Abstract
This retrospective study aimed to evaluate radiation-induced pneumonitis (RIP) and a related condition that we define in this report—prolonged minimal RIP (pmRIP)—after stereotactic body radiotherapy (SBRT) for Stage I primary lung cancer in patients with chronic obstructive pulmonary disease (COPD). We assessed 136 Stage I lung cancer patients with COPD who underwent SBRT. Airflow limitation on spirometry was classified into four Global Initiative for Chronic Obstructive Lung Disease (GOLD) grades, with minor modifications: GOLD 1 (mild), GOLD 2 (moderate), GOLD 3 (severe) and GOLD 4 (very severe). On this basis, we defined two subgroups: COPD-free (COPD −) and COPD-positive (COPD +). There was no significant difference in overall survival or cause-specific–survival between these groups. Of the 136 patients, 44 (32%) had pmRIP. Multivariate analysis showed that COPD and the Brinkman index were statistically significant risk factors for the development of pmRIP. COPD and the Brinkman index were predictive factors for pmRIP, although our findings also indicate that SBRT can be tolerated in early lung cancer patients with COPD.
Keywords: stereotactic body radiotherapy (SBRT), radiation-induced pneumonitis (RIP), prolonged minimal radiation-induced pneumonitis (pmRIP), Stage I lung cancer, chronic obstructive pulmonary disease (COPD)
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a significant risk factor for lung cancer, and smoking is a common risk factor for both lung cancer and COPD [1–3]. Since COPD also carries a high risk of post-operative complications, surgical treatment is not indicated for patients with lung cancer comorbid with severe COPD [4]. Stereotactic body radiotherapy (SBRT) is recognized as a highly effective treatment for early-stage lung cancer [5], but not all patients with idiopathic interstitial pneumonitis are candidates for radiotherapy. Also, not all early-stage lung cancer patients with severe COPD develop radiation-induced pneumonitis (RIP) after SBRT. Indeed, lung cancer patients with severe emphysema tolerated SBRT well, based on our previous experience. In this study, we retrospectively analyzed the clinical results of using SBRT to treat Stage I lung cancer patients with COPD at a single institution, focusing on patient survival and RIP. This is the first report concerning prolonged minimal RIP (pmRIP), which is defined as minimal pneumonitis without significantly diffuse infiltration or consolidation-associated ground-glass opacity in the irradiated volume lasting 6 months or more after SBRT for Stage I lung cancer associated with COPD.
MATERIALS AND METHODS
The institutional review board approved the study protocol, including the chart review. We obtained written informed consent from each patient for SBRT during the first examination.
Patients
Between June 2007 and October 2013, 220 patients with Stage I lung cancer were treated with SBRT at our institute. The inclusion criteria were: availability of follow-up computed tomography (CT) images at least 12 months after SBRT, no previous overlapping radiation exposure, and availability of spirometry measurement results; 84 patients were excluded for not meeting some of these criteria. The remaining 136 patients met the above criteria, and the median follow-up time for these patients was 33 months (range, 12–85 months).
The study included 101 men and 35 women, with a median age of 76 years (range, 55–98 years). COPD was defined according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria [1, 2], and on this basis patients were divided into two groups, COPD-free (COPD −) and COPD-positive (COPD +) (Table 1). Of the 74 patients (66%) in the COPD – group, 49 had a Brinkman index (BI, calculated as cigarettes/day × years) of 400 or more, while 54 of the 62 patients (87%) in the COPD + group had a BI of 400 or more (χ2 = 8.0038, P = 0.0047). The other 10 items were evenly distributed between the two groups. The most common histological type was adenocarcinoma (56 cases, 41%), followed by squamous cell carcinoma (24 cases, 18%). A different histology was present in 8 cases (6%), and the remaining 48 cases (35%) lacked a definitive histological diagnosis due to older age of the patient, the location of tumor, or complications such as severe or very severe COPD that prevented biopsy. A total of 74 patients were classified as having an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0; and 43, 9 and 10 patients had a PS of 1, 2 and 3, respectively. More than half the patients (56%) had multiple cancers; 48 had 2, 15 had 3, 10 had 4, 2 had 5, and 1 had 6 cancers. Of the 122 cases of multiple cancers, 51 (42%) were in the lung, 11 were in the prostate and large intestine, 9 were in the stomach and the head and neck regions, 8 were in the liver, 6 were in the kidney, 3 were in the urinary bladder, 3 were in the esophagus, 3 were malignant lymphoma, 2 were in the breast and skin, and 1 each was in the gall bladder, small intestine, adrenal gland, and thymus. When patients without a definitive histological diagnosis had multiple cancers, the primary or metastatic nature of the lung lesions was determined based on clinical and imaging findings.
