Abstract
Aims: NDC80/Hec1, one of four proteins of the outer kinetochore NDC80 complex, is involved in the tumorigenesis of a variety of cancers. In this study, we focused on that NDC80 is overexpressed in human pancreatic cancer and investigates the role of NDC80-knockdown in pancreatic cancer cells proliferation. Materials and methods: We determined the expression levels of NDC80 on both mRNA and protein levels in fresh pancreatic cancer tissues and cells by quantitative real-time polymerase chain reaction and immunoblotting, respectively. Furthermore, protein level of NDC80 was identified using immunochemistry in paraffin-embedded tumor specimen, with correlation between NDC80 expression and various clinicopathological parameters evaluated. The role of NDC80 in pancreatic cancer cells (Panc-1) growth was investigated by lentivirus-mediated silencing of NDC80. The effect of NDC80 deletion on cell proliferation was analyzed by MTT assay and clone formation assay, while cell cycle distributions and apoptosis were analyzed by flow cytometry. Results: The mRNA and protein of NDC80 were overexpressed in pancreatic cancer tissues and cells. The statistical analysis based on immunohistochemical evaluation suggested that NDC80 overexpression was signifi cantly associated with clinicopathological parameters including pathological T staging and N staging, which may be served as an predictor for poor outcomes. The silencing of NDC80 in Panc-1 cells could suppress cell proliferation and colony formation. Furthermore, the NDC80-siRNA infected Panc-1 cells lead to cell cycle arrest at G2/M phase and induction of apoptosis. Conclusion: These results demonstrated that NDC80 plays an essential role in the tumorigenesis of pancreatic cancer, and might serve as potential prognostic and therapeutic target for treatment of pancreatic cancer.
Keywords: NDC80, pancreatic cancer, proliferation, apoptosis
Introduction
The precise distribution of the replicated genome during cell division is critical for cellular viability and organismal development. Chromosomal instability leading to an abnormal number of chromosomes (aneuploidy) is a hallmark of cancers [1,2]. This observation has triggered a series of research for molecular determinants and mechanism that regulate proper chromosome segregation and their abnormalities as mediators of tumorigenesis [1,3].
Regulatory proteins and mechanisms safeguard against erroneous chromosome segregation, and hence, increase the fidelity of mitosis [4]. For example, erroneous attachment configurations on kinetochores will be destabilized and eliminated, and unattached kinetochores are the primary signal to launch the spindle assembly checkpoint (SAC), a cell cycle surveillance pathway that delays exit from mitosis. As core components of the kinetochore, the Constitutive Centromere-Associated Network (CCAN) and the Knl1/Mis12/Ndc80 complex (KMN) network bind centromeric DNA and microtubules respectively [5,6]. Across eukaryotes, the kinetochore-localized KMN network, in particular the Ndc80 complex, is essential for both microtubule binding and SAC signaling [4]. The Ndc80 tetrameric complex consists of Hec1 (Highly expressed in cancer 1/also called Ndc80), Nuf2 (Nuclear filamentous 2), Spc24 (Spindle pole component) and Spc25. NDC80/Nuf2 and Spc24/Spc25 heterodimers assemble into a dumbbell-like structure at either end, interacting via coiled-coil domains [7,8]. The microtubule-binding interface of the kinetochore, which is created from multiple Ndc80 complexes binding around each kinetochore fiber, is of central importance in chromosome segregation. Moreover, the Ndc80 complex plays an essential role in relaying microtubule-binding status via recruiting of the SAC proteins Mad1, Mad2, and Mps1 to the kinetochore [9].
NDC80/Hec1, a kinetochore outer layer component and spindle checkpoint regulator, is highly expressed in a variety of human cancers. The depletion of NDC80 impairs chromosome compression and leads to the activation of the SAC, resulting in apoptotic cell death [9,10]. The N-terminal region of NDC80 contains several sites of phosphorylation by the mitotic Aurora B/Ipl1 kinase [11]. Regulation of NDC80 by nek2 and Aurora B is critical for its mitotic function and cell survival [5]. Overexpression of NDC80 was found to induce tumor formation in a mouse model by activating mitotic checkpoint [12]. Moreover, overexpression of NDC80 was determined to be associated with poor clinical prognosis in breast cancer and other cancers [13,14]. Together, these results highlight a crucial role of NDC80 in tumorigenesis and emerge as a potential mitotic target for cancer intervention.
Pancreatic cancer is a lethal malignancy, corresponding to the fourth leading cause of cancer related death worldwide [15]. A majority of patients have been diagnosed with locally advanced or metastatic because of the lack of easily observable symptoms, and only 15%-20% of the patients have the chance for surgery and the rest could receive adjuvant therapies [16]. Even though new antitumor drugs and techniques have been developed, the prognosis for these patients remains poor [17]. Therefore, further research of molecule mechanisms and novel therapeutic strategies should be urgently required. Here, we examined the NDC80 expressions on both mRNA and protein levels in pancreatic cancer tissues and paratumoral tissues, and analyzed the correlations between NDC80 expressions and various clinicopathological parameters. Furthermore, we knockdown NDC80 via lentivirus mediated siRNA infection in pancreatic cells (Panc-1) to explore the role of NDC80 in pancreatic tumorigenesis.
