Abstract
Cancer stem cells (CSCs) are a subpopulation of tumor cells that possess unique self-renewal activity and mediate tumor initiation and propagation. The PI3K/Akt/mTOR signaling pathway can be considered as a master regulator for cancer. More and more recent studies have shown the links between PI3K/Akt/mTOR signaling pathway and CSC biology. Herein, we provide a comprehensive review on the role of signaling components upstream and downstream of PI3K/Akt/mTOR signaling in CSC. In addition, we also summarize various classes of small molecule inhibitors of PI3K/Akt/mTOR signaling pathway and their clinical potential in CSC. Overall, the current available data suggest that the PI3K/Akt/mTOR signaling pathway could be a promising target for development of CSC-target drugs.
Keywords: Cancer stem cells, PI3K/Akt/mTOR signaling pathway, self-renew, tumor initiation, rapamycin
Introduction
In the past decades, cancer stem-like cells (CSCs) have been identified in several types of solid tumors, such as those of the lung, breast, colon and liver [1-4]. CSCs are a unique subpopulation not only a renewable source of tumor cells, but also a source of tumor resistance leading to tumor recurrence, metastasis, and progression [5]. Some key signaling pathways, including Wnt/β-catenin, STAT3 and TGF-β, have been implicated in the maintenance of CSCs [6-8]. The phosphatidylinositol-3-kinase (PI3K)/Akt and the mammalian target of rapamycin (mTOR) signaling pathways are crucial to many physiological and pathological conditions, such as cell proliferation, angiogenesis, metabolism, differentiation and survival [9]. Activate mTOR are frequently improperly regulated in most human cancers. For example, PI3K/Akt/mTOR pathway is activated in approximately 70% of ovarian cancers [10]. Tapia et al. [11] also found that the PI3K/Akt/mTOR pathway is activated in tumor tissues from patients with advanced gastric cancer compare with that in nontumor gastric mucosa. Apart from the attention on cancer cell, more and more recent studies have shown the links between PI3K/Akt/mTOR signaling and CSC biology [12,13]. In the study of Sunayama et al [14], mTOR signaling has been shown to maintain the self-renewal and tumorigenicity of glioblastoma stem-like cells. In sharp contrast, mTOR inhibition by rapamycin has been shown to significantly increase CD133 expression in gastrointestinal cancer cells [15].
The objective of the present work is to review the evidence about the roles of the PI3K/Akt/mTOR pathway in cancer stem cells and to solve the controversy among these reports.
Pathogenesis of cancer and the PI3K/Akt/mTOR pathway
Previous studies showed the importance of mTOR pathway in cancer pathogenesis. PIK3 is overexpressed in ovarian [16] and cervical cancer [17]. Its mutations have been observed in breast cancer, glioblastoma and gastric cancer [18]. Akt1 overexpression has been detected in gastric carcinoma [19], and Akt2 overexpression has been observed in ovarian and pancreatic cancer [20,21]. Although mutation of Akt itself is rare, Carpten et al. [22] described somatic mutations occurring in Akt1 in a small percentage of human breast, ovarian, and colorectal cancers. mTOR complex 1 (mTORC1) could increase mRNA translation, protein synthesis and cellular proliferation [23]. Activation of a second mTOR complex (mTORC2), involved in regulation of the cytoskeleton, is probably an effect of Akt loop feedback [23]. Balsara et al. [24] found that positive staining for mTOR was exhibited in 74% specimens from the patients with non-small cell lung cancer (NSCLC) by using tissue microarray (TMA). Rictor, a mTORC2 subunit, promoted mTORC2 assembly and activity and endowed glioma cells with increased proliferative and invasion potential [25].
Cancer stem cell and the PI3K/Akt/mTOR pathway
More and more studies showed the role of mTOR pathway in the maintenance of CSCs. Chang et al. [26] found that prostate cancer radioresistance is associated with epithelial-mesenchymal transition (EMT) and enhanced CSC phenotypes via activation of the PI3K/Akt/mTOR signaling pathway. Activation of the mTOR pathway in breast cancer stem-like cells is required for colony-formation ability in vitro and tumorigenicity in vivo [27]. mTOR suppression could decrease aldehyde dehydrogenase 1 (ALDH1) activity, which is a marker for colorectal cancer stem cells [28,29]. Inhibition of mTORC2 led to decrease a hepatic CSC marker (epithelial cell adhesion molecule, EpCAM) expression and little or no tumorigenicity in hepatocellular cancer stem cells [30]. Sunayama et al. [14] found that cross-inhibitory regulation between the MEK/ERK and PI3K/mTOR pathways contributed to the maintenance of the self-renewal and the tumorigenic capacity of glioblastoma cancer stem-like cells. Bleau et al. [31] found that Akt, but not its downstream target mTOR, regulates ATP binding cassette transporters (ABCG2) activity in glioma tumor stem-like cells. Corominas-Faja et al. [32] used Yamanaka’s stem cell technology in an attempt to create stable CSC research lines, and they found that the transcriptional suppression of mTOR repressors is an intrinsic process occurring in luminal-like breast cancer cells during the acquisition of CSC-like properties. Previous studies have indicated that CD133 is one of the markers for cancer stem cells [33-36]. Inhibition of mTOR signaling up-regulated CD133 expression in gastrointestinal cancer cells [15]. The results of Yang et al. [37] showed that mTOR inhibition increase the CD133+ subpopulations, and trigger the conversion of CD133- to CD133+ liver tumor cells. These two results indicated that inhibition of mTOR signaling could induce the generation of CSC cells. However, the main reason for the discrepancy is different cellular contexts. CD133 expression mRNA and protein levels were elevated under hypoxic conditions [38].
