Abstract
Background: Prostate cancer (PCa) is one of the most prevalent malignant tumors, PCa-related death is mainly due to the high probability of metastasis. MicroRNAs (miRNAs) play an important role in cancer initiation, progression and metastasis by regulating their target genes. Methods: real-time PCR was used to detected the expression of microRNA-497. The molecular biological function was investigated by using cell proliferation assays, cell cycle assay, and migration and invasion assay. We used several Algorithms and confirmed that IKKβ is directly regulated by miR-497. Results: Here, we found miR-497 is downregulated in human prostate cancer (PCa) and inhibites the proliferation activity, migration and invasion of PC3-AR cells. Subsequently, IKKβ is confi rmed as a target of miR-497. Furthermore, knockdown of IKKβ expression resulted in decreased proliferation activity, migration and invasion. Finally, similar results was found after treatment with a novel IKK-β inhibitor (IMD-0354) in PC3-AR cells. CDK8, MMP-9, and PSA were involved in all these process. Conclusion: Taken together, our results show evidence that miR-497 may function as a tumor suppressor genes by regulating IKK-β in PCa, and may provide a strategy for blocking PCa metastasis.
Keywords: MiR-497, PCa, IKKβ, IMD-0354, migration, invasion
Introduction
Prostate cancer (PCa) is one of the most prevalent malignant tumors and the second major cause of cancer-related mortality in American men [1]. PCa-related death is mainly due to its high metastatic probability to bone and/or other organs [2,3]. In spite of significant progress, the prevention and treatment of metastasis of PCa is still a key challenge as the concrete molecular mechanisms of metastasis and invasion of PCa are not studied thoroughly. Prostate-specific antigen (PSA) is the most widely used cancer biomarker for prevention, diagnosis, and monitoring of patients with PCa [4]. However, PSA in the serum was pointless in high-risk populations for monitoring clinical progression and improving mortality. Thus, more effective biomarkers to discriminate between high-risk and low-risk patients with PCa to optimize and individualize therapy strategies at early clinical stage are urgently needed.
As a class of small non-coding RNAs, microRNAs (miRNAs) silence their targets by cleavage of mRNA, translational repression, destabilization of mRNA, or a combination of these mechanisms [5]. miRNAs play a key role in cell metastasis, likely owing to their effect on post-transcriptional regulation of gene networks important for cell motility, migration, and invasion [6]. A number of studies have noted the potential role of miRNAs in PCa. Nevertheless, little is known about the manner in which miRNAs act on the progression and metastasis of PCa. Recently, several studies have demonstrated that the expression of some miRNAs in body fluids may act as effective biomarkers for early diagnosis of cancer [7].
Nuclear factor-kappaB (NF-κB) is a transcriptional factor with pleiotropic activity due to the key roles it plays in various biological processes [8,9]. Aberrant activation of NF-κB has been discovered in the progression of several human cancers including PCa. Furthermore, NF-κB has been found to be involved in the metastasis and invasion of PCa through matrix metalloproteinase-9 (MMP-9) [10]. In the classical pathway, inhibitors of NF-κB (IκBs) kinase β (IKKβ) activate NF-κB through the phosphorylation of IκBs, which leads to the translocation of cytoplasmic NF-κB into the nucleus [8,9,11]. However, the molecular mechanisms through which miRNAs regulate the IKKβ pathway in PCa remain mostly unclear.
In this study, miR-497 was selected for the analysis because of its aberrant expression in PCa [12]. Similarly, it was found that the expression of miR-497 was deviant in 20 matched serum samples from patients with PCa and healthy control subjects. Then, it was showed that the aberrant expression of miR-497 changed cellular proliferation, migration, and invasion in PCa cell line PC3-AR cells by directly targeting IKKβ. The knockdown of IKKβ also suppressed cell proliferation, migration and invasion. Similar results were obtained when the NF-κB signaling pathway was inhibited with a novel IKKβ inhibitor, IMD-0354. The expression of cyclin-dependent kinase 8 (CDK8), MMP-9, and PSA were detected aberrant in all these processes. Altogether, the ability of miR-497 to regulate PC3-AR cells suggests that targeting this miRNA may have significant therapeutic potential.
Materials and methods
Specimens
In total, 20 serum samples from patients with PCa and 20 matched samples from healthy control subjects were obtained from Xinhua Hospital, Shanghai Jiaotong University after receiving informed consent and ethical approval. The blood samples were centrifuged at room temperature using the Capricorn CEP2000 for 20 min at 2200 rcf. Sera samples were stored at -80°C.