Table 1.
Patients and tumor characteristics by COPD status
| Item | Category | COPD − (n = 74) | COPD + (n = 62) |
|---|---|---|---|
| Age | Median age (range) | 77 (58–94) | 76 (55–98) |
| Gender | Male : Female | 52:22 | 49:13 |
| Performance Status | 0:1:2:3 | 46:15:7:6 | 28:28:2:4 |
| Multiple cancers | None:2:3:4:5:6 | 31:30:7:5:0:1 | 29:18:8:5:2:0 |
| Brinkman index | <400: ≥400 | 25:49 | 8:54 |
| Chemotherapy | (−):(+) | 57:17 | 49:13 |
| Thoracic surgery | (−):(+) | 50:24 | 48:14 |
| Stage | IA:IB | 63:11 | 48:14 |
| Pathology | Ad:Sq:Others:No proof | 32:11:6:5 | 24:14:2:22 |
| Target location (lobe) | Upper:Middle:Lower | 41:5:28 | 40:2:20 |
| Laterality | Left:Right | 24:50 | 24:38 |
COPD = chronic obstructive pulmonary disease, Brinkman index = cigarettes/day × years, Ad = adenocarcinoma, Sq = squamous cell carcinoma.
Treatment methods
Treatment planning and procedures have been described in detail elsewhere [6]. In brief, a 6-MV X-ray Novalis unitTM (BrainLAB AG, Germany) was used for treatment, BrightSpeedTM (GE, USA) as CT-simulator, and BrainSCANTM and iPlan RT ImageTM (BrainLAB AG) for treatment planning. The pencil beam (PB) algorithm was used for dose calculation before September 2010, after which the Monte Carlo (MC) algorithm was used. Vac-Lok cushionsTM and HipFix ThermoplasticsTM (CIVCO, USA) were used as immobilization devices, and the ExacTrac X-ray positioning systemTM and 6-axis Robotic couchTM (BrainLAB AG) were used for fine localization. For respiratory motion suppression, the Air-Bag SystemTM (Niigata Megatoronics Co. Ltd, Japan) was used, both during the acquisition of treatment-planning CT images and during SBRT [6, 7].
The most common dose fractionation was 48 Gy/4 fractions to the PTV (n = 59), followed by 44 Gy/4 fractions to the GTV (n = 51). The former prescription was calculated using the PB algorithm and the latter using the MC algorithm. The other fractionation patterns were as follows: 12 patients were administered 60 Gy/10 fractions, 7 were administered 50 Gy/5 fractions, 2 were administered 54 Gy/9 fractions, and 1 each received 57.6 Gy/16 fractions, 58.5 Gy/9 fractions, 60 Gy/8 fractions, 60 Gy/12 fractions, and 70 Gy/14 fractions. Previous studies have shown that dose prescription to the GTV using the MC algorithm was more highly optimized than that to the PTV using the PB algorithm, and a lowest dose to 95% of the PTV (PTV D95) of 48 Gy using the PB algorithm was equivalent to a near-minimum dose to 99% of the GTV (GTV D99) of 44 Gy using the MC algorithm. Therefore, our current protocol has changed to a prescribed dose of 44 Gy in 4 fractions to the GTV using the MC algorithm from September 2010 [8, 9]. We generally used a treatment schedule with a larger number of fractions for central lesions and fewer fractions for peripheral lesions.
The probabilities of survival, local control (LC), and adverse events were calculated using the Kaplan–Meier method. Multiple logistic regression analysis was used for multivariate analyses. The National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) ver. 3 and 4 were used to determine the grade of SBRT-related adverse events [10, 11]. Airflow limitation of severity in COPD was defined according to the GOLD criteria [1, 2], with minor modification, such as exclusion of the estimation based on the post-bronchodilator forced expiratory volume in 1 s (FEV1). Airflow limitation on spirometry was classified into four grades (GOLD 1, mild; GOLD 2, moderate; GOLD 3, severe; GOLD 4, very severe). A total of 74 patients were classified as without COPD, 16 as having GOLD 1, 28 as having GOLD 2, 11 as having GOLD 3, and 7 as having GOLD 4.
We asked all patients to visit our clinic every 3 months after SBRT. Follow-up CT scans were acquired at each visit and were used to detect RIP and radiation-induced pulmonary fibrosis (RIPF). pmRIP was defined as minimal pneumonitis without significantly diffuse infiltration or consolidation-associated ground-glass opacity in the irradiated volume lasting 6 months or more after SBRT administered in this study.
