Abstract
Current management of radiation-induced liver injury is limited. Sinusoidal endothelial cell (SEC) apoptosis and inflammation are considered to be initiating events in hepatic damage. We hypothesized that mesenchymal stem cells (MSCs) possess anti-apoptotic and anti-inflammatory actions during hepatic irradiation, acting via paracrine mechanisms. This study aims to examine whether MSC-derived bioactive components are protective against radiation-induced liver injury in rats. MSC-conditioned medium (MSC-CM) was generated from rat bone marrow–derived MSCs. The effect of MSC-CM on the viability of irradiated SECs was examined by flow cytometric analysis. Activation of the Akt and ERK pathways was analyzed by western blot. MSC-CM was also delivered to Sprague–Dawley rats immediately before receiving liver irradiation, followed by testing for pathological features, changes in serum hyaluronic acid, ALT, and inflammatory cytokine levels, and liver cell apoptosis. MSC-CM enhanced the viability of irradiated SECs in vitro and induced Akt and ERK phosphorylation in these cells. Infusion of MSC-CM immediately before liver irradiation provided a significant anti-apoptotic effect on SECs and improved the histopathological features of injury in the irradiated liver. MSC-CM also reduced the secretion and expression of inflammatory cytokines and increased the expression of anti-inflammatory cytokines. MSC-derived bioactive components could be a novel therapeutic approach for treating radiation-induced liver injury.
Keywords: sinusoidal endothelial cell, radiation-induced liver injury, mesenchymal stem cell, apoptosis
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most common malignancy worldwide and accounts for nearly 10% of cancer deaths annually [1]. However, the majority of HCC patients present with advanced-stage disease. In these patients, radiotherapy (RT) is one of the major methods for palliative treatment. However, the major limitation of RT is the high risk of potentially lethal liver injury, later termed radiation-induced liver disease (RILD) [2, 3]. Current RILD management is mainly supportive, and the mortality rates exceed 75% [4, 5]. Developing new approaches to protect the liver from injury after irradiation is necessary and important.
The liver is a highly radiosensitive organ, and the threshold dose for whole-liver irradiation is reportedly between 20 and 30 Gy [6]. However, hepatocytes are considered more radioresistant than other cells [7–11]. Thus, hepatocytes are not the direct and initial target in radiation-induced liver injury. RILD is characterized by early veno-occlusive disease (VOD) [12, 13], and sinusoidal endothelial cell (SEC) injury has been traditionally postulated as the initiating lesion of VOD in RILD [13]. Yamanouchi et al. [14] reported that the number of apoptotic SECs increased significantly 6 h after 30 Gy of liver irradiation in a rat model; by contrast, apoptotic hepatocytes were only occasionally seen. SEC injury results in microcirculatory blood flow disturbances and secondary injury to hepatocytes, causing liver dysfunction. Inflammation is also important in RILD development. The inflammatory cytokines tumor necrosis factor α (TNF-α), interleukin 1β (IL-β), and IL-6, as well as numerous chemokines, have been implicated in early-phase RILD development [15–18]. Therefore, SECs are the primary targets in liver radiation, and inflammation enhances this process.
Mesenchymal stem cells (MSCs) show significant potential for clinical utility, due to their convenient isolation and culture, low immunogenicity, regenerative and multiple differentiation abilities, and potent anti-apoptotic and immunosuppressive effects [19]. Recent studies have shown that MSCs can ameliorate tissue injury resulting from radiation, including radiation-induced lung injury and radiation-induced intestinal injury [20–24]. The mechanisms of MSC action responsible for preventing radiation-induced tissue damage may involve paracrine secretory mechanisms [25]. Paracrine factors expressed by MSCs include different types of cytokines, such as anti-apoptotic factors, growth factors, and both anti-inflammatory and inflammatory factors [26–30]. Chang et al. [24] reported that bone marrow transplantation rescued intestinal mucosa after whole-body irradiation by secreting cytokines that enhance angiogenesis and chemotaxis. Therefore, systemic infusion of MSC-conditioned medium (MSC-CM) into recipients with irradiated livers may be an effective and appealing approach to reducing liver injury.
This study investigated whether systemic infusion of MSC-CM could protect SECs after radiation, which might eventually mitigate RILD. We injected MSC-CM into rats before liver irradiation. Terminal nucleotidyl transferase–mediated dUTP nick end labeling (TUNEL) was used to assess the apoptosis in liver tissue. Using an in vitro assay, we demonstrate that MSC secretory products have a direct inhibitory effect on SEC apoptosis and provide evidence that the Akt and ERK pathways may mediate this effect.
MATERIALS AND METHODS
Animals
Nine-week-old inbred male Sprague–Dawley rats (Animal Center of Zhongshan Hospital, Fudan University, Shanghai, China) were used in this study. Rats were maintained under controlled conditions (24 ± 2°C temperature, 40–70% relative humidity, and 12-h light/12-h dark cycle) and given a normal laboratory diet and water ad libitum, in accordance with the criteria of the Guide for the Care and Use of Laboratory Animals of Fudan University.
Cell culture and MSC-CM preparation
Rat liver SECs were purchased from PriCells Company (Wuhan, China). SECs were seeded in low DMEM medium (Invitrogen, Cergy Pontoise, France) supplemented with 15% fetal bovine serum (Gibco-BRL, Grand Island, NY), 10 ng/ml epidermal growth factor, 2 ng/ml hydrocortisone, 2 mmol/l L-glutamine, 100 units/ml penicillin and 100 ng/ml streptomycin, referred to as EC complete medium.
