Abstract
Incidence and mortality of intrahepatic cholangiocarcinoma (ICC) are increasing. However, its prognostic predictive system associated with outcome after surgery remains poorly defined. In this study, we conducted retrospective survival analyses in a primary cohort of 370 patients who underwent partial hepatectomy for ICC (2005 and 2009). We found that seven variables were significantly independent predictors for overall survival (OS): serum prealbumin (hazard ratio [HR]: 1.447; p = 0.015), carbohydrate antigen 19-9 (HR: 1.438; p = 0.009), carcinoembryonic antigen (HR: 1.732; p = 0.002), tumor number (HR: 1.781; p < 0.001), vascular invasion (HR: 1.784; p < 0.001), regional lymphatic metastasis (HR: 2.003; p < 0.001) and local extrahepatic metastasis (HR: 1.506; p = 0.008). Using these independent predictors, we created a simple clinicopathologic prognostic staging system for predicting survival of ICC patients after resection. The validity of the prognostic staging system was prospectively assessed in 115 patients who underwent partial hepatectomy between January 2010 and December 2010 at the same institution. The prognostic power was quantified using likelihood ratio test and Akaike information criteria. Compared with the 6th and 7th AJCC staging systems, the new staging system in the primary cohort had a higher predictive accuracy for OS in terms of homogeneity and discriminatory ability. In the validation cohort, the homogeneity and discrimination of the new staging system were also superior to the two other staging systems. Conclusions: The new staging system based on clinicopathologic features may provide relatively higher accuracy in prognostic prediction for ICC patients after tumor resection.
Keywords: Intrahepatic cholangiocarcinoma, clinicopathologic features, prognosis, staging system, partial
Introduction
Intrahepatic cholangiocarcinoma (ICC), a bile duct carcinoma arising from either the second-order or more peripheral branches of the intrahepatic bile duct, occurs more rarely than does hepatocellular carcinoma (HCC) [1,2]. Although the incidence of ICC is very low, it is the second most common primary liver cancer after HCC, and its incidence and mortality have been increasing over the past several decades worldwide [3-8]. Partial hepatectomy is considered the standard curative treatment option for ICC [2,9,10]. However, despite advances in surgical modalities, prognosis after tumor resection remains very poor, primarily due to the persistent high incidence of recurrence and/or distant metastases [11-13]. HCC and ICC have distinct mechanisms of carcinogenesis and biologic behaviors [12,14], and the oncologic nature of the two primary cancers vary. There are also important epidemiologic, etiologic, biologic, and therapeutic differences between ICC and hilar or distal bile duct cholangiocarcinoma [15]. As such, there has been an increasing realization of the importance of establishing a distinct staging system for ICC.
Unfortunately, because the incidence of ICC is very low, and due to a lack of symptoms until late in the course of disease [16], with the majority of ICC patients having unresectable tumors at diagnosis, little data exist on the clinicopathologic factors associated with outcomes following surgical resection of ICC. Currently, only few studies have proposed distinct staging systems for patients with ICC. Two studies were based exclusively on limited numbers of patients from Japan [17,18]. In the AJCC/UICC liver cancer staging systems, ICC is staged using a TNM classification. The widely used 6th edition of TNM classification, which is based on data exclusively derived from HCC patients, is not sufficiently accurate for ICC [19]. Recently, a large population-based Western cohort of patients with ICC was reported. In the study, Nathan, et al. found that tumor size had no effect on survival, either in overall or in multiple subgroup analyses, and therefore proposed a simplified staging system based on the number of ICC tumors, vascular invasion, and the presence of metastatic disease in the regional lymph node basin or at distant sites [20]. The recent publication of the 7th edition AJCC/UICCA staging system which is a distinct staging system for ICC adopts the proposals of Nathan and colleagues [20]. ICC is characterized by a variety of risk factors. Previous studies have suggested various etiologies involving distinct molecular pathways in ICC development [21], and the potential involvement of different prognoses after partial hepatectomy [22-25]. There is a very significant difference in the distribution of risk factors for ICC development between China and Japan, or compared to the majority of Western countries. China is an HBV-endemic area and HBV is the predominant cause of ICC. Given this, the aforementioned staging systems based exclusively on data from Japan, or essentially based on Western data, may not provide a precise prognostic prediction for ICC patients from Chinese populations or HBV-endemic area. Fortunately, recently, two prognostic systems for ICC based on data from China, and one from Eastern and Western countries, were developed. The first staging system was a prognostic scoring system based on clinical features of ICC patients from the Zhongshan Hospital (Shanghai, China), which may provide a relatively accurate prognostic prediction for ICC patients from a Chi-nese population [26]. However, the scoring system was only based on clinical features and did not include pathologic information. Moreover, it excluded ICC patients with alpha-fetoprotein (AFP) above 20 μg/L (above one fifth of ICC patients from Chinese populations) [21,24,27]. The second was a prognostic nomogram based on clinicopathologic features of ICC from the Eastern Hepatobiliary Surgery Hospital (Shanghai, China), which showed relatively more accurate prognostic prediction for patients with ICC [28]. The last system was also a prognostic nomogram based on the clinicopathologic features of ICC, but the data were from 13 major hepatobiliary centers in the United States, Europe, and Asia [29]. However, the two prognostic nomograms are complex and can be methodologically challenging to create, so their utilization in clinical practice may be significantly limited. Therefore, a simple but comprehensive staging system for ICC has yet to be developed using Chinese or HBV-endemic area data.
The goal of the present study was to identify clinicopathologic determinants of survival following resection of ICC, and in turn develop a new staging system with specific relevance to ICC. We also evaluated the prognostic validity of the AJCC 6th edition TNM classification and the newly released the 7th edition TNM classification and compared them with that of the new staging system.
Materials and methods
Patient cohort
We identified all hospitalized patients who were admitted with a primary diagnosis of ICC and received a partial hepatectomy (Admission Code: M81600/3) at the Eastern Hepatobiliary Surgery Hospital of the Second Military Medical University (Shanghai, China) between January 2005 and December 2009.
Only patients who completed resection of liver tumors and were histopathologically proven ICC were included in our study cohort. We applied the following exclusion criteria: history of other malignancies, history of previous anticancer therapy, extrahepatic or hilar cholangiocarcinoma, tumors of uncertain origin or probable metastatic liver tumor, mixed type of primary liver cancer as confirmed histopathologically. Pa-tients with incomplete clinicopathologic data or who died perioperatively (within 30 days of surgery) or who were lost to follow-up were also excluded. Death before the recurrence of ICC was defined as competing mortality, and such patients were also excluded.
From January 2010 to December 2010, an independent cohort of consecutive patients who underwent a partial hepatectomy for ICC in the same institution was prospectively studied, using the same inclusion and exclusion criteria. These patients formed the validation cohort of this study.
