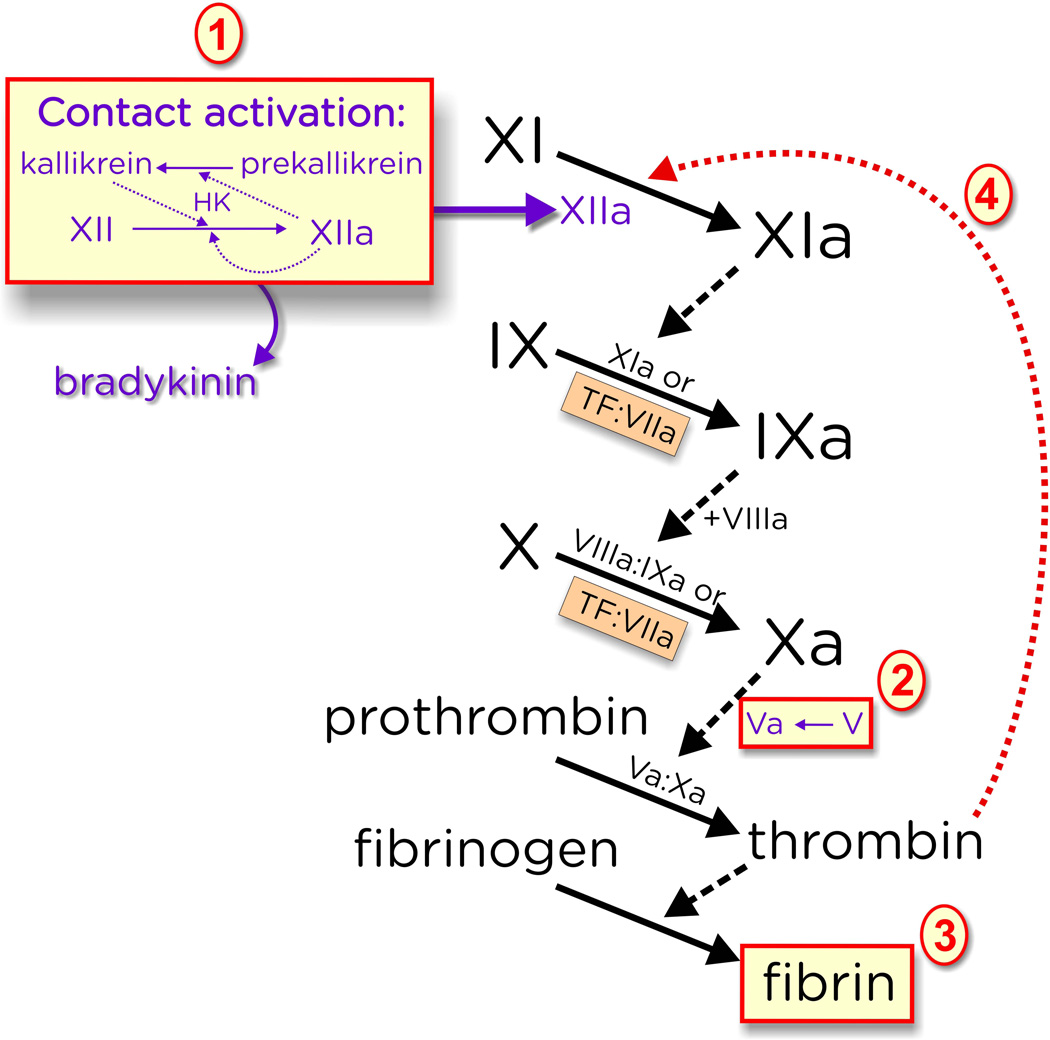
Figure 2.