Dubrovska et al. [5] found that PTEN/PI3K/Akt pathway is critical for prostate cancer stem-like cell maintenance and that targeting PI3K signaling may be beneficial in prostate cancer treatment by eliminating prostate cancer stem-like cells. Activated PI3K upregulated ABCG2 expression and elevated percentage of cancer stem-like cells in both acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL) [39]. However, in the study of Airiau et al. [40], they found that mTOR inhibition showed no effect on chronic myeloid leukemia (CML) stem cells (CD34+/CD38-), while PI3K inhibition restored the cell line sensitivity to nilotinib, a second generation tyrosine kinase inhibitor (TKI). Abnormal activation of PI3K/Akt/mTOR signaling pathway leads to enhanced expression of chemokine (C-X-C motif) receptor 4 (CXCR4), which in turn promotes CXCR4-mediated STAT3 signaling that might be responsible for maintenance of stemness in NSCLC cells [41]. Chang et al. [42] found that insulin-like growth factor-1 receptor (IGF-1R) and its signaling via PI3K/Akt/mTOR pathway are attractive targets for therapy directed against breast cancer stem cells. Cyclin G1-induced liver tumor-initiating cells expansion contributes to the recurrence and chemoresistance of hepatoma via Akt/mTOR signaling [43]. Decreased mTOR activity in response to hypoxia-inducible factor 1α (HIF-1α) upregulation inhibits proliferation and promotes survival of prostate cancer stem cells through the PI3K feedback loop [44].
As discussed above, a link between the PI3K/Akt/mTOR pathway and cancer stem cell is clearly evident and the components of this pathway are viable candidates for therapeutic intervention (Figure 1).
Figure 1.
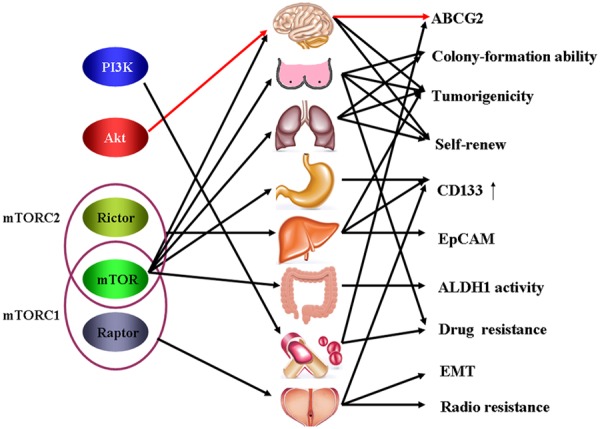
Schematic representation of the PI3K/Akt/mTOR signaling pathway and CSC biology.
PI3K/Akt/mTOR is a target for cancer stem cells therapy
The Food and Drug Administration (FDA) approved temsirolimus for the treatment of advanced stage renal cell carcinoma in 2007. Temsirolimus became the first mTOR inhibitor approved for cancer therapy [45]. From then on, three generations of compounds targeting PI3K/mTOR have already been developed. The first-generation of PI3K inhibitors, also being called “pan-inhibitors”, were able to bind all class I PI3Ks [46]. The second-generation inhibitors are characterized by greater and isoform-specific selective activity [46]. The third generation inhibitors, “dual PI3K/mTOR inhibitors”, not only inhibits all PI3K class I isoforms, but also mTORC1 and mTORC2 [47].
The mTOR antagonist everolimus has effective inhibitory effects on HER2-overexpressing breast cancer stem cells in vitro and in vivo by reducing the expression of Akt1 and p-Akt [47]. Liu et al. [48] found that everolimus in combination with letrozole inhibit human breast cancer MCF-7 stem cells via PI3K/mTOR pathway.
Mendiburu-Eliçabe et al. [49] found that rapamycin reduced cell proliferation and tumorigenic potential, led to the loss of CD133+ population and increased the level of p-Akt in glioblastoma cells. Wang et al. [50] found that depletion of F-box and WD repeat domain containing 7 (FBXW7) in colon cancer cells induces EMT and cancer stem cell-like characteristics, which can be suppressed by mTOR inhibitor, rapamycin. Rapamycin also has been demonstrated that could target the self-renewal and vascular differentiation potential in patient-derived hemangioma stem cells [51].
