Abstract
Purpose:
We prepared and characterized topical ophthalmic formulations containing brimonidine-loaded bioadhesive cationic chitosan or anionic alginate nanoparticles (NPs) for sustained release of brimonidine as once daily regimen for management of glaucoma.
Methods:
Nanoparticles were prepared using a spontaneous emulsification solvent diffusion method. Different concentrations of polymers, emulsifiers, and NPs stabilizers were used for formulation optimization. Nanoparticles were characterized regarding particle size, zeta potential, morphology, and drug content. Brimonidine-loaded NPs were incorporated into eye drops, a temperature-triggered in situ gelling system, and a preformed gel. They then were characterized regarding their pH, viscosity, uniformity of drug content, in vitro release characteristics, in vitro cytotoxicity, and in vivo intraocular pressure (IOP) lowering effects.
Results:
Characteristics of optimized brimonidine-loaded chitosan and alginate NPs, respectively, are: particle size, 115.67 ± 3.58 and 157.67 ± 5.53 nm; zeta potential, +35.27 ± 3.39 and −37.8 ± 3.77 mV; encapsulation efficiency, 74.34% ± 2.05% and 70.40% ± 2.77%; drug loading, 11.81% ± 0.67% and 13.14% ± 0.90%; and yield, 87.91% ± 5.92% and 76.53% ± 3.32%. Transmission electron microscope (TEM) analyses revealed that NPs have spherical shapes with a dense core and distinct coat. Formulations possessed uniform drug content. Furthermore, pH and viscosity were compatible with the eye. Formulations showed a sustained release without any burst effect with a Higuchi non-Fickian diffusion mechanism. Cytotoxicity studies revealed that all formulations are biocompatible. Importantly, all formulations possessed a sustained IOP lowering effect compared to the marketed brimonidine tartrate eye drops.
Conclusions:
These formulations provide a great improvement in topical ocular brimonidine delivery. The application of a single drop is sufficient to provide extended IOP reduction, which should improve patient compliance.
Translational Relevance:
We have developed a novel biocompatible topical delivery system for brimonidine, a first line glaucoma medication. Once daily application should have positive effects on patient compliance and, therefore, preservation of vision.
Keywords: chitosan, alginate, biocompatibility, ophthalmic, brimonidine, nanoparticles, controlled release, cyctotoxicity, intraocular pressure
Introduction
Nanocarrier design has become a very attractive focus of modern drug delivery research due to its many advantages, which include minimization of drug degradation, increase in cellular uptake,1 a long shelf life, and the ability to pass important mucosal barriers.2 In addition, nanoformulations have the additional benefit of allowing for superior control of drug release to the target tissue, which is a much-needed characteristic. Mucoadhesive nanocarrier drug delivery systems are a promising strategy toward the treatment of various ophthalmic disorders, as they have the ability to avoid major drawbacks of conventional topical ophthalmic drug delivery systems, such as the rapid and extensive loss through the drainage via the nasolacrimal duct.3 Several investigations attempted to improve corneal drug penetration through the development of various colloidal drug delivery systems, such as liposomes,4 nanoparticles (NPs),5 and nanocapsules.6 Unfortunately, these colloidal drug delivery systems were not able to solve the problem of rapid loss of dosage form from the eye surface and still have a short residence time.