RESULTS
Survivals and local control rate
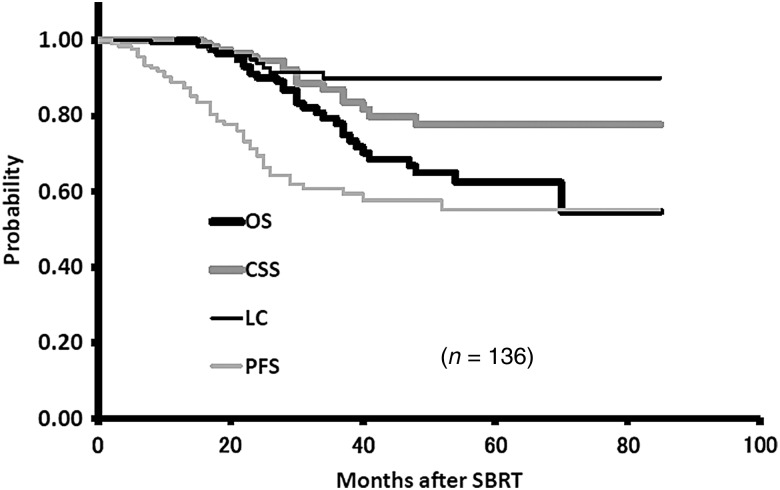
The 3-year overall survival (OS), cause-specific survival (CSS) and progression-free survival (PFS) rates were 78.0% (95% CI, 69.4–86.6%), 87.0% (95% CI, 80.0–94.1%), and 60.9% (95% CI, 69.4–86.6%), respectively. The corresponding LC rate was 90.0% (95% CL, 83.9–96.1%) (Fig. 1).
Fig. 1.
The 3-year overall survival (OS), cause-specific survival (CSS), and progression-free survival (PFS) rates were 77.7%, 86.9% and 60.9%, respectively. The corresponding local control (LC) rate was 89.8%.
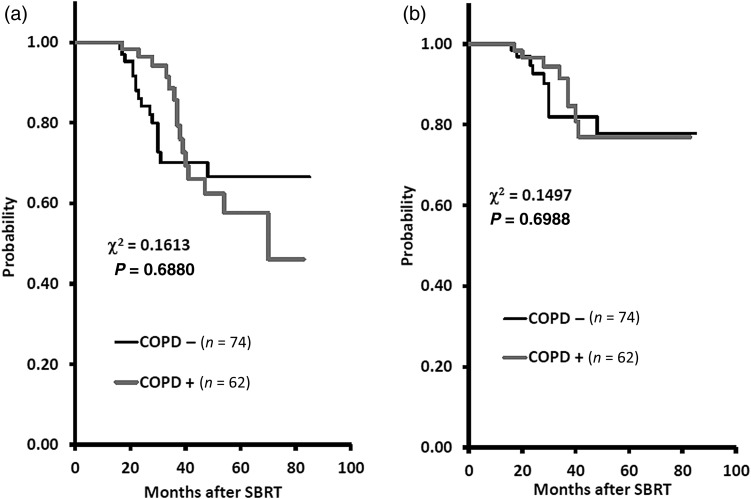
The 3-year OS rates were 70.1% (95% CI, 57.1–83.1%) and 85.6% (95% CI, 74.7–96.6%) for the COPD − and COPD + groups, respectively (P = 0.6880). The corresponding CSS rates were 81.9% (95% CI, 70.2–93.6%) and 91.4% (95% C, 83.1–99.7%) for the COPD − and COPD + groups, respectively (P = 0.6988). In the COPD − group, eight patients died of lung cancer, three of other cancers, and five of intercurrent disease, and in the COPD + group, nine died of lung cancer, four of other cancers, and three of intercurrent disease. There was no significant difference in OS or CSS between the COPD − and COPD + groups (Fig. 2).
Fig. 2.
The 3-year overall survival (OS) rates were 70.1% and 85.6% for the COPD − and COPD + groups, respectively (P = 0.6880). The corresponding 3-year cause-specific survival (CSS) rates were 81.9% and 91.4% for the COPD − and COPD + groups, respectively (P = 0.6988).
RIP, pmRIP and RIPF
Of the 136 patients, 44 (32%) suffered pmRIP. The median duration of pmRIP resulting from SBRT was 10 months (range, 6–32 months). Multivariate analysis showed that COPD and BI were statistically significant risk factors for the development of the pmRIP, with odds ratios of 5.6027 (95% CI, 2.2283–14.0871; P = 0.0002) and 4.8670 (95% CI, 1.1025–21.4856; P = 0.0367), respectively. Patients in the COPD + group had a higher incidence of pmRIP (52%) than those in the COPD − group (16%). Patients with a BI of ≥400 also had a higher incidence of pmRIP (40%) compared with those with a BI of <400, for whom the incidence of pmRIP was only 9% (Table 2).