The project was approved by the Eastern Hepatobiliary Surgery Hospital Ethical Commi-ttee, China. The data do not contain any information that could identify the patients.
Clinicopathologic investigation
The following demographics and clinicopathologic information were retrospectively obtained from patients’ medical records: age, gender, alanine transaminase (ALT), aspartate transaminase (AST), albumin (ALB), prealbumin (PA), total bilirubin (TBIL), r-glutamyltransferase (r-GT), alkaline phosphatase (ALP), AFP, carbohydrate antigen 19-9 (CA19-9), carcinoembryonic antigen (CEA), HBV infection (represented by positive HBsAg (hepatitis B surface antigen) in serum or liver tissue, or detectable HBV DNA in serum), tumor location, tumor size (main tumor or the largest one), tumor capsule formation, tumor histologic type, tumor differentiation (according to the WHO classification system of tumor: well, moderately or poorly differentiated; when histological diversity was observed in a tumor, the higher grade was taken as the overall grade), major portal vein invasion, microvascular invasion, vascular invasion (included major portal vein invasion, hepatovein invasion, and microvascular invasion), lymphatic metastasis (included regional lympha node metastasis and distant lympha node metastasis) (1), tumor number (satellite nodules, multifocal primary cholangiocarcinomas, and intrahepatic metastases are not distinguished and are considered multiple tumors), extrahepatic metastasis (tumor directly metastasized extrahepatic tissues or organs), operative procedures, postoperative complication, and mode of recurrence.
TNM stage
Tumors were staged using the 6th and 7th edition of the AJCC/UICC staging manual [1,19].
Follow-up
All patients were regularly followed up for CEA, CA 19-9 and AFP measurements, ultrasonography (USG), and/or a computed tomography (CT) or magnetic resonance imaging (MRI) scan every one to two months for the first six months after operation, and every three months after that. Tumor recurrence was suspected when there was a progressive elevation of serum CEA, CA19-9 or AFP and/or ultrasonographic evidence of a new hepatic lesion that was confirmed by dynamic CT scan, MRI or position emission tomography (PET). Disease-free survival was measured from the date of surgery to the date of recurrence. Overall survival (OS) was defined as the period from the date of hepatectomy to the date of death. Follow-up of patients was continued until death, or Dec 5, 2013, whichever occurred first.
Statistical analysis
Statistical analyses were performed using SPSS, version 16.0 for Windows (SPSS Inc., Chicago, IL, USA). OS was calculated using the Kaplan-Meier method. Clinicopathological pro-gnostic factors were evaluated using the univariate Kaplan-Meier method and compared with the log-rank test to identify the prognostic predictors for survival. Multivariate regression analysis was performed using Cox proportional hazards models to identify the independent prognostic factors for survival. Variables to be entered into the multivariate analysis were selected on the basis of the results of univariate analyses (p < 0.1). The performance of staging systems was assessed according to homogeneity (smaller differences in survival among patients in the same stage within each system) and discriminatory ability (greater differences in survival among patients in the different stages within each system) [30]. To evaluate homogeneity within each staging system, the likelihood ratio test was performed-that is, whether or not the difference in survival time is small among patients classified into the same group by that system. The likelihood ratio test can also estimate the monotonicity of gradient; the mean survival time for a group classified as favorable by that system is always longer than the survival times noted in less favorable groups. The Akaike information criteria were also used to measure the discriminatory ability of each staging system. Generally, more accurate stages showed lower Akaike information criteria and higher likelihood ratio χ2 values. Comparisons bet-ween groups were conducted using the chi-squared test for categorical variables and the t-test for discrete variables. A value of p < 0.05 was considered statistically significant.
Results
Demographics and clinicopathologic characteristics
Using our inclusion criteria and exclusion criteria, in the primary cohort, a total of 370 patients with surgically treated, pathologically confirmed ICCs were enrolled in the study. There were 236 men (63.8%) and 134 women (36.2%) enrolled, with a male-to-female ratio of 1.8:1. The median age was 55.0 (range, 27-85) years old. Of the 370 patients, 187 (50.5%) had HBV infection (26 cases were positive for HBsAg only in liver tissue; 54 cases were only seropositive for HBsAg; 106 cases were positive for HBsAg in both serum and liver tissue; one case was negative for HBsAg but had detectable HBV-DNA in serum), 115 (31.1%) had cirrhosis (96 with cirrhosis related to HBV, 3 with cirrhosis related to HCV, 1 with alcoholic liver cirrhosis, 1 with non-alcoholic fatty liver cirrhosis, 6 with liver schistosomiasis-associated cirrhosis and 8 with occult cirrhosis), and 36 (9.7%) had hepatolithiasis. For the validation cohort, we prospectively analyzed 115 consecutive patients (75 men and 40 women with a male-to-female ratio of 1.9:1). The demographics and clinicopathologic characteristics of patients in the primary and validation cohorts are listed in Table 1. No significant differences in demographics or clinicopathologic characteristics were found between the primary and the validation cohorts.
Table 1.