Cell culture and reagents
PC3-AR cells were maintained in high-glucose Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, GrandIsland, NY), containing 10% fetal bovine serum (FBS) (Gibco) and incubated in a humidified chamber with 5% CO2 at 37°C.
The miR-497 mimics (miR-497), the miR-497 inhibitor (anti-miR-497) sequences were synthesized by Integrated Biotech Solutions Company (Shanghai, China), siR-IKKβ was synthesized by Genechem Company (Shanghai, China), and miR-NC and siR-NC were used as negative controls. Transfection was performed with Lipofectamine 2000 (Invitrogen, Carlsbad, CA) following the manufacturer’s instructions. IMD-0354, a novel inhibitor of IKK-β, was molecularly designed, synthesized, and obtained from Tocris Bioscience, (R&D Systems, Bristol, UK). Before use, IMD-0354 was dissolved (10 mg/mL) in dimethylsulfoxide (DMSO; Sigma-Aldrich, Milan, Italy), aliquoted, and stored at -20°C. The culture medium added with the same amount of drug-free DMSO was used as negative control in all experiments.
Cell proliferation assays
PC3-AR cells were seeded onto a 96-well plate at 5000 cells per well and incubated for 72 h. Cell viability was determined at 24, 48 and 72 h after transfection or treatment with IMD-0354 using the Cell Counting Kit-8 (CCK-8) (Dojindo Laboratories, Kumamoto, Japan). The absorbance of each well was measured with a microplate reader set at 450 nM.
Cell cycle assay
For cell cycle analysis, 24 and 48 h after transfection or treatment with IMD-0354, PC3-AR cells were collected and fixed with 70% ethanol at -20°C for 18 h, propidium iodide (BD Biosciences, Franklin, US) was then added to the cells. Samples were analyzed by flow cytometry on FACScalibur (Becton Dickinson).
Migration and invasion assay
Transwell chambers coated with or without 40 μL of 1 mg/mL Matrigel (BD Biosciences) were used to examine in vitro migration and invasion capability of the PC3-AR cells. After transfection or treatment with IMD-0354, 5×104 cells were seeded onto the upper chamber containing 200 μL DMEM with 1% FBS, and the lower chamber was filled with 500 μL of DMEM with 20% FBS as a chemoattractant. Transwell chambers were then incubated at 37°C for 48 h. Cells adhering to the lower membrane of the inserts were counted as previously described [13]. Images of different fields were taken.
RNA extraction and qRT-PCR
Total RNA was extracted from sera with Trizol reagent according to the manufacturer’s instructions (Invitrogen). The level of miR-497 was quantified by quantitative reverse transcription (RT)-polymerase chain reaction (PCR) using TaqMan MicroRNA Assay kits (Applied Biosystems, Foster City, CA) with miR-1228 as an internal normalized reference as previously described [14]. Their relative levels were measured in triplicate on a Prism 7300 real-time PCR machine (Applied Biosystems). After transfection with miR-497, anti-miR-497, and NC, the expression levels of miR-497 mRNAs in PC3-AR cells were also quantified using qRT-PCR.
Western blot
At 48 h after transfection or treatment with IMD-0354, cells were lysed at 4°C for 30 min using radio immunoprecipitation assay buffer. Total protein was quantified using bicinchoninic acid assay and separated by electrophoresis in sodium dodecyl sulfate-polyacrylamide gels before transferring to nitrocellulose membranes (Bio-Rad, Hercules, US). They were then blocked in 5% skimmed milk in Tris buffered saline with Tween 20. Immune complexes were formed by incubation of membranes with anti-IKKβ (1:1000) (Cell Signaling, Boston, US), anti-CDK8 (1:1000) (Cell Signaling), anti-MMP-9 (1:1000) (Cell Signaling), anti-PSA (1:1000) (Cell Signaling), and anti-β-actin (1:1000) (Cell Signaling) antibodies overnight at 4°C. Blots were washed and incubated for 1 h with anti-rabbit secondary antibody. Immunoreactive protein bands were detected with an Odyssey Scanning system.
Luciferase reporter assay
The putative targets of miR-497 were predicted using the TargetScan, PicTar, and miRanda algorithms. Only common targets were considered for experimental analyses. The IKKβ 3’-untranslated mRNA region (3’-UTR) containing the predicted miR-497-binding sites were cloned into the pMIR-REPORT vector (Ambion, Austin, TX) using PCR-generated fragments (WT-UTR). A mutant luciferase vector with miR-497 one pairing site deleted (DEL-UTR) was also constructed. When PC3-AR cells reached 60-70% conf1uence in 24-well plate, 100 ng of Luciferase plasmid was cotransfected with 50 ng of Renilla plasmid (Ambion) and 650 ng of miR-497 mimics or NC using Lipofectamine 2000. After 48 h, luciferase activities were measured using a dual-luciferase reporter assay system (Promega, Madison, US) according to the manufacturer’s instructions.