Rat bone marrow MSCs were isolated, cultured and identified as previously described [31]. To generate MSC-CM, passage 3–4 MSC cells were grown to 80–90% confluence in 25-cm2 flasks, washed thoroughly, and cultured in serum-free low-glucose DMEM for 24 h. MSC-CM was collected by filtering through a 50-μm mesh.
Experimental design and irradiation procedure
For in vitro experiments, irradiated SECs (15 Gy) were seeded in the bottom of 6-well plates and incubated with DMEM, or MSC-CM (2 ml, MSC-CM was collected from 1×105 MSCs) for 2 h. After incubation, apoptosis in SECs was examined by fluorescence-activated cell sorting (FACS). When confirming activation of the Akt and ERK pathways, PI3 K/Akt inhibitor (LY294002) and MEK/ERK inhibitor (U0126) were respectively added 2.5 h before irradiation, and the apoptotic SECs were counted by FACS.
For in vivo experiments, MSC-CM was concentrated using an Amicon Ultra Centrifugal Filter Device (Millipore). Animals were allocated into one of three experimental groups: (i) Control group, (ii) RT + DMEM group, and (iii) MSC-CM + RT group. Because the effect of MSC-CM alone on normal liver was not the focus of this study, we excluded MSC-CM with normal liver in the Control group. As described previously [32], rats were anesthetized, and a 20-Gy dose of X-rays was delivered to rats' livers in a single fraction using a linear accelerator (Oncor; Siemens, Munich, Germany). Organs near the liver were protected by lead shielding. A total of 1.0 ml MSC-CM (equal numbers of 1 × 106 MSCs) or medium was injected into the penile vein immediately before liver irradiation. Liver tissues and blood samples were collected at different times. Parts of the tissues were fixed, and the remaining tissues and serum samples were stored at −80°C.
Apoptosis determination
For in vitro studies, apoptosis was measured by flow cytometric analysis. SECs were pretreated with MSC-CM or DMEM for 2 h prior to 15-Gy irradiation. Cells were harvested at 24 h after irradiation. Apoptosis was determined using an Annexin V-FITC/7-oxaloacetic acid (OAA) fluorescent cell staining kit (KeyGEN, BioTECH, Nanjing, China), according to the manufacturer's instructions.
For in vivo apoptosis studies, liver tissue samples were formalin-fixed and paraffin-embedded, and 4-µm sections were stained with a TUNEL protocol (KeyGEN, BioTECH, Nanjing, China).
Serum analysis
Serum levels of TNF-α, IL1-β, IL-6, IL-10 and hyaluronic acid (HA) were quantified using enzyme-linked immunosorbent assays (ELISAs) as per the manufacturer's instructions (R&D Systems Inc., Minneapolis, MN). Alanine aminotransferase (ALT) was measured using an enzymatic assay (Biotron Diagnostics, Inc., Hemet, CA), according to the manufacturer's instructions.
Real-Time quantitative Reverse Transcription-PCR
Total RNA was isolated from the liver tissue was used for real-time quantitative reverse transcription-polymerase chain reaction (RT-qPCR) (Toyobo, Osaka, Japan) using the QuantiTect SYBR Green Reverse Transcriptase-Polymerase Chain Reaction kit (Qiagen, Valencia, CA). The sequences of the specific primers for TNF-α, IL1-β, IL-6, IL-10 and β-actin are shown in Supplemental Table e1.
Liver histology
Formalin-fixed, paraffin-embedded liver tissues were sectioned (4-µm thick), and sections were stained with H&E and observed by light microscopy.
Protein isolation and western blot
SECs were grown to confluence in six-well culture plates, and media were replaced with serum-free DMEM medium (2 ml/well). After 2 h, media were replaced with MSC-CM i, and SECs were incubated for an additional 45 min before harvesting for cellular protein analysis. For western blot analysis, equal amounts of SEC cellular proteins were resolved by 10% SDS–PAGE and transferred to PVDF membranes. Antibodies against the following proteins used to probe membranes were used as primary antibodies: phospho-Akt and phospho-ERK1/2 (all from Cell Signaling, Beverly, MA).
Statistics
Data are expressed as means ± standard deviation (SD). Comparisons among multiple groups were performed by non-parametric analysis of variance (ANOVA). Differences were considered to be statistically significant when P < 0.05.
RESULTS
MSC-CM inhibits radiation-induced SEC apoptosis in vitro
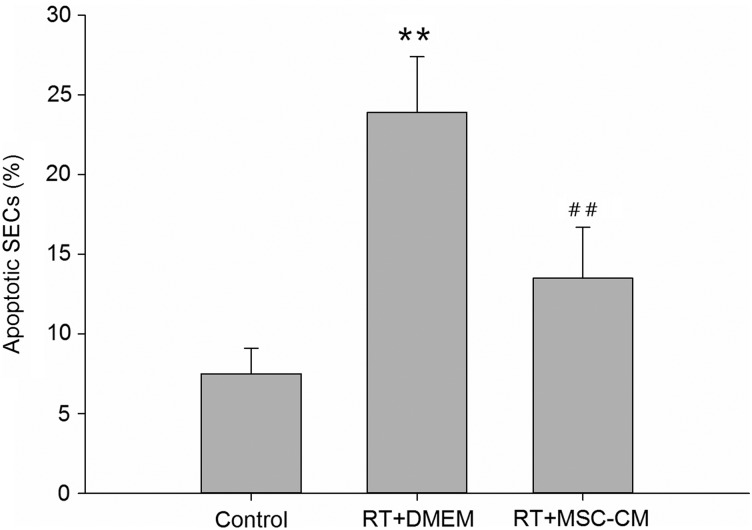
To determine whether secretory products in MSC-CM decrease radiation-induced SEC apoptosis, we performed (FACS) analyses in combination with annexin V staining. Pretreatment with MSC-CM decreased the number of apoptotic SECs 24 h after 15 Gy irradiation to 13.5 ± 2.9% of the total SEC population versus 23.6 ± 3.2% when incubated in DMEM only (P < 0.01; Fig. 1). The results confirm that MSC-CM therapy effectively reduces the SEC apoptosis that is induced by irradiation.