Metformin (1,1-dimethylbiguanide hydrochloride), the most widely prescribed drug for treatment of type 2 diabetes, inhibition of CSCs was first showed in 2009 in preclinical breast cancer models [52]. Interestingly, metformin preferentially kills CSCs over NSCCs (non-stem cancer cells) derived from human breast tumors, and it inhibits growth of mammospheres derived from these tumors [53]. These results were subsequently extended to pancreatic cancer cell line, metformin decreased CSC markers, CD44, CD133, ALDH1, and EPCAM and modulation of the mTOR signaling pathway [13]. Metformin eradicates radioresistant cancer stem cells in mouse fibrosarcoma cell (FSaII) and human mammary adenocarcinoma cell (MCF-7) by activating AMP-activated protein kinase (AMPK) and suppressing mTOR [54]. Furthermore, the proliferation of breast cancer stem cells was markedly suppressed by metformin that leading to inactivation of mTOR [55].
Salinomycin is a monocarboxylic polyether antibiotic used to prevent coccidiosis in poultry [56]. Gupta et al. [57] showed that salinomycin selectively kills human breast CSCs in 2009. A series of followed studies showed similar effects of salinomycin in other types of CSCs, such as pancreatic cancer [58], colorectal cancer [59] and lung cancer [60]. Many mechanisms of salinomycin have been identified in CSC cells [58-60]. One of the mechanisms is that salinomycin induces cell death and differentiation in head and neck squamous cell carcinoma stem cells by activation of EMT and Akt [61]. Metformin in combination with salinomycin could be a promising treatment option for five NSCLC cells and their stem cells (HCC4006, NCI-H1975, NCI-H2122, HCC95 and NCI-H3122) [62].
The radiosensitization efficiency of NVP-BEZ235, a novel dual PI3K/mTOR inhibitor, is achieved in human glioma stem cells by its cumulative antitumor effects, including induction of autophagy, apoptosis, cell cycle arrest, and prevention of DNA repair [63]. Dubrovska et al. [64] also found that NVP-BEZ235 leads to a decrease in the population of CD133+/CD44+ prostate cancer progenitor cells in vivo. Blockage of the PI3K/mTOR pathway inhibited the in vitro proliferation of colorectal cancer stem cells and in vivo xenograft tumor growth by using a dual PI3K/mTOR inhibitor, PF-04691502 [65]. The apoptosis-inducing mTOR inhibitor, Torin-1, hindered growth, motility, invasion, and survival of colorectal cancer stem cell in vitro, and suppressed tumor growth in vivo [66]. A novel dual mTORC2/mTORC1 inhibitor, OSI-027, suppresses primitive leukemic precursors from AML patients and is much more effective than rapamycin in eliciting antileukemic effects in vitro [67]. The anthracycline daunorubicin (DNR) is one of the major antitumor agents widely used in the treatment of AML [68]. PI-103, a dual inhibitor of PI3K and mTOR sensitizes AML stem cells to daunorubicin-induced cytotoxicity [69]. Hong et al. [70] also found that arsenic disulfide (As2S2) synergizes with PI-103 eradicated AML stem cells by targeting the PI3K/Akt/mTOR pathway. Silibinin, a flavonoid compound, inhibits colon CSCs self-renewal and sphere formation by suppressing the PP2A/Akt/mTOR pathway [71]. Quinoline imidoselenocarbamate EI201 reduces the CSC population and inhibits tumor growth in an in vivo model of prostate cancer by suppressing Akt/mTOR pathway [72]. Rottlerin is a plant derived chemotherapeutic agent and has been used as a protein kinase C-∆ signaling pathway inhibitor [73]. Singh et al. [74] found that rottlerin induces autophagy which leads to apoptotic cell death through inhibition of PI3K/Akt/mTOR pathway in human pancreatic cancer stem cells. Kumar et al. [75,76] found that ro signaling pathway.
Based on the previous studies as described above, better understanding of PI3K/Akt/mTOR signaling should create novel therapeutic opportunities in treating cancer stem cells (Figure 2).
Figure 2.
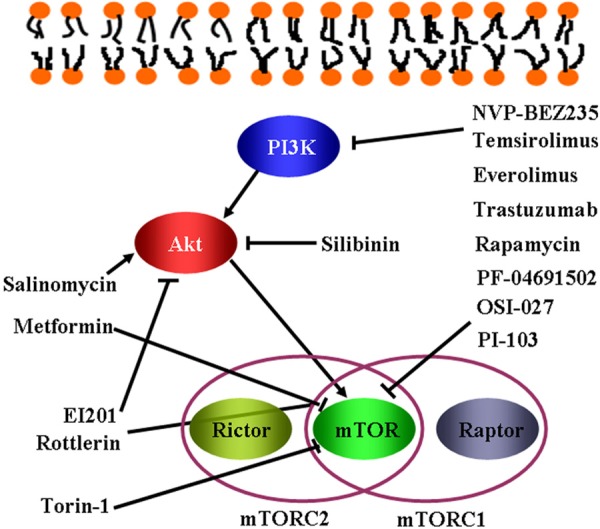
Schematic representation of the action PI3K/Akt/mTOR pathway inhibitors in CSC.
Perspective
In summary, the PI3K/AktmTOR pathway is a very complicated intracellular network, we are only at the beginning of understanding the precise role of PI3K/Akt/mTOR signaling in regulating cancer stem cells. Our current understanding of the precise mechanisms through PI3K/Akt/mTOR signaling is still extremely limited. Importantly, it remains to be determined how broadly useful such molecules will be in the clinical setting. We believe that these data will greatly impact the development of new therapies being designed to eradicate CSC.