Statistical analysis
All experiments were done in triplicate. Continuous variables were expressed as mean±standard deviation, *P<0.05, **P<0.01. The two-tailed t-test was used to draw a comparison between groups. All statistical analyses were performed using SPSS 19.0 (SPSS Inc., Chicago, IL).
Results
miRNA-497-5p is downregulated in PCa
In former studies, the expression of miR-497 was found to be downregulated in PCa samples compared with the benign prostrate hyperplasia samples [12]. In this study, 20 pairs of serum samples were collected from patients with PCa and healthy control subjects for further confirming the pattern of expression of miR-497 in PCa. qRT-PCR analysis showed that the expression level of miR-497 was downregulated in PCa serum samples, compared with samples from healthy control subjects (P<0.05, Figure 1A), suggesting that aberrant expression of miR-497 might be involved in the initiation and development of PCa.
Figure 1.
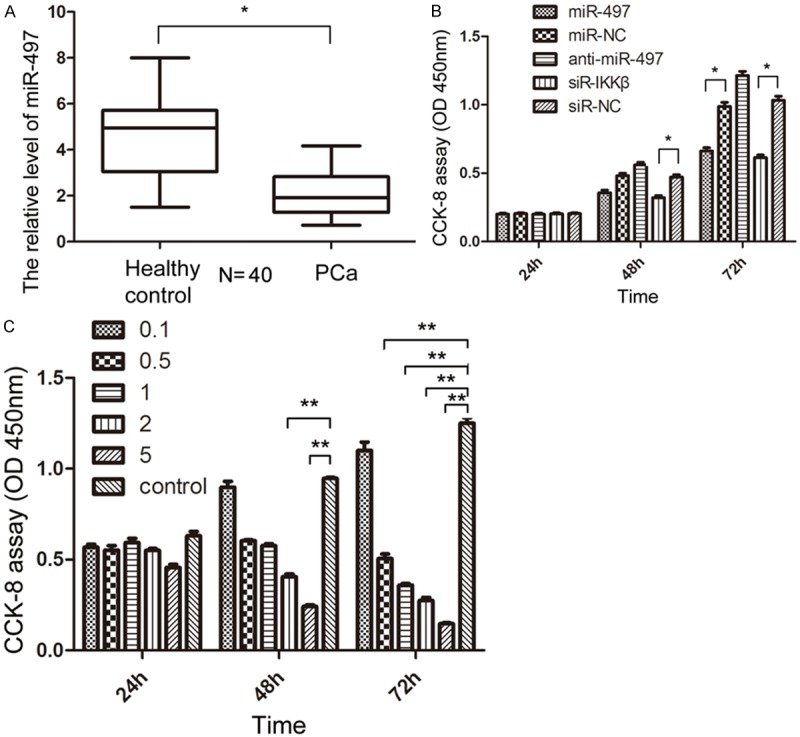
The expression of miR-497 in PCa and the CCK-8 assay in PC3-AR cells. A. The relative expression of miR-497 in 20 pairs of serum samples from patients with PCa and healthy control subjects were analyzed by qRT-PCR. B. CCK-8 assay of PC3-AR cells transfection with miR-497, miR-NC, anti-miR-497, siR-IKKβ, and siR-NC. C. CCK-8 assay of PC3-AR cells treatment with IMD-0354 in different concentrations (μM).
Overexpression of miRNA-497 in PC3-AR cells inhibits cell proliferation by inducing G0/G1 arrest
The downregulation of miR-497 in PCa tissue and blood prompted us to determine whether miR-497 functioned as a tumor suppressor. Therefore, whether overexpression of miR-497 could affect the ability of cell growth in PC3-AR cells was examined. The CCK-8 assay showed that miR-497 mimics significantly inhibited the proliferation of PC3-AR cells by 28.43±1.73% and 38.65±2.41% (P<0.05) at 48 and 72 h, respectively. Conversely, anti-miR-497 transfection in PC3-AR cells could promote cell proliferation (Figure 1B). As cell-cycle distribution is a parameter reflecting the growth of cells, the function of miR-497 on cell-cycle profile of PC3-AR cells at different time points was assessed. Cell cycle analysis found that overexpression of miR-497 resulted in S and G0/G1 phase cell cycle arrest in PC3-AR cells after 24 and 48 h of exposure, respectively (Figure 2). On the contrary, anti-miR-497 transfection suppressed the effect.
