Abstract
OSCP1/NOR1 (organic solute carrier partner 1/oxidored-nitro domain-containing protein 1) is known as a transporter of various organic solutes into cells and also is reported to act as a tumor suppressor protein. Although overexpression of OSCP1 has been shown to play multiple roles in mammalian cell lines, its biological significance in living organisms is not fully understood. To explore the effects of OSCP1/NOR1 on development, we performed genetic studies in flies featuring overexpression of its Drosophila orthologue, dOSCP1. Overexpression of dOSCP1 in eye imaginal discs induced a rough eye phenotype in adult flies, likely resulting from a delay in S phase progression and induction of caspase-dependent apoptosis followed by compensatory proliferation. However, it did not appear to be involved in differentiation of R7 photoreceptor cells. We also found that overexpression of dOSCP1 caused endoplasmic reticulum stress in salivary gland cells. These results indicate that overexpression of dOSCP1 exerts effects on various biological processes during Drosophila development.
Keywords: OSCP1/NOR1, apoptosis, proliferation, ER stress
Introduction
Tumor cells employ various mechanisms to support their requirements for nutrients. Regulation of nutrient transporters in the tumor cells has been reviewed elsewhere [1,2]. For instance, tumor cells enhance glucose uptake via induction of GLUT1 and SGLT1 transporters [3-6]. At the same time, SMCT1 (SLC5A8), the Na+-coupled lactate transporter that was originally identified as a tumor suppressor in colon [7], is silenced in cancers of a variety of tissues [7-10]. Understanding the action of these transporters may help to improve drug delivery or drug design for cancer chemotherapy.
Organic solute carrier partner 1/oxidored-nitro domain-containing protein 1 (OSCP1/NOR1) is known as a polyspecific solute carrier protein [11-13]. When expressed in Xenopus laevis oocytes, human OSCP1 (hOSCP1), mouse OSCP1 (mOSCP1) and rat OSCP1 (rOSCP1) were found to mediate high affinity transport of p-aminohippurate (PAH), tetraethylammonium (TEA), and a wide range of structurally diverse organic compounds including estron sulfate, glutarate, L-leucine, L-ascorbic acid and tetracycline [11-13]. These data suggest that OSCP1 facilitates the transport of various organic solutes into cells and that its properties are conserved among vertebrates.
On the other hand, hOSCP1 is also reported to be down-expressed in several kinds of cancers, such as examples in the nasopharynx, lung, gastric, colon, rectum and cervix [14-19]. In fact, the promoter region of OSCP1 is frequently methylated in nasopharyngeal carcinomas (NPCs) tissue samples and cancer cell lines [17], as well as in leukemia cell lines and in acute myeloid leukemia (ALM) patients [20]. Since promoter hypermethylation of tumor suppressor genes is a common hallmark of human cancers, these data suggest that OSCP1 play a role in their genesis. However, the precise mechanisms of OSCP1 actions need to be elucidated in more detail.
Overexpression of hOSCP1 results in inhibition of proliferation, colony forming ability and promotion of apoptosis of NPCs, Hela cervical cancer (CCA) cells [14,17,19,21]. However, effects of overexpression of OSCP1 in living organisms have not been examined. Drosophila contains a single orthologue of OSCP1 (dOSCP1) that shares 58% homology with its human counterpart [22]. dOSCP1 localizes in the plasma membrane, endoplasmic reticulum, Golgi apparatus, mitochondria and even nuclei, but not in cytosol, suggesting that it plays multiple regulatory roles [22]. In the present study, we established transgenic fly lines to explore the consequence of overexpression of dOSCP1 during development. In the eye imaginal discs the result was induction of a rough eye phenotype in adult flies, due to caspase-dependent apoptosis followed by compensatory proliferation and a delay in S phase progression. Furthermore, we also found that overexpression of dOSCP1 caused endoplasmic reticulum (ER) stress in salivary gland cells. These data indicate that overexpression of dOSCP1 affects multiple processes during Drosophila development.
Materials and methods
Fly stocks
Fly stocks were maintained at 25°C on standard food containing 0.7% agar, 5% glucose and 7% dry yeast. Canton S was used as the wild type. The transgenic fly line carrying the glass minimal response element GMR-GAL4 on X chromosome was as described previously [23]. All other stocks used in this study were from the Bloomington Drosophila Stock Center (Bloomington, UAS) or Drosophila Genetic Resource Center (Kyoto, Japan).
Plasmid construction and establishment of transgenic flies
To construct the plasmid pUAST-dOSCP1, the full coding sequence of dOSCP1 cDNA (accession number NM_136887) corresponding to amino acids (aa) 1 to 302 was amplified by PCR with primers carrying EcoRI and XhoI sites (dOSCP1FEcoRI: 5’-GGGAATTGGGAATTCATGC-TCTACGTGATCGATCAG, dOSCP1RXhoI: 5’-AGA-GGTACCCTCGAGTCAAGGCATTTTGCTGTACAG). The PCR products were digested with EcoRI and XhoI and inserted into plasmid pUAST [24]. P element mediated germ line transformation by injecting pUAS-dOSCP1 into embryos was carried out as described earlier [25]. F1 transformants were selected on the basis of white eye color rescue [26].
Immunohistochemistry
For immunohistochemistry, third instar larvae were dissected and fixed in 4% paraformaldehyde (PFA) in phosphate buffer saline (PBS) for 15 min at 25°C. After washing with 0.3% Triton X-100 in PBS (PBS-T), the samples were blocked with PBS containing 0.15% Triton X-100 and 10% normal goat serum for 20 min at 25°C and incubated with primary antibodies for 16 h at 4°C. The following primary antibodies were used: guinea pig anti-dOSCP1 (1:100), rabbit anti-cleaved caspase-3 (1:500) (BD Biosciences), mouse anti-β-galactosidase (1:500) (DSHB) and mouse anti-KDEL antibody (Enzo Life Sciences). After washing with PBST, samples were incubated with secondary antibodies labeled with either Alexa 594 or Alexa 488 (1:400) (Invitrogen) for 3 h at 25°C. After washing with PBS-T followed by PBS, the samples were mounted in Vectashield Mounting Medium (Vector laboratories) and inspected under a confocal laser scanning microscope (OLYMPUS Fluoview FV10i).