Fig. 1.
Anti-apoptotic effect of MSC-CM on SECs in vitro. Pretreatment with MSC-CM decreased the number of apoptotic SECs. Apoptosis was determined by fluorescence-activated cell sorting (FACS) analysis. Data are shown as means ± SD. **P < 0.01 (DMEM group versus control group). ##P < 0.01 (MSC-CM group versus DMEM group).
MSC-CM inhibits radiation-induced SEC apoptosis in vivo
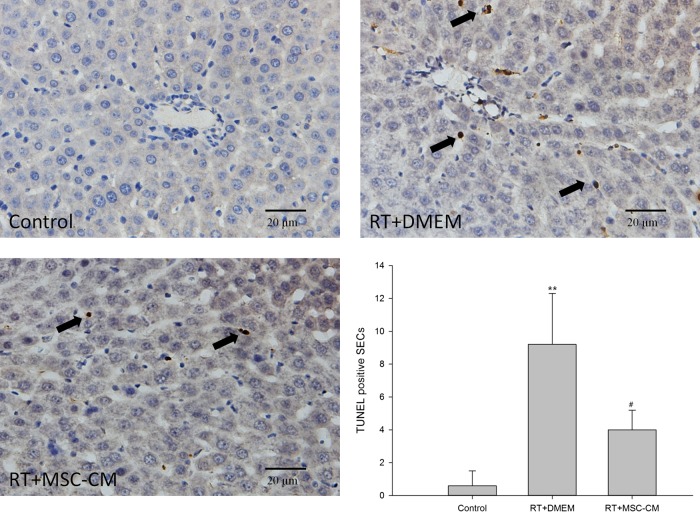
We used the TUNEL assay to evaluate SEC apoptosis in liver sections generated in an irradiated rat model. Liver irradiation significantly increased the number of apoptotic SECs from 6 h after RT compared with the non-RT control group (Fig. 2). There were 9.2 ± 3.1 TUNEL-positive SECs per high-power field, compared with 0.6 ± 0.9 cells per high-power field in the control group (P < 0.05) at 6 h. Treatment with MSC-CM significantly reduced the number of apoptotic SECs induced by RT (4.0 ± 1.2, P < 0.05). These data demonstrate that MSC-CM specifically inhibits radiation-induced SEC apoptosis in vivo. Unlike SECs, the number of TUNEL-positive hepatocytes was few in irradiated rat liver sections.
Fig. 2.
Effect of MSC-CM pretreatment on radiation-induced SEC apoptosis in rat liver. Irradiation initiated SEC apoptosis in rat liver SECs, which was significantly decreased by a pre-RT intravenous injection of MSC-CM. Five randomly selected areas were viewed by light microscopy (×400 total magnification) to enumerate apoptotic SECs positive for terminal nucleotidyl transferase-mediated nick end labeling (TUNEL) staining. Arrows indicate apoptotic SECs. Data are shown as means ± SD. **P < 0.01 (DMEM group versus control group). #P < 0.05 (MSC-CM group versus DMEM group).
Serum HA and ALT levels after radiation and MSC-CM therapy in vivo
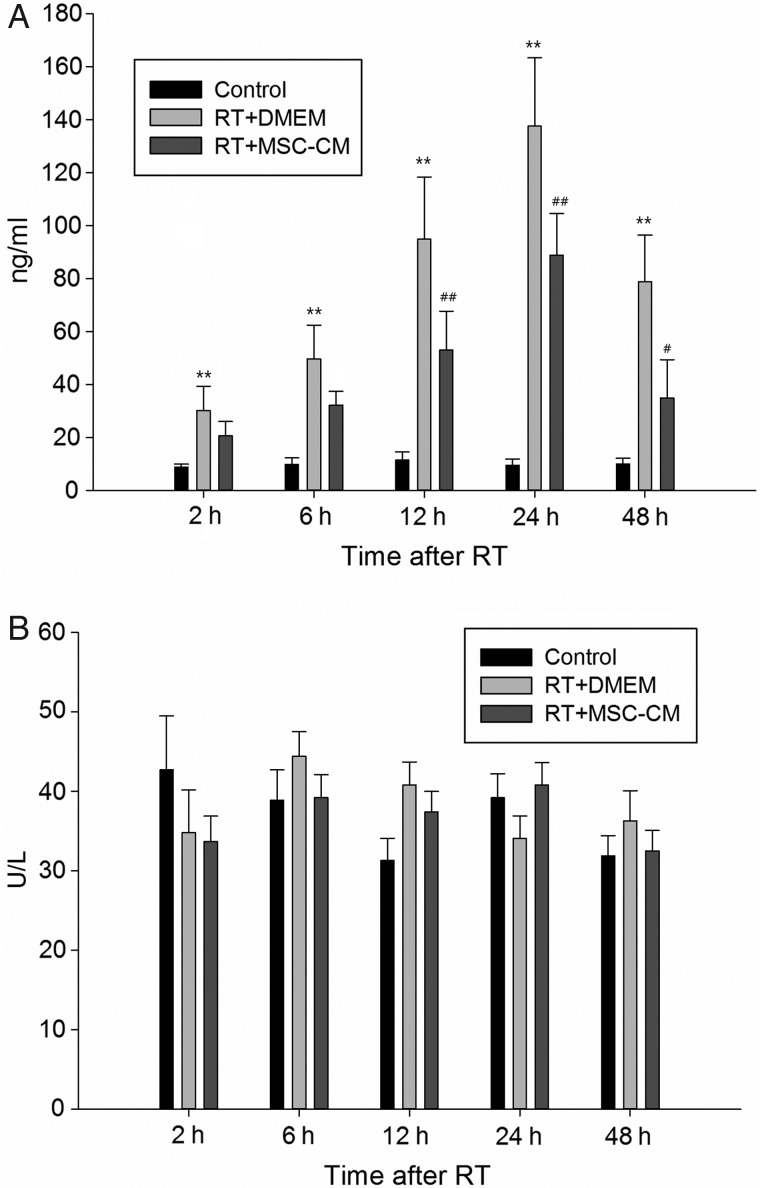
Elevated serum HA and ALT levels are markers of SEC and hepatocyte injury, respectively. Serum HA levels in liver-irradiated rats were significantly increased at 6 h after RT compared with the non-RT control group, and this effect was maximal at 24 h (control group, 9.6 ± 2.3 ng/ml versus RT group, 137.6 ± 25.8 ng/ml; P < 0.01) (Fig. 3A). Pretreatment with systemic MSC-CM significantly alleviated SEC damage in liver-irradiated rats, evidenced by a significant reduction in serum HA levels to 88.9 ± 15.7 ng/ml at 24 h (Fig. 3A). However, serum ALT serum did not significantly increase throughout the 48-h experiment (Fig. 3B). These data together indicate that the acute effect of RT on hepatocyte death is minimal; however, SECs are much more sensitive to radiation-induced damage and killing, and soluble factors in MSC-CM have a protective effect on these cells.