PolyP modulates blood clotting at the indicated points. (1) Very long-chain polyP (of the type found in microbes) is a potent trigger of the contact pathway of blood clotting, leading to generation of factor XIIa and release the inflammatory mediator, bradykinin, via proteolysis from high molecular weight kininogen (HK); (2) PolyP accelerates factor V activation; (3) PolyP enhances fibrin clot structure and renders clots resistant to fibrinolysis; and (4) PolyP greatly accelerates factor XI activation by thrombin. (Not shown is the ability of polyP to antagonise the anticoagulant activity of TFPI, nor the ability of polyP to stimulate factor XI autoactivation.) While efficient triggering of the contact pathway requires very long-chain polyP (step 1), the other three reactions are also promoted by polyP of the size secreted by activated platelets (about 60 to100 phosphates long).
Although dispensable for haemostasis, the contact pathway is thought to contribute to at least some types of thrombosis. In multiple animal models, factor XII deficiency has been shown to protect against arterial and venous thrombosis [24, 25]. Severe hereditary factor XI deficiency in humans is associated with a mild bleeding tendency. Interestingly, these patients are significantly protected against ischaemic stroke and deep-vein thrombosis, but not against myocardial infarction [26, 27]. Furthermore, epidemiologic studies have shown that elevated factor XI correlates with risk of ischaemic stroke and venous thromboembolism (reviewed by He et al. [28]). Taken together, results from both humans and experimental animals argue that inhibitors that target factor XI and/or factor XIa may reduce thrombotic risk with lower bleeding side-effects compared to conventional anticoagulants that target the common pathway of blood clotting [29].
Although some of the best-known activators of the contact pathway are artificial surfaces and substances such as glass, ground-up clay, diatomaceous earth and ellagic acid, humans clearly did not evolve this pathway to respond to such artificial activators. Considerable attention has therefore been addressed to identifying the true (patho)physiologic activators of the contact pathway. In recent years, a number of candidate activators have been identified, including extracellular nucleic acids [30], misfolded proteins [31], and specific microbial proteins (reviewed by Nickel and Renné [32]).
We recently reported that polyP is a highly potent activator of the contact pathway in vitro and in vivo (see Fig. 2) [5, 15], and that polyP binds with high affinity to multiple proteins in the contact pathway [5, 33, 34]. Importantly, the ability of polyP to trigger clotting via the contact pathway is highly dependent on polymer length, with a specific activity that increases essentially exponentially with polymer length [34]. Very long-chain polyP is frequently found in microbes, so it is tempting to speculate that activation of the contact pathway via microbial polyP could play a role in host response to pathogens. Administering high levels of polyP intravenously in mice led to lethal pulmonary embolism, while factor XII-deficient mice, or mice administered a factor XIIa inhibitor, survived the polyP challenge [15]. Long-chain polyP can therefore be thrombogenic in vivo, in a factor XII-dependent manner [15].
Conversely, we found that polyP of the size released by activated platelets possessed a small, but measurable ability to activate clotting via the contact pathway [34]. A number of studies over the years have reported that activated human platelets are weakly able to trigger the contact pathway in a factor XII-dependent manner [35]. Faxäl et al. [36] in particular have pointed out that platelet-size polyP has very little ability to trigger factor XII activation, consistent with our report that the ability of polyP to trigger the contact pathway of blood clotting increases essentially exponentially with polymer length [34]. These findings are consistent with the notion that platelets are good at accelerating clotting reactions but poor at initiating them.
PolyP, the contact pathway, and inflammation
The contact pathway is known to contribute to inflammatory processes. Activation of the contact pathway (perhaps better called the plasma kallikrein-kinin system [37]) results in kallikrein-mediated release of bradykinin via proteolysis of high molecular weight kininogen. Bradykinin has potent vasoactive function [38]. Furthermore, kallikrein can directly activate complement components C3 and C5 [39, 40], while factor XIIa can trigger the classical complement cascade [41].
As with other contact activators, polyP promotes bradykinin release [15]. Subcutaneous injection of long-chain polyP induces localised capillary leak in a factor XII-dependent manner [15, 42], while injecting polyP intraperitoneally can induce a lethal, systemic reduction in blood pressure that is dependent on factor XII and bradykinin [15].