Flip-out experiments
Overexpression clones in Drosophila larval salivary glands were generated with a flip-out system [27]. Female flies with hs-flp; Act5C>FRT y FRT>GAL4, UAS-GFP were crossed with male flies carrying UAS-dOSCP1. Clones were evaluated by the presence of green fluorescent protein (GFP) expressed under control of the Act5C promoter. Flip-out was induced by heat shock (60 min at 37°C) at 24-48 h after egg laying.
EdU (5-ethynyl-2’-deoxyuridine) labeling
Cells in S phase were detected by using an EdU-labeling kit from Invitrogen (Click-iT EdU Alexa Fluor 594 Imaging Kit). Third instar larvae were dissected in PBS and the imaginal discs were suspended in Grace’s insect medium in the presence of 10 μM EdU for 60 min at 25°C. The samples were then fixed with 3.7% formaldehyde in PBS for 15 min at 25°C, washed with 3% BSA in PBS and permeabilized in 0.5% Triton X-100 in PBS for 20 min at 25°C. Thereafter, samples were washed with 3% BSA in PBS and incubated with Click-iT reaction cocktails for 30 min at 25°C. After washing with 3% BSA in PBS and PBS alone, samples were mounted and assessed as described in the immunohistochemistry section.
Scanning electron microscopy
Adult flies were anesthetized, mounted on stages and observed with a VE-7800 (Keyence Inc.) scanning electron microscope in the low vacuum mode. In every experiment, the eye phenotype of at least five adult flies of each line was simultaneously examined by scanning electron microscopy, and these experiments were repeated 3 times. In the experiments, no significant variation in eye phenotype among the groups of five individuals was observed.
Quantification and statistical analysis
The dOSCP1, EdU signals in the region posterior to the MF were counted and measured from eye imaginal discs using MetaMorph software (Molecular Devices). The experiments were repeated three times. Statistical analysis was conducted, as indicated in the figure legends, using GraphPad Prism 6 (MDF).
Results
Overexpression of dOSCP1 in eye imaginal discs induces a rough eye phenotype in adult flies
We established seven independent transgenic fly lines carrying UAS-dOSCP1. The established lines with their linkages of the transgene in different chromosomes are summarized in Table 1. In order to investigate the effect of overexpression of dOSCP1 on Drosophila development, the transgenic flies were crossed with different GAL4 driver strains, featuring expression of GAL4 in different developmental stages and various tissues [24]. Morphological aberration was observed in the adult compound eye (Table 2). Moreover, the most prominent phenotype (the rough eye phenotype) was observed with the GMR-GAL4 driver. Furthermore, all progeny showed this phenotype. For that reason, we focused on analysis of the compound eye phenotype with GMR-GAL4. Careful examination under the scanning electron microscope revealed that the compound eyes of adult flies carrying the GMR-GAL4 alone showed normal eye morphology (Figure 1A), while flies carrying UAS-dOSCP1 (GMR-GAL4; UAS-dOSCP1) showed a severe rough eye phenotype with fusion of ommatidia (Figure 1B, 1C).
Table 1.
Transgenic fly lines established in this study and their chromosome linkages
| P element plasmid | Strains | Chromosome linkages |
|---|---|---|
| pUAST-dOSCP1 | 5 | II |
| 17 | II | |
| 27 | II | |
| 29 | III | |
| 30 | II | |
| 33 | X | |
| 47 | II |
Table 2.
Summary of effects of dOSCP1 expression with several GAl4 driver lines
| GAl4 line | Chromosome linkages | Phenotype |
|---|---|---|
| GMR | X | Rough eye |
| Eyeless | II | Weak rough eye |
| 91Y | II | Weak rough eye |
| Arm | II | ND |
| C179 | II | ND |
| dpp | III | ND |
| Act5C | III | ND |
| Dll | II | ND |
| 48Y | II | ND |
| Pnr | II | ND |
| En | II | ND |
ND: Not detectable.
Figure 1.
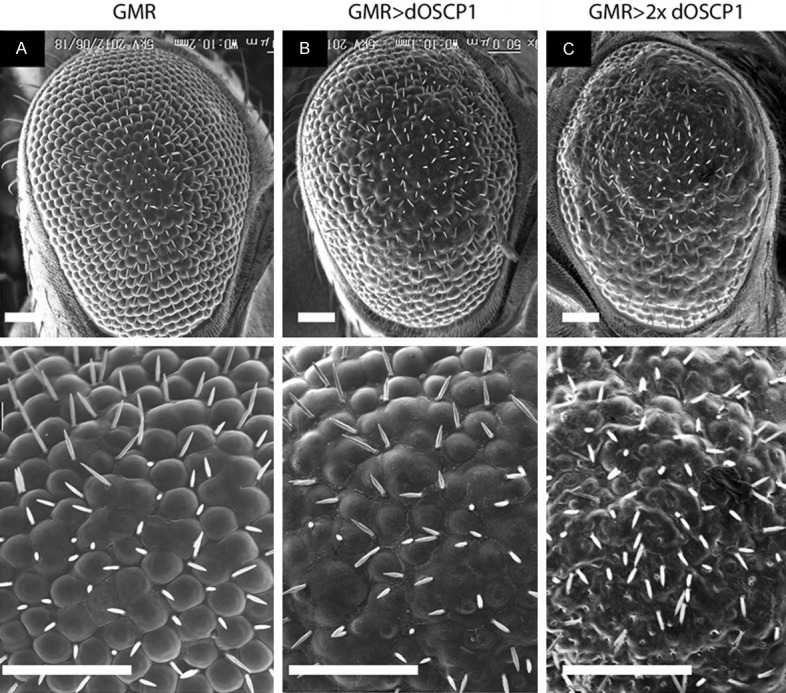
Overexpression of dOSCP1 in eye imaginal discs induces a rough eye phenotype in adult flies. A-C Scanning electron micrographs of adult compound eyes. Magnification x200 (top), x700 (bottom). A. GMR-GAL4. B. GMR-GAL4; UAS-dOSCP1/+. C. GMR-GAL4; UAS-dOSCP1. The flies were reared at 28°C. Bars indicate 50 μm.