Demographics and clinicopathologic characteristics of patients with ICC
| Primary Cohort (n=370) | Validation cohort (n=115) | P value | |
|---|---|---|---|
|
|
|||
| No. of patients (%) | No. of patients (%) | ||
| Gender | 0.780 | ||
| male | 236 (63.8) | 75 (65.2) | |
| female | 134 (36.2) | 40 (34.8) | |
| Age (≥ 65 years) | 81 (21.9) | 19 (16.5) | 0.214 |
| HBV infection | 187 (50.5) | 51 (44.3) | 0.246 |
| Seropositive anti-HCV | 10 (2.7) | 2 (1.7) | 0.740 |
| Hepatolithiasis | 36 (9.7) | 11 (9.6) | 0.958 |
| Liver schistosomiasis | 22 (5.9) | 7 (6.1) | 0.956 |
| ALT (> 41 U/L) | 103 (27.8) | 29 (25.2) | 0.581 |
| AST (> 37 U/L) | 110 (29.7) | 30 (26.1) | 0.451 |
| PA (< 170 mg/L) | 71 (19.2) | 29 (25.2) | 0.163 |
| TBIL (> 20 µmol/L) | 59 (15.9) | 15 (13.0) | 0.450 |
| r-GT (> 61 U/L) | 214 (57.8) | 60 (52.2) | 0.285 |
| ALP (> 129 U/L) | 129 (34.9) | 34 (29.6) | 0.293 |
| AFP (> 20 µg/L) | 74 (20.0) | 16 (13.9) | 0.142 |
| CA19-9 (> 39 U/mL) | 200 (54.1) | 58 (50.4) | 0.497 |
| CEA (> 10 µg/L) | 55 (14.9) | 19 (16.5) | 0.666 |
| Tumor size (cm) | 0.353 | ||
| < 5 | 130 (35.1) | 35 (30.4) | |
| ≥ 5 | 240 (64.9) | 80 (69.6) | |
| Tumor number | 0.944 | ||
| single | 233 (63.0) | 72 (62.6) | |
| multiple | 137 (37.0) | 43 (37.4) | |
| Cirrhosis | 115 (31.1) | 29 (25.2) | 0.229 |
| Capsule formation | 32 (8.6) | 6 (5.2) | 0.232 |
| Tumor differentiation | 0.825 | ||
| well to moderately | 334 (90.3) | 103 (89.6) | |
| poorly | 36 (9.7) | 12 (10.4) | |
| Vascular invasion | 112 (30.3) | 29 (25.2) | 0.297 |
| Regional lymphatic metastasis | 70 (18.9) | 27 (23.5) | 0.286 |
| Local extrahepatic metastasis | 67 (18.1) | 16 (13.9) | 0.297 |
| 6th TNM | 0.327 | ||
| Stage I | 148 (40.0) | 40 (34.8) | |
| Stage II | 31 (8.4) | 16 (13.9) | |
| Stage III | 158 (42.7) | 48 (41.7) | |
| Stage IV | 33 (8.9) | 11 (9.6) | |
| 7th TNM | 0.232 | ||
| Stage I | 146 (39.5) | 39 (33.9) | |
| Stage II | 105 (28.4) | 40 (34.8) | |
| Stage III | 23 (6.2) | 3 (2.6) | |
| Stage IV | 96 (25.9) | 33 (28.7) | |
ICC: intrahepatic cholangiocarcinoma; HBV: hepatitis B virus; HCV: hepatitis C virus; M: male; F: female; Prealbumin: PA; TBIL: total bilirubin; alanine transaminase (ALT); aspartate transaminase (AST); ALP: alkaline phosphatase; r-GT: r-glutamyltransferase; AFP: Alpha-fetoprotein; CA19-9: carbohydrate antigen 19-9, CEA: carcinoembryonic antigen.
Recurrence and overall survival (OS) in the primary cohort
The median follow-up time was 26.9 months (range 3.8-106.0 months) for all patients, 20.4 months (range 3.8-94.7 months) for the 285 patients who died, and 58.3 months (range 48.3-106.0 months) for the 85 patients who remained alive. The median recurrence-free survival (RFS) time was 5.6 months (range 1 to 106.0 months), and the 1-, 3-, and 5-year RFS rates after tumor resection were 33.1%, 18.2% and 16.9%, respectively. The median survival time was 14.8 months, with 106 patients surviving more than 3 years. The cumulative 1-, 3-, and 5-year survival rates were 55.7%, 28.6% and 20.7%, respectively (Figure 1).
Figure 1.
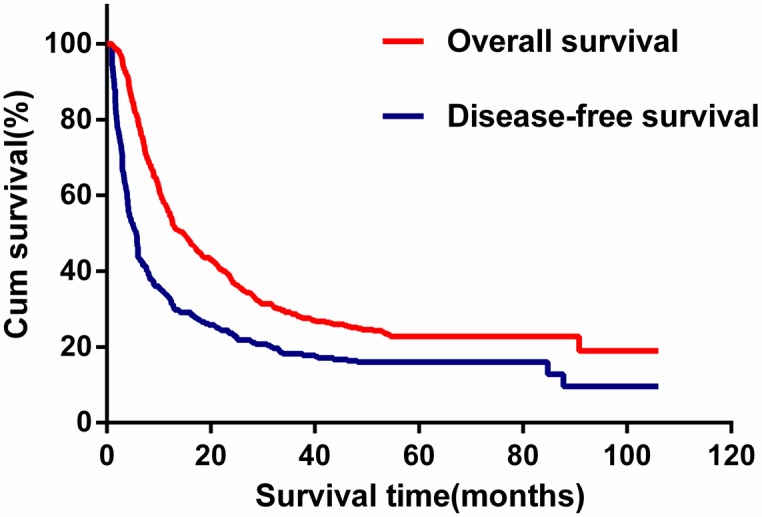
Kaplan-Meier estimated overall survival and disease-free survival curves of patients with intrahepatic cholangiocarcinoma in the primary cohort.
Univariate and multivariate predictors for OS
The univariate analysis demonstrated that significant prognostic factors for relatively poorer survival included the absence of HBV infection (p = 0.003), hepatolithiasis (p < 0.001), AST > 37 U/L (p = 0.017), PA < 170 mg/L (p < 0.001), r-GT > 61 U/L (p < 0.001), ALP > 129 U/L (p < 0.001), CA19-9 > 39 U/mL (p < 0.001), CEA >10 µg/L (p < 0.001), multiple tumors (p < 0.001), tumor size ≥ 5 cm (p < 0.001), absence of capsule formation (p = 0.012), vascular invasion (p < 0.001), regional lymphatic metastasis (p < 0.001), and local extrahepatic metastasis (p < 0.001). Further multivariate analyses showed that seven variables were significantly independent predictors for OS: serum PA (hazard ratio [HR]: 1.447), CA19-9 (HR: 1.438), CEA (HR: 1.732), tumor number (HR: 1.781), vascular invasion (HR: 1.784), regional lymphatic metastasis (HR: 2.003) and local extrahepatic metastasis (HR: 1.506) (Table 2). Sex, age, anti-HCV, cirrhosis, liver schistosomiasis, ALT, TBIL, AFP, and tumor differentiation did not significantly correlate with OS following hepatic resection.
Table 2.