Materials and methods
Cells culture
The human pancreatic adenocarcinoma cell lines Panc-1, BxPC-3, Sw1900 and Hek293 (human embryonic kidney cell line) were purchased from the Cell Bank of Chinese Academy of Sciences (Shanghai, China), and cultured in RPMI-1640 medium (GIBCO, New York, USA), containing 10% fetal bovine serum (FBS) and 100 U/mL penicillin/streptomycin. All cell lines were maintained in a 5% CO2 humidified atmosphere at 37°C.
Tissue samples
We retrospectively analyzed clinicopathological data of 122 patients with pancreatic cancer and underwent surgical pancreatic resection undergoing surgery at the Department of General surgery, Shanghai Sixth People’s Hospital Affiliated to Shanghai Jiaotong University from January 2009 to October 2012. All specimens were determined as pancreatic ductal adenocarcinoma by pathological examinations. Informed consent was obtained from each patient before tissue specimens were collected. Tumors were classified histologically according to TNM staging criteria from American Joint Committee on Cancer (AJCC), and tumor grade was evaluated according to the WHO criteria.
Immunohistochemistry analysis
Briefly, sections of paraffin-embedded tissue in 4 μm thickness were deparaffinized with xylene and rehydrated in serially diluted alcohol solutions. Microwave heating was used to retrieve antigen. Endogenous peroxidase was blocked by incubation in 0.3% hydrogen peroxide. Then, the slides were incubated with the primary anti-NDC80 antibody (1:1000, Abcam) overnight at 4°C, followed by incubation of second antibody for 30 minutes. Finally, all slides were stained with diaminobenzidine and then counterstained with hematoxylin. The slides incubated with PBS instead of primary antibody were applied as the negative control.
A combined scoring system, multiplying the staining intensity (SI) and the percentage of positive cells (PP), was used to evaluate immunoreactivity for NDC80 expression. Scores from 0-3 were given for SI or PP as follows respectively: score of 0 (negative or < 5%); score of 1 (weak or 6-25%); score of 2 (moderate or 26-50%); score of 3 (strong or 51-70%) and score of 4 (only 71-100%). Then, a final decision for NDC80 expression was made according to the standard (low expression: score < 4; high expression: score ≥ 4).
Construction of recombinant lentivirus and infection
The shRNA (5’-CATTCTTGACCAGAAATTA-3’) for human NDC80 gene was inserted into the lentivirus expression plasmid pFH-L (Shanghai Genechem, Shanghai, China) and non-silencing shRNA (5’-TTCTCCGAACGTGTCACGT-3’) was used as a negative control. For virus packaging, NDC80-shRNA lentivirus or control lentivirus together with pHelper 1.0 and pHelper 2.0 (pVSVG-I and pCMVΔR 8.92 plasmids, respectively), were added to 293T cells with Lipofectamine TM 2000 (Invitrogen, Shanghai, China) according to the manufacturer’s instructions. After 48 h of transfection, viral supernatants were collected and centrifuged to get rid of cell debris, and then filtered through 0.45 µm polyvinylidene fluoride membranes. Panc-1 cells were infected with NDC80-siRNA lentivirus or control lentivirus at multiplicity of infection (MOI) of 2. The infected cells expressing GFP protein were observed using fluorescence microscopy 2009 (CKX41, Olympus, Tokyo, Japan) in order to determine the infection efficiency. Then, cells were collected to perform qRT-PCR analysis and western blot analysis after lentivirus infection.
RNA extraction and quantitative real-time polymerase chain reaction
Total RNA was extracted from tumor tissues and cultured cells using Trizol reagent (Invitrogen, USA) as described in the manufacturer’s instructions. Complementary DNA (cDNA) was generated using Superscript III Reverse Transcriptase (Promega, USA). Expression of mRNA was determined by quantitative real-time polymerase chain reaction (qRT-PCR). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA was amplified as an internal standard for quantification. The following primers used for qRT-PCR analysis: NDC80 expression were 5’-CCTCTCCATGCAGGAGTTAAGA-3’ (forward) and 5’-GGTCTCGGGTCCTTGATTTTCT-3’ (reverse); GAPDH expression were 5’-TGAC-TTCAACAGCGACACCCA-3’ (forward) and 5’-CAC CCTGTTGCTGTAGCCAAA-3’ (reverse). The PCR amplification consisted of 40 cycles (95°C for 15s and 55°C for 15s) after an initial denaturation step (95°C for 10s). Relative expression of Tbx3 was calculated against β-actin mRNA level by using the 2-ΔΔCt method.