PolyP has additional proinflammatory effects beyond to its ability to trigger contact activation. Extracellular histones are strongly proinflammatory and also exhibit procoagulant activities [43]. PolyP binds to histones and increases their ability to induce platelet activation, and to accelerate thrombin generation in a factor XII-independent manner [44]. PolyP was also shown to increase barrier permeability and apoptosis in cultured endothelial cells in a mechanism involving NF-κB activation [45]. PolyP was further shown to amplify the ability of histone H4- and HMGB1 to mediate inflammatory signalling in human umbilical vein endothelial cells via interaction with cell-surface receptors, RAGE and P2Y1 [46]. On the other hand, polyP can exhibit what appear to be anti-inflammatory actions: long-chain polyP suppresses complement via binding to and destabilising C5b,6, thereby inhibiting the membrane attack complex [47].
Amplification of thrombin generation by polyP
PolyP of the size secreted by activated platelets acts at several steps in the clotting cascade to influence the rate of thrombin generation (Fig. 2): it enhances the conversion of factor V to Va, it greatly accelerates factor XI activation, and it strongly antagonises the anticoagulant activity tissue factor pathway inhibitor (TFPI) [34]. Thus, an important effect of polyP on blood clotting is a shortening of the time to the thrombin burst [5]. Platelets from Hermansky-Pudlak syndrome patients (which lack dense granules) support thrombin generation less robustly, but this can be rectified by exogenously added polyP [15].
Factor Va occupies a key, central role in the clotting cascade as the essential protein cofactor for factor Xa in the final common part of the clotting pathway. We found that polyP of the size secreted by activated platelets was able to accelerate the rate of factor V activation by factor Xa, thrombin [5, 34] and factor XIa [48].
PolyP accelerates factor XI activation
In the classic waterfall or cascade model of blood clotting, factor XI is activated by factor XIIa. However, this mechanism of factor XI is clearly irrelevant to haemostasis, since persons with severe factor XII deficiency do not have any bleeding tendency, while severe factor XI-deficient patients can exhibit clinically significant bleeding [26]. This suggests that, for it to function in normal haemostasis, factor XI must be activated by a protease other than factor XIIa. A possible answer to this conundrum was proposed by two groups in 1991, when it was shown that thrombin could feed back and activate factor XI [49, 50] in a feedback reaction that was proposed to lead to more extended thrombin generation. However, since this reaction is very slow, its highly unfavourable kinetics made it unlikely to be significant in plasma [51, 52]. We recently discovered that platelet-sized polyP is a highly potent cofactor for the activation of factor XI by thrombin [53], accelerating the rate of this reaction some 3000-fold [53, 54] (Fig. 2). PolyP also markedly accelerated factor XI autoactivation [53] and the rate of factor V activation by factor XIa [48]. PolyP may therefore be the “missing” cofactor that can explain the otherwise puzzling role of factor XI in haemostasis.
PolyP antagonises TFPI anticoagulant function
TFPI is a Kunitz-type protease inhibitor that circulates in plasma and is found in other compartments in vivo, including on the surface of endothelial cells and in platelets. It binds to and inhibits both factor Xa and the factor VIIa/tissue factor complex [55]. We found that polyP completely abrogates the anticoagulant function of TFPI in plasma clotting assays [5], and furthermore, that polyP secreted by platelets strongly inhibits TFPI function [5, 15]. It has been shown that any factor Xa that is already bound to its protein cofactor, factor Va, is resistant to inhibition by TFPI, especially in the presence of prothrombin [56]. The ability of polyP to accelerate factor V activation may therefore accelerate the rate at which newly-generated factor Xa is bound by factor Va and is thereby protected from inhibition by TFPI. More recent work has shown that TFPI can still inhibit factor Xa in complex with factor Va, provided the factor Va is only partially activated (i.e., retains portions of the B domain) as is observed when factor Xa activates factor V, or when partially activated factor V is released from activated platelets [57].
In addition to its ability to antagonise the anticoagulant activity of TFPI, polyP has also been shown to antagonise the anticoagulant activity of a variety of anticoagulant drugs, including heparins and direct inhibitors of thrombin and factor Xa [58]. PolyP, or polyP-based haemostatic agents, may therefore have utility as general agents to treat bleeding, and perhaps as reversal agents that can antagonise the action of anticoagulant drugs.