Table 2.
Multivariate analysis for pmRIP
| pmRIP |
Multivariate analysis |
||||||
|---|---|---|---|---|---|---|---|
| Item | Category | (−) | (+) | Total | OR | 95% Cl | P value |
| Age | <77 | 45 | 24 | 69 | 0.9734 | 0.3868–2.4501 | 0.9544 |
| ≥77 | 47 | 20 | 67 | ||||
| Gender | Male | 62 | 39 | 101 | 0.2861 | 0.0785–1.0426 | 0.0579 |
| Female | 30 | 5 | 35 | ||||
| Stage | IA | 75 | 35 | 110 | 0.4708 | 0.1482–1.4954 | 0.2014 |
| IB | 17 | 9 | 26 | ||||
| Pathology | NSCLC | 58 | 32 | 90 | 0.3593 | 0.1315–0.9819 | 0.0460* |
| No proof | 34 | 12 | 46 | ||||
| Multiple cancer | (−) | 37 | 23 | 60 | 0.3331 | 0.1179–0.9414 | 0.0381* |
| (+) | 55 | 21 | 76 | ||||
| PS | 0–1 | 78 | 37 | 115 | 1.4900 | 0.3728–5.9549 | 0.5726 |
| 2–4 | 14 | 7 | 21 | ||||
| Laterality | Left | 33 | 15 | 48 | 1.2130 | 0.4588–3.2069 | 0.6971 |
| Right | 59 | 29 | 88 | ||||
| Target location (lobe) | Upper/Middle | 54 | 34 | 88 | 0.3842 | 0.1463–1.0085 | 0.0520 |
| Lower | 38 | 10 | 48 | ||||
| Chemotherapy | (−) | 73 | 33 | 106 | 1.9233 | 0.6683–5.5350 | 0.2253 |
| (+) | 19 | 11 | 30 | ||||
| Thoracic surgery | (−) | 64 | 34 | 98 | 2.1142 | 0.6464–6.9148 | 0.2156 |
| (+) | 28 | 10 | 38 | ||||
| COPD | (−) | 62 | 12 | 74 | 5.6027 | 2.2283–14.0871 | 0.0002** |
| (+) | 30 | 32 | 62 | ||||
| Brinkman index | <400 | 30 | 3 | 33 | 4.8670 | 1.1025–21.4856 | 0.0367* |
| ≥400 | 62 | 41 | 103 | ||||
| Total | 92 | 44 | 136 | ||||
pmRIP = prolonged minimal RIP, OR = odds ratio, CI = confidence interval, NSCLC = non-small cell lung cancer, PS = performance status, COPD = chronic obstructive pulmonary disease, Brinkman index = cigarettes/day × years. *P value < 0.05, **P value < 0.01.
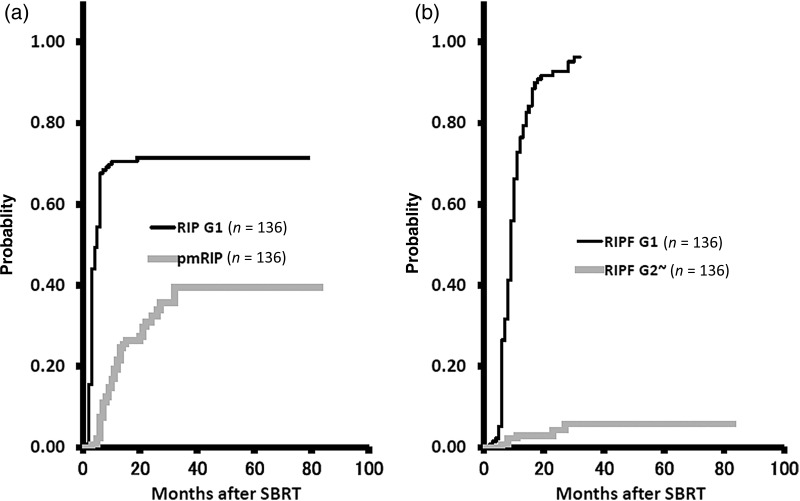
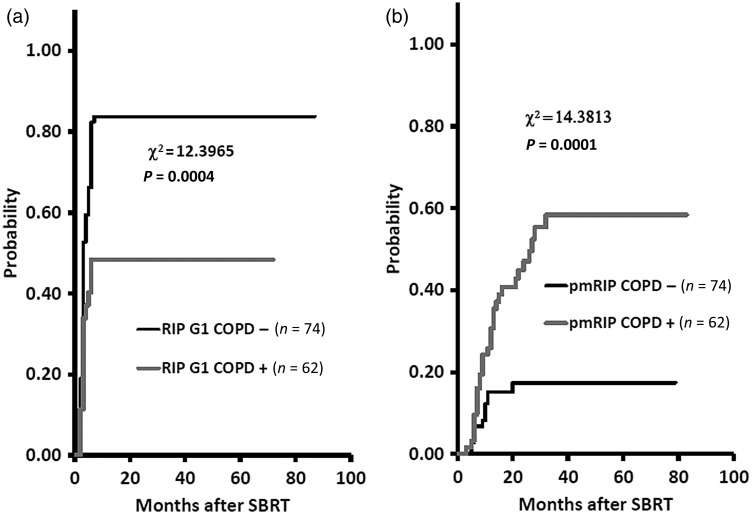
The 1-year cumulative probabilities were 70.6% (95% CI, 62.9–78.3%) and 21.5% (95% CI, 14.6–28.4%) for the development of Grade 1 RIP and for continuing pmRIP, respectively, and 76.5% (95% CI, 69.3–83.6%) and 3.0% (95% CI, 0.1–5.8%) for Grade 1 RIPF and Grade 2 or worse RIPF, respectively (Fig. 3). The 1-year cumulative probabilities of developing Grade 1 RIP were 83.8% (95% CI, 75.4–92.2%) and 48.4% (95% CI, 36.0–60.8%) for the COPD − and COPD + groups, respectively (P = 0.0004), and for continuing pmRIP were 15.1% (95% CI, 6.9–23.3%) and 30.7% (95% CI, 19.2–42.1%), respectively (P = 0.0001) (Fig. 4).