Figure 2.
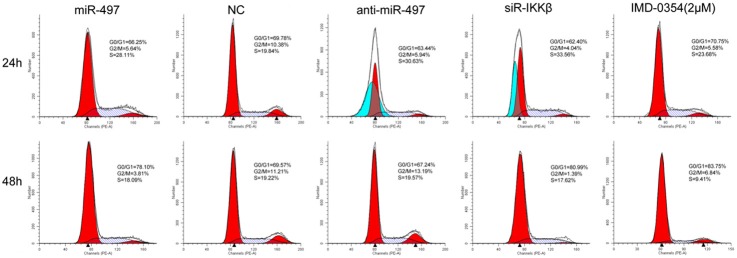
Cell cycle analysis showed that there was significant cell-cycle transition in PC3-AR cells after 24 and 48 h of treatment with miR-497, control, anti-miR-497, siR-IKKβ, and IMD-0354.
Since the regulatory role of miR-497 in the cell-cycle transition of PC3-AR cells was found, the possibility that cell cycle regulator might be modulated by miR-497 was further investigated. Western blot analysis revealed that overexpression of miR-497 significantly downregulated the protein levels of CDK8 (Figure 4A). Taken together, these results indicated that miR-497 could transcriptionally regulate the expression of CDK8, and therefore, suppress the proliferation of PC3-AR cells.
Figure 4.
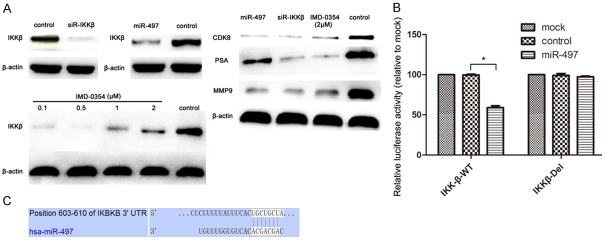
IKKβ is a target gene of miR-497 in PC3-AR cells. A. Western blot analysis was used to detect the expression of IKKβ, CDK8, PSA, and MMP9 in PC3-AR cells after treatment with siR-IKKβ, miR-497, IMD-0354, and control. β-actin served as an internal control. B. Relative luciferase activity of the IKKβ WT-UTR and the DEL-UTR luciferase constructs in PC3-AR cells transfected with miR-497 or control. C. IKKβ was predicted to be a candidate target of miR-497 using the bioinformatics algorithm TargetScan.
Effects of miR-497 on cell migration and invasion of PC3-AR cells
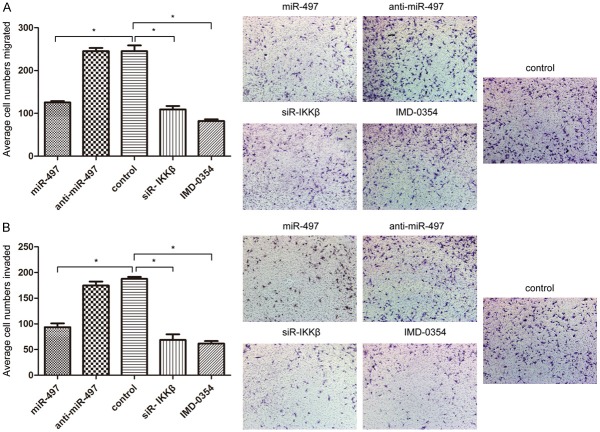
Transwell assay with or without Matrigel was used to detect the effects of miR-497 on the migratory and invasive potential of PC3-AR cells. As shown in Figure 2B, Transwell assays without Matrigel illustrated that the forced expression of miR-497 resulted in a 51.3±5.1% decrease in the migratory ability of PC3-AR cells when compared to the control cells (P<0.05, Figure 3A). Transwell assays with Matrigel showed that overexpression of miR-497 decreased the invasive ability of PC3-AR cells by 53.7±4.5%, compared with the control cells (P<0.05, Figure 3B). There was no statistically significant difference between anti-miR-497 transfection and control PC3-AR cells in both migration and invasion assays. All these results support the fact that overexpression of miR-497 inhibits the migration and invasion of PC3-AR cells.