Univariate and multivariate analyses of prognostic factors on overall Survival
| Factor | N | Survival rate (%) | Univariate analysis (P value) | multivariate analysis (P value) | Hazard ratio | 95% CI | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| 1-year | 3-year | ||||||
| HBV infection | 0.003 | 0.463 | 0.908 | 0.702-1.175 | |||
| Yes | 187 | 59.9 | 35.8 | ||||
| No | 183 | 51.4 | 21.3 | ||||
| Hepatolithiasis | < 0.001 | 0.173 | 1.328 | 0.883-1.998 | |||
| Yes | 36 | 38.9 | 8.3 | ||||
| No | 334 | 57.5 | 30.8 | ||||
| AST | 0.017 | 0.588 | 1.088 | 0.802-1.476 | |||
| ≤ 37 U/L | 260 | 58.5 | 31.9 | ||||
| > 37 U/L | 110 | 49.1 | 20.9 | ||||
| PA | < 0.001 | 0.015 | 1.447 | 1.076-1.947 | |||
| < 170 mg/L | 71 | 40.8 | 11.3 | ||||
| ≥ 170 mg/L | 299 | 59.2 | 32.8 | ||||
| r-GT | < 0.001 | 0.557 | 1.094 | 0.810-1.477 | |||
| ≤ 61 U/L | 156 | 67.3 | 41.0 | ||||
| > 61 U/L | 214 | 47.2 | 19.6 | ||||
| ALP | < 0.001 | 0.349 | 1.149 | 0.859-1.538 | |||
| ≤ 129 U/L | 241 | 64.7 | 36.1 | ||||
| > 129 U/L | 129 | 38.8 | 14.7 | ||||
| CA19-9 | < 0.001 | 0.009 | 1.438 | 1.096-1.886 | |||
| ≤ 39 U/mL | 170 | 71.2 | 42.4 | ||||
| > 39 U/mL | 200 | 42.5 | 17.0 | ||||
| CEA | < 0.001 | 0.002 | 1.732 | 1.224-2.451 | |||
| ≤ 10 µg/L | 315 | 60.3 | 33.0 | ||||
| > 10 µg/L | 55 | 29.1 | 3.6 | ||||
| Tumor number | < 0.001 | < 0.001 | 1.781 | 1.381-2.298 | |||
| single | 233 | 66.5 | 36.9 | ||||
| multiple | 137 | 37.2 | 14.6 | ||||
| Tumor size | < 0.001 | 0.557 | 1.091 | 0.817-1.456 | |||
| < 5 cm | 130 | 70.0 | 42.3 | ||||
| ≥ 5 cm | 240 | 47.9 | 21.3 | ||||
| Capsule formation | 0.012 | 0.210 | 0.732 | 0.450-1.192 | |||
| Yes | 32 | 75.0 | 46.9 | ||||
| No | 338 | 53.8 | 26.9 | ||||
| Vascular invasion | < 0.001 | < 0.001 | 1.784 | 1.370-2.323 | |||
| Yes | 112 | 41.1 | 13.4 | ||||
| No | 258 | 62.0 | 35.3 | ||||
| Regional lymphatic metastasis | < 0.001 | < 0.001 | 2.003 | 1.490-2.693 | |||
| Yes | 70 | 27.1 | 5.7 | ||||
| No | 300 | 62.3 | 34.0 | ||||
| Local extrahepatic metastasis | < 0.001 | 0.008 | 1.506 | 1.112-2.039 | |||
| Yes | 67 | 28.4 | 6.0 | ||||
| No | 303 | 61.7 | 33.7 | ||||
ICC: intrahepatic cholangiocarcinoma; HBV: hepatitis B virus; alanine transaminase (ALT); aspartate transaminase (AST); PA: pre-albumin; ALP: alkaline phosphatase; r-GT: r-glutamyltransferase; CA 19-9: carbohydrate antigen 19-9; CEA: carcinoembryonic antigen.
New staging system for OS
A new staging system was devised by assigning a linear score (0/1) to the seven independent predictors for OS: stage I disease was defined as meeting 0 of total risk score, stage II disease was defined as meeting 1 of total risk score, stage III disease was defined as meeting 2 or 3 of total risk score, and stage IV disease was defined as meeting 4 or more of total risk score (Table 3). The survival curve for the 370 patients in the primary cohort was calculated by the Kaplan-Meier method (Figure 2A), according to the new staging system. For the four stages of patients (stage I to stage IV), the 1-year survival rates were 97.1%, 69.9%, 42.1%, and 19.3% for stage I, II, III, and IV patients, respectively; the 3-year survival rates were 70.6%, 37.6%, 14.5%, and 1.8%, respec-tively.
Table 3.
The new staging system of intrahepatic cholangiocarcinoma
| Independent predictor | Risk score | ||
|
| |||
| Serum PA level (mg/L) | |||
| < 170 | 1 | ||
| ≥ 170 | 0 | ||
| Serum CA19-9 level (U/ml) | |||
| > 39 | 1 | ||
| ≤ 39 | 0 | ||
| Serum CEA level (µg/L) | |||
| > 10 | 1 | ||
| ≤ 10 | 0 | ||
| Tumor number | |||
| Solitary | 0 | ||
| Multiple | 1 | ||
| Vascular invasion | |||
| Yes | 1 | ||
| No | 0 | ||
| Regional lymphatic metastasis | |||
| Yes | 1 | ||
| No | 0 | ||
| Local extrahepatic metastasis | |||
| Yes | 1 | ||
| No | 0 | ||
|
| |||
| New stage groupings | Total risk score | HR (95% CI) | P |
|
| |||
| Stage I | 0 | 1 | < 0.001 |
| Stage II | 1 | 2.306 (1.495-3.558) | < 0.001 |
| Stage III | 2 or 3 | 4.736 (3.166-7.085) | < 0.001 |
| Stage IV | ≥ 4 | 10.392 (6.561-16.460) | < 0.001 |
PA: pre-albumin; CA 19-9: carbohydrate antigen 19-9; CEA: carcinoembryonic antigen; HR: hazard ratio; 95% CI: confidence interval.
Figure 2.
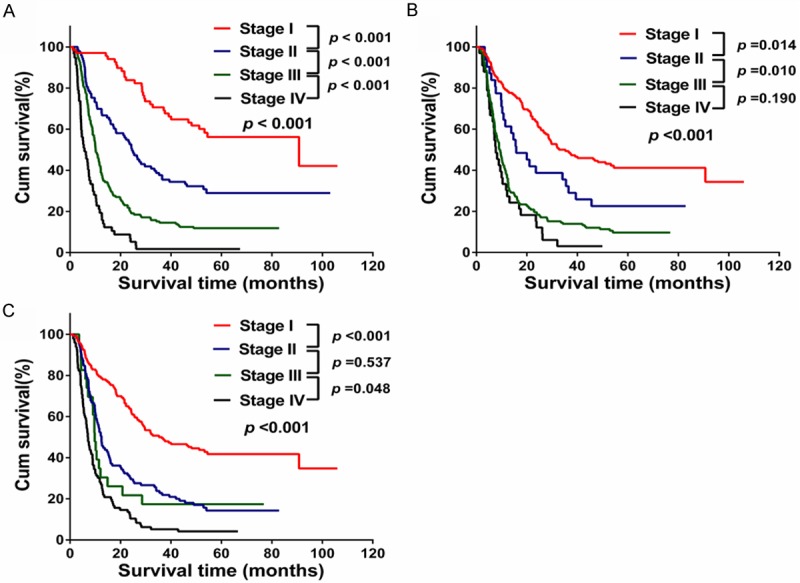
Overall survival curves according to the three staging systems in the primary cohort. A. New staging system; B. The sixth AJCC staging system; C. The seventh AJCC staging system.
Prognostic performance of each staging system in the primary cohort
Patient distribution and OS according to each staging system is shown in Table 4. According to the 7th edition of the AJCC staging system, most of patients (67.9%) were either stage I or stage II. However, according to the 6th edition and new staging system, most patients (6th edition: 51.6%; new staging system: 56.5%) were in stage III and stage IV. The 1- or 3-year survival rate of patients in stage I or stage II of the new staging system was higher than that found by the AJCC 6th edition or 7th edition. In contrast, 1- or 3-year survival rates of patients in stage IV of the new staging system were lower than that of the AJCC 6th edition or 7th edition.