Fig. 3.
The 1-year cumulative probabilities were 70.6% and 21.5% for development of Grade 1 radiation-induced pneumonitis (RIP) and for continuing prolonged minimal RIP (pmRIP), respectively (a). The 1-year cumulative probabilities were 76.5% and 3.0% for development of Grade 1 radiation-induced pulmonary fibrosis (RIPF) and Grade 2 or worse RIPF, respectively (b).
Fig. 4.
The 1-year cumulative probabilities for development of Grade 1 radiation-induced pneumonitis (RIP) were 83.8% and 48.4% for the COPD − and COPD + groups, respectively (a). The 1-year cumulative probabilities for continuation of prolonged minimal RIP (pmRIP) were 15.1% and 30.7% for the COPD − and COPD + groups, respectively (b).
Case reports
Two typical cases of pmRIP are presented as follows.
Case 1
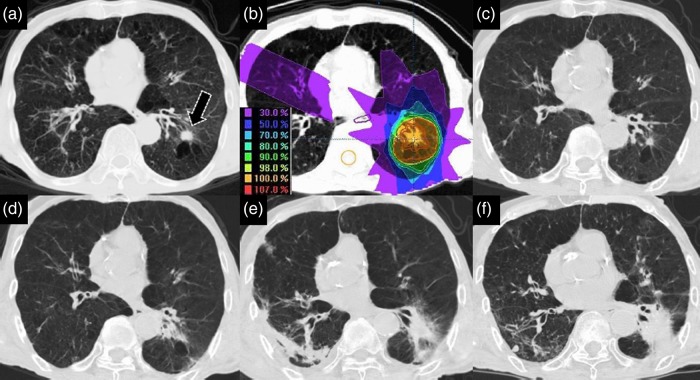
A 73-year-old man with adenocarcinoma of the left lower lobe (LLL), c T1aN0M0 and Stage IA had a history of COPD, pulmonary tuberculosis, chronic bronchiolitis and chronic hepatitis. His BI was 550, and his ECOG PS was 1. Spirometry examination showed that the FEV1/forced vital capacity (FVC) was 52.26%, and %FEV1 was 39.3%. He was classified as having GOLD 3 COPD. At the time of the follow-up CT examination of his COPD in 2007, a small round nodule was detected in the LLL (S9) associated with emphysema, which showed a significant increase in size 3 months later. Transbronchial biopsy under fiberscopic examination revealed adenocarcinoma. Because of his severe COPD, he underwent SBRT consisting of 50 Gy in 5 fractions over 5 days. He did not develop RIP within 12 months after SBRT, but pmRIP persisted for 27 months after SBRT. He developed RIPF 30 months later, and bilateral aspiration pneumonitis 49 months later. He is still alive at the time of reporting with a denser RIPF with post-pneumonitis in LLL 77 months after SBRT (Fig. 5).