Fig. 3.
Effect of liver irradiation on serum hyaluronic acid (HA) and ALT levels in rats. (A) Circulating HA levels (SEC-derived) were time-dependently increased in rats after liver irradiation versus non-irradiated controls, and this effect was decreased by intravenous infusion of MSC-CM. (B) Irradiation did not alter circulating ALT levels (hepatocyte-derived). Data are shown as means ± SD. **P < 0.01 (DMEM group versus control group). ##P < 0.01 and #P < 0.05 (MSC-CM group versus DMEM group).
MSC-CM therapy modulates the inflammatory response to liver irradiation in vivo
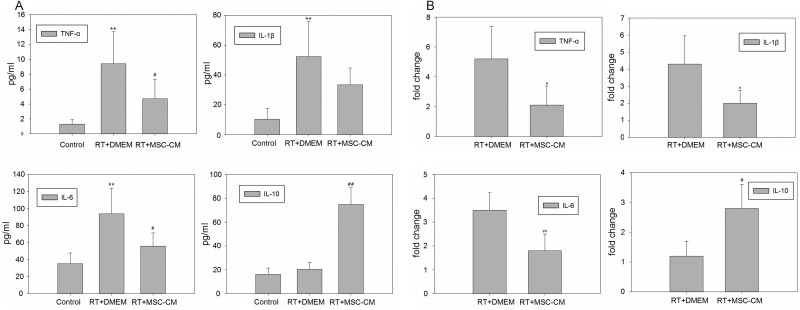
Inflammatory cytokines are known to be upregulated during liver injury [11]. Liver irradiation in rats significantly increased serum concentrations of the inflammatory cytokines TNF-α, IL-β and IL-6, and systemic MSC-CM treatment inhibited this response (P < 0.05 for TNF-α and IL-6, Fig. 4A). Conversely, levels of the anti-inflammatory cytokine IL-10 were increased in the MSC-CM treated group (P < 0.01). Real-time RT-qPCR was used to assess the local inflammatory reaction in liver tissue 6 h after liver irradiation (Fig. 4B). Compared with the radiation-only group, systemic MSC-CM administration significantly reduced the mRNA expression levels of TNF-α, IL-1β and IL-6, and increased IL-10 mRNA expression of IL-10 in irradiated rat livers (P < 0.05 for TNF-α and IL-10, P < 0.01 for IL-1β and IL-6). Taken together, these data demonstrate that intravenous infusion of MSC-secreted molecules can modulate local and systemic inflammatory responses associated with RILD.
Fig. 4.
Expression levels of inflammatory and anti-inflammatory cytokines in peripheral blood and liver tissue. Samples were collected 6 h after liver irradiation. Enzyme-linked immunosorbent assay (ELISA) (A) and Real-Time quantitative Reverse Transcription-PCR (RT-qPCR) (B) analyses showed that TNF-α, IL-β and IL-6 were all significantly increased after RT, and these effects were partially decreased by MSC-CM administration. Expression of the anti-inflammatory cytokine IL-10 was significantly increased by MSC-CM treatment. Data are shown as means ± SD. **P < 0.01 (DMEM group versus control group). ##P < 0.01 and #P < 0.05 (MSC-CM group versus DMEM group).