PolyP modulates fibrin clot structure and stability
Purified fibrinogen can be converted to fibrin by the proteolytic action of thrombin. We found that fibrin clots formed in the presence of polyP are more turbid, contain thicker fibrin fibrils, are more resistant to elastic stretching, and are more resistant to fibrinolysis than are fibrin clots formed in the absence of polyP [59]. It appears that polyP may be incorporated directly into fibrin clots. Interestingly, pyrophosphate (PPi) abrogates the ability of polyP to enhance fibrin clot structure but has no measurable effect on fibrin clots formed in the absence of polyP [34]. Platelet dense granules also contain substantial quantities of pyrophosphate [7], but little is known about its function. While the mechanisms by which polyP causes fibrin clots to be resistant to fibrinolysis are not completely understood, it has been shown that forming fibrin clots in the presence of polyP results in inhibition of binding of tissue-type plasminogen activator and plasminogen to fibrin, possibly by masking C-terminal lysine residues [60].
PolyP inhibitors as novel antithrombotic agents
Proof-of-principal polyP inhibitors have recently been identified, including cationic proteins, small molecules and dendrimers [42, 61]. These anti-polyP agents were thromboprotective in mouse models of arterial and venous thrombosis, while having fewer bleeding side effects than heparin. On the other hand, all of these compounds have significant toxicities, making them unlikely starting points for developing new drugs. Most recently, we reported that a new class of nontoxic, dendrimer-like cationic compounds are potent polyP blockers and are effective in protecting mice against thrombosis [62]. These compounds had substantially reduced bleeding side effects compared with heparin.
Conclusions
Recent studies from our lab and others have now shown that both platelet and microbial polyP are potent modulators of the blood clotting cascade, resulting in accelerated thrombin generation, reversal of the anticoagulant activity of a variety of both natural anticoagulants (TFPI) and anticoagulant drugs (heparins and direct inhibitors of factor Xa and thrombin), enhanced fibrin clot structure, and resistance of clots to fibrinolysis. PolyP or suitable derivatives might therefore have utility as haemostatic agents to treat bleeding. Recent work also implicates polyP (especially, long-chain polyP) as contributing to host-pathogen interactions via its ability to trigger the contact pathway. PolyP blockers may therefore have utility as novel antithrombotic/anti-inflammatory agents with reduced bleeding side effects compared with conventional anticoagulant drugs that target the final common pathway of blood clotting. Doubtless, much remains to be discovered regarding the roles of polyP in haemostasis, thrombosis, and inflammation/innate immunity.
Acknowledgements
The authors’ studies were supported by grant R01 HL047014 from the National Heart, Lung and Blood Institute of the National Institutes of Health.
Footnotes
Disclosure of Conflicts of Interest
J. H. Morrissey and S. A. Smith report grants from National Institutes of Health, during the conduct of the study. Both authors have patent applications on medical uses of polyphosphate and polyphosphate inhibitors pending. J. H. Morrissey reports personal fees from rEVO Biologics, Biogen Idec and other from KeraFAST, outside the submitted work.