Fig. 5.
A 73-year-old man with adenocarcinoma of the left lower lobe (LLL, S9), cT1aN0M0, Stage IA. A small round nodule (black arrow) was detected in the LLL, and was found to have enlarged significantly 3 months later (a). He underwent stereotactic body radiotherapy (SBRT) of 50 Gy in five fractions over 5 days in March 2008 (b). He did not develop radiation-induced pneumonitis (RIP) in the first 12 months following SBRT (c). He continued to show prolonged minimal RIP (pmRIP) during the following 27 months after SBRT (d). He developed radiation-induced pulmonary fibrosis (RIPF) 30 months later and suffered from bilateral aspiration pneumonitis 49 months later (e). He was still alive 77 months following SBRT (f).
Case 2
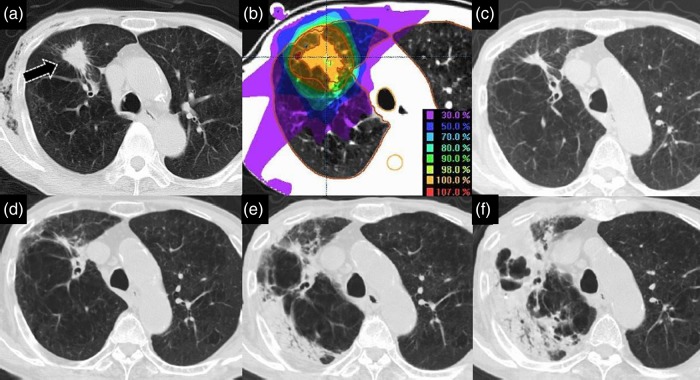
An 89-year-old man with squamous cell carcinoma of the right upper lobe (RUL), cT2aN0M0 and Stage IB, had a history of COPD and pulmonary tuberculosis. His BI was 1950, and his ECOG PS was 1. Spirometry examination showed FEV1/FVC was 46.67%, and %FEV1 was 62.9%. He was classified as having COPD GOLD 2. In 2009, a plain chest X-ray film showed an abnormal shadow and emphysema in the RUL (S3), and the former was found to have enlarged significantly 3 months later. He underwent a CT-guided pulmonary biopsy that revealed squamous cell carcinoma of the lung. Because this patient was elderly and had COPD, he underwent SBRT consisting of 50 Gy in 5 fractions over 5 days in June 2009. He developed pmRIP that persisted for 25 months following SBRT. He developed pulmonary tuberculosis 28 months after SBRT, and died of pulmonary tuberculosis 39 months later (Fig. 6).
Fig. 6.
An 89-year-old man with squamous cell carcinoma of the right upper lobe (RUL; S3), cT2aN0M0, Stage IB. Examination of a chest X-ray film revealed an abnormal irregular shadow (black arrow) in the RUL (a). He underwent stereotactic body radiotherapy (SBRT) of 50 Gy in five fractions over 5 days in June 2009 (b). He did not develop radiation-induced pneumonitis (RIP) in the first 12 months following SBRT (c). He continued to show prolonged minimal RIP (pmRIP) over the 25 months following SBRT (d). He suffered from pulmonary tuberculosis 28 months after SBRT (e), and died of this condition 39 months later (f).
DISCUSSION
SBRT is recognized as a highly effective treatment for early-stage lung cancer [5, 12]. This is particularly relevant for many elderly patients as they often have severe intercurrent diseases, and thus cannot undergo surgery.
The clinical effects of radiation on pulmonary tissue were first noted in 1923. Thereafter, many investigators reported these phenomena variously as radiation pneumonitis, radiation pleuropneumonitis, radiation reaction in the lung, post-radiation pulmonary changes, radiation lung fibrosis or scarring, or irradiation injury or effects on the lung [13]. The term radiation pneumonitis is customarily used for late effects of the lung, not only for side effects but also for adverse events, and we use ‘radiation-induced pneumonitis’ in a narrow sense to exclude radiation-related adverse events in this paper.
In this study, 47 of the 136 patients (35%) lacked a definitive histological diagnosis due to old age, tumor location, or complications such as severe or very severe grade COPD preventing biopsy. The proportion of elderly lung cancer patients is growing, and as a result it is now internationally accepted that these cases are suitable for inclusion in clinical trials. The lack of a pre-treatment histological diagnosis is more common in medically inoperable patients who may be at higher risk of complications following a transthoracic needle biopsy. However, this has been addressed to an extent by the use of repeated CT studies, PET-CT scans, and tumor marker evaluation, which can establish whether SBRT is possible [12].