Disclosure of conflict of interest
None.
References
- 1.Chen DQ, Huang JY, Feng B, Pan BZ, De W, Wang R, Chen LB. Histone deacetylase 1/Sp1/MicroRNA-200b signaling accounts for maintenance of cancer stem-like cells in human lung adenocarcinoma. PLoS One. 2014;9:e109578. doi: 10.1371/journal.pone.0109578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergamaschi A, Madak-Erdogan Z, Kim Y, Choi YL, Lu H, Katzenellenbogen BS. The forkhead transcription factor FOXM1 promotes endocrine resistance and invasiveness in estrogen receptor-positive breast cancer by expansion of stem-like cancer cells. Breast Cancer Res. 2014;16:436. doi: 10.1186/s13058-014-0436-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiener Z, Högström J, Hyvönen V, Band AM, Kallio P, Holopainen T, Dufva O, Haglund C, Kruuna O, Oliver G, Ben-Neriah Y, Alitalo K. Prox1 promotes expansion of the colorectal cancer stem cell population to fuel tumor growth and ischemia resistance. Cell Rep. 2014;8:1943–1956. doi: 10.1016/j.celrep.2014.08.034. [DOI] [PubMed] [Google Scholar]
- 4.Chai S, Tong M, Ng KY, Kwan PS, Chan YP, Fung TM, Lee TK, Wong N, Xie D, Yuan YF, Guan XY, Ma S. Regulatory role of miR-142-3p on the functional hepatic cancer stem cell marker CD133. Oncotarget. 2014;5:5725–5735. doi: 10.18632/oncotarget.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dubrovska A, Kim S, Salamone RJ, Walker JR, Maira SM, García-Echeverría C, Schultz PG, Reddy VA. The role of PTEN/Akt/PI3K signaling in the maintenance and viability of prostate cancer stem-like cell populations. Proc Natl Acad Sci U S A. 2009;106:268–273. doi: 10.1073/pnas.0810956106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curtin JC, Lorenzi MV. Drug discovery approaches to target Wnt signaling in cancer stem cells. Oncotarget. 2010;1:563–577. doi: 10.18632/oncotarget.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou J, Yi L, Ouyang Q, Xu L, Cui H, Xu M. Neurotensin signaling regulates stem-like traits of glioblastoma stem cells through activation of IL-8/CXCR1/STAT3 pathway. Cell Signal. 2014;26:2896–2902. doi: 10.1016/j.cellsig.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 8.Anido J, Saez-Borderias A, Gonzalez-Junca A, Rodon L, Folch G, Carmona MA, Prieto-Sánchez RM, Barba I, Martínez-Sáez E, Prudkin L, Cuartas I, Raventós C, Martínez-Ricarte F, Poca MA, García-Dorado D, Lahn MM, Yingling JM, Rodón J, Sahuquillo J, Baselga J, Seoane J. TGF-beta receptor inhibitors target the CD44(high)/Id1(high) glioma-initiating cell population in human glioblastoma. Cancer Cell. 2010;18:655–668. doi: 10.1016/j.ccr.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 9.Porta C, Paglino C, Mosca A. Targeting PI3K/Akt/mTOR signaling in cancer. Front Oncol. 2014;4:64. doi: 10.3389/fonc.2014.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li H, Zeng J, Shen K. PI3K/AKT/mTOR signaling pathway as a therapeutic target for ovarian cancer. Arch Gynecol Obstet. 2014;290:1067–1078. doi: 10.1007/s00404-014-3377-3. [DOI] [PubMed] [Google Scholar]
- 11.Tapia O, Riquelme I, Leal P, Sandoval A, Aedo S, Weber H, Letelier P, Bellolio E, Villaseca M, Garcia P, Roa JC. The PI3K/AKT/mTOR pathway is activated in gastric cancer with potential prognostic and predictive significance. Virchows Arch. 2014;465:25–33. doi: 10.1007/s00428-014-1588-4. [DOI] [PubMed] [Google Scholar]
- 12.Honjo S, Ajani JA, Scott AW, Chen Q, Skinner HD, Stroehlein J, Johnson RL, Song S. Metformin sensitizes chemotherapy by targeting cancer stem cells and the mTOR pathway in esophageal cancer. Int J Oncol. 2014;45:567–574. doi: 10.3892/ijo.2014.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohammed A, Janakiram NB, Brewer M, Ritchie RL, Marya A, Lightfoot S, Steele VE, Rao CV. Antidiabetic drug metformin prevents progression of pancreatic cancer by targeting in part cancer stem cells and mTOR signaling. Transl Oncol. 2013;6:649–659. doi: 10.1593/tlo.13556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sunayama J, Matsuda KI, Sato A, Tachibana K, Suzuki K, Narita Y, Shibui S, Sakurada K, Kayama T, Tomiyama A, Kitanaka C. Crosstalk between the PI3K/mTOR and MEK/ERK pathways involved in the maintenance of self-renewal and tumorigenicity of glioblastoma stem-like cells. Stem Cells. 2010;28:1930–1939. doi: 10.1002/stem.521. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto K, Arao T, Tanaka K, Kaneda H, Kudo K, Fujita Y, Tamura D, Aomatsu K, Tamura T, Yamada Y, Saijo N, Nishio K. mTOR signal and hypoxia-inducible factor-1 alpha regulate CD133 expression in cancer cells. Cancer Res. 2009;69:7160–7164. doi: 10.1158/0008-5472.CAN-09-1289. [DOI] [PubMed] [Google Scholar]