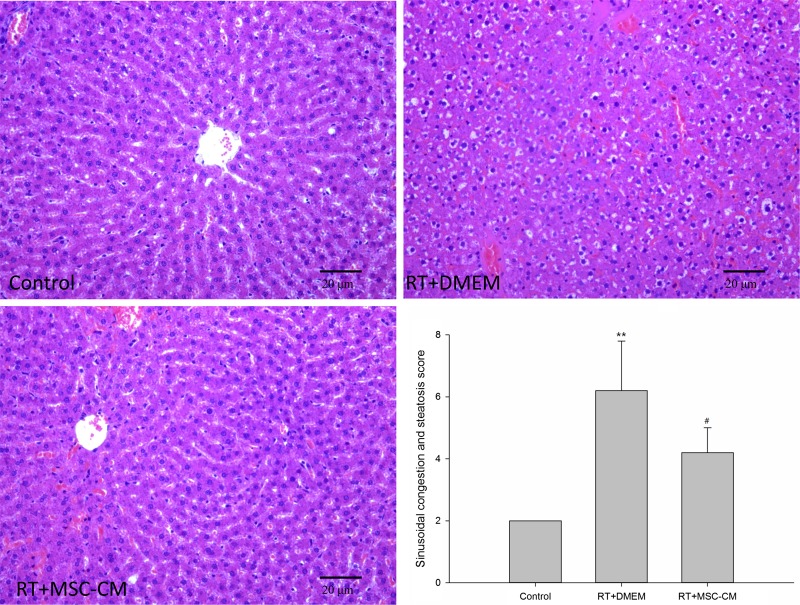
MSC-CM therapy improves liver histopathology
Histological examination of liver biopsy specimens was used to assess long-term injury. Liver irradiation resulted in typical sinusoidal congestion and hepatic steatosis in otherwise normal livers; at 4 weeks after RT, the sinusoidal congestion and steatosis score for RT+DMEM group was 6.2 ± 1.6 (Fig. 5); however, these changes were much less pronounced in rats pretreated with MSC-CM (4.2 ± 0.8, P < 0.05).
Fig. 5.
Effect of MSC-CM on radiation-induced liver injury. Liver histopathology showed radiation-induced sinusoidal congestion and hepatic steatosis at 4 weeks after RT (original magnification ×200). These features were improved by systemic MSC-CM administration. Data are shown as means ± SD. **P < 0.01 (DMEM group versus control group). #P < 0.01 (MSC-CM group versus DMEM group).
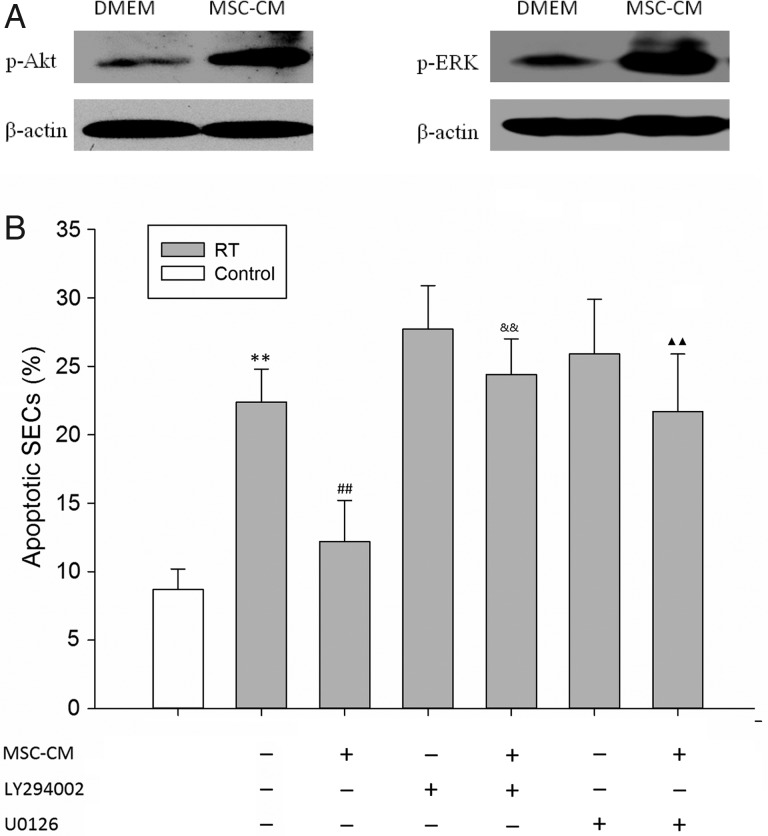
Effects of MSC-CM on Akt and ERK phosphorylation in SECs
Phosphorylation of the intracellular signaling molecules Akt and ERK is an important trigger of their anti-apoptosis and cell survival activities [33, 34]. Thus, we examined whether MSC-CM treatment affected Akt and ERK phosphorylation in cultured SECs. Treating serum-starved SECs with MSC-CM markedly induced Akt phosphorylation at 45 min (Fig. 6A). Similarly, MSC-CM induced ERK1/2 phosphorylation (Fig. 6B). To confirm the role of Akt and ERK activation in MSC-CM-mediated SEC survival, we used the Akt inhibitor (LY290004) and ERK inhibitor (U0126) to test the survival of SECs (Fig. 6C). LY290004 and U0126 inhibited MSC-CM–mediated radioprotection (apoptotic SECs, 24.4 ± 2.6% and 21.7 ± 4.2% after pretreatment with LY290004 and U0126, respectively).
Fig. 6.
