Abstract
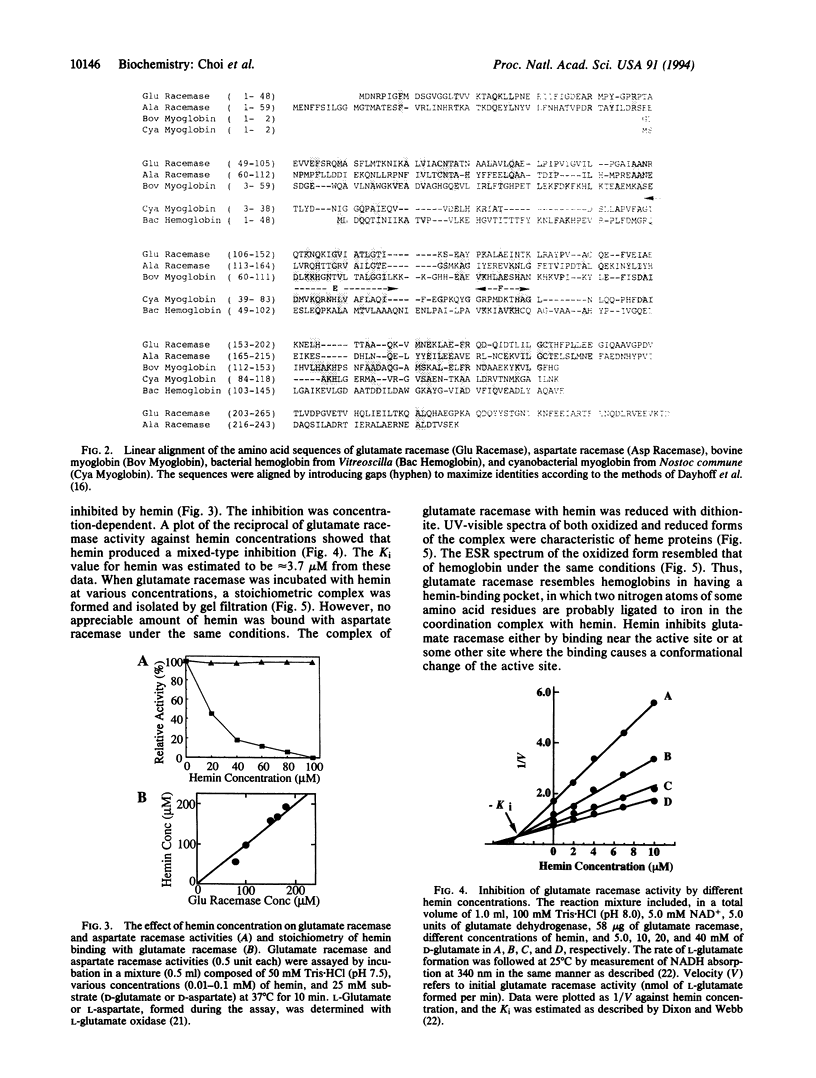
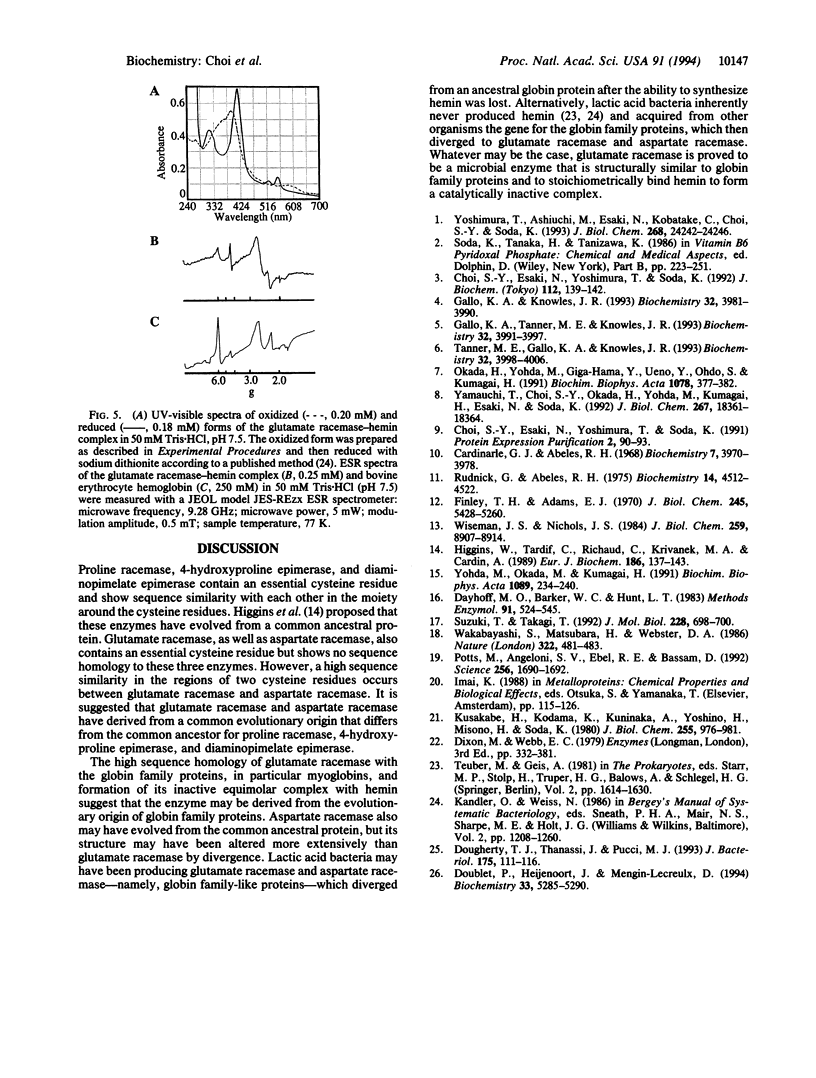
Glutamate racemase (EC 5.1.1.3), an enzyme of microbial origin, shows significant sequence homology with mammalian myoglobins, in particular in the regions corresponding to the E and F helices, which constitute the heme binding pocket of myoglobins. Glutamate racemase binds tightly an equimolar amount of hemin, leading to loss of racemase activity. Although this enzyme shows homology with aspartate racemase, the latter does not bind hemin. The glutamate racemase gene of Pediococcus pentosaceus has a 795-nt open reading frame and encodes 265-amino acid residues, which form a monomeric protein (M(r) 29,000). Neither racemase has cofactors, but they contain essential cysteine residues [Yohda, M., Okada, H. & Kumagai, H. (1991) Biochim. Biophys. Acta 1089, 234-240].
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cardinale G. J., Abeles R. H. Purification and mechanism of action of proline racemase. Biochemistry. 1968 Nov;7(11):3970–3978. doi: 10.1021/bi00851a026. [DOI] [PubMed] [Google Scholar]
- Choi S. Y., Esaki N., Yoshimura T., Soda K. Overproduction of glutamate racemase of Pediococcus pentosaceus in Escherichia coli clone cells and its purification. Protein Expr Purif. 1991 Feb;2(1):90–93. doi: 10.1016/1046-5928(91)90016-c. [DOI] [PubMed] [Google Scholar]
- Choi S. Y., Esaki N., Yoshimura T., Soda K. Reaction mechanism of glutamate racemase, a pyridoxal phosphate-independent amino acid racemase. J Biochem. 1992 Jul;112(1):139–142. doi: 10.1093/oxfordjournals.jbchem.a123853. [DOI] [PubMed] [Google Scholar]
- Dayhoff M. O., Barker W. C., Hunt L. T. Establishing homologies in protein sequences. Methods Enzymol. 1983;91:524–545. doi: 10.1016/s0076-6879(83)91049-2. [DOI] [PubMed] [Google Scholar]