References
- 1.Ault-Riché D, Fraley CD, Tzeng CM, Kornberg A. Novel assay reveals multiple pathways regulating stress-induced accumulations of inorganic polyphosphate in Escherichia coli. J Bacteriol. 1998;180:1841–1847. doi: 10.1128/jb.180.7.1841-1847.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown MR, Kornberg A. Inorganic polyphosphate in the origin and survival of species. Proc Natl Acad Sci U S A. 2004;101:16085–16087. doi: 10.1073/pnas.0406909101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kornberg A, Rao NN, Ault-Riché D. Inorganic polyphosphate: a molecule of many functions. Annu Rev Biochem. 1999;68:89–125. doi: 10.1146/annurev.biochem.68.1.89. [DOI] [PubMed] [Google Scholar]
- 4.Moreno SN, Docampo R. Polyphosphate and its diverse functions in host cells and pathogens. PLoS Path. 2013;9:e1003230. doi: 10.1371/journal.ppat.1003230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith SA, Mutch NJ, Baskar D, Rohloff P, Docampo R, Morrissey JH. Polyphosphate modulates blood coagulation and fibrinolysis. Proc Natl Acad Sci U S A. 2006;103:903–908. doi: 10.1073/pnas.0507195103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Docampo R, Moreno SN. Acidocalcisomes. Cell Calcium. 2011;50:113–119. doi: 10.1016/j.ceca.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruiz FA, Lea CR, Oldfield E, Docampo R. Human platelet dense granules contain polyphosphate and are similar to acidocalcisomes of bacteria and unicellular eukaryotes. J Biol Chem. 2004;279:44250–44257. doi: 10.1074/jbc.M406261200. [DOI] [PubMed] [Google Scholar]
- 8.Moreno-Sanchez D, Hernandez-Ruiz L, Ruiz FA, Docampo R. Polyphosphate is a novel pro-inflammatory regulator of mast cells and is located in acidocalcisomes. J Biol Chem. 2012;287:28435–28444. doi: 10.1074/jbc.M112.385823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pisoni RL, Lindley ER. Incorporation of [32P]orthophosphate into long chains of inorganic polyphosphate within lysosomes of human fibroblasts. J Biol Chem. 1992;267:3626–3631. [PubMed] [Google Scholar]
- 10.Kumble KD, Kornberg A. Inorganic polyphosphate in mammalian cells and tissues. J Biol Chem. 1995;270:5818–5822. doi: 10.1074/jbc.270.11.5818. [DOI] [PubMed] [Google Scholar]
- 11.Azevedo C, Saiardi A. Functions of inorganic polyphosphates in eukaryotic cells: a coat of many colours. Biochem Soc Trans. 2014;42:98–102. doi: 10.1042/BST20130111. [DOI] [PubMed] [Google Scholar]
- 12.Dean GE, Fishkes H, Nelson PJ, Rudnick G. The hydrogen ion-pumping adenosine triphosphatase of platelet dense granule membrane. Differences from F1F0- and phosphoenzyme-type ATPases. J Biol Chem. 1984;259:9569–9574. [PubMed] [Google Scholar]
- 13.White JG. The dense bodies of human platelets: inherent electron opacity of the serotonin storage particles. Blood. 1969;33:598–606. [PubMed] [Google Scholar]
- 14.Fukami MH, Dangelmaier CA, Bauer JS, Holmsen H. Secretion, subcellular localization and metabolic status of inorganic pyrophosphate in human platelets. A major constituent of the amine-storing granules. Biochem J. 1980;192:99–105. doi: 10.1042/bj1920099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Müller F, Mutch NJ, Schenk WA, Smith SA, Esterl L, Spronk HM, Schmidbauer S, Gahl WA, Morrissey JH, Renné T. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell. 2009;139:1143–1156. doi: 10.1016/j.cell.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gunay-Aygun M, Huizing M, Gahl WA. Molecular defects that affect platelet dense granules. Semin Thromb Hemost. 2004;30:537–547. doi: 10.1055/s-2004-835674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernández-Ruiz L, Sáez-Benito A, Pujol-Moix N, Rodríguez-Martorell J, Ruiz FA. Platelet inorganic polyphosphate decreases in patients with delta storage pool disease. J Thromb Haemost. 2009;7:361–363. doi: 10.1111/j.1538-7836.2008.03238.x. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh S, Shukla D, Suman K, Lakshmi BJ, Manorama R, Kumar S, Bhandari R. Inositol hexakisphosphate kinase 1 maintains hemostasis in mice by regulating platelet polyphosphate levels. Blood. 2013;122:1478–1486. doi: 10.1182/blood-2013-01-481549. [DOI] [PubMed] [Google Scholar]