(A) Phospho-AKT and Phospho-ERK1/2 expression by western blot in SECs treated with or without MSC-CM for 45 min. (B) SECs were cultured in the presence of the PI3 K/Akt inhibitor (LY294002) and MEK/ERK inhibitor (U0126) with or without RT and MSC-CM, and cell apoptosis was evaluated by fluorescence-activated cell sorting (FACS) analysis. Data are shown as means ± SD. **P < 0.01 (DMEM group versus control group). ##P < 0.01 (MSC-CM group versus DMEM group). &&P < 0.01 (MSC-CM group versus MSC-CM + LY294002 group). Two closed triangles: P < 0.01 (MSC-CM group versus MSC-CM + U0126 group).
DISCUSSION
SEC apoptosis and inflammation are initial events in RILD. This study provided the first clear evidence that delivery of MSC secretory products has the potential to dramatically reduce SEC death in the acutely radiation-injured liver. In vivo, rat liver irradiation increased circulating HA levels, SEC apoptosis, and inflammatory cytokine expression, and all of these effects were inhibited by pre-RT treatment with intravenous MSC-CM injection, ultimately reducing RILD. These observations suggest that MSC-CM contains soluble factors that are capable of attenuating liver injury. Identification of these soluble factors secreted by MSCs may yield new therapeutic options for RILD.
We demonstrated that apoptosis after liver irradiation occurs in SECs, but not hepatocytes, in the early stage after RT. Radiation-induced SEC apoptosis, measured by TUNEL staining of liver sections, occurred within 6 h of irradiation. This effect is comparable with apoptosis of the small intestinal microvascular endothelium, which underwent apoptosis within 4 h of whole-body irradiation in mice [35]. However, fewer apoptotic SECs were observed in irradiated rat livers after pretreatment with intravenous MSC-CM infusion.
We also determined serum HA and ALT levels in rats with irradiated livers, as respective markers of SEC and hepatocyte integrity. HA is an unbranched glycosaminoglycan present in the extracellular matrix. More than 90% of circulating HA is removed by hepatic SECs via HA-specific receptor-mediated endocytosis [36]. Increased circulating HA serves as a biomarker of dysfunction of hepatic SECs following liver injury [37]. Elevated circulating ALT is a known marker of liver damage, specifically of hepatocytes. We found that serum HA levels reached peak values within 24 h of liver RT, but serum ALT levels did not significantly change during the 48 h post-irradiation. Reducing injury by maintaining SEC integrity can prevent further hepatocyte damage [38, 39]. Ma et al. reported that: in vitro, adipose-derived stem cells prevent apoptosis of freshly isolated liver SECs by secreting VEGF; in vivo, implanted syngeneic adipose-derived stem cells attenuated small-for-size liver graft injuries in a rat model [40].
In in vitro experiments, we also found that MSC-CM pretreatment can protect cultured SECs from radiation injury–induced apoptosis. This protective mechanism may involve activation of the Akt and ERK1/2 signaling pathways. The PI3 K/Akt and ERK1/2 pathways are activated by growth factors and are both important cell survival signaling pathways [35, 36]. In the present study, Akt and ERK1/2 phosphorylation was induced by MSC-CM, which suggests that the protective effect of MSC-CM may be mediated by Akt and ERK1/2 activation. This effect may be caused by some growth factors secreted by MSCs. It has been reported that MSCs express many diverse biological factors, including VEGF, βFGF, PDGF, IGF-1 and S1P, all of which have anti-apoptotic activity.
MSCs are emerging as a therapeutic modality for various inflammatory diseases because of their anti-inflammatory and immunomodulatory properties. It has been reported that MSC-CM reduces the inflammatory response in both ischemia–reperfusion injury and D-galactosamine–induced acute liver injury models [41, 42]. In other organ injury models, MSC transplantation downregulates inflammatory cytokines and upregulates anti-inflammatory cytokines such as IL-10 [43, 44]. In this study, we found that systemic MSC-CM administration decreased TNF-α, IL-β and IL-6, and increased IL-10 expression, both in peripheral blood and liver tissue after hepatic irradiation. Secretion of the anti-inflammatory cytokine IL-10 by MSCs has been investigated in several studies [23, 45, 46]. In a model of radiation-induced intestinal injury, Chang et al. also demonstrated a significantly increased IL-10 concentration in response to human MSC administration [23].
RILD has been confirmed as a VOD with sinusoidal congestion of the central portion of the liver lobules, and ultimate atrophy of hepatocytes and fibrosis of the pericentral lobule regions [3]. Our study suggests that sinusoidal congestion emerges at 4 weeks after liver RT, and systemic MSC-CM pretreatment before irradiation can mitigate this damage. We also detected the appearance of hepatic steatosis in addition to sinusoidal congestion in rat livers following 30-Gy radiation. Steatosis often occurs in alcoholic liver disease and other diverse toxin-mediated liver diseases, in which oxidative stress reactions mediate liver injury [47]. We speculate that hepatic steatosis might also result from radiation-induced liver toxicity, and additionally, administration of MSC-CM can improve radiation-induced hepatic steatosis.
Most reports have employed cell transplantation as the primary mode of MSC therapy. However, the liver is a large organ that is not suitable for local MSC injection, and portal vein injection to circumvent pulmonary lodging of transplanted cells and subsequent embolus formation is invasive and often lethal. For these reasons, systemic infusion of MSC-CM represents an effective alternative for delivering the therapeutic effects of MSCs in acute liver injury. Systemic infusion of MSC-CM is a reasonable and effective procedure for delivering the protective effects of MSCs in RILD in in vivo models. However, systematic proteomic analyses combined with fractionation studies of MSC-CM are necessary to identify not only the key therapeutic components, but also any potentially harmful secretory products.