- Doublet P., van Heijenoort J., Mengin-Lecreulx D. The glutamate racemase activity from Escherichia coli is regulated by peptidoglycan precursor UDP-N-acetylmuramoyl-L-alanine. Biochemistry. 1994 May 3;33(17):5285–5290. doi: 10.1021/bi00183a035. [DOI] [PubMed] [Google Scholar]
- Dougherty T. J., Thanassi J. A., Pucci M. J. The Escherichia coli mutant requiring D-glutamic acid is the result of mutations in two distinct genetic loci. J Bacteriol. 1993 Jan;175(1):111–116. doi: 10.1128/jb.175.1.111-116.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay T. H., Adams E. Kinetic and structural studies of hydroxyproline 2-epimerase. J Biol Chem. 1970 Oct 25;245(20):5248–5260. [PubMed] [Google Scholar]
- Gallo K. A., Knowles J. R. Purification, cloning, and cofactor independence of glutamate racemase from Lactobacillus. Biochemistry. 1993 Apr 20;32(15):3981–3990. doi: 10.1021/bi00066a019. [DOI] [PubMed] [Google Scholar]
- Gallo K. A., Tanner M. E., Knowles J. R. Mechanism of the reaction catalyzed by glutamate racemase. Biochemistry. 1993 Apr 20;32(15):3991–3997. doi: 10.1021/bi00066a020. [DOI] [PubMed] [Google Scholar]
- Higgins W., Tardif C., Richaud C., Krivanek M. A., Cardin A. Expression of recombinant diaminopimelate epimerase in Escherichia coli. Isolation and inhibition with an irreversible inhibitor. Eur J Biochem. 1989 Dec 8;186(1-2):137–143. doi: 10.1111/j.1432-1033.1989.tb15187.x. [DOI] [PubMed] [Google Scholar]
- Kusakabe H., Kodama K., Kuninaka A., Yoshino H., Misono H., Soda K. A new antitumor enzyme, L-lysine alpha-oxidase from Trichoderma viride. Purification and enzymological properties. J Biol Chem. 1980 Feb 10;255(3):976–981. [PubMed] [Google Scholar]
- Okada H., Yohda M., Giga-Hama Y., Ueno Y., Ohdo S., Kumagai H. Distribution and purification of aspartate racemase in lactic acid bacteria. Biochim Biophys Acta. 1991 Jul 12;1078(3):377–382. doi: 10.1016/0167-4838(91)90159-w. [DOI] [PubMed] [Google Scholar]