Figure 3.
miR-497 suppresses the migration and invasion of PC3-AR cells. A. The migratory activity of PC3-AR cells was detected using a transwell migration assay treated with miR-497, anti-miR-497, control, siR-IKKβ, and IMD-0354. Representative images are shown on the right, the values shown are expressed as mean±SD. B. The invasive activity of PC3-AR cells was detected using a transwell invasion assay.
miR-497 directly targets IKKβ
To explore the mechanism of involvement of miR-497 in PCa, three bioinformatic algorithms (TargetScan, PicTar, and miRanda) were used to identify putative targets. Among many candidates, IKKβ was selected for further analysis, because IKKβ is known as the key kinase in the canonical NF-κB signaling pathways that control the expression of genes involved in cell proliferation, angiogenesis, cell survival, metastasis, invasion, and the epithelial-mesenchymal transition processes in the progression of cancer [15,16]. The binding sites of miR-497 were predicted at positions 603-610 in the 3’-UTR of IKKβ mRNA (Figure 4C). To validate IKKβ as a bona fide target of miR-497, luciferase reporter assays were performed to check binding of miR-497 to the 3’-UTR of IKKβ mRNA. Data showed that the luciferase activity was significantly inhibited by transfection with miR-497 and a vector carrying the wild-type 3’-UTR of IKKβ (P<0.05, Figure 4B), whereas transfection with deletion vectors blocked the decrease in luciferase activity. These data suggested that miR-497 bound directly to specific site in the 3’-UTR of IKKβ mRNA. Next, it was determined whether endogenous expression of IKKβ could be downregulated by miR-497. To address this issue, Western blot analysis was used to evaluate the expression of IKKβ at the protein level after transfection with miR-497 mimics and NC inPC3-AR cells. The results showed that the overexpression of miR-497 significantly decreased the expression levels of IKKβ protein (Figure 4A). Collectively, these luciferase and Western blot results showed that miR-497 acted as negative regulators of expression of IKKβ.
In addition, the protein levels of MMP-9 and PSA showed a decrease after the overexpression of miR-497 (Figure 4A). These results indicated that miR-497 may regulate the cell migration and invasion of PC3-AR cells by targeting NF-κB/IKKβ/MMP-9 signaling. Furthermore, miR-497 is likely to be a new diagnostic marker to replace or complement PSA in PCa.
Knockdown of IKKβ suppresses cell proliferation, migration, and invasion in vitro
To further verify whether the role of miR-497 in PCa was mediated by suppressing the expression of IKKβ, expression of IKKβ was knocked down via siRNA in PC3-AR cells. Western blot analysis revealed that the expression of IKKβ in PC3-AR cells was significantly decreased after transfection with siR-IKKβ, compared to that with NC (Figure 4A). Knockdown of IKKβ significantly suppressed the cell proliferation at 48 and 72 h in CCK-8 assays (P<0.05, Figure 1B), and significantly decreased the proportion of cells in G2/M phase and increased the proportion of cells in G1/G0 phase (Figure 2), similar to that observed in the overexpression of miR-497. In the transwell assays, as shown in Figure 3A and 3B, knockdown of IKKβ markedly inhibited the migration and invasion of PC3-AR cells, compared to NC (P<0.05). Furthermore, the protein levels of CDK8, MMP-9, and PSA showed varying degrees of reduction after transfected with siR-IKKβ in PC3-AR cells (Figure 4A). Therefore, the data indicated further that the tumor suppressive roles of miR-497 in PCa were partially mediated by targeting IKKβ/CDK8/MMP-9.
IMD-0354 suppresses cell proliferation, migration, and invasion in vitro
Given the considerable contribution of NF-κB to the development of PCa, inhibition of NF-κB may have a therapeutic potential for the control of progression of tumor. However, IMD-0354, a novel IKK-β inhibitor, its study in PCa was still vacant. Here, PC3-AR cells were incubated in the presence or absence of indicated doses of each IMD-0354. IMD-0354 suppressed the proliferation of PC3-AR cells in a dose-dependent manner after 48 h (Figure 1C). As shown in Figure 2, cell cycle was arrested at the G0/G1 phase after treatment with 2 μmol/L IMD-0354 in PC3-AR cells. In the transwell assays, as shown in Figure 3A and 3B, IMD-0354 significantly inhibited the cell migration and invasion of PC3-AR cells at 2 μmol/L concentration, compared to NC (P<0.05). Similar to our previous studies, the protein levels of CDK8, MMP-9, and PSA also showed a decrease at different levels after treatment with 2 μmol/L IMD-0354 in PC3-AR cells (Figure 4A). Therefore, all these results further validated our previous hypothesis.