Table 4.
Distribution of patients and survival according to each staging system in the primary cohort.
| Staging system | N (%) | Median survival time (months) | Deaths (%) | Survival rate (%) | |
|---|---|---|---|---|---|
|
| |||||
| 1-year | 3-year | ||||
| 6th pAJCC system | |||||
| I | 148 (40.0) | 34.0 | 87 (58.8) | 79.1 | 49.3 |
| II | 31 (8.4) | 15.8 | 24 (77.4) | 61.3 | 32.3 |
| III | 158 (42.7) | 9.3 | 142 (89.9) | 38.0 | 13.9 |
| IV | 33 (8.9) | 7.7 | 32 (97.0) | 30.3 | 3.0 |
| 7th pAJCC system | |||||
| I | 146 (39.5) | 35.9 | 85 (58.2) | 79.5 | 50.0 |
| II | 105 (28.4) | 12.5 | 89 (84.8) | 52.4 | 22.9 |
| III | 23 (6.2) | 9.8 | 19 (82.6) | 34.8 | 17.4 |
| IV | 96 (25.9) | 7.1 | 92 (95.8) | 28.1 | 5.2 |
| New staging system | |||||
| I | 68 (18.4) | 53.0 | 30 (44.1) | 97.1 | 70.6 |
| II | 93 (25.1) | 24.6 | 65 (69.9) | 69.9 | 37.6 |
| III | 152 (41.1) | 10.2 | 134 (88.2) | 42.1 | 14.5 |
| IV | 57 (15.4) | 5.7 | 56 (98.2) | 19.3 | 1.8 |
AJCC: American Joint Commission for Cancer Staging.
Figure 2 depicts the Kaplan-Meier estimated OS curves according to the three different staging systems. All three staging systems in our study showed a significant progressive decre-ase in OS from the earliest to the most ad-vanced stages (log-rank test, all p < 0.001) (Figure 2). In Figure 2, although the AJCC sixth edition (Figure 2B) or seventh edition (Figure 2C) showed good prognostic stratification for patients between stage I and stage II or later in the primary cohort, the AJCC sixth edition was unsatisfactory in stratifying patients between stage III and stage IV (p = 0.190), and the AJCC seventh was unsatisfactory in stratifying patients between stage II and III (p = 0.537). Our new staging system displayed better accuracy between each stage grouping in the primary cohort (Figure 2A) (p < 0.001). The results indicate that the new staging system was a useful predictor for survival of ICC patients in the primary cohort.
Finally, the predictive accuracy of the three various staging systems were evaluated. Compared with the 6th AJCC staging system, the 7th AJCC staging system had a higher discriminatory ability for different stages and a better homogeneity in the same stages, as shown in Table 5 (likelihood ratio χ2 values, 104.395 versus 97.045; Akaike information criteria values, 2949.771 versus 2959.120). However, the new staging system had the highest prognostic power among the three staging systems (likelihood ratio χ2 value, 148.857; Akaike information criteria value, 2909.309).
Table 5.
Prognostic performance of each staging system
| Staging system | Likelihood Ratio (χ2) | Akaike Information Criteria |
|---|---|---|
| In the primary | ||
| 6th AJCC system | 97.045 | 2959.120 |
| 7th AJCC system | 104.395 | 2949.771 |
| New stage system | 148.857 | 2909.309 |
| In the validation | ||
| 6th AJCC system | 34.646 | 716.091 |
| 7th AJCC system | 34.130 | 714.607 |
| New stage system | 40.928 | 711.809 |
Note: Regarding discriminatory ability, homogeneity, and monotonicity of gradients, the model with the higher χ2 by the likelihood ratio test was considered the better model. Furthermore, the lower value of Akaike information criteria is considered the better model for discriminatory ability. American Joint Commission for Cancer Staging: AJCC.
Validation of the predictive accuracy of the new staging system
In the validation cohort, the median follow-up time was 15.0 months (range 3.8-47.4 months) for all patients, 11.9 months (range 3.8-38.9 months) for the 88 patients who died, and 39.2 months (range 36.0-47.4 months) for the 27 patients who remained alive for the duration of the follow-up. Median disease-free survival time was 4.8 months (range, 1 to 47.4 months), and the 1- and 3-year RFS rates after operation were 25.6% and 17.8%, respectively. The median survival time was 12.6 months; 28 patients survived more than 3 years. The cumulative 1- and 3-year survival rates were 52.2% and 24.3%, respectively. As shown in Figure 3, although the AJCC 6th edition (Figure 3B) and 7th edition (Figure 3C) also showed good prognostic stratification for patients between stages I and II, or even later in the validation cohort, the 6th AJCC edition was unsatisfactory in stratifying patients between stage II and III (p = 0.449), between stages III and IV (p = 0.995), and the AJCC 7th edition was unsatisfactory in stratifying patients between stage II and III (p = 0.129), between stage III and stage IV (p = 0.658). Our new staging system displayed better accuracy between each stage grouping in the validation cohort (Figure 3A) (p < 0.05).
Figure 3.
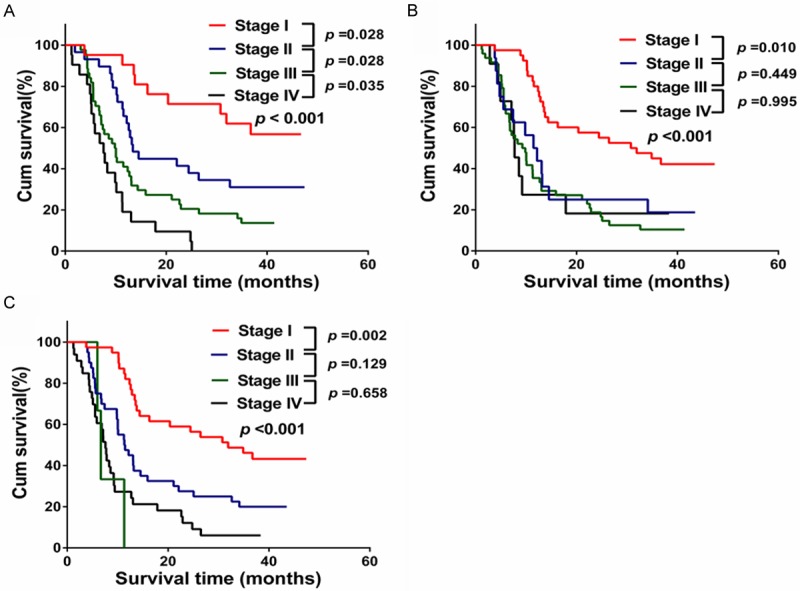
Overall survival curves according to the three staging systems in the validation cohort. A. New staging system; B. The sixth AJCC staging system; C. The seventh AJCC staging system.