Protein extraction and immunoblotting analysis
Cells were seeded into six-well-plates, cultured until 70-80% confluency, and washed twice with cold phosphate-buffered saline (PBS). Then, total cellular protein was extracted from cells using RIPA buffer (50 mM Tris-HCl, 150 mM NaCl, 1% Nonidet P-40, 1% sodium deoxycholate, and 0.1% sodium dodecyl sulfate) containing protease inhibitors. Next, 60 μl lysis buffer was added to each well, and the cell lysate was collected into a tube using a scraper. The supernatant was collected after centrifugation at 10,000×g for 10 min and separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA). The membrane was incubated with primary anti-NDC80 monoclonal antibody (1:1000, Abcam) at 4 overnight, washed with Tris-buffered saline with Tween 20 (TBST) 3 times, and incubated with HRP- conjugated goat anti-mouse secondary antibody (1:5000, Santa Cruz Biotechnology) for 2 hours at room temperature. The GAPDH was used as endogenous control.
MTT assay of cell growth inhibition
Both of infected and noninfected Panc-1 cells were seeded in 96-well plates at a density of 2×103 cells/well and incubated at 37°C for 1, 2, 3, 4, and 5 days, respectively. Then, cells were washed two times with PBS and 3-(4,5-dimethyl-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution (5 mg/mL) was added to each well. After 4 hours of incubation, supernatants in each well were removed and then 100 μL of dimethyl sulfoxide (DMSO) was added to solubilize the formazan salt. Ten minutes later, the optical density (OD) was measured at 490 nm by using a microplate reader.
Clone formation assay
Both of infected and noninfected Panc-1 cells (200 cells/well) were seeded in six-well plates. Culture medium was changed at regular time intervals. After incubation at 37°C for 14 days, adherent cells were washed twice with PBS, fixed with 4% paraformaldehyde for 30 min. The colonies were stained with Giemsa solution for 10 min, then washed twice with double distilled water. The number of colonies (> 50 cells/colony) were counted using a fluorescence microscopy (CKX41, Olympus, Tokyo, Japan).
Cell cycle analysis
Approximately 3×104 of Panc-1 cells were seeded in six-well culture plates and incubated in complete medium to 80% confluence at 37°C. The harvested cells were fixed with 70% cold ethanol at 4°C overnight, washed twice with ice-cold PBS, and incubated with 10 mg/ml RNase at 37°C. Cell cycle was monitored by using propidium iodide (PI) staining of nuclei. The fluorescence of DNA-bound PI in cells was measured with a flow cytometry (FACSCalibur, Becton Dickinson), and the cell populations in different phases of the cell cycle were analyzed with ModFit 3.0 software.
Apoptotic assay
ApoScreen Annexin V Apoptosis Kit (Southern Biotech) were used for labeling of apoptotic cells according to the manufacturer’s protocol. Cells were harvested at an exponential growth phase, centrifuged and resuspended in binding buffer. Then, cells (100 μL of the cell suspension solution) were stained by Annexin V-APC, incubated at room temperature for 15 min, and subjected to flow cytometry (FACSCalibur, Becton Dickinson).
Statistical analysis
All experiments were performed in triplicate. SPSS version 21.0 software (Chicago, IL) was used for all statistical analyses. Data were expressed as mean ± standard deviation of three independent determinations. Differences were compared using Student’s t test. The χ2 test and Fisher exact test were used to test the significance of the difference in expression of NDC80 between tumor and paratumor samples. For all analysis, a P value of less than 0.05 was considered to be statistically significant.
Results
Overexpression of NDC80 in pancreatic cancer tissues and cell lines
The mRNA levels of NDC80 were determined in 20 fresh pancreatic cancer tissues and paratumor tissues using qRT-PCR. As shown in Figure 1A, NDC80 mRNA levels in the tumor tissue was higher than in the control tissue and this difference was statistically significant (4.208 ± 0.0556 vs. 1.129 ± 0.0746, P < 0.01). Furthermore, NDC80 was widely expressed in 3 pancreatic cancer cell lines including Panc-1, BxPC-3, and Sw1990, rather than Hek293 cell line (Figure 1B). The similar trend on NDC80 protein levels was observed as on its mRNA levels by immunoblotting analysis (Figure 1C).
Figure 1.
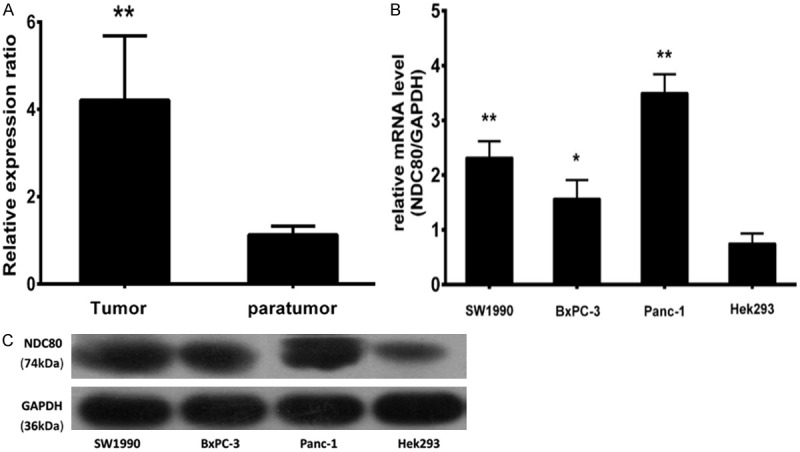
Expression of NDC80 in pancreatic cancer tissues and cell lines. The NDC80 mRNA expression in the tumor tissues and corresponding paratumor tissues was evaluated by qRT-PCR (A). The mRNA and protein levels of NDC80 expressed in 3 pancreatic cancer cell lines and Hek293 cell line, were analysised by qRT-PCR and immunoblotting, respectively (B and C). GAPDH was used as an internal control. *P < 0.05, **P < 0.01 as compared to the non-tumor group.