To examine whether the overexpression of dOSCP1 truly resulted in the eye phenotype, we carried out immunostaining of eye imaginal discs from third instar larvae with specific anti-dOSCP1 antibodies [22]. The eye discs of the control GMR-GAL4 line showed ubiquitous signals of endogenous dOSCP1 (Figure 2A), whereas the signal of dOSCP1 was increased from the morphogenetic furrow (MF) to the posterior end region of eye imaginal discs in GMR-GAL4; UAS-dOSCP1 flies (Figure 2B). The significance of these results was further quantified by MetaMorph software (Figure 2C). The data indicated that overexpression of dOSCP1 results in abnormal eye morphology.
Figure 2.
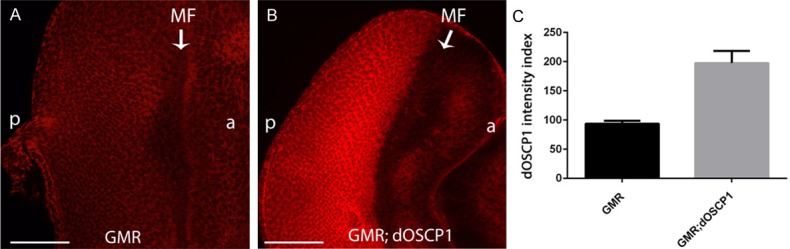
Confirmation of increased dOSCP1 levels in the posterior region of eye imaginal discs. Immunostaining with anti-dOSCP1 antibodies. A. GMR-GAL4. B. GMR-GAL4; UAS-dOSCP1. C. Quantification of dOSCP1 signals. Intensity of dOSCP1 signals were quantified in the region posterior to the MF and anterior region. Mean intensities with standard deviation from seven discs are shown: p < 0.005 by Welch’s t-test. Arrows/MF morphogenetic furrow. (p) posterior. (a) anterior. The flies were reared at 28°C. Bars indicate 50 μm.
Overexpression of dOSCP1 induces caspase 3-dependent cell death in eye imaginal discs
To gain insight into mechanisms, we first examined whether apoptosis is induced by overexpression of dOSCP1 in eye imaginal discs, since the rough eye phenotype is sometimes accompanied by increased apoptosis [28,29]. The rough eye phenotype was strongly suppressed by co-expression of an inhibitor of apoptosis, DIAP1 [30-32] (Figure 3D, 3D’). However, such suppression was not observed on co-expression of control LacZ (Figure 3C, 3C’). Ectopic expression of DIAP1 alone exerted no effect on the compound eye morphology (Figure 3E, 3E’). These data suggest that the rough eye phenotype is accompanied by induction of apoptosis.
Figure 3.
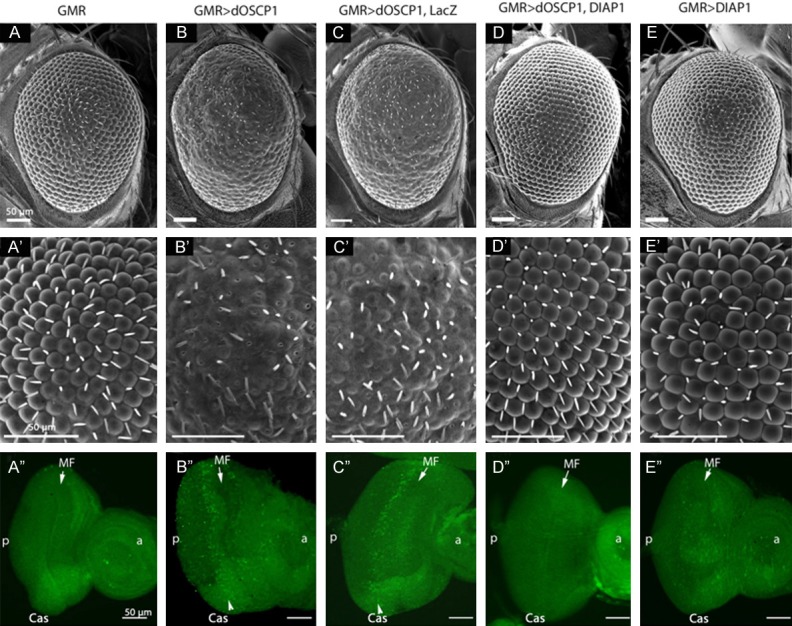
Overexpression of dOSCP1 induces caspase-dependent apoptosis in eye imaginal discs. A-E. Scanning electron micrographs of adult compound eyes, magnification x200. A’-E’. Magnification x700. A’’-E’’. Immunostaining of eye imaginal discs with anti-active 3 antibodies. A, A’’. GMR-GAL4. B, B’’. GMR-GAL4; UAS-dOSCP1. C, C’’. GMR-GAL4; UAS-dOSCP1; UAS-LacZ. D, D’’. GMR-GAL4; UAS-dOSCP1; UAS-DIAP1. E, E’’. GMR-GAL4; +; UAS-DIAP1. The flies were reared at 28°C. Arrows/MF morphogenetic furrow. (p) posterior. (a) anterior. Bars indicate 50 μm.