- 19.Hernandez-Ruiz L, González-García I, Castro C, Brieva JA, Ruiz FA. Inorganic polyphosphate and specific induction of apoptosis in human plasma cells. Haematologica. 2006;91:1180–1186. [PubMed] [Google Scholar]
- 20.Jimenez-Nuñez MD, Moreno-Sanchez D, Hernandez-Ruiz L, Benítez-Rondán A, Ramos-Amaya A, Rodríguez-Bayona B, Medina F, Brieva JA, Ruiz FA. Myeloma cells contain high levels of inorganic polyphosphate which is associated with nucleolar transcription. Haematologica. 2012;97:1264–1271. doi: 10.3324/haematol.2011.051409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reusch RN, Huang R, Kosk-Kosicka D. Novel components and enzymatic activities of the human erythrocyte plasma membrane calcium pump. FEBS Lett. 1997;412:592–596. doi: 10.1016/s0014-5793(97)00863-6. [DOI] [PubMed] [Google Scholar]
- 22.Reusch RN. Transmembrane ion transport by polyphosphate/poly-(R)-3-hydroxybutyrate complexes. Biochemistry (Mosc) 2000;65:280–295. [PubMed] [Google Scholar]
- 23.Colman RW, Schmaier AH. Contact system: a vascular biology modulator with anticoagulant, profibrinolytic, antiadhesive, and proinflammatory attributes. Blood. 1997;90:3819–3843. [PubMed] [Google Scholar]
- 24.Gailani D, Renné T. Intrinsic pathway of coagulation and arterial thrombosis. Arterioscler Thromb Vasc Biol. 2007;27:2507–2513. doi: 10.1161/ATVBAHA.107.155952. [DOI] [PubMed] [Google Scholar]
- 25.Müller F, Renné T. Novel roles for factor XII-driven plasma contact activation system. Curr Opin Hematol. 2008;15:516–521. doi: 10.1097/MOH.0b013e328309ec85. [DOI] [PubMed] [Google Scholar]
- 26.Seligsohn U. Factor XI deficiency in humans. J Thromb Haemost. 2009;7(Suppl 1):84–87. doi: 10.1111/j.1538-7836.2009.03395.x. [DOI] [PubMed] [Google Scholar]
- 27.Salomon O, Steinberg DM, Zucker M, Varon D, Zivelin A, Seligsohn U. Patients with severe factor XI deficiency have a reduced incidence of deep-vein thrombosis. Thromb Haemost. 2011;105:269–273. doi: 10.1160/TH10-05-0307. [DOI] [PubMed] [Google Scholar]
- 28.He R, Chen D, He S. Factor XI: hemostasis, thrombosis, and antithrombosis. Thromb Res. 2012;129:541–550. doi: 10.1016/j.thromres.2011.11.051. [DOI] [PubMed] [Google Scholar]
- 29.Renné T, Oschatz C, Seifert S, Müller F, Antovic J, Karlman M, Benz PM. Factor XI deficiency in animal models. J Thromb Haemost. 2009;7(Suppl 1):79–83. doi: 10.1111/j.1538-7836.2009.03393.x. [DOI] [PubMed] [Google Scholar]
- 30.Kannemeier C, Shibamiya A, Nakazawa F, Trusheim H, Ruppert C, Markart P, Song Y, Tzima E, Kennerknecht E, Niepmann M, von Bruehl ML, Sedding D, Massberg S, Gunther A, Engelmann B, Preissner KT. Extracellular RNA constitutes a natural procoagulant cofactor in blood coagulation. Proc Natl Acad Sci U S A. 2007;104:6388–6393. doi: 10.1073/pnas.0608647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maas C, Govers-Riemslag JW, Bouma B, Schiks B, Hazenberg BP, Lokhorst HM, Hammarstrom P, ten Cate H, de Groot PG, Bouma BN, Gebbink MF. Misfolded proteins activate factor XII in humans, leading to kallikrein formation without initiating coagulation. J Clin Invest. 2008;118:3208–3218. doi: 10.1172/JCI35424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nickel KF, Renné T. Crosstalk of the plasma contact system with bacteria. Thromb Res. 2012;130(Suppl 1):S78–S83. doi: 10.1016/j.thromres.2012.08.284. [DOI] [PubMed] [Google Scholar]