Compared with 6th AJCC staging system, the 7th AJCC staging system still had a higher discriminatory ability for different stages (Akaike information criteria values, 714.607 versus 716.091), but had a similar homogeneity in the same stages (likelihood ratio χ2 values, 34.130 versus 34.646), as shown in Table 5. However, the new staging system had the highest prognostic power among the three staging systems (likelihood ratio χ2 value, 40.928; Akaike information criteria value, 711.809). The results further suggest that the new staging system was a useful predictor for survival of patients with ICC.
Discussion
In the present study, a new staging system based on clinicopathologic features has been developed and shown to have more satisfactory predictive power than the 6th or 7th AJCC editor staging system for OS of patients with ICC after a partial hepatectomy. More importantly, the new staging system can be easily implemented in clinical practice.
A staging system for primary liver cancer was first published in 1987 by the AJCC/UICC in the third version of the TNM classification. This staging system included both HCC and ICC. In the previous 6th edition of the AJCC/UICC manual, ICC was staged identically to HCC, largely due to HCC representing more than 90% of primary liver cancers, and the paucity of prognostic data available for ICC. Nonetheless, HCC and ICC differ significantly in pathogenesis, tumor behavior and prognosis after surgical resection. Therefore, after two decades, the development of a separate staging with specific relevance to ICC was critical because information derived from staging not only provides data regarding prognosis but also dictates patient stratification in clinical research. Based on the analysis of data obtained from The Surveillance, Epidemiology, and End Results (SEER) database on 598 unselected patients who underwent surgery for ICC between 1988 and 2004, Nathan and colleagues proposed a new staging schema that was adopted in the 7th edition of the TNM Staging Manual [20]. The AJCC/UICC 7th edition staging system proposed a distinct staging system for ICC [1]. The staging system omitted tumor size due to a lack of prognostic discrimination of this characteristic, instead using the following independent predictors of survival derived from the SEER database: tumor number, vascular invasion, lymph node status, and presence of metastatic disease [20]. Indeed, in contrast to HCC, the prognostic importance of tumor size in patients with ICC is controversial. Data from a number of Japanese and Nathan et al. studies have suggested that tumor size may not be independently associated with prognosis in ICC [17,20,31-34]. However, recently, three studies (one study from 13 major hepatobiliary centers including the United States, European, and Asia; two studies from Chinese) reported that tumor size is an independent predictor of OS for patients with ICC [26,28,29]. In both our former and present studies, although a tumor size ≥ 5 cm showed a relatively poorer survival in univariate analysis, it was not an independent predictor of OS in multivariate analyses [22]. In contrast, vascular invasion, tumor number, regional lymph node status, and local extrahepatic metastasis were decidedly predictors of OS. The four parameters may be more accurate morphologic indicators of the biologic behavior of a tumor than of tumor size [17].
Although the AJCC 7th edition staging system for ICC in some Western countries [35,36] was found be superior to the 2 Western [19] and 2 Japanese pTNM classifications that had previously been used [17,18], the exact predictive power of the staging system for OS of ICC patients from Chinese populations remains unclear. Jiang and colleagues found that the AJCC 7th edition staging system provided a better prognostic discrimination than the 6th edition [26], whereas, in more recently, Wang et al. reported prognostic discrimination of the two staging system was similar (C-indices: 0.65 vs. 0.65) [28]. In the present study, our results showed the AJCC 7th edition staging system had a higher discriminatory ability for different stages and a better homogeneity in same stages than the 6th edition in the primary cohort, had a higher discriminatory ability for different stages and similar homogeneity in the same stage than did the 6th edition in the validation. The exactly superiority, compared with the previous edition, of the AJCC 7th edition ICC staging system among Chinese patients with ICC needs further confirmation.
The new staging system performed well in predicting survival by the Kaplan-Meier method, and its prediction was further supported by the likelihood ratio test and Akaike information criteria. When compared with the AJCC 6th and 7th edition staging systems, the new staging system showed better predictive accuracy for survival. The new staging system also includes two tumor markers (CA 19-9 and CEA) and one parameter of liver function (PA), which were not been included as variables in the AJCC or the two Japanese staging systems. High serum levels of CEA or CA 19-9 has been suggested to be independently associated with poor prognosis of ICC [26,28,37]. Although tumor markers have not previously been included in ICC staging systems, their role in predictive performance has been observed in HCC staging systems [38,39]. PA is a protein that is made in the liver and released in the blood. It is an important marker for assessing protein deficiency, the status of a patient’s nutrition, and the level of liver function. Serum PA combined with the model for end-stage liver disease (MELD) can more accurately predict the prognosis of patients with decompensated cirrhosis than a MELD score alone [40]. Recent studies also found that low perioperative serum PA predicts early recurrence after curative pulmonary resection for non-small-cell lung cancer [41] and short-term survival after hepatectomy for HCC [42]. In the present study, we found that low PA level was independently associated with poor prognosis for ICC patients.
There are two limitations to the present study. First, the staging system was established based on data obtained from a single institution in China. Second, HBV infection was the predominant cause of ICC patients in present study (49.1%) and only 10 patients (2.7%) in primary cohort and 2 patients (1.7%) in the validation cohort had seropositive anti-HCV. Because HCV infection and primary sclerosing cholangitis (PSC) are important risk factors of ICC development in western countries, whether this new staging system is applicable to ICC patients of western countries is still unclear.
Conclusions
Overall, compared with the existing AJCC 6th edition staging system for primary liver cancer and the 7th edition ICC staging system, the proposed new staging system is simpler and has a relatively higher predictive accuracy for OS of ICC patients after surgical resection. Prospective validation of this new staging system through a multicenter collaboration would confirm its utility.
Acknowledgements
This project is supported by National key scientific instrument and equipment development grant 2012YQ220113 (H.H.) and Natural Science Foundation of China (NSFC) grants 81372672 (H.H.).
Disclosure of conflict of interest
None.