Discussion
Circulating miRNAs profusely exist in various body fluids. Their high stability and approachability in the circulation make them promising biomarkers for clinical monitoring of presymptomatic diseases in at-risk patients, especially the early stage of cancer. Previous studies have shown that miR-497 was downregulated in PCa [12]; here it was found that the expression level of miR-497 was downregulated in PCa serum samples, compared with healthy controls. Hence, miR-497 was likely to be potential diagnostic and prognostic biomarkers for PCa.
To our knowledge, the miRNA family plays a key role in the regulation of gene expression networks, further involved in multiple signaling pathways [17]. miRNAs can exert positive or negative control over the expression of oncogenes or tumor-suppressor genes to effect tumor growth [18]. Until recently, only a limited number of studies have been performed to assess the effects of miRNA in PCa, and only a few of these studies successfully determined any miRNA targets that were specifically modulated in PCa. The present study showed that overexpression of miR-497 inhibited the cell proliferation by inducing G0/G1 cell cycle arrest in PC3-AR cells. Obviously, uncontrolled cell proliferation due to aberrant regulation of cell cycle can result in the development of cancer. For the first time, it was found that the protein level of CDK8, a gene encoding the CDK component of the mediator complex (known as a colon cancer oncogene), was downregulated with the transfection of miR-497. Furthermore, the migratory and invasive ability was significantly decreased after transfection with miR-497.
Metastasis is a complex process that involves many genes and signaling pathways. It is believed that advances about the relationship between miRNAs and target genes are necessary for a comprehensive understanding of the regulatory mechanism of miRNAs in cancer metastasis and invasion. A single miRNA can modulate a signaling network by targeting genes with multiple functions [19]. It has been identified that the concrete role of miRNA strictly relies on its spatiotemporal expression pattern and its targeted genes [20]. Prediction by bioinformatic analysis and the results of the present study determined that IKKβ was a bona fide target of miR-497. Furthermore, it was found that miR-497 was involved in modulating the NF-κB/MMP-9/PSA signaling pathway in PCa, because the expression of MMP-9 and PSA detected varying degrees of reduction after transfection with miR-497 in PC3-AR cells. To our knowledge, multiple genes regulated by NF-κB, including interleukin-6 (IL-6), IL-8, and MMP-9, were involved in tumorigenesis and had been recognized in the progression of PCa [21]. Among them, levels of IL-8 and MMP-9 had been reported to be associated with the increased metastatic potential [22,23].
Until now, there has been no report about whether IKKβ regulated by miRNA affects proliferation, migration, and invasion in PCa yet. It is for the first the new relationship of miRNA and IKKβ in PCa was uncovered. In our studies, the expression of IKKβ in PC3-AR cells was knocked down via siRNA. Similar to transfection with miR-497, the proliferation was suppressed by inducing G0/G1 cell cycle arrest, and there was also a decrease in the protein level of CDK8. Therefore, it could be concluded that miR-497 may repress the expression of IKKβ to downregulate the transcriptional activity of CDK8, leading to conversion of PC3-AR cell cycle. In addition, knockdown of IKKβ significantly inhibited the cell migration and invasion of PC3-AR cells. The expression of MMP-9 and PSA was also decreased in the process.
In lots of cancers, including prostate cancer, the activation of the IKK complex leads to the constitutive release of NF-κB, affecting expression of genes with important functions in tropism, cell cycle progression, and cell migration [24,25]. It was found that the expression of PSA significantly increased with overexpression of NF-κB in PCa LNCaP and DU145 cells, which was closely related to the progression of PCa [26]. Considering the considerable contribution of NF-κB to the development of PCa, inhibition of NF-κB may have a therapeutic prospect for the control of progression of PCa. As a novel IKK-β inhibitor, IMD-0354 had not been researched in PCa until now. Hence, PC3-AR cell, an invasive PCa cell line, was selected for this study here. As expected, IMD-0354 suppressed the proliferation of PC3-AR cells in a dose-dependent manner, along with G0/G1 cell cycle arrest and significantly inhibited the cell migration and invasion. Moreover, the expression of CDK8, MMP-9, and PSA decreased in different degrees after treatment with IMD-0354. The earlier studies showed that inhibition of NF-κB activity with IMD-0354 decreased the expression of CDK4/6, cyclinD1, and D3, leading to cell cycle arrest and apoptosis [27,28]. For the first time, CDK8 was found involved in the process of cell cycle transition through miR-372/p65/NF-κB pathway in PCa; the specific mechanisms needed further study.