Correlation between NDC80 expression and clinicopathological parameters in pancreatic cancer
Immunochemistry analysis was used to identify the expression of NDC80 in 122 paraffin-embedded pancreatic cancer tissues. NDC80 staining was positive in more than 90% of cancer tissues, among which 73 (60%) were NDC80-high expression. Detailed analysis revealed that NDC80 were mainly located in the cytoplasm of carcinoma cells. Representative immunohistochemical results were shown in Figure 2.
Figure 2.
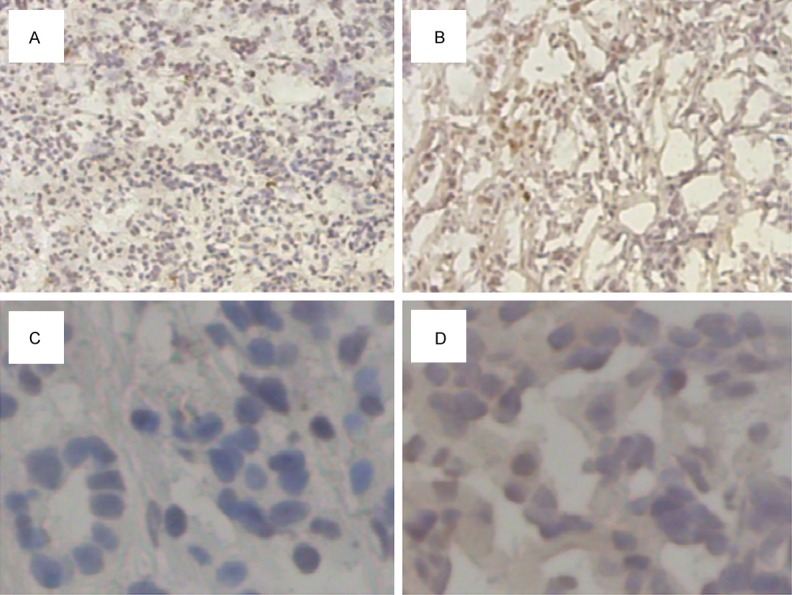
Representative immunohistochemical staining for NDC80 in tumor tissues and paratumoral tissues. Original magnification ×100 and ×400: NDC80 expression in paratumoral tissues (A and C) and tumor tissues (B and D), respectively.
Table 1 shows the association of NDC80 expression and clinicopathological parameters. There was no significant correlation between NDC80 expression and gender (P = 0.132), age (P = 0.830), tumor position (0.849) or differentiation (P = 0.397). However, overexpression of NDC80 was significant associated with pathological T staging (P = 0.009) and pathological N staging (P = 0.022).
Table 1.
The associations of NDC80 expression with clinicopathological parameters in 122 patients with resectable pancreatic cancer
| Clinicopathological parameters | N | NDC80 expression | P value | |
|---|---|---|---|---|
|
| ||||
| High | Low | |||
| Gender | ||||
| Male | 73 | 48 | 25 | 0.132 |
| Female | 49 | 25 | 24 | |
| Age (years) | ||||
| ≥ 65 | 92 | 56 | 36 | 0.830 |
| < 65 | 30 | 17 | 13 | |
| Tumor position | ||||
| Proximal | 78 | 46 | 32 | 0.849 |
| Distal | 44 | 27 | 17 | |
| Differentiation | ||||
| Well/moderate | 91 | 52 | 39 | 0.397 |
| Poor | 31 | 21 | 10 | |
| Pathological T staging | ||||
| T1-2 | 85 | 44 | 41 | 0.009 |
| T3 | 37 | 29 | 8 | |
| Pathological N staging | ||||
| N0 | 33 | 14 | 19 | 0.022 |
| N1 | 89 | 59 | 30 | |
Expression of NDC80 was suppressed by infection of lentivirus-mediated siRNA in pancreatic cancer cells
The fluorescence level of GFP protein were observed to determine the infection efficiency. After NDC80 siRNA infection, a high infection efficiency was confirmed though that more than 90% of cells were expressing GFP (Figure 3A). Furthermore, the mRNA and protein expression level of NDC80 after siRNA infection was evaluated by qRT-PCR analysis and Immunoblotting analysis, respectively. As shown in Figure 3B and 3C, the mRNA and protein expression levels of NDC80 were significantly suppressed compared to control group, respectively (P < 0.01). Therefore, lentiviral-mediated siRNA infection was an effective way to knockdown NDC80 in pancreatic cancer cells.
Figure 3.