Among the natural bioadhesive and biodegradable polymers that are used in the preparation of ophthalmic formulations, chitosan (CS) and sodium alginate (ALG)7 are the most widely preferred. Chitosan possesses unique properties that are responsible for its superiority, including bioadhesiveness, biodegradability, biocompatibility,8 antibacterial activity,9 and its penetration enhancing properties.10 Recently, CS has been proposed as a material with a good potential for topical ocular use due to its ability to prolong the corneal residence time for at least 24 hours.11 Alginates also are biodegradable, biocompatible, and mucoadhesive polymers.1 They do not accumulate in any major organs and show evidence of in vivo degradation.12 Sodium alginate is considered an excellent polymer for the preparation of NPs for sustained ophthalmic drug delivery.13
Glaucoma is a significant public health problem worldwide.14 Although high intraocular pressure (IOP) is not the only risk factor associated with glaucoma, most of the current treatment strategies focus on lowering IOP. Another dangerous risk factor of glaucoma is retinal ganglion cell degeneration, which is a leading cause of blindness.15 Brimonidine is a potent and relatively selective α2-adrenergic receptor agonist.16 It is available in two chemically different forms: brimonidine tartrate salt, which has a good water solubility (34 mg/mL), and brimonidine free-base, which has a negligible water solubility.16 Brimonidine is commercially available as 0.1% brimonidine tartrate eye drops (Alphagan P; Allergan, Inc., Irvine, CA). It provides efficient management for glaucoma, but it suffers from poor patient compliance, because it requires multiple dosing per day because of its short precorneal retention time.17
Brimonidine has two proposed mechanisms by which it treats glaucoma. The first is by decreasing IOP by reducing the production of aqueous humor and increasing uveoscleral outflow.18 It also acts as a neuroprotective agent that can promote the survival of injured retinal ganglion nerve cells and prevent its further damage.19 Therefore, brimonidine is considered a highly useful therapeutic approach in glaucoma management as it targets IOP reduction and neuroprotection.15 The preparation of brimonidine in the form of a bioadhesive, biodegradable, and sustained-release topical formulation would be advantageous, as it would limit its application to once daily, thereby increasing patient compliance. Several studies have prepared brimonidine preparations to increase its precorneal retention time and decrease its dosing frequency.16,17,20,21 However, the main limitation of all of these investigations was a short duration of action; that is, up to four hours. In our investigation, we sought to formulate a sustained release brimonidine topical ophthalmic formulation to be administered once daily for the treatment of glaucoma.
Materials and Methods
Materials
Brimonidine (U104), hydroxypropylmethylcellulose (HPMC), methylcellulose (Methocel, MC), polyvinyl alcohol (PVA), Triton X-100, methyl thiazol tetrazolium (MTT), absolute ethyl alcohol, acetone, and dichloromethane (DCM) were purchased from Sigma-Aldrich (St. Louis, MO). Chitosan (molecular weight, 100,000–300,000 Da) was purchased from Acros Organics, (Fair Lawn, NJ). Sodium alginate (medium viscosity) was purchased from MP Biomedicals (Solon, OH). Soybean L-α-Lecithin (98% phosphotidyl choline) was purchased from Calbiochem (San Diego, CA). Poloxamer 188 (molecular weight 8400 Da) was purchased from Spectrum Chemical Mfg. Corp., (New Brunswick, NJ). Dimethyl sulfoxide (DMSO) was purchased from ThermoScientific Co. (Rockford, IL). Eagle's minimal essential cell culture medium (EMEM) was purchased from ATCC Co. (Manassas, VA). Isothesia (Isoflurane USP) was purchased from Butler Schein Animal Health (Dublin, OH).
Animals
All experimental protocols were approved by the Animal Care and Use review board of the University of Tennessee Health Science Center. Mice were handled in a manner consistent with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, the Public Health Service Policy on Humane Care and Use of Laboratory Animals). Mice strains BXD29 and BXD96 were generously provided by Dr. Robert W. Williams (The University of Tennessee Health Science Center). Both strains were selected from a large recombinant inbred family of mice. Mouse strain BXD29 has spontaneously elevated IOP, while BXD96 has an IOP in the normal range.22 All mice were aged 3 to 5 months and weighed 25 to 35 grams. They were healthy and free from any clinically observable abnormalities. Moreover, all eyes were free from apparent injury and the IOP difference between eyes of the same mouse did not exceed 2 mm Hg. The basal IOP was 18 ± 2 mm Hg for BXD29 and 15 ± 2 mm Hg for BXD96.
Preparation of Plain and Brimonidine-Loaded NPs
Plain NPs were prepared using a spontaneous emulsification solvent diffusion technique23 as described in our published methods.22 Table 1 summarizes the formulation parameters used to synthesize our optimized brimonidine-loaded NPs. Unique features of the present study include the use of two natural polymers (CS and ALG) to generate NPs. Chitosan was dissolved in a minimal amount of 1% acetic acid and brought to volume with deionized water (DIW), after which its pH was adjusted to 4.5, while ALG was dissolved in DIW alone. Poloxamer 188 or PVA was dissolved in the polymer solution. Drug and lecithin were dissolved in DCM followed by the addition of acetone. The organic solution then was injected into the polymer solution. The produced emulsion was sonicated and stirred overnight. The prepared NPs were separated by ultracentrifugation for 2 hours at 60,000 revolutions per minute (rpm) followed by washing and ultracentrifugation 3 times to remove the free unincorporated drug. Our methods for optimizing our NPs have been described in detail previously.22,24 In brief, characterization methods include measurement of particle size and charge, evaluation of morphology using transmission electron microscopy (TEM), and determination of drug loading capacity, encapsulation efficiency, and NP yield.