To further examine the involvement of dOSCP1 in induction of caspase-dependent apoptosis, we monitored cell death by immunostaining eye imaginal discs from third instar larvae with anti-active caspase 3 antibodies. In eye imaginal discs of flies expressing GAL4 alone, very few apoptosis cells were detected (Figure 3A’’). However, eye imaginal discs of GMR-GAL4>UAS-dOSCP1 and GMR-GAL4>UAS-dOSCP1; UAS-LacZ flies showed significantly increased levels of caspase 3 signals in the posterior region to the morphogenetic furrow (Figure 3B’’, 3C’’). In addition, co-expression of dOSCP1 and DIAP1 could suppress the dOSCP1-induced apoptosis in eye imaginal discs (Figure 3D’’). These results indicated that overexpression of dOSCP1 can indeed induce caspase-dependent apoptosis in eye imaginal discs.
Apoptosis-induced proliferation is activated in eye imaginal discs of dOSCP1-overexpressing flies to counteract the induced apoptosis
In imaginal discs of Drosophila third instar larvae, the morphogenetic furrow (MF) moves slowly in a posterior to anterior direction. In front of the MF, cells proliferate asynchronously. In the MF, cells are arrested synchronously in the G1 phase. Then they undergo one cell cycle in a highly synchronized way that produces an S-phase zone (the second mitotic wave-SMW) followed by differentiation [24].
In eye discs of dOSCP1 overexpressing flies, even though apoptotic signals were detected behind the MF (Figure 3C’’), sizes of the adult compound eyes were not significantly reduced (Figures 1 and 3). Previous studies have indicated that during development, to maintain the proper size of organs in multi-cellular organisms requires a balance between cell proliferation and cell death [33-35]. This suggests that the induced apoptosis in dOSCP1-overexpressing eye imaginal discs might also be associated with the apoptosis-induced cell proliferation. To assess this possibility, an EdU incorporation assay was performed to monitor S phase cells. In the eye imaginal discs of GMR-GAL4; UAS-dOSCP1 flies, increased EdU positive cells were observed in the region posterior to the synchronized phase zone behind the MF (Figure 4B, 4D), as compared with the control line carrying GMR-GAL4 alone (Figure 4A, 4D). Ectopic mitotic signals were reduced by co-expression of dOSCP1 and DIAP1 (Figure 4C, 4D). Quantification of the EdU positive cells in the region posterior to the MF further confirmed that the results were statistically significant (Figure 4D). These finding indicated that overexpression of dOSCP1 induces apoptosis which is followed by compensatory proliferation.
Figure 4.
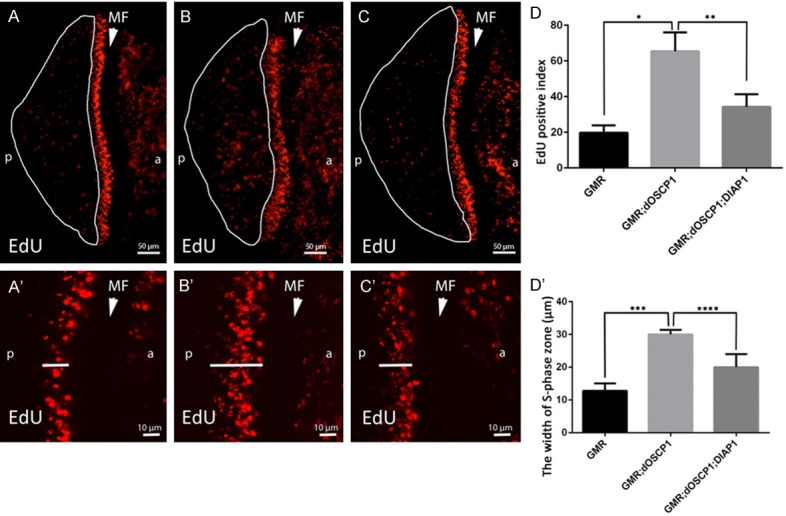
Overexpression of dOSCP1 induces ectopic mitosis in eye imaginal discs depending on apoptosis function. Eye discs labeled with EdU. A, A’. GMR-GAL4. B, B’. GMR-GAL4; UAS-dOSCP1. C, C’. GMR-GAL4; UAS-dOSCP1; UAS-DIAP1. A-C. Regions with ectopic EdU positive cells marked with crescents. A’-C’. Arrows show the measured position. D, D’. Quantification of EdU positive cells and width of the S phase zone in the posterior region. Mean intensities with standard deviation from seven discs are shown: *p < 0.0001, **p < 0.001, ***p < 0.001, ****p < 0.005 by Wech’s t-test. The flies were reared at 28°C. Arrow heads/MF morphogenetic furrow. (p) posterior. (a) anterior. Bars indicate 50 μm (A-C) or 10 μm (A’-C’).
In addition, we measured the zone of synchronized S phase in eye imaginal discs to examine whether there is any delay in overexpressing flies. In comparison with control flies (Figure 4A’), a wider zone of S phase cells was observed in dOSCP1 overexpressing flies (Figure 4B’). This phenotype was rescued by co-expressing DIAP1 (Figure 4C’). The results were further confirmed by statistical analysis (Figure 4D’), the data suggesting that a delay in S phase progression is also linked to induction of apoptosis in dOSCP1 overexpressing flies. Taken together, our observations suggest that the overexpression of dOSCP1 induces a delay in S phase progression and apoptosis followed by compensatory proliferation.
dOSCP1 is not involved in differentiation of R7 photoreceptor cells
It is well known that photoreceptor cells are generated sequentially: R8 is generated first, with movement posterior from the MF, then cells are added pair wise (R2 and R5, R3 and R4, and R1 and R6), R7 being the last photoreceptor to be added to the precluster [36]. Several enhancer trap lines have been developed to mark photoreceptor cells. To examine photoreceptor cell differentiation in eye imaginal discs of dOSCP1-overexpressing flies, we utilized enhancer trap line P82 (inserted in deadpan) [37] and B38 (inserted in klingon) [38], which specifically express the β-gala-ctosidase marker in photoreceptor cells of R3/R4/R7 and R7, respectively. After mating of each enhancer trap line with dOSCP1-overexpressing flies, eye imaginal discs of F1 larvae were immunostained with anti-β-galactosidase antibody. The immunostaining data revealed that R3/R4/R7 signals were detected in the eye imaginal discs of both P82 control flies (Figure 5A, 5A’) and dOSCP1 overexpressing flies (Figure 5B, 5B’). No apparent difference in R7 differentiation was detected in the eye discs of B38 control flies (Figure 5C, 5C’), and with overexpressing dOSCP1 (Figure 5D, 5D’). These results indicate that dOSCP1 is not involved in development of R7 photoreceptor cells.