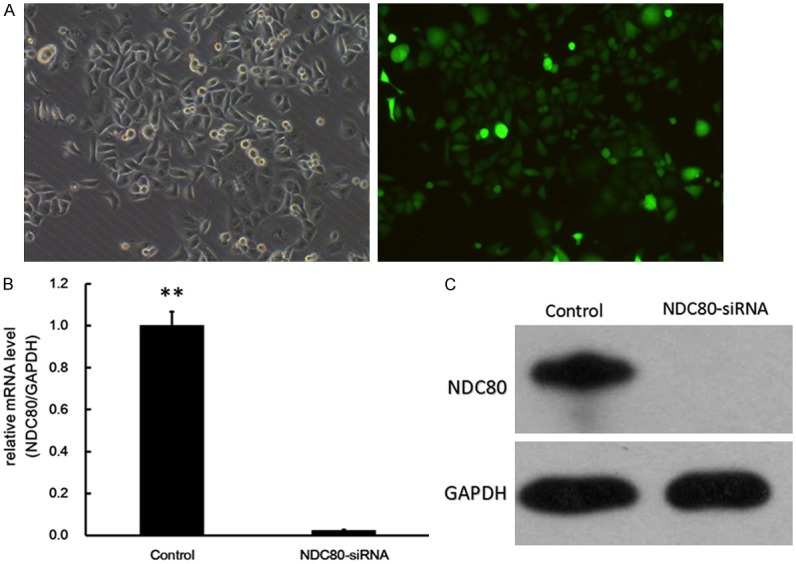
Expression of NDC80 was suppressed by infection of lentivirus-mediated siRNA in pancreatic cancer cells. Bright and fluorescence field of Panc-1 cells after infection with lentivirus containing NDC80 at magnification of ×100 (A). qRT-PCR and immunoblotting analysis of the NDC80 expression levels after siRNA infection compared with the control group (B and C). GAPDH was used as internal control. **P < 0.01 as compared to the control group.
NDC80 knockdown suppressed cell proliferation and colony formation in pancreatic cancer cells
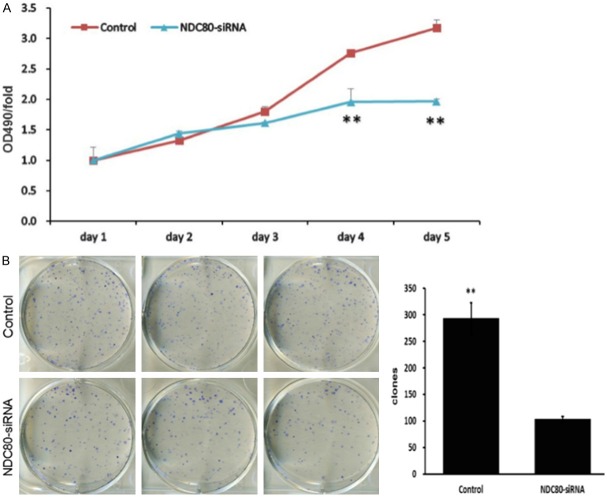
The effect of NDC80 knockdown on the proliferation ability of Panc-1 cells was determined by MTT assay and clone formation assay. As depicted in Figure 4A, the cellular proliferative rate of NDC80-siRNA group was remarkably reduced with respect to control group. Moreover, Figure 4B showed that NDC80 knockdown cells, compared with control cells, had a significant supression in their ability to form colonies (P < 0.01). Hence, it is demonstrated that the silencing of NDC80 could suppress tumorigenicity of pancreatic cancer cells.
Figure 4.
NDC80-siRNA suppressed cell proliferation and colony formation in pancreatic cancer cells. The proliferation curve of siRNA infected Panc-1 cells compared with control group, was determined by MTT assay (A). The similar trends was observed in clone formation assay (B). **P < 0.01 as compared to the control group.
NDC80 knockdown arrested the cell cycle progression and induced apoptosis in pancreatic cancer cells
The inhibition of cell proliferation in pancreatic cancer cells might be due to arrested the cell cycle progression or induced apoptosis, which was analyzed by flow cytometry. As shown in Figure 5, cell cycle analysis reveals that NDC80-siRNA cells showed the proportion of cells in G1 phase (41.47% ± 0.20% vs. 48.40% ± 0.39%, P < 0.01), S phase (30.14% ± 0.54% vs. 45.81% ± 0.50%, P < 0.01) and G2/M (28.38% ± 0.65% vs. 5.79% ± 0.17%, P < 0.01), compared to control group. Furthermore, the apoptosis analysis following Annexin V staining showed that the apoptosis rate of NDC80 knockdown cells was significantly increased with respect to control group (Figure 6, 4.11% ± 0.15% vs. 6.04% ± 0.25%, P < 0.01). Therefore, these results revealed that silencing of NDC80 in Panc-1 cells could lead to cell cycle arrest at G2/M phase and induction of apoptosis.
Figure 5.