Table 1.
Composition of Brimonidine-Loaded NPs
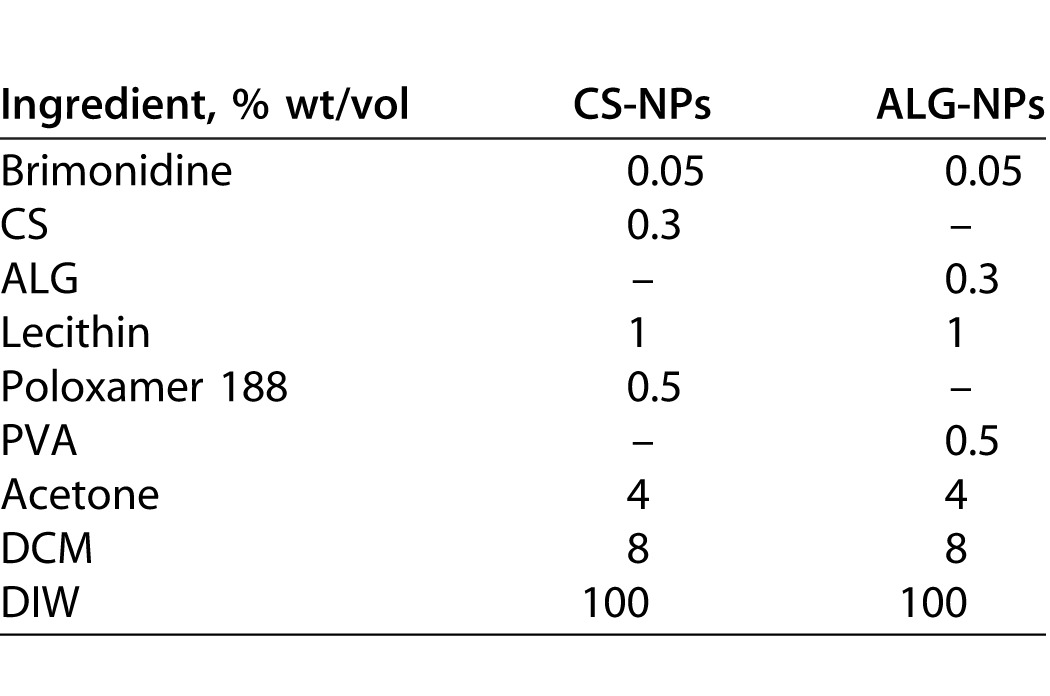
Preparation of the Topical Ophthalmic Formulations
Chitosan and ALG-NPs were incorporated into three vehicles to increase the retention time and allow for long-term release of brimonidine. These formulations were eye drops, an in situ gelling system, and a preformed gel. Details of the synthesis of each formulation are provided in our previous publications.22,24,25 Brimonidine-loaded NPs were incorporated in such plain vehicles to give a final concentration of brimonidine equivalent to 0.1% wt/vol.
In Vitro Evaluation of the Formulations
The pH of each formulation was determined using standard methods and a pH meter (Corning pH meter 440; Corning, Inc., Corning, NY). The viscosity and temperature-induced gelation properties were measured using a cone (1.5°) and plate rotary viscometer (Brookfield DV-II+ programmable viscometer; Brookfield Engineering Laboratories, Inc., Middleboro, MA) and our previously published methods.22,24,25 All studies were performed in triplicate and results were calculated as mean ± SD. The drug content of each formulation was assayed using our established protocols.22,24,25 The clear solution was assayed spectrophotometrically at 261 nm for its drug content with appropriate negative controls. The experiments were repeated three times and the results were calculated as mean ± SD.
Drug release studies were performed using 1500 μL fast micro-equilibrium dialyzers with semipermeable regenerated cellulose membranes (25,000 Da weight cut-off; Harvard Apparatus Co., Holliston, MA), as we previously published.24 Samples were analyzed spectrophotometrically at 248 nm for their drug content. Control experiments were performed using plain NPs incorporated into plain dosage form vehicles. The experiments were repeated three times and the concentrations were calculated from the standard curve as mean ± SD. The release data were statistically analyzed using 1-way analysis of variance (ANOVA) test followed by Tukey-Kramer multiple comparisons test26 using GraphPad Prism-5 software (GraphPad Software, Inc., La Jolla, CA).