- 16.Shayesteh L, Lu YL, Kuo WL, Baldocchi R, Godfrey T, Collins C, Pinkel D, Powell B, Mills GB, Gray JW. PIK3CA is implicated as an oncogene in ovarian cancer. Nat Genet. 1999;21:99–102. doi: 10.1038/5042. [DOI] [PubMed] [Google Scholar]
- 17.Ma YY, Wei SJ, Lin YC, Lung JC, Chang TC, Whang-Peng J, Liu JM, Yang DM, Yang WK, Shen CY. PIK3CA as an oncogene in cervical cancer. Oncogene. 2000;19:2739–2744. doi: 10.1038/sj.onc.1203597. [DOI] [PubMed] [Google Scholar]
- 18.Samuels Y, Wang ZH, Bardelli A, Silliman N, Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ, Willson JK, Markowitz S, Kinzler KW, Vogelstein B, Velculescu VE. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554–554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 19.Staal SP. Molecular-cloning of the Akt oncogene and its human homologs Akt1 and Akt2-amplification of Akt1 in a primary human gastric adenocarcinoma. Proc Natl Acad Sci U S A. 1987;84:5034–5037. doi: 10.1073/pnas.84.14.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng JQ, Ruggeri B, Klein WM, Sonoda G, Altomare DA, Watson DK, Testa JR. Amplification of AKT2 in human pancreatic cancer cells and inhibition of ATK2 expression and tumorigenicity by antisense RNA. Proc Natl Acad Sci U S A. 1996;93:3636–3641. doi: 10.1073/pnas.93.8.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruggeri BA, Huang LY, Wood M, Cheng JQ, Testa JR. Amplification and overexpression of the AKT2 oncogene in a subset of human pancreatic ductal adenocarcinomas. Mol Carcinog. 1998;21:81–86. [PubMed] [Google Scholar]
- 22.Carpten JD, Faber AL, Horn C, Donoho GP, Briggs SL, Robbins CM, Hostetter G, Boguslawski S, Moses TY, Savage S, Uhlik M, Lin A, Du J, Qian YW, Zeckner DJ, Tucker-Kellogg G, Touchman J, Patel K, Mousses S, Bittner M, Schevitz R, Lai MH, Blanchard KL, Thomas JE. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007;448:439–444. doi: 10.1038/nature05933. [DOI] [PubMed] [Google Scholar]
- 23.Tasian SK, Teachey DT, Rheingold SR. Targeting the PI3K/mTOR pathway in pediatric hematologic malignancies. Front Oncol. 2014;4:108. doi: 10.3389/fonc.2014.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balsara BR, Pei J, Mitsuuchi Y, Page R, Klein-Szanto A, Wang H, Unger M, Testa JR. Frequent activation of AKT in non‑small cell lung carcinomas and preneoplastic bronchial lesions. Carcinogenesis. 2004;25:2053–2059. doi: 10.1093/carcin/bgh226. [DOI] [PubMed] [Google Scholar]
- 25.Fan QW, Weiss WA. Inhibition of PI3K-Akt-mTOR signaling in glioblastoma by mTORC1/2 inhibitors. Methods Mol Biol. 2012;821:349–359. doi: 10.1007/978-1-61779-430-8_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang L, Graham PH, Hao J, Ni J, Bucci J, Cozzi PJ, Kearsley JH, Li Y. Acquisition of epithelial-mesenchymal transition and cancer stem cell phenotypes is associated with activation of the PI3K/Akt/mTOR pathway in prostate cancer radioresistance. Cell Death Dis. 2013;4:e875. doi: 10.1038/cddis.2013.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou J, Wulfkuhle J, Zhang H, Gu P, Yang Y, Deng J, Margolick JB, Liotta LA, Petricoin E 3rd, Zhang Y. Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proc Natl Acad Sci U S A. 2007;104:16158–16163. doi: 10.1073/pnas.0702596104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Douville J, Beaulieu R, Balicki D. ALDH1 as a functional marker of cancer stem and progenitor cells. Stem Cells Dev. 2009;18:17–25. doi: 10.1089/scd.2008.0055. [DOI] [PubMed] [Google Scholar]