Certainly, a clear phased advancement was witnessed during the recent two decades after the wide application of PSA testing. Nevertheless, the significance of PSA in the monitoring and prognosis of PCa is still being questioned, as population-based PSA screening can lead to over diagnosis and overtreatment of PCa as well as ineffectiveness to identify men with aggressive forms of PCa [29-31]. miRNAs may be potential biomarkers as their relatively small size protects them from RNase attack and these are secreted within protective exosomes; furthermore, the expression patterns of miRNA are more reliable and sensitive to alteration in cell biology [32]. In the present study, the exciting discovery that miR-497 independently repressed MMP-9 and PSA protein level by targeting IKKβ/NF-κB in PC3-AR cells was made. The consistent decrease in miR-497 in the human prostate tumor tissues and blood samples increases the feasibility of using them as a signature for the progression of PCa. Furthermore, considering the inhibitory effect of cell proliferation, migration, and invasion with miR-497, siR-IKKβ, and IMD-0354, it can be concluded that miR-497 participates in the development and progression of human PCa by targeting the IKKβ-mediated NF-κB/MMP-9/PSA signaling pathway. Taken together, all these results suggest that targeting the miR-497/IKKβ interaction or perturbing the expression of miR-497 may prove to be a new insight into prevention, diagnosis, and treatment of patients with PCa.
Acknowledgements
This study was supported by grants from the National Natural Science Funds (No. 81101931) and the Shanghai Science and Technology Funds (No. 134119a0600 and 14430720800).
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 3.Corey E, Brown LG, Kiefer JA, Quinn JE, Pitts TE, Blair JM, Vessella RL. Osteoprotegerin in prostate cancer bone metastasis. Cancer Res. 2005;65:1710–1718. doi: 10.1158/0008-5472.CAN-04-2033. [DOI] [PubMed] [Google Scholar]
- 4.Diamandis EP. Prostate cancer screening with prostate-specific antigen testing: more answers or more confusion? Clin Chem. 2010;56:345–351. doi: 10.1373/clinchem.2009.140046. [DOI] [PubMed] [Google Scholar]
- 5.Dykxhoorn DM. MicroRNAs and metastasis: little RNAs go a long way. Cancer Res. 2010;70:6401–6406. doi: 10.1158/0008-5472.CAN-10-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalmay T, Edwards DR. MicroRNAs and the hallmarks of cancer. Oncogene. 2006;25:6170–6175. doi: 10.1038/sj.onc.1209911. [DOI] [PubMed] [Google Scholar]
- 7.Cortez MA, Bueso-Ramos C, Ferdin J, Lopez-Berestein G, Sood AK, Calin GA. MicroRNAs in body fluids--the mix of hormones and biomarkers. Nat Rev Clin Oncol. 2011;8:467–477. doi: 10.1038/nrclinonc.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(Suppl):S81–96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 9.Chen LF, Greene WC. Shaping the nuclear action of NF-kappaB. Nat Rev Mol Cell Biol. 2004;5:392–401. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
- 10.Suh J, Rabson AB. NF-kappaB activation in human prostate cancer: important mediator or epiphenomenon? J Cell Biochem. 2004;91:100–117. doi: 10.1002/jcb.10729. [DOI] [PubMed] [Google Scholar]
- 11.Annunziata CM, Davis RE, Demchenko Y, Bellamy W, Gabrea A, Zhan F, Lenz G, Hanamura I, Wright G, Xiao W, Dave S, Hurt EM, Tan B, Zhao H, Stephens O, Santra M, Williams DR, Dang L, Barlogie B, Shaughnessy JD Jr, Kuehl WM, Staudt LM. Frequent engagement of the classical and alternative NF-kappaB pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell. 2007;12:115–130. doi: 10.1016/j.ccr.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porkka KP, Pfeiffer MJ, Waltering KK, Vessella RL, Tammela TL, Visakorpi T. MicroRNA expression profiling in prostate cancer. Cancer Res. 2007;67:6130–6135. doi: 10.1158/0008-5472.CAN-07-0533. [DOI] [PubMed] [Google Scholar]
- 13.Peter ME. Targeting of mRNAs by multiple miRNAs: the next step. Oncogene. 2010;29:2161–2164. doi: 10.1038/onc.2010.59. [DOI] [PubMed] [Google Scholar]