A total of 76 patients (56%) developed 122 other primary carcinomas in this study cohort. Among previous reports, one stated that nearly 10% of the patients in the study cohort with hypopharyngeal and glottic cancers developed other primary carcinomas. In that study, more advanced therapeutic and diagnostic techniques, together with the field carcinogenesis phenomenon, resulted in a high incidence of additional primary carcinomas [14, 15]. Advances in cancer diagnosis have greatly increased early cancer detection over the last 40 years, and this may have resulted in the high incidence of other primary carcinomas in this series.
RIP in almost all cases was asymptomatic and of NCICTCAE Grade 1; it was unexpectedly detected during the follow-up CT for early-stage lung cancer. However, we found that some patients did not develop significant RIP, and we define pmRIP as minimal pneumonitis without significantly diffuse infiltration or consolidation-associated ground-glass opacity in the irradiated volume lasting 6 months or more after SBRT administered in this study. Most of these patients had severe emphysema, and we therefore analyzed the results of the spirometry examination to assess the relationship between airflow limitation severity in COPD and pmRIP. We also investigated whether survival rates after SBRT for early-stage lung cancer were affected by the presence of COPD in the current study.
In Japan, SBRT is generally indicated if patients are unable to undergo surgery. Accordingly, older patients (median age, 76 years; range, 55–98 years) were included in the present study. Significant differences between the characteristics of primary and metastatic lung cancer patients treated with SBRT have previously been reported; most notably, patients with primary lung cancers are older than those with metastatic tumors [6]. Excellent clinical outcomes have recently been reported in elderly patients with co-existent severe COPD [4].
Cigarette smoke is a well-established risk factor for the development of lung cancer and COPD [1–3]. In the present study, the median patient BI was 900 (range, 0–7050). Forty-nine of 74 (66%) patients in the COPD − group and 54 of 62 (87%) of patients in the COPD + group had a BI of 400 or more. Multivariate analysis showed that COPD was a significant risk factor for the development of the pmRIP. In Japan, many cases of COPD are of the bronchiole-central emphysematous type, which involves an abnormal enlargement of the air-lumen associated with destruction of the alveolar cell wall from the terminal bronchiole to its peripheral portion. Accordingly, there is a significant loss of the cellular alveoli components. In contrast, the alveolar epithelium of the lung consists of type I and II pneumocytes [3]. Many type I pneumocytes are lost after radiotherapy, at which point the type II alveolar cells begin to proliferate and produce surfactant apoproteins to repair the surrounding damage, which results in an inflammatory cytokine cascade and vasculature changes [4]. Despite the reduced inflammatory cascade due to cellular loss in the emphysematous lung after SBRT, this pathological process still seems to result in the development of pmRIP.
Serious infections, especially respiratory infections, are frequently seen in patients with COPD. Moreover, elderly patients with COPD are susceptible to aspiration pneumonitis. For post-SBRT lung cancer patients with COPD, it is important to also treat comorbidities such as cardiovascular diseases, skeletal muscle dysfunction, and tuberculosis, which may have a significant impact on prognosis [1, 2].
In the future, not only spirometry examination but also a topological study such as V/Q single-photon emission computed tomography would be required for the meticulous treatment planning of SBRT for early-stage lung cancer patients with severe COPD [16, 17].
There were no significant differences in OS or CSS between the COPD − and COPD + groups in this study. This is an important finding, as it suggests that COPD does not need to be a contraindication for SBRT in early-stage lung cancer patients. This is further supported by the review of Palma et al., who found that survival at 1 and 3 years was similar with SBRT and surgery-based treatments [18].
Emami's classic study on the tolerance of normal tissue did not seem appropriate for advanced precision radiotherapy [19], and consequently, Marks et al. proposed a new Quantitative Analysis of Normal Tissue Effects in the Clinic (QUANTEC) model to help predict clinical outcomes [20]. However, they described the tolerance level based on the endpoint of symptomatic pneumonitis; accordingly, they did not consider the pmRIP condition that we describe here.
To the best of our knowledge, this is the first report concerning pmRIP after SBRT for early-stage lung cancer associated with COPD. We found that both COPD and BI are statistically significant risk factors for the development of pmRIP after SBRT for early-stage lung cancer patients. Both OS and CSS were comparable between the COPD − and COPD + patient groups. Therefore, aggressive SBRT treatment for early-stage lung cancer patients with COPD should be acceptable. However, smoking cessation is still very important to reduce the mortality from both lung cancer and COPD, and an anti-smoking campaign is currently an urgent issue in Japan.