- 29.Huang EH, Hynes MJ, Zhang T, Ginestier C, Dontu G, Appelman H, Fields JZ, Wicha MS, Boman BM. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Res. 2009;69:3382–3389. doi: 10.1158/0008-5472.CAN-08-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishitani S, Horie M, Ishizaki S, Yano H. Branched chain amino acid suppresses hepatocellular cancer stem cells through the activation of mammalian target of rapamycin. PLoS One. 2013;8:e82346. doi: 10.1371/journal.pone.0082346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bleau AM, Hambardzumyan D, Ozawa T, Fomchenko EI, Huse JT, Brennan CW, Holland EC. PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell. 2009;4:226–235. doi: 10.1016/j.stem.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corominas-Faja B, Cufí S, Oliveras-Ferraros C, Cuyàs E, López-Bonet E, Lupu R, Alarcón T, Vellon L, Iglesias JM, Leis O, Martín ÁG, Vazquez-Martin A, Menendez JA. Nuclear reprogramming of luminal-like breast cancer cells generates Sox2-overexpressing cancer stem-like cellular states harboring transcriptional activation of the mTOR pathway. Cell Cycle. 2013;12:3109–3124. doi: 10.4161/cc.26173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei X, Wang J, He J, Ma B, Chen J. Biological characteristics of CD133 (+) cancer stem cells derived from human laryngeal carcinoma cell line. Int J Clin Exp Med. 2014;7:2453–2462. [PMC free article] [PubMed] [Google Scholar]
- 34.Kozovska Z, Gabrisova V, Kucerova L. Colon cancer: Cancer stem cells markers, drug resistance and treatment. Biomed Pharmacother. 2014;68:911–916. doi: 10.1016/j.biopha.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 35.Guo Z, Li LQ, Jiang JH, Ou C, Zeng LX, Xiang BD. Cancer stem cell markers correlate with early recurrence and survival in hepatocellular carcinoma. World J Gastroenterol. 2014;20:2098–2106. doi: 10.3748/wjg.v20.i8.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xia P. Surface markers of cancer stem cells in solid tumors. Curr Stem Cell Res Ther. 2014;9:102–111. doi: 10.2174/1574888x09666131217003709. [DOI] [PubMed] [Google Scholar]
- 37.Yang Z, Zhang L, Ma A, Liu L, Li J, Gu J, Liu Y. Transient mTOR inhibition facilitates continuous growth of liver tumors by modulating the maintenance of CD133+ cell populations. PLoS One. 2011;6:e28405. doi: 10.1371/journal.pone.0028405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mak AB, Blakely KM, Williams RA, Penttilä PA, Shukalyuk AI, Osman KT, Kasimer D, Ketela T, Moffat J. CD133 Nglycosylation processing contributes to cell-surface recognition of the primitive cell marker AC133. J Biol Chem. 2011;286:41046–41056. doi: 10.1074/jbc.M111.261545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang FF, Wu DS, Zhang L, Yu YH, Yuan XY, Li WJ, Chen XP, Zhao XL, Chen FP, Zeng H. Inactivation of PTEN increases ABCG2 expression and the side population through the PI3K/Akt pathway in adult acute leukemia. Cancer Lett. 2013;336:96–105. doi: 10.1016/j.canlet.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 40.Airiau K, Mahon FX, Josselin M, Jeanneteau M, Belloc F. PI3K/mTOR pathway inhibitors sensitize chronic myeloid leukemia stem cells to nilotinib and restore the response of pro genitors to nilotinib in the presence of stem cell factor. Cell Death Dis. 2013;4:e827. doi: 10.1038/cddis.2013.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jung MJ, Rho JK, Kim YM, Jung JE, Jin YB, Ko YG, Lee JS, Lee SJ, Lee JC, Park MJ. Upregulation of CXCR4 is functionally crucial for maintenance of stemness in drug-resistant non-small cell lung cancer cells. Oncogene. 2013;32:209–221. doi: 10.1038/onc.2012.37. [DOI] [PubMed] [Google Scholar]
- 42.Chang WW, Lin RJ, Yu J, Chang WY, Fu CH, Lai A, Yu JC, Yu AL. The expression and significance of insulin-like growth factor-1 receptor and its pathway on breast cancer stem/progenitors. Breast Cancer Res. 2013;15:R39. doi: 10.1186/bcr3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wen W, Han T, Chen C, Huang L, Sun W, Wang X, Chen SZ, Xiang DM, Tang L, Cao D, Feng GS, Wu MC, Ding J, Wang HY. Cyclin G1 expands liver tumor-initiating cells by Sox2 induction via Akt/mTOR signaling. Mol Cancer Ther. 2013;12:1796–1804. doi: 10.1158/1535-7163.MCT-13-0099. [DOI] [PubMed] [Google Scholar]
- 44.Marhold M, Tomasich E, El-Gazzar A, Heller G, Spittler A, Horvat R, Krainer M, Horak P. HIF-1alpha regulates mTOR signaling and viability of prostate cancer stem cells. Mol Cancer Res. 2015;13:556–64. doi: 10.1158/1541-7786.MCR-14-0153-T. [DOI] [PubMed] [Google Scholar]
- 45.Bergmann L, Maute L, Guschmann M. Temsirolimus for advanced renal cell carcinoma. Expert Rev Anticancer Ther. 2014;14:9–21. doi: 10.1586/14737140.2014.864562. [DOI] [PubMed] [Google Scholar]