References
- 1.Washington MK, Berlin J, Branton PA, Burgart LJ, Carter DK, Compton CC, Frankel WL, Jessup JM, Kakar S, Minsky B, Nakhleh RE, Vauthey JN. Protocol for the examination of specimens from patients with carcinoma of the intrahepatic bile ducts. Arch Pathol Lab Med. 2010;134:e14–e18. doi: 10.5858/134.4.e14. [DOI] [PubMed] [Google Scholar]
- 2.Dodson RM, Weiss MJ, Cosgrove D, Herman JM, Kamel I, Anders R, Geschwind JF, Pawlik TM. Intrahepatic cholangiocarcinoma: management options and emerging therapies. J Am Coll Surg. 2013;217:736–750. doi: 10.1016/j.jamcollsurg.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 3.Chang KY, Chang JY, Yen Y. Increasing incidence of intrahepatic cholangiocarcinoma and its relationship to chronic viral hepatitis. J Natl Compr Canc Netw. 2009;7:423–427. doi: 10.6004/jnccn.2009.0030. [DOI] [PubMed] [Google Scholar]
- 4.Shaib YH, Davila JA, McGlynn K, El-Serag HB. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol. 2004;40:472–477. doi: 10.1016/j.jhep.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 5.Mouzas IA, Dimoulios P, Vlachonikolis IG, Skordilis P, Zoras O, Kouroumalis E. Increasing incidence of cholangiocarcinoma in Crete 1992-2000. Anticancer Res. 2002;22:3637–3641. [PubMed] [Google Scholar]
- 6.Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33:1353–1357. doi: 10.1053/jhep.2001.25087. [DOI] [PubMed] [Google Scholar]
- 7.Patel T. Worldwide trends in mortality from biliary tract malignancies. Bmc Cancer. 2002;2:10. doi: 10.1186/1471-2407-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Welzel TM, Graubard BI, El-Serag HB, Shaib YH, Hsing AW, Davila JA, McGlynn KA. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population-based case-control study. Clin Gastroenterol Hepatol. 2007;5:1221–1228. doi: 10.1016/j.cgh.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan JC, Coburn NG, Baxter NN, Kiss A, Law CH. Surgical management of intrahepatic cholangiocarcinoma--a population-based study. Ann Surg Oncol. 2008;15:600–608. doi: 10.1245/s10434-007-9627-x. [DOI] [PubMed] [Google Scholar]
- 10.Lang H, Sotiropoulos GC, Sgourakis G, Schmitz KJ, Paul A, Hilgard P, Zopf T, Trarbach T, Malago M, Baba HA, Broelsch CE. Operations for intrahepatic cholangiocarcinoma: single-institution experience of 158 patients. J Am Coll Surg. 2009;208:218–228. doi: 10.1016/j.jamcollsurg.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 11.Endo I, Gonen M, Yopp AC, Dalal KM, Zhou Q, Klimstra D, D’Angelica M, DeMatteo RP, Fong Y, Schwartz L, Kemeny N, O’Reilly E, Abou-Alfa GK, Shimada H, Blumgart LH, Jarnagin WR. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248:84–96. doi: 10.1097/SLA.0b013e318176c4d3. [DOI] [PubMed] [Google Scholar]
- 12.Nathan H, Pawlik TM, Wolfgang CL, Choti MA, Cameron JL, Schulick RD. Trends in survival after surgery for cholangiocarcinoma: a 30-year population-based SEER database analysis. J Gastrointest Surg. 2007;11:1488–1496. 1496–1497. doi: 10.1007/s11605-007-0282-0. [DOI] [PubMed] [Google Scholar]
- 13.Borie F, Niampa H, Bouvier AM, Faivre J, Launoy G, Delafosse P, Velten M, Buemi A, Peng J, Grosclaude P, Tretarre B. [Current management and prognosis of intrahepatic cholangiocarcinoma in France] . Gastroenterol Clin Biol. 2009;33:971–976. doi: 10.1016/j.gcb.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 14.Paik KY, Jung JC, Heo JS, Choi SH, Choi DW, Kim YI. What prognostic factors are important for resected intrahepatic cholangiocarcinoma? J Gastroenterol Hepatol. 2008;23:766–770. doi: 10.1111/j.1440-1746.2007.05040.x. [DOI] [PubMed] [Google Scholar]
- 15.Petrowsky H, Hong JC. Current surgical management of hilar and intrahepatic cholangiocarcinoma: the role of resection and orthotopic liver transplantation. Transplant Proc. 2009;41:4023–4035. doi: 10.1016/j.transproceed.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Lang H, Sotiropoulos GC, Fruhauf NR, Domland M, Paul A, Kind EM, Malago M, Broelsch CE. Extended hepatectomy for intrahepatic cholangiocellular carcinoma (ICC): when is it worthwhile? Single center experience with 27 resections in 50 patients over a 5-year period. Ann Surg. 2005;241:134–143. doi: 10.1097/01.sla.0000149426.08580.a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okabayashi T, Yamamoto J, Kosuge T, Shimada K, Yamasaki S, Takayama T, Makuuchi M. A new staging system for mass-forming intrahepatic cholangiocarcinoma: analysis of preoperative and postoperative variables. Cancer. 2001;92:2374–2383. doi: 10.1002/1097-0142(20011101)92:9<2374::aid-cncr1585>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 18.Yamasaki S. Intrahepatic cholangiocarcinoma: macroscopic type and stage classification. J Hepatobiliary Pancreat Surg. 2003;10:288–291. doi: 10.1007/s00534-002-0732-8. [DOI] [PubMed] [Google Scholar]
- 19.Vauthey JN, Lauwers GY, Esnaola NF, Do KA, Belghiti J, Mirza N, Curley SA, Ellis LM, Regimbeau JM, Rashid A, Cleary KR, Nagorney DM. Simplified staging for hepatocellular carcinoma. J. Clin. Oncol. 2002;20:1527–1536. doi: 10.1200/JCO.2002.20.6.1527. [DOI] [PubMed] [Google Scholar]
- 20.Nathan H, Aloia TA, Vauthey JN, Abdalla EK, Zhu AX, Schulick RD, Choti MA, Pawlik TM. A proposed staging system for intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2009;16:14–22. doi: 10.1245/s10434-008-0180-z. [DOI] [PubMed] [Google Scholar]
- 21.Zhou H, Wang H, Zhou D, Wang H, Wang Q, Zou S, Tu Q, Wu M, Hu H. Hepatitis B virus-associated intrahepatic cholangiocarcinoma and hepatocellular carcinoma may hold common disease process for carcinogenesis. Eur J Cancer. 2010;46:1056–1061. doi: 10.1016/j.ejca.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Zhou HB, Wang H, Li YQ, Li SX, Wang H, Zhou DX, Tu QQ, Wang Q, Zou SS, Wu MC, Hu HP. Hepatitis B virus infection: a favorable prognostic factor for intrahepatic cholangiocarcinoma after resection. World J Gastroenterol. 2011;17:1292–1303. doi: 10.3748/wjg.v17.i10.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang L, Cai JQ, Zhao JJ, Bi XY, Tan XG, Yan T, Li C, Zhao P. Impact of hepatitis B virus infection on outcome following resection for intrahepatic cholangiocarcinoma. J Surg Oncol. 2010;101:233–238. doi: 10.1002/jso.21488. [DOI] [PubMed] [Google Scholar]