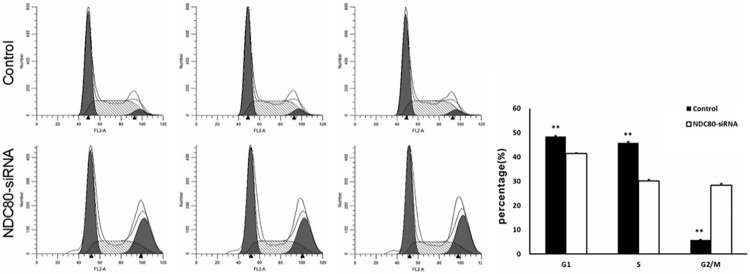
NDC80 knockdown induced cell cycle arrest at G2/M phase. Flow cytometry analysis of cell cycle reveals that NDC80-siRNA cells showed the proportion of cells in G1 phase (41.47% ± 0.20% vs. 48.40% ± 0.39%), S phase (30.14% ± 0.54% vs. 45.81% ± 0.50%) and G2/M (28.38% ± 0.65% vs. 5.79% ± 0.17%), compared to the control. **P < 0.01 as compared to the control group.
Figure 6.
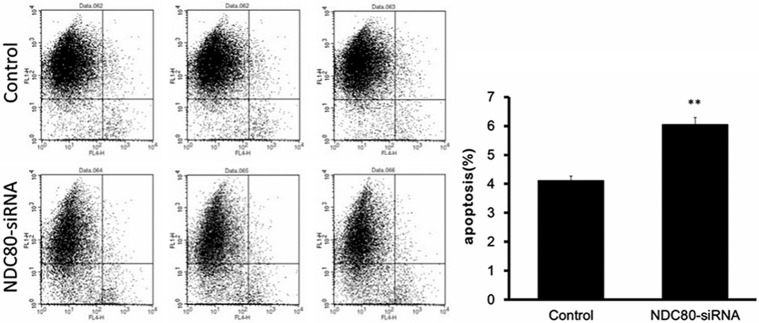
The apoptosis of Panc-1 cells was induced by si-NDC80 infection. Flow cytometry analysis of cell apoptosis reveals that the apoptosis rate of NDC80 knockdown cells was significantly increased with respect to control group (4.11% ± 0.15% vs. 6.04% ± 0.25%). **P < 0.01 as compared to the control group.
Discussion
Chromosomal instability has long been considered as a primary contributor to tumorigenesis, including two forms: microsatellite instability characterized by a high mutational load and genetic instability resulting in aneuploidy or aberrant chromosome numbers [1]. There is evidence suggesting that defects in the spindle checkpoint might promote aneuploidy and tumorigenesis [18]. NDC80/Hec1 (coded by the gene Hec1), which localizes to kinetochore in cell mitosis, plays an essential role in chromosome segregation by over activation of the mitotic checkpoint. Furthermore, NDC80 is highly expressed in a variety of tumors and also shown to correlate with tumor grade and prognosis [19,20]. The present study described that the mRNA levels of NDC80 in pancreatic cancer tissues and cancer cell lines were significantly higher than in paratumoral tissues and Hek293 cell line. By immunoblotting analysis, we observed similar trends on NDC80 protein levels as on its mRNA levels. To further investigate its clinical significance, the immunochemistry was applied and the results suggested that the protein expression of NDC80 was significantly associated with prognosis-related clinical parameters, including pathological T staging and pathological N staging, which may be linked with poor prognosis of patients with pancreatic cancer.
Many studies have reported a role of NDC80 in mitosis. The depletion of NDC80 in different organisms leads to the mislocalization of several kinetochore proteins, including components of the SAC and the Nup107-160 nuclear pore complex [21,22]. NDC80 plays an important role in the maintenance of G2/M checkpoint function by driving G2 to M phase transition [9]. Silencing of NDC80 recovered the SAC activity, induced accumulation of cells in G2/M phase, and prolonged mitotic arrest [23]. Recent work from Qu Y. and coworkers has demonstrated that cell cycle arrest of NDC80 knockdown gastric cancer cells at G2/M phase lead to increase apoptosis owing to severe dysfunction of the mitotic checkpoint [20]. In the present study, with the lentivirus-mediated infection of siRNA, we obtained an efficient knockdown of NDC80 in PANC-1 cells which were subjected to investigate the biological role of NDC80 in cell growth. Knockdown of the NDC80 from PANC-1 cells induced significant growth inhibition, decreased colony formation ability and also led to apoptosis by arresting the cell cycle at G2/M phase. In agreement with previous findings, our data showed that NDC80 plays an essential role in regulating PANC-1 cells proliferation. The block of G2/M phase was corroborated with decreased levels of cyclin A and cyclin B1. As the key molecule for G2/M phase transition during the cell cycle, Cyclin B1 is indispensable for the initiation of mitosis and Cyclin B1 knockdown could lead to cell apoptosis [24]. NUF2, similar to NDC80, is part of a molecular linker between the kinetochore attachment site and tubulin subunits [25]. Cell cycle analysis from Hu P. and coworkers showed that Cyclin B1, Cdc2 and Cdc25A were suppressed in pancreatic cell lines after NUF2 silencing, inducing cell cycle arrest at G0/G1 phase [26]. However, the mechanism through which NDC80 regulates pancreatic cancer cell growth still needs further investigation.