Figure 5.
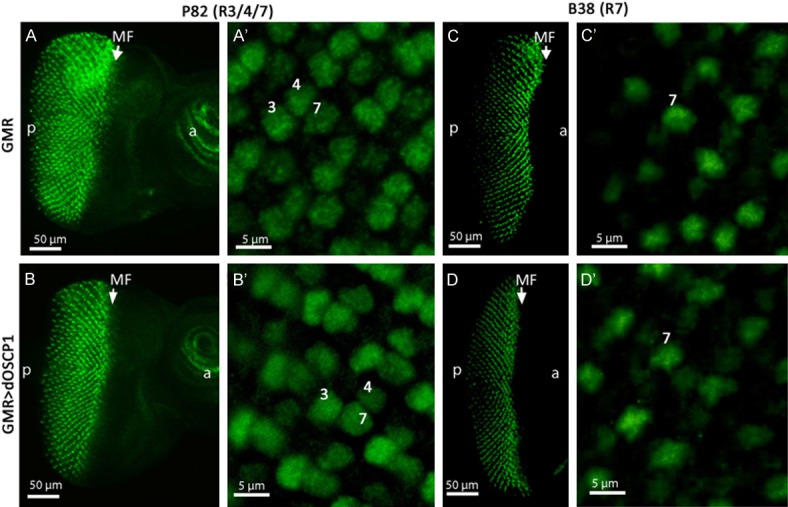
Overexpression of dOSCP1 is not involved in differentiation of R7 photoreceptor cells. Confocal images of eye imaginal discs immunostained with anti-β-galactosidase antibodies. The flies were reared at 28°C. A, A’. GMR-GAL4; P82/+; +. B, B’. GMR-GAL4; P82/UAS-dOSCP1; +. C, C’. GMR-GAL4; B38/+; +. D, D’. GMR-GAL4; B38/UAS-dOSCP1; +. Numbers R3, R4 and R7 photoreceptor cells. Arrows/MF morphogenetic furrow. Bars indicate 50 μm (A-D) or 5 μm (A’-D’).
Overexpression of dOSCP1 induces ER stress in salivary gland cells
In a previous study, we found that dOSCP1 localizes in endoplasmic reticulum (ER) in salivary gland cells [22]. To evaluate its significance in the ER, we investigated whether overexpression of dOSCP1 induces ER stress and unfolded protein response (UPR) activation in salivary gland cells. A flip-out experiment was employed to make a somatic clone overexpressing dOSCP1 in the salivary glands. Double immunostaining with anti-dOSCP1 and anti-KDEL antibodies was performed. The anti-KDEL antibody has been used [39] to detect ER chaperones Grp78 and Grp94 [40]. In the overexpressing dOSCP1 clones marked with GFP, the levels of dOSCP1 and KDEL were specifically increased (Figure 6). It is well known that the ER stress sensors activate downstream signaling to induce ER chaperones [41,42]. These results indicated that overexpression of dOSCP1 indeed induces ER stress in salivary gland cells.
Figure 6.
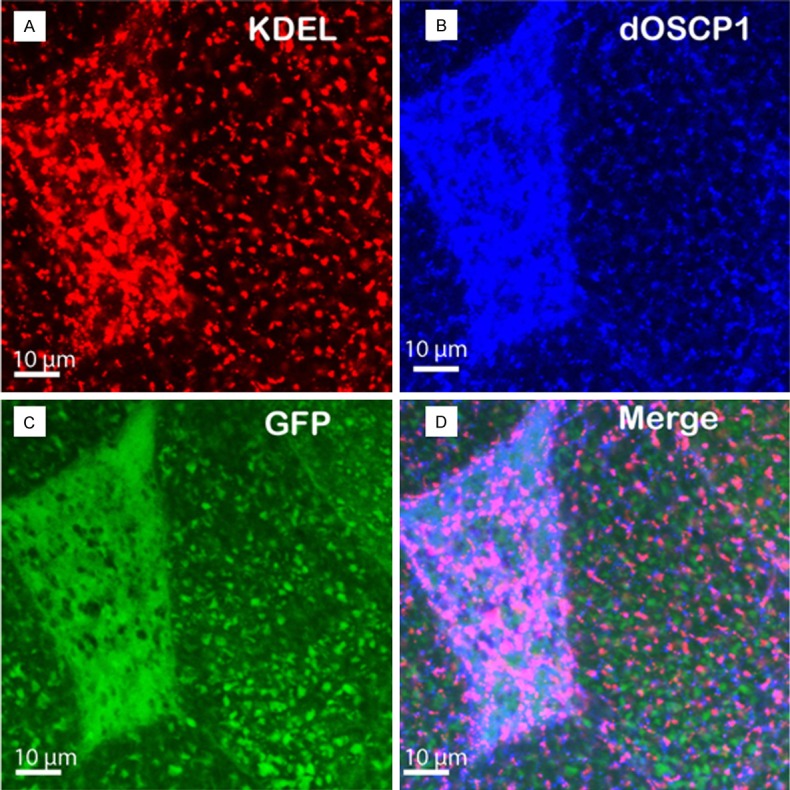
Overexpression of dOSCP1 induces ER stress in the salivary gland. A-D. Image of flip-out experiment. A. ER marker KDEL. B. OSCP1. C. GFP. D. Merged image. Bars indicate 10 μm.
Discussion
In this study, we utilized a Drosophila model to explore the biological action of overexpression of OSCP1 in living organisms. Overexpression of dOSCP1, a Drosophila homologue of human OSCP1 in living flies, clearly demonstrated that this protein can affect various biological processes, such as apoptosis, cell cycling, and ER stress during the development of Drosophila.