- 33.Choi SH, Collins JN, Smith SA, Davis-Harrison RL, Rienstra CM, Morrissey JH. Phosphoramidate end labeling of inorganic polyphosphates: facile manipulation of polyphosphate for investigating and modulating its biological activities. Biochemistry. 2010;49:9935–9941. doi: 10.1021/bi1014437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith SA, Choi SH, Davis-Harrison R, Huyck J, Boettcher J, Reinstra CM, Morrissey JH. Polyphosphate exerts differential effects on blood clotting, depending on polymer size. Blood. 2010;116:4353–4359. doi: 10.1182/blood-2010-01-266791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caen J, Wu Q. Hageman factor, platelets and polyphosphates: early history and recent connection. J Thromb Haemost. 2010;8:1670–1674. doi: 10.1111/j.1538-7836.2010.03893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Faxalv L, Boknas N, Strom JO, Tengvall P, Theodorsson E, Ramstrom S, Lindahl TL. Putting polyphosphates to the test: evidence against platelet-induced activation of factor XII. Blood. 2013;122:3818–3824. doi: 10.1182/blood-2013-05-499384. [DOI] [PubMed] [Google Scholar]
- 37.Schmaier AH. Assembly, activation, and physiologic influence of the plasma kallikrein/kinin system. Int Immunopharmacol. 2008;8:161–165. doi: 10.1016/j.intimp.2007.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Björkqvist J, Jämsä A, Renné T. Plasma kallikrein: the bradykinin-producing enzyme. Thromb Haemost. 2013;110:399–407. doi: 10.1160/TH13-03-0258. [DOI] [PubMed] [Google Scholar]
- 39.DiScipio RG. The activation of the alternative pathway C3 convertase by human plasma kallikrein. Immunology. 1982;45:587–595. [PMC free article] [PubMed] [Google Scholar]
- 40.Wiggins RC, Giclas PC, Henson PM. Chemotactic activity generated from the fifth component of complement by plasma kallikrein of the rabbit. J Exp Med. 1981;153:1391–1404. doi: 10.1084/jem.153.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghebrehiwet B, Randazzo BP, Dunn JT, Silverberg M, Kaplan AP. Mechanisms of activation of the classical pathway of complement by Hageman factor fragment. J Clin Invest. 1983;71:1450–1456. doi: 10.1172/JCI110898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith SA, Choi SH, Collins JN, Travers RJ, Cooley BC, Morrissey JH. Inhibition of polyphosphate as a novel strategy for preventing thrombosis and inflammation. Blood. 2012;120:5103–5110. doi: 10.1182/blood-2012-07-444935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Semeraro N, Ammollo CT, Semeraro F, Colucci M. Sepsis, thrombosis and organ dysfunction. Thromb Res. 2012;129:290–295. doi: 10.1016/j.thromres.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 44.Semeraro F, Ammollo CT, Morrissey JH, Dale GL, Friese P, Esmon NL, Esmon CT. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood. 2011;118:1952–1961. doi: 10.1182/blood-2011-03-343061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bae JS, Lee W, Rezaie AR. Polyphosphate elicits pro-inflammatory responses that are counteracted by activated protein C in both cellular and animal models. J Thromb Haemost. 2012;10:1145–1151. doi: 10.1111/j.1538-7836.2012.04671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dinarvand P, Hassanian SM, Qureshi SH, Manithody C, Eissenberg JC, Yang L, Rezaie AR. Polyphosphate amplifies proinflammatory responses of nuclear proteins through interaction with receptor for advanced glycation end products and P2Y1 purinergic receptor. Blood. 2014;123:935–945. doi: 10.1182/blood-2013-09-529602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wat JM, Foley JH, Krisinger MJ, Ocariza LM, Lei V, Wasney GA, Lameignere E, Strynadka NC, Smith SA, Morrissey JH, Conway EM. Polyphosphate suppresses complement via the terminal pathway. Blood. 2014;123:768–776. doi: 10.1182/blood-2013-07-515726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi SH, Smith SA, Morrissey JH. Polyphosphate accelerates factor V activation by factor XIa. Thromb Haemost. 2014;113 doi: 10.1160/TH14-06-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Naito K, Fujikawa K. Activation of human blood coagulation factor XI independent of factor XII. Factor XI is activated by thrombin and factor XIa in the presence of negatively charged surfaces. J Biol Chem. 1991;266:7353–7358. [PubMed] [Google Scholar]