Any potential cytotoxicity of our formulations was evaluated by an MTT assay with minor modifications27 that included the use of HEK293 cells per our published methods.24 Optical absorbance was measured at 570 nm with the use of a microplate reader and converted to percent cell viability relative to the control (untreated) cells. Cell viability data were statistically analyzed using 1-way ANOVA test followed by Tukey-Kramer multiple comparisons test.26 Each experiment was performed using six biological replicates and the result calculated as mean ± SD.
In Vivo Evaluation of the Ophthalmic Formulations
The study was conducted using a single dose response design. Animals were lightly sedated by isoflurane inhalation. Ten microliters of the test formulations or the control preparation (i.e., Alphagan P) were instilled into the inferior conjunctival sac of the right eyes of mice while the untreated left eyes served as untreated controls (n = 5). Eyes were held open for at least 20 seconds to allow adequate ocular surface contact with each formulation. We measured the IOP of both eyes using a Tonolab tonometer (Colonial Medical Supply, Franconia, NH) immediately before the formulations application (baseline) and at different time intervals after application until the IOP returned to baseline. All formulation applications began on the same time of the day (8 AM). Intraocular pressure was measured three times in each eye at each time interval.
Evaluation of each ophthalmic formulation was based on pharmacodynamic parameters, including maximum decrease in IOP (Imax), the time required to reach maximum decrease in IOP (Tmax), the time required for IOP to return again to its baseline (i.e., end of drug effect; Tend), the total area under the IOP-versus-time curve (AUCtotal), and the relative area under the IOP-versus-time curve (AUCrel), which can be estimated from the quotient of the values of AUCtotal of the test formulations and that of the control preparation. Values of AUCtotal were determined using a linear trapezoidal method.17 Results were reported as mean ± SEM. Statistical analyses of the obtained results were performed using 1-way ANOVA with Tukey-Kramer multiple comparisons test.26 All pharmacodynamic parameters as well as the statistical analysis of the results were calculated using GraphPad Prism-5 software.
Results
Characterization of Brimonidine-Loaded NPs
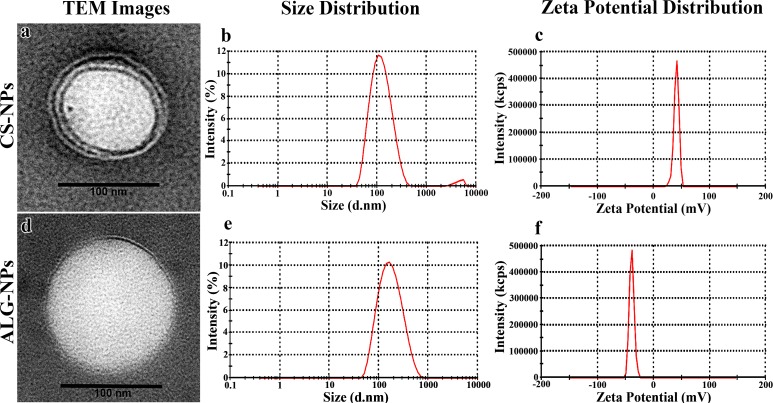
Optimization of our plain NP formulations has been described previously.25 The optimal CS-NPs that has the smallest particle size and the highest zeta potential contained 0.3% CS, 1% lecithin, and 0.5% poloxamer 188, while the optimal ALG-NPs contained 0.3% ALG, 1% lecithin, and 0.5% PVA. Moreover, optimal NPs were obtained in the presence of acetone after 10 minutes of sonication (Table 1).25 Compared to the particle size of plain NPs, there was a significant increase in the mean particle size of ALG-NPs (P < 0.05) and a nonsignificant increase in CS-NPs (P > 0.05) after incorporation of brimonidine (Table 2). In addition, the incorporation of brimonidine into NPs resulted in a small but nonsignificant increase in the positive zeta potential of CS-NPs, and a similar decrease in the negative zeta potential of ALG-NPs (P > 0.05). The encapsulation efficiency of brimonidine was quite high (74.34% and 70.40% for CS- and ALG-NPs, respectively; Table 2) with a final drug loading of 11.81% and 13.14% for CS- and ALG-NPs, respectively. Figure 1 shows the TEM images of brimonidine-loaded CS- and ALG-NPs, as well as the particle size and zeta potential distribution curves obtained by the zetasizer. The TEM images show that our NPs have a distinct spherical shape with solid dense polymeric core and surrounded by thin evenly distributed coat. The zetasizer curves for particle size and zeta potential show normal distribution around the mean size (115.67 and 157.67 nm for CS- and ALG-NPs, respectively) and zeta potential (38.27 and −33.80 mV for CS- and ALG-NPs, respectively).