In conclusion, systemic MSC-CM therapy has profound inhibitory effects on SEC death and inflammatory response modulation, and it ultimately improves VOD in rats undergoing radiation-induced liver injury. Furthermore, MSC-CM has direct anti-apoptotic effects on cultured SECs, which possibly involve activation of the Akt and ERK cell survival signaling pathways. These findings highlight a potential new therapeutic avenue for protecting against RILD.
FUNDING
Funding to pay the Open Access publication charges for this article was provided by the Specialized Research Fund for the Doctoral Program of Higher Education of China (20120071110065) and the Research Fund from the Shanghai Municipal Science and Technology Committee (14140902303, 12XD1401800).
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the Specialized Research Fund for the Doctoral Program of Higher Education of China (20120071110065) and the Research Fund from the Shanghai Municipal Science and Technology Committee (14140902303, 12XD1401800).
REFERENCES
- 1.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet 2003;362:1907–17. [DOI] [PubMed] [Google Scholar]
- 2.Guha C, Kavanagh BD. Hepatic radiation toxicity: avoidance and amelioration. Semin Radiat Oncol 2011;21:256–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawrence TS, Robertson JM, Anscher MS, et al. Hepatic toxicity resulting from cancer treatment. Int J Radiat Oncol Biol Phys 1995;31:1237–48. [DOI] [PubMed] [Google Scholar]
- 4.Cheng JC, Wu JK, Huang CM, et al. Radiation-induced liver disease after radiotherapy for hepatocellular carcinoma: clinical manifestation and dosimetric description. Radiother Oncol 2002;63:41–5. [DOI] [PubMed] [Google Scholar]
- 5.Liang SX, Zhu XD, Xu ZY, et al. Radiation-induced liver disease in three-dimensional conformal radiation therapy for primary liver carcinoma: the risk factors and hepatic radiation tolerance. Int J Radiat Oncol Biol Phys 2006;65:426–34. [DOI] [PubMed] [Google Scholar]
- 6.Jirtle RL, Anscher MS, Alati T. Radiation sensitivity of the liver. Adv Radiat Biol 1990;14:269–311. [Google Scholar]
- 7.Alati T, Eckl P, Jirtle RL. An in vitro micronucleus assay for determining the radiosensitivity of hepatocytes. Radiat Res 1989;119:562–8. [PubMed] [Google Scholar]
- 8.Alati T, Van Cleeff M, Strom SC, et al. Radiation sensitivity of adult human parenchymal hepatocytes. Radiat Res 1988;115:152–60. [PubMed] [Google Scholar]
- 9.Jirtle RL, Michalopoulos G, Mclain JR, et al. Transplantation system for determining the clonogenic survival of parenchymal hepatocytes exposed to ionizing radiation. Cancer Res 1981;41:3512–8. [PubMed] [Google Scholar]
- 10.Jirtle RL, Michalopoulos G, Strom SC, et al. The survival of parenchymal hepatocytes irradiated with low and high LET radiation. Br J Cancer 1984;6:S197–201. [PMC free article] [PubMed] [Google Scholar]
- 11.Christiansen H, Saile B, Neubauer-Saile K, et al. Irradiation leads to susceptibility of hepatocytes to TNF-alpha mediated apoptosis. Radiother Oncol 2004;72:291–6. [DOI] [PubMed] [Google Scholar]
- 12.Reed GB, Jr, Cox AJ., Jr The human liver after radiation injury: a form of veno-occlusive disease. Am J Pathol 1966;48:597–611. [PMC free article] [PubMed] [Google Scholar]
- 13.Fajardo LF, Colby TV. Pathogenesis of veno-occlusive liver disease after radiation. Arch Pathol Lab Med 1980;104:584–8. [PubMed] [Google Scholar]
- 14.Yamanouchi K, Zhou H, Roy-Chowdhury N, et al. Hepatic irradiation augments engraftment of donor cells following hepatocyte transplantation. Hepatology 2009;49:258–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malik IA, Moriconi F, Sheikh N, et al. Single-dose gamma irradiation induces up-regulation of chemokine gene expression and recruitment of granulocytes into the portal area but not into other regions of rat hepatic tissue. Am J Pathol 2010;176:1801–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moriconi F, Christiansen H, Raddatz D, et al. Effect of radiation on gene expression of rat liver chemokines: in vivo and in vitro studies. Radiat Res 2008;169:162–9. [DOI] [PubMed] [Google Scholar]
- 17.Moriconi F, Malik I, Ahmad G, et al. Effect of irradiation on gene expression of rat liver adhesion molecules: in vivo and in vitro studies. Strahlenther Onkol 2009;185:460–8. [DOI] [PubMed] [Google Scholar]
- 18.Christiansen H, Sheikh N, Saile B, et al. X-irradiation in rat liver: consequent upregulation of hepcidin and downregulation of hemojuvelin and ferroportin-1 gene expression. Radiology 2007;242:189–97. [DOI] [PubMed] [Google Scholar]
- 19.Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell 2009;4:206–16. [DOI] [PubMed] [Google Scholar]
- 20.Gao Z, Zhang Q, Han Y, et al. Mesenchymal stromal cell–conditioned medium prevents radiation-induced small intestine injury in mice. Cytotherapy 2012;14:267–73. [DOI] [PubMed] [Google Scholar]
- 21.Wang H, Yang YF, Zhao L, et al. Hepatocyte growth factor gene-modified mesenchymal stem cells reduce radiation-induced lung injury. Hum Gene Ther 2013;24:343–53. [DOI] [PubMed] [Google Scholar]