- 50.Gailani D, Broze GJ., Jr Factor XI activation in a revised model of blood coagulation. Science. 1991;253:909–912. doi: 10.1126/science.1652157. [DOI] [PubMed] [Google Scholar]
- 51.Pedicord DL, Seiffert D, Blat Y. Feedback activation of factor XI by thrombin does not occur in plasma. Proc Natl Acad Sci U S A. 2007;104:12855–12860. doi: 10.1073/pnas.0705566104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scott CF, Colman RW. Fibrinogen blocks the autoactivation and thrombin-mediated activation of factor XI on dextran sulfate. Proc Natl Acad Sci U S A. 1992;89:11189–11193. doi: 10.1073/pnas.89.23.11189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi SH, Smith SA, Morrissey JH. Polyphosphate is a cofactor for the activation of factor XI by thrombin. Blood. 2011;118:6963–6970. doi: 10.1182/blood-2011-07-368811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Geng Y, Verhamme IM, Smith SA, Cheng Q, Sun M, Sheehan JP, Morrissey JH, Gailani D. Factor XI anion-binding sites are required for productive interactions with polyphosphate. J Thromb Haemost. 2013;11:2020–2028. doi: 10.1111/jth.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Broze GJ., Jr Tissue factor pathway inhibitor and the current concept of blood coagulation. Blood Coagul Fibrinolysis. 1995;6(Suppl 1):S7–S13. doi: 10.1097/00001721-199506001-00002. [DOI] [PubMed] [Google Scholar]
- 56.Mast AE, Broze GJ., Jr Physiological concentrations of tissue factor pathway inhibitor do not inhibit prothrombinase. Blood. 1996;87:1845–1850. [PubMed] [Google Scholar]
- 57.Wood JP, Bunce MW, Maroney SA, Tracy PB, Camire RM, Mast AE. Tissue factor pathway inhibitor-alpha inhibits prothrombinase during the initiation of blood coagulation. Proc Natl Acad Sci U S A. 2013;110:17838–17843. doi: 10.1073/pnas.1310444110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith SA, Morrissey JH. Polyphosphate as a general procoagulant agent. J Thromb Haemost. 2008;6:1750–1756. doi: 10.1111/j.1538-7836.2008.03104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith SA, Morrissey JH. Polyphosphate enhances fibrin clot structure. Blood. 2008;112:2810–2816. doi: 10.1182/blood-2008-03-145755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mutch NJ, Engel R, Uitte de Willige S, Philippou H, Ariens RA. Polyphosphate modifies the fibrin network and down-regulates fibrinolysis by attenuating binding of tPA and plasminogen to fibrin. Blood. 2010;115:3980–3988. doi: 10.1182/blood-2009-11-254029. [DOI] [PubMed] [Google Scholar]
- 61.Jain S, Pitoc GA, Holl EK, Zhang Y, Borst L, Leong KW, Lee J, Sullenger BA. Nucleic acid scavengers inhibit thrombosis without increasing bleeding. Proc Natl Acad Sci U S A. 2012;109:12938–12943. doi: 10.1073/pnas.1204928109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Travers RJ, Shenoi RA, Kalathottukaren MT, Kizhakkedathu JN, Morrissey JH. Nontoxic polyphosphate inhibitors reduce thrombosis while sparing hemostasis. Blood. 2014;124:3183–3190. doi: 10.1182/blood-2014-05-577932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brown MR, Kornberg A. The long and short of it – polyphosphate, PPK and bacterial survival. Trends Biochem Sci. 2008;33:284–290. doi: 10.1016/j.tibs.2008.04.005. [DOI] [PubMed] [Google Scholar]