Our data showed increased apoptosis signals detected in the eye imaginal discs of dOSCP1 overexpressing flies, the results indicating participation of OSCP1 in apoptosis in vivo. In agreement with this finding, overexpression of hOSCP1 has been reported to induce apoptosis in nasopharyngeal carcinoma cells, Hela cells [14], and prostate cancer cells [43]. Furthermore, overexpression of hOSCP1 down-regulated Bcl-2 and Bcl-xl anti-apoptotic and up-regulated Bax and Bak pro-apoptotic proteins [14,21,43]. The data imply that OSCP1 induced apoptosis is mediated by a mitochondria-dependent pathway, compatible with the described localization of OSCP1 in mitochondria [14,22]. However, the role of OSCP1 in the mitochondria-dependent apoptosis pathway needs to be further studied. Genetic screening to identify mutations that can modify the dOSCP1 induced rough eye phenotype might give us clues to clarifying the link between dOSCP1 and apoptosis.
In addition, our findings provided evidence that overexpression of dOSCP1 caused a delay in S phase progression in the eye imaginal discs, consistent with a previous report that overexpression of hOSCP1 in NPC and CCA cells arrested them in S phase [14]. Moreover, we also found that the overexpression of dOSCP1 induced S phase delay was related to induction of apoptosis. Similar results were also obtained in overexpression studies of hOSCP1 in NPC and CCA cell lines [14,19]. In addition, we found that the induced apoptosis is followed by compensatory proliferation to maintain a constant size of compound eyes. This process is commonly observed in various Drosophila tissues and organs, including the wing imaginal discs [44] and the eye discs [34,35]. The results suggest that induction of apoptosis is a primary effect in dOSCP1 overexpressing flies that is followed by not only delay in S phase progression, but also the activation of compensatory proliferation in the eye imaginal discs.
Earlier observation of CCA cells showed an ER localization of hOSCP1 [14], as also found for dOSCP1 in Drosophila salivary gland tissues [22], indicating that OSCP1 may play a role in ER function. Since the contribution of OSCP1 in the ER is as yet unknown, in the present study we examined ER chaperon signals with overexpression of dOSCP1 in salivary gland tissues. In multicellular organisms, the ER responds to the accumulation of unfolded proteins in its lumen (ER stress) by activating the unfolded protein response (UPR). Under UPR, unfolded proteins may undergo refolding through chaperon pathways [41,42,45]. The present data indicate that overexpression of dOSCP1 can cause ER stress and activate UPR in the Drosophila salivary gland tissues. Further analyses are necessary to clarify the exact significance of OSCP1 in the ER stress response.
Taken together, our findings suggest that the roles of OSCP1 are highly conserved between hOSCP1 and dOSCP1, with involvement in multiple biological processes such as apoptosis, proliferation and the ER stress response. Fly models of human diseases provide several unique features, including powerful genetics control, highly conserved disease pathways, and low comparative costs [46,47]. OSCP1 is known to be a mediator of transport of various organic solutes into cells [11-13]. Previous studies have suggested that it may play important roles in the progression of various cancers [14-18], and leukemia [20]. Moreover, nutrient transporters contribute to the survival of tumor cells. Understanding actions of such transporters is valuable for development of new approaches to interference with tumor metabolism and blocking tumorigenesis. Thus, the dOSCP1-overexpression flies established in this study might also be suitable for identifying natural substances and chemical compounds that can alter the actions of dOSCP1. This might be a first step in finding drug candidates to treat diseases or cancers in which OSCP1 is involved.
Acknowledgements
We thank Dr. M. Moore for advice in English usage. This study was supported by Grants-in-Aid from the Ministry of Education, Science, Sports and Culture of Japan, JST and JSPS Core-to-Core Program, B. Asia-Africa Science Platforms.
Disclosure of conflict of interest
None.
References
- 1.Jones RG, Thompson CB. Tumor suppressors and cell metabolism: a recipe for cancer growth. Genes Dev. 2009;23:537–548. doi: 10.1101/gad.1756509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ganapathy V, Thangaraju M, Prasad PD. Nutrient transporters in cancer: relevance to Warburg hypothesis and beyond. Pharmacol Ther. 2009;121:29–40. doi: 10.1016/j.pharmthera.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Baer SC, Casaubon L, Younes M. Expression of the human erythrocyte glucose transporter Glut1 in cutaneous neoplasia. J Am Acad Dermatol. 1997;37:575–577. doi: 10.1016/s0190-9622(97)70174-9. [DOI] [PubMed] [Google Scholar]
- 4.Smith TA. Facilitative glucose transporter expression in human cancer tissue. Br J Biomed Sci. 1999;56:285–292. [PubMed] [Google Scholar]
- 5.Macheda ML, Rogers S, Best JD. Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J Cell Physiol. 2005;202:654–662. doi: 10.1002/jcp.20166. [DOI] [PubMed] [Google Scholar]
- 6.Airley RE, Mobasheri A. Hypoxic regulation of glucose transport, anaerobic metabolism and angiogenesis in cancer: novel pathways and targets for anticancer therapeutics. Chemotherapy. 2007;53:233–256. doi: 10.1159/000104457. [DOI] [PubMed] [Google Scholar]