Table 2.
Characteristics of Plain and Brimonidine-Loaded NPs
Figure 1.
NP size, charge and morphology. (a) Transmission electron microscopy (TEM) image of brimonidine-loaded CS-NPs. (b) Size distribution curve for brimonidine-loaded CS-NPs. (c) Zeta potential distribution curve for brimonidine-loaded CS-NPs. (d) TEM image of brimonidine-loaded ALG-NPs. (e) Size distribution curves for brimonidine-loaded ALG-NPs. (f) Zeta potential distribution curve for brimonidine-loaded ALG-NPs.
In vitro Evaluation of the Formulations
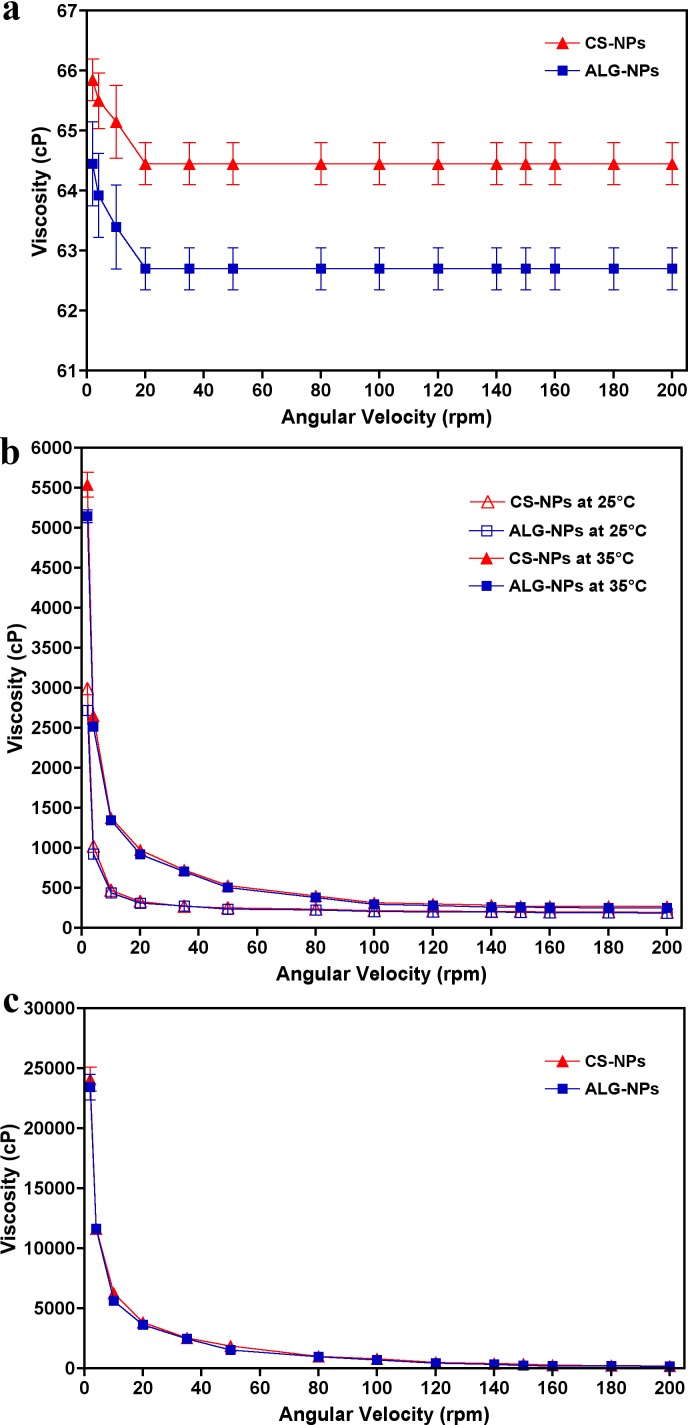
Table 3 lists the pH, drug content, and the viscosity at 35°C and 10 rpm of each prepared ophthalmic formulation. The corresponding rheological profiles of each ophthalmic formulation are shown in Figure 2. The pH of the prepared ophthalmic formulations ranged from 7.67 to 7.81 and the drug content ranged from 99.77% to 102.67%. From the rheological profiles it is apparent that the viscosity of eye drops did not change by increasing the shearing rate (i.e., Newtonian flow behavior). In contrast, the viscosity of the in situ gelling system and preformed gel decreased when the shearing rate was increased (i.e., non-Newtonian pseudoplastic flow behavior). Figure 2B shows the temperature-induced gelation of the in situ gelling systems. These data show that the viscosities of the formulations increased when the temperature at which the measurements were taken increased from the non-physiological temperature (25°C) to the physiological temperature (35°C).