FUNDING
There are no specific funding sources to acknowledge.
ACKNOWLEDGEMENTS
We would like to thank Editage (www.editge.jp) for English language editing.
REFERENCES
- 1.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for diagnosis, management, and prevention of chronic obstructive pulmonary disease (Updated 2014). http://www.goldcopd.com/uploads/users/files/GOLD_Report_2014_Oct30.pdf (3 December 2014, date last accessed).
- 2.Committee for the Second Edition of the COPD Guidelines of the Japanese Respiratory Society. Guidelines for the Diagnosis and Treatment of COPD (Chronic Obstructive Pulmonary Disease). 2nd edn, Pocket Guide Tokyo: The Japanese Society, 2004. [Google Scholar]
- 3.Colby TV, Koss MN, Travis WD. Tumors of the lower respiratory tract. In: Rosai J, Sobin LH. (eds). Atlas of Tumor Pathology. Third Series Fascicle 13. Washington, DC: AFIP, 1995, 3–30 and 91–106. [Google Scholar]
- 4.Palma JD, Zaorosky NG, Witek M, et al. Molecular markers to predict clinical outcome and radiation-induced toxicity in lung cancer. J Thorac Dis 2014;6:387–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onishi H, Araki T, Shirato H, et al. Stereotactic hypofractionated high-dose irradiation for stage I non-small cell lung carcinoma: clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer 2004;101:1623–31. [DOI] [PubMed] [Google Scholar]
- 6.Inoue T, Oh R-J, Shiomi H, et al. Stereotactic body radiotherapy for pulmonary metastases. Prognostic factors and adverse respiratory events. Strahlenther Onkol 2013;189:285–92. [DOI] [PubMed] [Google Scholar]
- 7.Miura H, Oh R, Masai N, et al. Lung stereotactic body radiotherapy using an abdominal compression system. “Air-Bag System”. Int J Med Phys Clin Eng Radiat Oncol 2014;3:98–106. [Google Scholar]
- 8.Miura H, Masai N, Oh R, et al. Approach to dose definition of the gross tumor volume for lung cancer with respiratory tumor motion. J Radiat Res 2013;54:140–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miura H, Masai N, Oh R, et al. Clinical introduction of Monte Carlo treatment planning for lung stereotactic body radiotherapy. J Appl Clin Med Phys 2014;15:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events (CTCAE) Ver. 3.0, DCTD, NCI, NIH, DHHS, Published: 9 August 2006.
- 11.Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0 Published: 28 May 2009 (Ver. 4.03: 14 June 2010) http://www.jcog.jp/doctor/CTCAEv4J_20111217_version.pdf (3 December 2014, date last accessed). [Google Scholar]
- 12.Senan S, Palma DA, Lagerwaard FJ. Stereotactic ablative radiotherapy for stage I NSCLC: recent advances and controversies. J Thorac Dis 2011;3:189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubin P, Casarett GW. Chapter 12, Respiratory system. In: Rubin P, Casarett GW. (eds). Clinical Radiation Pathology. Vol. 1 Philadelphia: W.B. Saunders Co, 1968, 423–70. [Google Scholar]
- 14.Inoue T, Shigematsu Y, Sato T. Treatment of carcinoma of the hypopharynx. Cancer 1973;31:649–55. [DOI] [PubMed] [Google Scholar]
- 15.Inoue T, Shigematsu Y, Sato T. Results of radiation therapy of early carcinoma of the vocal cords. Acta Radiol Ther Phys Biol 1975;14:318–24. [DOI] [PubMed] [Google Scholar]
- 16.Yuan ST, Frey KA, Gross MD, et al. Semiquantification and classification of local pulmonary function by V/Q single photon emission computed tomography in patients with non-small cell lung cancer: potential indication for radiotherapy planning. J Thorac Oncol 2011;6:71–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meng X, Fey K, Matuszak M, et al. Changes in functional lung regions during the course of radiation therapy and their potential impact on lung dosimetry for non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2014;89:145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palma D, Lagerwaard F, Rodrigues G, et al. Curative treatment of Stage I non-small-cell lung cancer in patients with severe COPD: stereotactic radiotherapy outcomes and systemic review. Int J Radiat Oncol Biol Phys 2012;82:1149–56. [DOI] [PubMed] [Google Scholar]
- 19.Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 1991;15:109–22. [DOI] [PubMed] [Google Scholar]
- 20.Marks LB, Yorke ED, Jackson A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys 2010;76:S10–9. [DOI] [PMC free article] [PubMed] [Google Scholar]