- 46.Martelli AM, Chiarini F, Evangelisti C, Cappellini A, Buontempo F, Bressanin D, Fini M, McCubrey JA. Two hits are better than one: targeting both phosphatidylinositol 3-kinase and mammalian target of rapamycin as a therapeutic strategy for acute leukemia treatment. Oncotarget. 2012;3:371–394. doi: 10.18632/oncotarget.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu Y, Zhang X, Liu Y, Zhang S, Liu J, Ma Y, Zhang J. Antitumor effect of the mTOR inhibitor everolimus in combination with trastuzumab on human breast cancer stem cells in vitro and in vivo. Tumour Biol. 2012;33:1349–1362. doi: 10.1007/s13277-012-0383-6. [DOI] [PubMed] [Google Scholar]
- 48.Liu Y, Zhang X, Liu J, Hou G, Zhang S, Zhang J. Everolimus in combination with letrozole inhibit human breast cancer MCF-7/Aro stem cells via PI3K/mTOR pathway: an experimental study. Tumour Biol. 2014;35:1275–1286. doi: 10.1007/s13277-013-1170-8. [DOI] [PubMed] [Google Scholar]
- 49.Mendiburu-Eliçabe M, Gil-Ranedo J, Izquierdo M. Efficacy of rapamycin against glioblastoma cancer stem cells. Clin Transl Oncol. 2014;16:495–502. doi: 10.1007/s12094-013-1109-y. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y, Liu Y, Lu J, Zhang P, Wang Y, Xu Y, Wang Z, Mao JH, Wei G. Rapamycin inhibits FBXW7 loss-induced epithelial-mesenchymal transition and cancer stem cell-like characteristics in colorectal cancer cells. Biochem Biophys Res Commun. 2013;434:352–356. doi: 10.1016/j.bbrc.2013.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Greenberger S, Yuan S, Walsh LA, Boscolo E, Kang KT, Matthews B, Mulliken JB, Bischoff J. Rapamycin suppresses self-renewal and vasculogenic potential of stem cells isolated from infantile hemangioma. J Invest Dermatol. 2011;131:2467–2476. doi: 10.1038/jid.2011.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hirsch HA, Iliopoulos D, Tsichlis PN, Struhl K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 2009;69:7507–7511. doi: 10.1158/0008-5472.CAN-09-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iliopoulos D, Hirsch HA, Struhl K. Metformin decreases the dose of chemotherapy for prolonging tumor remission in mouse xenografts involving multiple cancer cell types. Cancer Res. 2011;71:3196–3201. doi: 10.1158/0008-5472.CAN-10-3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Song CW, Lee H, Dings RP, Williams B, Powers J, Santos TD, Choi BH, Park HJ. Metformin kills and radiosensitizes cancer cells and preferentially kills cancer stem cells. Sci Rep. 2012;2:362. doi: 10.1038/srep00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee H, Park HJ, Park CS, Oh ET, Choi BH, Williams B, Lee CK, Song CW. Response of breast cancer cells and cancer stem cells to metformin and hyperthermia alone or combined. PLoS One. 2014;9:e87979. doi: 10.1371/journal.pone.0087979. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56.Fuchs D, Heinold A, Opelz G, Daniel V, Naujokat C. Salinomycin induces apoptosis and overcomes apoptosis resistance in human cancer cells. Biochem Biophys Res Commun. 2009;390:743–749. doi: 10.1016/j.bbrc.2009.10.042. [DOI] [PubMed] [Google Scholar]
- 57.Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lander ES. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang GN, Liang Y, Zhou LJ, Chen SP, Chen G, Zhang TP, Kang T, Zhao YP. Combination of salinomycin and gemcitabine eliminates pancreatic cancer cells. Cancer Lett. 2011;313:137–144. doi: 10.1016/j.canlet.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 59.Dong TT, Zhou HM, Wang LL, Feng B, Lv B, Zheng MH. Salinomycin selectively targets ‘CD133+’ cell subpopulations and decreases malignant traits in colorectal cancer lines. Ann Surg Oncol. 2011;18:1797–1804. doi: 10.1245/s10434-011-1561-2. [DOI] [PubMed] [Google Scholar]
- 60.Wang Y. Effects of salinomycin on cancer stem cell in human lung adenocarcinoma A549 cells. Med Chem. 2011;7:106–111. doi: 10.2174/157340611794859307. [DOI] [PubMed] [Google Scholar]