Although the chemotherapy drugs are widely used, such as gemcitabine, it just prolongs the survival of several months [27]. As part of the cancer chemotherapeutic compounds, drugs that interfere with mitosis currently obtain better results in clinical practice, focusing on inhibition of the mitotic spindle through interactions with microtubules [28]. NDC80 is a component of the kinetochore and is overexpressed in a variety of human cancers, which make it as an attractive molecular target for anticancer therapeutics. Lee W. and colleagues first discovered INH1, a small molecule compound targeting the NDC80/Nek2 pathway, which inhibited the proliferation of breast cancer cell lines and retarded tumor growth in a nude mouse model [10]. Recently, TAI-95, the potential of a NDC80 inhibitor, may represent a promising novel approach for the treatment of primary liver cancers [29]. Therefore, inhibition of NDC80 by small molecule compound for the treatment of pancreatic cancer is required for further validation.
In summary, our findings indicate that NDC80 is highly expressed in human pancreatic cancer tissues and cell lines, and closely correlated with the clinical feature of patients with pancreatic cancer. The silencing of NDC80 expression suppressed the proliferation and cell cycle progression in pancreatic cancer lines. Hence, these results suggested that NDC80 could be an effective potential therapeutic target for treatment of pancreatic cancer. The regulation mechanisms of NDC80 in pancreatic tumorigenesis still require more studies to ensure an eventual conversion into clinical practices.
Acknowledgements
We thank all the patients and clinical investigators who were involved in this study and this study was funded by the Science and Technology Commission of Shanghai Municipality (NO. 12DZ1940808), Shanghai, China.
Disclosure of conflict of interest
None.
References
- 1.Bharadwaj R, Yu H. The spindle checkpoint, aneuploidy, and cancer. Oncogene. 2004;23:2016–2027. doi: 10.1038/sj.onc.1207374. [DOI] [PubMed] [Google Scholar]
- 2.Jallepalli PV, Lengauer C. Chromosome segregation and cancer: cutting through the mystery. Nat Rev Cancer. 2001;1:109–117. doi: 10.1038/35101065. [DOI] [PubMed] [Google Scholar]
- 3.Wassmann K, Benezra R. Mitotic checkpoints: from yeast to cancer. Curr Opin Genet Dev. 2001;11:83–90. doi: 10.1016/s0959-437x(00)00161-1. [DOI] [PubMed] [Google Scholar]
- 4.McAinsh AD, Meraldi P. The CCAN complex: linking centromere specification to control of kinetochore-microtubule dynamics. Semin Cell Dev Biol. 2011;22:946–952. doi: 10.1016/j.semcdb.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 5.Cheeseman IM, Chappie JS, Wilson-Kubalek EM, Desai A. The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell. 2006;127:983–997. doi: 10.1016/j.cell.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 6.Foley EA, Kapoor TM. Microtubule attachment and spindle assembly checkpoint signalling at the kinetochore. Nat Rev Mol Cell Biol. 2013;14:25–37. doi: 10.1038/nrm3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciferri C, Pasqualato S, Screpanti E, Varetti G, Santaguida S, Dos Reis G, Maiolica A, Polka J, De Luca JG, De Wulf P, Salek M, Rappsilber J, Moores CA, Salmon ED, Musacchio A. Implications for kinetochore-microtubule attachment from the structure of an engineered Ndc80 complex. Cell. 2008;133:427–439. doi: 10.1016/j.cell.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei RR, Schnell JR, Larsen NA, Sorger PK, Chou JJ, Harrison SC. Structure of a central component of the yeast kinetochore: the Spc24p/Spc25p globular domain. Structure. 2006;14:1003–1009. doi: 10.1016/j.str.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Martin-Lluesma S, Stucke VM, Nigg EA. Role of Hec1 in spindle checkpoint signaling and kinetochore recruitment of Mad1/Mad2. Science. 2002;297:2267–2270. doi: 10.1126/science.1075596. [DOI] [PubMed] [Google Scholar]
- 10.Wu G, Qiu XL, Zhou L, Zhu J, Chamberlin R, Lau J, Chen PL, Lee WH. Small molecule targeting the Hec1/Nek2 mitotic pathway suppresses tumor cell growth in culture and in animal. Cancer Res. 2008;68:8393–8399. doi: 10.1158/0008-5472.CAN-08-1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeLuca JG, Gall WE, Ciferri C, Cimini D, Musacchio A, Salmon ED. Kinetochore microtubule dynamics and attachment stability are regulated by Hec1. Cell. 2006;127:969–982. doi: 10.1016/j.cell.2006.09.047. [DOI] [PubMed] [Google Scholar]