- 7.Li H, Myeroff L, Smiraglia D, Romero MF, Pretlow TP, Kasturi L, Lutterbaugh J, Rerko RM, Casey G, Issa JP, Willis J, Willson JK, Plass C, Markowitz SD. SLC5A8, a sodium transporter, is a tumor suppressor gene silenced by methylation in human colon aberrant crypt foci and cancers. Proc Natl Acad Sci U S A. 2003;100:8412–8417. doi: 10.1073/pnas.1430846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paroder V, Spencer SR, Paroder M, Arango D, Schwartz S Jr, Mariadason JM, Augenlicht LH, Eskandari S, Carrasco N. Na(+)/monocarboxylate transport (SMCT) protein expression correlates with survival in colon cancer: molecular characterization of SMCT. Proc Natl Acad Sci U S A. 2006;103:7270–7275. doi: 10.1073/pnas.0602365103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong C, Maunakea A, Jun P, Bollen AW, Hodgson JG, Goldenberg DD, Weiss WA, Costello JF. Shared epigenetic mechanisms in human and mouse gliomas inactivate expression of the growth suppressor SLC5A8. Cancer Res. 2005;65:3617–3623. doi: 10.1158/0008-5472.CAN-05-0048. [DOI] [PubMed] [Google Scholar]
- 10.Park JY, Zheng W, Kim D, Cheng JQ, Kumar N, Ahmad N, Pow-Sang J. Candidate tumor suppressor gene SLC5A8 is frequently down-regulated by promoter hypermethylation in prostate tumor. Cancer Detect and Prev. 2007;31:359–365. doi: 10.1016/j.cdp.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi Y, Shibusawa A, Saito H, Ohshiro N, Ohbayashi M, Kohyama N, Yamamoto T. Isolation and functional characterization of a novel organic solute carrier protein, hOSCP1. J Biol Chem. 2005;280:32332–32339. doi: 10.1074/jbc.M504246200. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi Y, Tsuchiya A, Hayashi T, Kohyama N, Ohbayashi M, Yamamoto T. Isolation and characterization of polyspecific mouse organic solute carrier protein 1 (mOscp1) Drug Metab Dispos. 2007;35:1239–1245. doi: 10.1124/dmd.107.014795. [DOI] [PubMed] [Google Scholar]
- 13.Izuno H, Kobayashi Y, Sanada Y, Nihei D, Suzuki M, Kohyama N, Ohbayashi M, Yamamoto T. Rat organic solute carrier protein 1 (rOscp1) mediated the transport of organic solutes in Xenopus laevis oocytes: isolation and pharmacological characterization of rOscp1. Life Sci. 2007;81:1183–1192. doi: 10.1016/j.lfs.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Ouyang J, Wu M, Huang C, Cao L, Li G. Overexpression of oxidored-nitro domain containing protein 1 inhibits human nasopharyngeal carcinoma and cervical cancer cell proliferation and induces apoptosis: Involvement of mitochondrial apoptotic pathways. Oncol Rep. 2013;29:79–86. doi: 10.3892/or.2012.2101. [DOI] [PubMed] [Google Scholar]
- 15.Xiang B, Yi M, Wang L, Liu W, Zhang W, Ouyang J, Peng Y, Li W, Zhou M, Liu H, Wu M, Wang R, Li X, Li G. Preparation of polyclonal antibody specific for NOR1 and detection of its expression pattern in human tissues and nasopharyngeal carcinoma. Acta Biochim Biophys Sin (Shanghai) 2009;41:754–762. doi: 10.1093/abbs/gmp064. [DOI] [PubMed] [Google Scholar]
- 16.Nie X, Zhang B, Li X, Xiang J, Xiao B, Ma J, Zhou M, Zhu S, Lu H, Gui R, Shen S, Li G. Cloning, expression, and mutation analysis of NOR1, a novel human gene down-regulated in HNE1 nasopharyngeal carcinoma cell line. J Cancer Res Clin Oncol. 2003;129:410–414. doi: 10.1007/s00432-003-0451-9. [DOI] [PubMed] [Google Scholar]
- 17.Li W, Li X, Wang W, Li X, Tan Y, Yi M, Yang J, McCarthy JB, Xiong W, Wu M, Ma J, Su B, Zhang Z, Liao Q, Xiang B, Li G. NOR1 is an HSF1-and NRF1-regulated putative tumor suppressor inactivated by promoter hypermethylation in nasopharyngeal carcinoma. Carcinogenesis. 2011;32:1305–1314. doi: 10.1093/carcin/bgr174. [DOI] [PubMed] [Google Scholar]
- 18.Xiang B, Wang W, Li W, Li X, Li X, Li G. Differential expression of oxidored nitro domain containing protein 1 (NOR1), in mouse tissues and in normal and cancerous human tissues. Gene. 2012;493:18–26. doi: 10.1016/j.gene.2011.11.039. [DOI] [PubMed] [Google Scholar]
- 19.Xiang B, Yi M, Li W, Wang W, Zheng P, Li X, Li G. Expression of oxidored nitro domain-containing protein 1(NOR1) impairs nasopharyngeal carcinoma cells adaptation to hypoxia and inhibits PDK1 expression. Mol Cell Biochem. 2014;393:293–300. doi: 10.1007/s11010-014-2072-9. [DOI] [PubMed] [Google Scholar]
- 20.Kroeger H, Jelinek J, Estecio MR, He R, Kondo K, Chung W, Zhang L, Shen L, Kantarjian HM, Bueso-Ramos CE, Issa JP. Aberrant CpG island methylation in acute myeloid leukemia is accentuated at relapse. Blood. 2008;112:1366–1373. doi: 10.1182/blood-2007-11-126227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li W, Li X, Wang W, Yi M, Zhou Y, Zheng P, Xiong W, Yang J, Peng S, McCarthy JB, Xiang B, Li G. Tumor suppressor gene Oxidored-nitro domain-containing protein 1 regulates nasopharyngeal cancer cell autophagy, metabolism,and apoptosis in vitro. Int J Biochem Cell Biol. 2013;45:2016–2026. doi: 10.1016/j.biocel.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 22.Huu NT, Yoshida H, Umegawachi T, Miyata S, Yamaguchi M. Structural characterization and subcellular localization of Drosophila organic solute carrier partner 1. BMC Biochem. 2014;15:11. doi: 10.1186/1471-2091-15-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirose F, Ohshima N, Shiraki M, Inoue YH, Taguchi O, Nishi Y, Matsukage A, Yamaguchi M. Ectopic expression of DREF induces DNA synthesis, apoptosis, and unusual morphogenesis in the Drosophila eye imaginal disc: Possible interaction with polycomb and trithorax group proteins. Mol Cell Biol. 2001;21:7231–7242. doi: 10.1128/MCB.21.21.7231-7242.