- 22.Xue J, Li X, Lu Y, et al. Gene-modified mesenchymal stem cells protect against radiation-induced lung injury. Mol Ther 2013;21:456–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang P, Qu Y, Liu Y, et al. Multi-therapeutic effects of human adipose-derived mesenchymal stem cells on radiation-induced intestinal injury. Cell Death Dis 2013;20(4):e685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang YH, Lin LM, Lou CW, et al. Bone marrow transplantation rescues intestinal mucosa after whole body radiation via paracrine mechanisms. Radiother Oncol 2012;105:371–7. [DOI] [PubMed] [Google Scholar]
- 25.Bi B, Schmitt R, Israilova M, et al. Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol 2007;18:2486e96. [DOI] [PubMed] [Google Scholar]
- 26.Lee JW, Fang X, Gupta N, et al. Allogenic human mesenchymal stem cells for treatment of E.coli endotoxin–induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci U S A 2009;106:16357e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yagi H, Soto-Gutierrez A, Navarro-Alvarez N, et al. Reactive bone marrow stromal cells attenuate systemic inflammation via sTNFR1. Mol Ther 2010;18:1857e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ionescu L, Byrne RN, van Haaften T, et al. Stem cell conditioned medium improves acute lung injury in mice: in vivo evidence for stem cell paracrine action. Am J Physiol Lung Cell Mol Physiol 2012;303:L967e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hung SC, Pochampally RR, Chen SC, et al. Angiogenic effects of human multipotent stromal cell conditioned medium activate the P13K-Akt pathway in hypoxic endothelial cells to inhibit apoptosis, increase survival, and stimulate angiogenesis. Stem Cells 2007;25:2363e70. [DOI] [PubMed] [Google Scholar]
- 30.Boomsma RA, Geenen DL. Mesenchymal stem cells secrete multiple cytokines that promote angiogenesis and have contrasting effects on chemotaxis and apoptosis. PLoS One 2012;7:e35685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eliopoulos N, Stagg J, Lejeune L, et al. Allogeneic marrow stromal cells are immune rejected by MHC class I- and class II-mismatched recipient mice. Blood 2005;106:4057. [DOI] [PubMed] [Google Scholar]
- 32.Wang TJ, Liu ZS, Zeng ZC, et al. Caffeine does not enhance radiosensitivity of normal liver tissue in vivo. Mol Biol Rep 2011;38:4359–67. [DOI] [PubMed] [Google Scholar]
- 33.Ozaki M, Haga S, Zhang HQ, et al. Inhibition of hypoxia/reoxygenation-induced oxidative stress in HGF-stimulated antiapoptotic signaling: role of PI3-K and Akt kinase upon rac1. Cell Death Differ 2003;10:508–15. [DOI] [PubMed] [Google Scholar]
- 34.Pearson G, Robinson F, Gibson TB, et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev 2001;22:153–83. [DOI] [PubMed] [Google Scholar]
- 35.Paris F, Fuks Z, Kang A, et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science 2001;293:293–7. [DOI] [PubMed] [Google Scholar]
- 36.Fraser JR, Alcorn D, Laurent TC, et al. Uptake of circulating hyaluronic acid by the rat liver. Cellular localization in situ. Cell Tissue Res 1985;242:505–10. [DOI] [PubMed] [Google Scholar]
- 37.George J, Stern R. Serum hyaluronan and hyaluronidase: very early markers of toxic liver injury. Clin Chim Acta 2004;348:189–97. [DOI] [PubMed] [Google Scholar]
- 38.Shimizu H, Miyazaki M, Wakabayashi Y, et al. Vascular endothelial growth factor secreted by replicating hepatocytes induces sinusoidal endothelial cell proliferation during regeneration after partial hepatectomy in rats. J Hepatol 2001;34:683. [DOI] [PubMed] [Google Scholar]
- 39.Ding BS, Nolan DJ, Butler JM, et al. Inductive angiocrine signals from sinusoidal endothelium is required for liver regeneration. Nature 2010;468:310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma T, Liu H, Chen W, et al. Implanted adipose-derived stem cells attenuate small-for-size liver graft injury by secretion of VEGF in rats. Am J Transplant 2012;12:620. [DOI] [PubMed] [Google Scholar]
- 41.Du Z, Wei C, Cheng K, et al. Mesenchymal stem cell–conditioned medium reduces liver injury and enhances regeneration in reduced-size rat liver transplantation. J Surg Res 2013;183:907–15. [DOI] [PubMed] [Google Scholar]
- 42.Van Poll D, Parekkadan B, Cho CH, et al. Mesenchymal stem cell–derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology 2008;47:1634–43. [DOI] [PubMed] [Google Scholar]
- 43.Togel F, Hu Z, Weiss K, et al. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol 2005;289:F31–42. [DOI] [PubMed] [Google Scholar]
- 44.Ortiz LA, Dutreil M, Fattman C, et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci U S A 2007;104:11002–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005;105:1815e22. [DOI] [PubMed] [Google Scholar]
- 46.Nemeth K, Leelahavanichkul A, Yuen PS, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med 2009;15:42e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gu K, Zhao JD, Ren ZG. A natural process of cirrhosis resolution and deceleration of liver regeneration after thioacetamide withdrawal in a rat model. Mol Biol Rep 2011;38:1687–96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.