- 12.Diaz-Rodríguez E, Sotillo R, Schvartzman JM, Benezra R. Hec1 overexpression hyperactivates the mitotic checkpoint and induces tumor formation in vivo. Proc Natl Acad Sci U S A. 2008;105:16719–16724. doi: 10.1073/pnas.0803504105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van’t Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, Peterse HL, van der Kooy K, Marton MJ, Witteveen AT, Schreiber GJ, Kerkhoven RM, Roberts C, Linsley PS, Bernards R, Friend SH. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 14.Glinsky GV, Berezovska O, Glinskii AB. Microarray analysis identifies a death-from-cancer signature predicting therapy failure in patients with multiple types of cancer. J Clin Invest. 2005;115:1503–1521. doi: 10.1172/JCI23412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 16.Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049–1057. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 17.Winter JM, Yeo CJ, Brody JR. Diagnostic, prognostic, and predictive biomarkers in pancreatic cancer. J Surg Oncol. 2013;107:15–22. doi: 10.1002/jso.23192. [DOI] [PubMed] [Google Scholar]
- 18.Bièche I, Vacher S, Lallemand F, Tozlu-Kara S, Bennani H, Beuzelin M, Driouch K, Rouleau E, Lerebours F, Ripoche H, Cizeron-Clairac G, Spyratos F, Lidereau R. Expression analysis of mitotic spindle checkpoint genes in breast carcinoma: role of NDC80/HEC1 in early breast tumorigenicity, and a two-gene signature for aneuploidy. Mol Cancer. 2011;10:23. doi: 10.1186/1476-4598-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayama S, Daigo Y, Kato T, Ishikawa N, Yamabuki T, Miyamoto M, Ito T, Tsuchiya E, Kondo S, Nakamura Y. Activation of CDCA1-KNTC2, members of centromere protein complex, involved in pulmonary carcinogenesis. Cancer Res. 2006;66:10339–10348. doi: 10.1158/0008-5472.CAN-06-2137. [DOI] [PubMed] [Google Scholar]
- 20.Qu Y, Li J, Cai Q, Liu B. Hec1/Ndc80 is overexpressed in human gastric cancer and regulates cell growth. J Gastroenterol. 2014;49:408–418. doi: 10.1007/s00535-013-0809-y. [DOI] [PubMed] [Google Scholar]
- 21.Liu ST, Rattner JB, Jablonski SA, Yen TJ. Mapping the assembly pathways that specify formation of the trilaminar kinetochore plates in human cells. J Cell Biol. 2006;175:41–53. doi: 10.1083/jcb.200606020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zuccolo M, Alves A, Galy V, Bolhy S, Formstecher E, Racine V, Sibarita JB, Fukagawa T, Shiekhattar R, Yen T, Doye V. The humanNup107-160 nuclear pore subcomplex contributes to proper kinetochore functions. EMBO J. 2007;26:1853–1864. doi: 10.1038/sj.emboj.7601642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mo QQ, Chen PB, Jin X, Chen Q, Tang L, Wang BB, Li KZ, Wu P, Fang Y, Wang SX, Zhou JF, Ma D, Chen G. Inhibition of Hec1 expression enhances the sensitivity of human ovarian cancer cells to paclitaxel. Acta Pharmacol Sin. 2013;34:541–548. doi: 10.1038/aps.2012.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menon VR, Peterson EJ, Valerie K, Farrell NP, Povirk LF. Ligand modulation of a dinuclear platinum compound leads to mechanistic differences in cell cycle progression and arrest. Biochem Pharmacol. 2013;86:1708–1720. doi: 10.1016/j.bcp.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nabetani A, Koujin T, Tsutsumi C, Haraguchi T, Hiraoka Y. A conserved protein, Nuf2, is implicated in connecting the centromere to the spindle during chromosome segregation: a link between the kinetochore function and the spindle checkpoint. Chromosoma. 2001;110:322–334. doi: 10.1007/s004120100153. [DOI] [PubMed] [Google Scholar]
- 26.Hu P, Chen X, Sun J, Bie P, Zhang LD. SiRNA-mediated Knockdown against NUF2 Suppresses Pancreatic Cancer Proliferation in Vitro and in Vivo. Biosci Rep. 2015;35:e00170. doi: 10.1042/BSR20140124. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Dakhel S, Padilla L, Adan J, Masa M, Martinez JM, Roque L, Coll T, Hervas R, Calvis C, Messeguer R, Mitjans F, Hernández JL. S100P antibody-mediated therapy as a new promising strategy for the treatment of pancreatic cancer. Oncogenesis. 2014;3:e92. doi: 10.1038/oncsis.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harrison MR, Holen KD, Liu G. Beyond taxanes: a review of novel agents that target mitotic tubulin and microtubules, kinases, and kinesins. Clin Adv Hematol Oncol. 2009;7:54–64. [PMC free article] [PubMed] [Google Scholar]
- 29.Huang LY, Chang CC, Lee YS, Huang JJ, Chuang SH, Chang JM, Kao KJ, Lau GM, Tsai PY, Liu CW, Lin HS, Gish RG, Lau JY. Inhibition of Hec1 as a novel approach for treatment of primary liver cancer. Cancer Chemother Pharmacol. 2014;74:511–520. doi: 10.1007/s00280-014-2540-7. [DOI] [PubMed] [Google Scholar]