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brand AH, Perrimon N. Targeted Gene-Expression as a Means of Altering Cell Fates and Generating Dominant Phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 25.Spradling AC, Rubin GM. Transposition of Cloned P Elements into Drosophila Germ Line Chromosomes. Science. 1982;218:341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- 26.Robertson HM, Preston CR, Phillis RW, Johnson-schlitz DM, Benz WK, Engels WR. A Stable Genomic Source of P-Element Transposase in Drosophila melanogaster . Genetics. 1988;118:461–470. doi: 10.1093/genetics/118.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun J, Tower J. FLP recombinase-mediated induction of Cu/Zn-superoxide dismutase transgene expression can extend the life span of adult Drosophila melanogaster flies. Mol Cell Biol. 1999;19:216–228. doi: 10.1128/mcb.19.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirose F, Ohshima N, Shiraki M, Inoue YH, Taguchi O, Nishi Y, Matsukage A, Yamaguchi M. Ectopic expression of DREF induces DNA synthesis, apoptosis, and unusual morphogenesis in the Drosophila eye imaginal disc: possible interaction with Polycomb and trithorax group proteins. Mol Cell Biol. 2001;21:7231–7242. doi: 10.1128/MCB.21.21.7231-7242.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolff T, Ready DF. Cell death in normal and rough eye mutants of Drosophila . Development. 1991;113:825–839. doi: 10.1242/dev.113.3.825. [DOI] [PubMed] [Google Scholar]
- 30.Muro I, Means JC, Clem RJ. Cleavage of the apoptosis inhibitor DIAP1 by the apical caspase DRONC in both normal and apoptotic Drosophila cells. J Biol Chem. 2005;280:18683–18688. doi: 10.1074/jbc.M501206200. [DOI] [PubMed] [Google Scholar]
- 31.Igaki T, Yamamoto-Goto Y, Tokushige N, Kanda H, Miura M. Down-regulation of DIAP1 triggers a novel Drosophila cell death pathway mediated by Dark and DRONC. J Biol Chem. 2002;277:23103–23106. doi: 10.1074/jbc.C200222200. [DOI] [PubMed] [Google Scholar]
- 32.Muro I, Hay BA, Clem RJ. The Drosophila DIAP1 protein is required to prevent accumulation of a continuously generated, processed form of the apical caspase DRONC. J Biol Chem. 2002;277:49644–49650. doi: 10.1074/jbc.M203464200. [DOI] [PubMed] [Google Scholar]
- 33.Fan Y, Bergmann A. Distinct mechanisms of apoptosis-induced compensatory proliferation in proliferating and differentiating tissues in the Drosophila eye. Dev Cell. 2008;14:399–410. doi: 10.1016/j.devcel.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ly LL, Suyari O, Yoshioka Y, Tue NT, Yoshida H, Yamaguchi M. dNF-YB plays dual roles in cell death and cell differentiation during Drosophila eye development. Gene. 2013;520:106–118. doi: 10.1016/j.gene.2013.02.036. [DOI] [PubMed] [Google Scholar]
- 35.Thao DT, An PN, Yamaguchi M, LinhThuoc T. Overexpression of ubiquitin carboxyl terminal hydrolase impairs multiple pathways during eye development in Drosophila melanogaster . Cell Tissue Res. 2012;348:453–463. doi: 10.1007/s00441-012-1404-x. [DOI] [PubMed] [Google Scholar]
- 36.Wolff T, Ready DF. Pattern formation in the Drosophila retina. In: Bate M, Martinez-Arias A, editors. The development of Drosophila melanogaster. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1993. pp. 1277–1316. [Google Scholar]
- 37.Kramer S, West SR, Hiromi Y. Cell fate control in the Drosophila retina by the orphan receptor seven-up: its role in the decisions mediated by the ras signaling pathway. Development. 1995;121:1361–1372. doi: 10.1242/dev.121.5.1361. [DOI] [PubMed] [Google Scholar]
- 38.Butler SJ, Ray S, Hiromi Y. Klingon, a novel member of the Drosophila immunoglobulin superfamily, is required for the development of the R7 photoreceptor neuron. Development. 1997;124:781–792. doi: 10.1242/dev.124.4.781. [DOI] [PubMed] [Google Scholar]
- 39.Charroux B, Royet J. Mutations in the Drosophila ortholog of the vertebrate Golgi pH regulator (GPHR) protein disturb endoplasmic reticulum and Golgi organization and affect systemic growth. Biol Open. 2014;3:72–80. doi: 10.1242/bio.20137187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Munro S, Pelham HR. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987;48:899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- 41.Yoshida H. ER stress and diseases. FEBS J. 2007;274:630–658. doi: 10.1111/j.1742-4658.2007.05639.x. [DOI] [PubMed] [Google Scholar]
- 42.Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 43.Shan Z, Hou Q, Zhang N, Guo L, Zhang X, Ma Y, Zhou Y. Overexpression of oxidored-nitro domain containing protein 1 induces growth inhibition and apoptosis in human prostate cancer PC3 cells. Oncol Rep. 2014;32:1939–1946. doi: 10.3892/or.2014.3407. [DOI] [PubMed] [Google Scholar]
- 44.Huh JR, Guo M, Hay BA. Compensatory proliferation induced by cell death in the Drosophila wing disc requires activity of the apical cell death caspase Dronc in a nonapoptotic role. Curr Biol. 2004;14:1262–1266. doi: 10.1016/j.cub.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 45.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 46.Pandey UB, Nichols CD. Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol Rev. 2011;63:411–436. doi: 10.1124/pr.110.003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bier E. Drosophila, the golden bug, emerges as a tool for human genetics. Nat Rev Genet. 2005;6:9–23. doi: 10.1038/nrg1503. [DOI] [PubMed] [Google Scholar]