- Potts M., Angeloni S. V., Ebel R. E., Bassam D. Myoglobin in a cyanobacterium. Science. 1992 Jun 19;256(5064):1690–1691. doi: 10.1126/science.256.5064.1690. [DOI] [PubMed] [Google Scholar]
- Rudnick G., Abeles R. H. Reaction mechanism and structure of the active site of proline racemase. Biochemistry. 1975 Oct 7;14(20):4515–4522. doi: 10.1021/bi00691a028. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Takagi T. A myoglobin evolved from indoleamine 2,3-dioxygenase. J Mol Biol. 1992 Nov 20;228(2):698–700. doi: 10.1016/0022-2836(92)90854-d. [DOI] [PubMed] [Google Scholar]
- Tanner M. E., Gallo K. A., Knowles J. R. Isotope effects and the identification of catalytic residues in the reaction catalyzed by glutamate racemase. Biochemistry. 1993 Apr 20;32(15):3998–4006. doi: 10.1021/bi00066a021. [DOI] [PubMed] [Google Scholar]
- Wakabayashi S., Matsubara H., Webster D. A. Primary sequence of a dimeric bacterial haemoglobin from Vitreoscilla. 1986 Jul 31-Aug 6Nature. 322(6078):481–483. doi: 10.1038/322481a0. [DOI] [PubMed] [Google Scholar]
- Wiseman J. S., Nichols J. S. Purification and properties of diaminopimelic acid epimerase from Escherichia coli. J Biol Chem. 1984 Jul 25;259(14):8907–8914. [PubMed] [Google Scholar]
- Yamauchi T., Choi S. Y., Okada H., Yohda M., Kumagai H., Esaki N., Soda K. Properties of aspartate racemase, a pyridoxal 5'-phosphate-independent amino acid racemase. J Biol Chem. 1992 Sep 15;267(26):18361–18364. [PubMed] [Google Scholar]
- Yohda M., Okada H., Kumagai H. Molecular cloning and nucleotide sequencing of the aspartate racemase gene from lactic acid bacteria Streptococcus thermophilus. Biochim Biophys Acta. 1991 Jun 13;1089(2):234–240. doi: 10.1016/0167-4781(91)90013-c. [DOI] [PubMed] [Google Scholar]
- Yoshimura T., Ashiuchi M., Esaki N., Kobatake C., Choi S. Y., Soda K. Expression of glr (murI, dga) gene encoding glutamate racemase in Escherichia coli. J Biol Chem. 1993 Nov 15;268(32):24242–24246. [PubMed] [Google Scholar]