- 14.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 15.Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34:D140–144. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mestdagh P, Lefever S, Pattyn F, Ridzon D, Fredlund E, Fieuw A, Ongenaert M, Vermeulen J, De Paepe A, Wong L, Speleman F, Chen C, Vandesompele J. The microRNA body map: dissecting microRNA function through integrative genomics. Nucleic Acids Res. 2011;39:e136. doi: 10.1093/nar/gkr646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uzzo RG, Crispen PL, Golovine K, Makhov P, Horwitz EM, Kolenko VM. Diverse effects of zinc on NF-kappaB and AP-1 transcription factors: implications for prostate cancer progression. Carcinogenesis. 2006;27:1980–1990. doi: 10.1093/carcin/bgl034. [DOI] [PubMed] [Google Scholar]
- 18.Chung TD, Yu JJ, Spiotto MT, Bartkowski M, Simons JW. Characterization of the role of IL-6 in the progression of prostate cancer. Prostate. 1999;38:199–207. doi: 10.1002/(sici)1097-0045(19990215)38:3<199::aid-pros4>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 19.Aalinkeel R, Nair MP, Sufrin G, Mahajan SD, Chadha KC, Chawda RP, Schwartz SA. Gene expression of angiogenic factors correlates with metastatic potential of prostate cancer cells. Cancer Res. 2004;64:5311–5321. doi: 10.1158/0008-5472.CAN-2506-2. [DOI] [PubMed] [Google Scholar]
- 20.Yang J, Richmond A. Constitutive IkappaB kinase activity correlates with nuclear factor-kappaB activation in human melanoma cells. Cancer Res. 2001;61:4901–4909. [PubMed] [Google Scholar]
- 21.Gasparian AV, Yao YJ, Kowalczyk D, Lyakh LA, Karseladze A, Slaga TJ, Budunova IV. The role of IKK in constitutive activation of NF-kappaB transcription factor in prostate carcinoma cells. J Cell Sci. 2002;115:141–151. doi: 10.1242/jcs.115.1.141. [DOI] [PubMed] [Google Scholar]
- 22.Chen CD, Sawyers CL. NF-kappa B activates prostate-specific antigen expression and is upregulated in androgen-independent prostate cancer. Mol Cell Biol. 2002;22:2862–2870. doi: 10.1128/MCB.22.8.2862-2870.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ezhevsky SA, Nagahara H, Vocero-Akbani AM, Gius DR, Wei MC, Dowdy SF. Hypo-phosphorylation of the retinoblastoma protein (pRb) by cyclin D:Cdk4/6 complexes results in active pRb. Proc Natl Acad Sci U S A. 1997;94:10699–10704. doi: 10.1073/pnas.94.20.10699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu DX, Greene LA. Neuronal apoptosis at the G1/S cell cycle checkpoint. Cell Tissue Res. 2001;305:217–228. doi: 10.1007/s004410100396. [DOI] [PubMed] [Google Scholar]
- 25.Lilja H, Ulmert D, Vickers AJ. Prostate-specific antigen and prostate cancer: prediction, detection and monitoring. Nat Rev Cancer. 2008;8:268–278. doi: 10.1038/nrc2351. [DOI] [PubMed] [Google Scholar]
- 26.Schroder FH. Review of diagnostic markers for prostate cancer. Recent Results Cancer Res. 2009;181:173–182. doi: 10.1007/978-3-540-69297-3_16. [DOI] [PubMed] [Google Scholar]
- 27.Lin K, Lipsitz R, Miller T, Janakiraman S U.S. Preventive Services Task Force. Benefits and harms of prostate-specific antigen screening for prostate cancer: an evidence update for the U. S. Preventive Services Task Force. Ann Intern Med. 2008;149:192–199. doi: 10.7326/0003-4819-149-3-200808050-00009. [DOI] [PubMed] [Google Scholar]
- 28.Weber JA, Baxter DH, Zhang S, Huang DY, Huang KH, Lee MJ, Galas DJ, Wang K. The microRNA spectrum in 12 body fluids. Clin Chem. 2010;56:1733–1741. doi: 10.1373/clinchem.2010.147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, Pilotti S, Pierotti MA, Daidone MG. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–5511. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 30.Hu J, Wang Z, Liao BY, Yu L, Gao X, Lu S, Wang S, Dai Z, Zhang X, Chen Q, Qiu SJ, Wu Y, Zhu H, Fan J, Zhou J, Wang J. Human miR-1228 as a stable endogenous control for the quantification of circulating microRNAs in cancer patients. Int J Cancer. 2014;135:1187–1194. doi: 10.1002/ijc.28757. [DOI] [PubMed] [Google Scholar]
- 31.Pasparakis M, Luedde T, Schmidt-Supprian M. Dissection of the NF-kappaB signalling cascade in transgenic and knockout mice. Cell Death Differ. 2006;13:861–872. doi: 10.1038/sj.cdd.4401870. [DOI] [PubMed] [Google Scholar]
- 32.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]