- 61.Kuo SZ, Blair KJ, Rahimy E, Kiang A, Abhold E, Fan JB, Wang-Rodriguez J, Altuna X, Ongkeko WM. Salinomycin induces cell death and differentiation in head and neck squamous cell carcinoma stem cells despite activation of epithelial-mesenchymal transition and Akt. BMC Cancer. 2012;12:556. doi: 10.1186/1471-2407-12-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiao Z, Sperl B, Ullrich A, Knyazev P. Metformin and salinomycin as the best combination for the eradication of NSCLC monolayer cells and their alveospheres (cancer stem cells) irrespective of EGFR, KRAS, EML4/ALK and LKB1 status. Oncotarget. 2014;5:12877–12890. doi: 10.18632/oncotarget.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang WJ, Long LM, Yang N, Zhang QQ, Ji WJ, Zhao JH, Qin ZH, Wang Z, Chen G, Liang ZQ. NVP-BEZ235, a novel dual PI3K/mTOR inhibitor, enhances the radiosensitivity of human glioma stem cells in vitro. Acta Pharmacol Sin. 2013;34:681–690. doi: 10.1038/aps.2013.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dubrovska A, Elliott J, Salamone RJ, Kim S, Aimone LJ, Walker JR, Watson J, Sauveur-Michel M, Garcia-Echeverria C, Cho CY, Reddy VA, Schultz PG. Combination therapy targeting both tumor-initiating and differentiated cell populations in prostate carcinoma. Clin Cancer Res. 2010;16:5692–5702. doi: 10.1158/1078-0432.CCR-10-1601. [DOI] [PubMed] [Google Scholar]
- 65.Fang DD, Zhang CC, Gu Y, Jani JP, Cao J, Tsaparikos K, Yuan J, Thiel M, Jackson-Fisher A, Zong Q, Lappin PB, Hayashi T, Schwab RB, Wong A, John-Baptiste A, Bagrodia S, Los G, Bender S, Christensen J, Vanarsdale T. Antitumor efficacy of the dual PI3K/mTOR inhibitor PF-04691502 in a human xenograft tumor model derived from colorectal cancer stem cells harboring a pik3ca mutation. PLoS One. 2013;8:e67258. doi: 10.1371/journal.pone.0067258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Francipane MG, Lagasse E. Selective targeting of human colon cancer stem-like cells by the mTOR inhibitor Torin-1. Oncotarget. 2013;4:1948–1962. doi: 10.18632/oncotarget.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Altman JK, Sassano A, Kaur S, Glaser H, Kroczynska B, Redig AJ, Russo S, Barr S, Platanias LC. Dual mTORC2/mTORC1 targeting results in potent suppressive effects on acute myeloid leukemia (AML) progenitors. Clin Cancer Res. 2011;17:4378–4388. doi: 10.1158/1078-0432.CCR-10-2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tallman MS, Gilliland DG, Rowe JM. Drug therapy for acute myeloid leukemia. Blood. 2005;106:1154–1163. doi: 10.1182/blood-2005-01-0178. [DOI] [PubMed] [Google Scholar]
- 69.Ding Q, Gu R, Liang J, Zhang X, Chen Y. PI-103 sensitizes acute myeloid leukemia stem cells to daunorubicin-induced cytotoxicity. Med Oncol. 2013;30:395. doi: 10.1007/s12032-012-0395-5. [DOI] [PubMed] [Google Scholar]
- 70.Hong Z, Xiao M, Yang Y, Han Z, Cao Y, Li C, Wu Y, Gong Q, Zhou X, Xu D, Meng L, Ma D, Zhou J. Arsenic disulfide synergizes with the phosphoinositide 3-kinase inhibitor PI-103 to eradicate acute myeloid leukemia stem cells by inducing differentiation. Carcinogenesis. 2011;32:1550–1558. doi: 10.1093/carcin/bgr176. [DOI] [PubMed] [Google Scholar]
- 71.Wang JY, Chang CC, Chiang CC, Chen WM, Hung SC. Silibinin suppresses the maintenance of colorectal cancer stem-like cells by inhibiting PP2A/AKT/mTOR pathways. J Cell Biochem. 2012;113:1733–1743. doi: 10.1002/jcb.24043. [DOI] [PubMed] [Google Scholar]
- 72.Ibáñez E, Agliano A, Prior C, Nguewa P, Redrado M, González-Zubeldia I, Plano D, Palop JA, Sanmartín C, Calvo A. The quinoline imidoselenocarbamate EI201 blocks the AKT/mTOR pathway and targets cancer stem cells leading to a strong antitumor activity. Curr Med Chem. 2012;19:3031–3043. doi: 10.2174/092986712800672076. [DOI] [PubMed] [Google Scholar]
- 73.Gschwendt M, Müller HJ, Kielbassa K, Zang R, Kittstein W, Rincke G, Marks F. Rottlerin, a novel protein-kinase inhibitor. Biochem Biophys Res Commun. 1994;199:93–98. doi: 10.1006/bbrc.1994.1199. [DOI] [PubMed] [Google Scholar]
- 74.Singh BN, Kumar D, Shankar S, Srivastava RK. Rottlerin induces autophagy which leads to apoptotic cell death through inhibition of PI3K/Akt/mTOR pathway in human pancreatic cancer stem cells. Biochem Pharmacol. 2012;84:1154–1163. doi: 10.1016/j.bcp.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 75.Kumar D, Shankar S, Srivastava RK. Rottlerin induces autophagy and apoptosis in prostate cancer stem cells via PI3K/Akt/mTOR signaling pathway. Cancer Lett. 2014;343:179–189. doi: 10.1016/j.canlet.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 76.Kumar D, Shankar S, Srivastava RK. Rottlerin-induced autophagy leads to the apoptosis in breast cancer stem cells: molecular mechanisms. Mol Cancer. 2013;12:171. doi: 10.1186/1476-4598-12-171. [DOI] [PMC free article] [PubMed] [Google Scholar]