- 24.Wu ZF, Yang N, Li DY, Zhang HB, Yang GS. Characteristics of intrahepatic cholangiocarcinoma in patients with hepatitis B virus infection: clinicopathologic study of resected tumours. J Viral Hepat. 2013;20:306–310. doi: 10.1111/jvh.12005. [DOI] [PubMed] [Google Scholar]
- 25.Yu TH, Yuan RH, Chen YL, Yang WC, Hsu HC, Jeng YM. Viral hepatitis is associated with intrahepatic cholangiocarcinoma with cholangiolar differentiation and N-cadherin expression. Mod Pathol. 2011;24:810–819. doi: 10.1038/modpathol.2011.41. [DOI] [PubMed] [Google Scholar]
- 26.Jiang W, Zeng ZC, Tang ZY, Fan J, Sun HC, Zhou J, Zeng MS, Zhang BH, Ji Y, Chen YX. A prognostic scoring system based on clinical features of intrahepatic cholangiocarcinoma: the Fudan score. Ann Oncol. 2011;22:1644–1652. doi: 10.1093/annonc/mdq650. [DOI] [PubMed] [Google Scholar]
- 27.Shen WF, Zhong W, Xu F, Kan T, Geng L, Xie F, Sui CJ, Yang JM. Clinicopathological and prognostic analysis of 429 patients with intrahepatic cholangiocarcinoma. World J Gastroenterol. 2009;15:5976–5982. doi: 10.3748/wjg.15.5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, Li J, Xia Y, Gong R, Wang K, Yan Z, Wan X, Liu G, Wu D, Shi L, Lau W, Wu M, Shen F. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J. Clin. Oncol. 2013;31:1188–1195. doi: 10.1200/JCO.2012.41.5984. [DOI] [PubMed] [Google Scholar]
- 29.Hyder O, Marques H, Pulitano C, Marsh JW, Alexandrescu S, Bauer TW, Gamblin TC, Sotiropoulos GC, Paul A, Barroso E, Clary BM, Aldrighetti L, Ferrone CR, Zhu AX, Popescu I, Gigot JF, Mentha G, Feng S, Pawlik TM. A Nomogram to Predict Long-term Survival After Resection for Intrahepatic Cholangiocarcinoma: An Eastern and Western Experience. JAMA Surg. 2014;149:432–438. doi: 10.1001/jamasurg.2013.5168. [DOI] [PubMed] [Google Scholar]
- 30.Kudo M, Chung H, Haji S, Osaki Y, Oka H, Seki T, Kasugai H, Sasaki Y, Matsunaga T. Validation of a new prognostic staging system for hepatocellular carcinoma: the JIS score compared with the CLIP score. Hepatology. 2004;40:1396–1405. doi: 10.1002/hep.20486. [DOI] [PubMed] [Google Scholar]
- 31.Nakagawa T, Kamiyama T, Kurauchi N, Matsushita M, Nakanishi K, Kamachi H, Kudo T, Todo S. Number of lymph node metastases is a significant prognostic factor in intrahepatic cholangiocarcinoma. World J Surg. 2005;29:728–733. doi: 10.1007/s00268-005-7761-9. [DOI] [PubMed] [Google Scholar]
- 32.Uenishi T, Yamazaki O, Yamamoto T, Hirohashi K, Tanaka H, Tanaka S, Hai S, Kubo S. Serosal invasion in TNM staging of mass-forming intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2005;12:479–483. doi: 10.1007/s00534-005-1026-8. [DOI] [PubMed] [Google Scholar]
- 33.Aishima S, Kuroda Y, Nishihara Y, Iguchi T, Taguchi K, Taketomi A, Maehara Y, Tsuneyoshi M. Proposal of progression model for intrahepatic cholangiocarcinoma: clinicopathologic differences between hilar type and peripheral type. Am J Surg Pathol. 2007;31:1059–1067. doi: 10.1097/PAS.0b013e31802b34b6. [DOI] [PubMed] [Google Scholar]
- 34.Shimada K, Sano T, Sakamoto Y, Esaki M, Kosuge T, Ojima H. Surgical outcomes of the mass-forming plus periductal infiltrating types of intrahepatic cholangiocarcinoma: a comparative study with the typical mass-forming type of intrahepatic cholangiocarcinoma. World J Surg. 2007;31:2016–2022. doi: 10.1007/s00268-007-9194-0. [DOI] [PubMed] [Google Scholar]
- 35.Farges O, Fuks D, Le Treut YP, Azoulay D, Laurent A, Bachellier P, Nuzzo G, Belghiti J, Pruvot FR, Regimbeau JM. AJCC 7th edition of TNM staging accurately discriminates outcomes of patients with resectable intrahepatic cholangiocarcinoma: By the AFC-IHCC-2009 study group. Cancer. 2011;117:2170–2177. doi: 10.1002/cncr.25712. [DOI] [PubMed] [Google Scholar]
- 36.Nathan H, Pawlik TM. Staging of intrahepatic cholangiocarcinoma. Curr Opin Gastroenterol. 2010;26:269–273. doi: 10.1097/MOG.0b013e328337c899. [DOI] [PubMed] [Google Scholar]
- 37.Cho SY, Park SJ, Kim SH, Han SS, Kim YK, Lee KW, Lee SA, Hong EK, Lee WJ, Woo SM. Survival analysis of intrahepatic cholangiocarcinoma after resection. Ann Surg Oncol. 2010;17:1823–1830. doi: 10.1245/s10434-010-0938-y. [DOI] [PubMed] [Google Scholar]
- 38.Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 39.Chevret S, Trinchet JC, Mathieu D, Rached AA, Beaugrand M, Chastang C. A new prognostic classification for predicting survival in patients with hepatocellular carcinoma. Groupe d’Etudeet de Traitement du Carcinome Hepatocellulaire. J Hepatol. 1999;31:133–141. doi: 10.1016/s0168-8278(99)80173-1. [DOI] [PubMed] [Google Scholar]
- 40.Liu F, Cai LY, Zhong L, Chen C, Xu F, Zhao ZX, Chen XM. Model for end-stage liver disease combined with serum prealbumin to predict the prognosis of patients with decompensated liver cirrhosis. J Dig Dis. 2010;11:352–357. doi: 10.1111/j.1751-2980.2010.00465.x. [DOI] [PubMed] [Google Scholar]
- 41.Kawai H, Ota H. Low perioperative serum prealbumin predicts early recurrence after curative pulmonary resection for non-small-cell lung cancer. World J Surg. 2012;36:2853–2857. doi: 10.1007/s00268-012-1766-y. [DOI] [PubMed] [Google Scholar]
- 42.Zhao WC, Zhang HB, Yang N, Fu Y, Qian W, Chen BD, Fan LF, Yang GS. Preoperative predictors of short-term survival after hepatectomy for multinodular hepatocellular carcinoma. World J Gastroenterol. 2012;18:3272–3281. doi: 10.3748/wjg.v18.i25.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]