Table 3.
Characteristics of Ophthalmic Formulations Containing Optimized Brimonidine-Loaded NPs
Figure 2.
Rheological profiles of eye drops containing brimonidine-loaded CS-NPs and ALG-NP formulations (a) eyedrops at 35°C, (b) in situ gelling systems at 25°C and 35°C, and (c) preformed gels at 35°C. Data are presented as mean ± SD; n = 3.
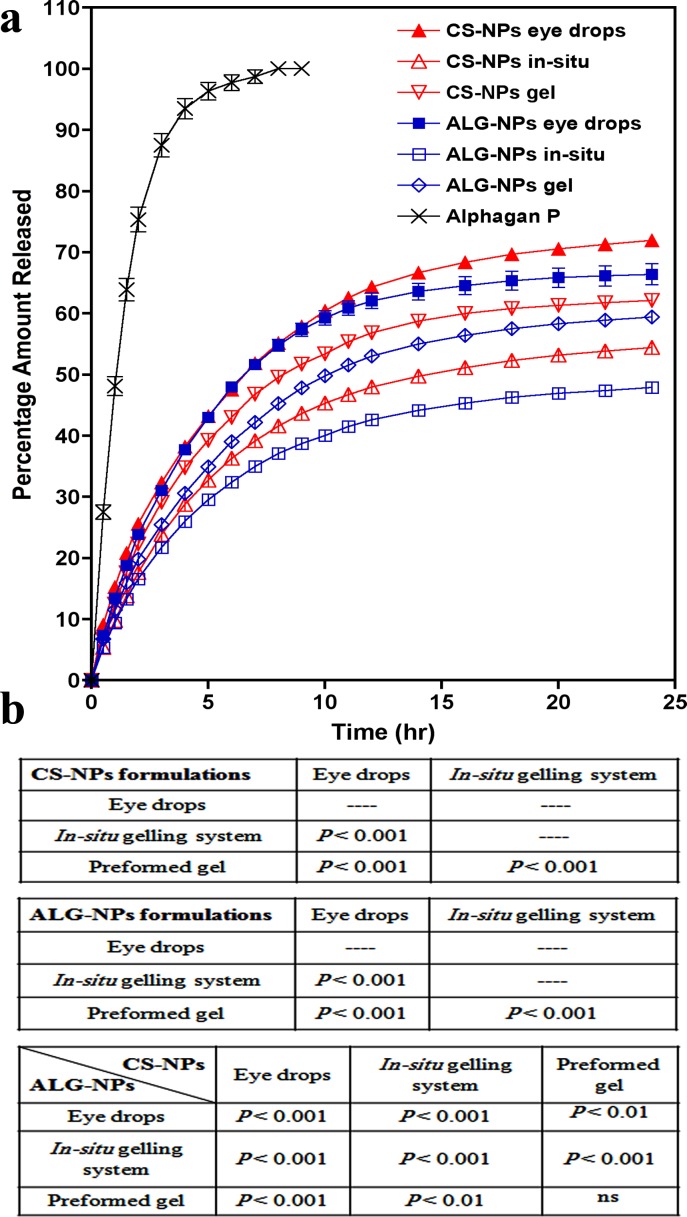
Figure 3A shows the release profiles of our ophthalmic formulations and that of the control eye drops (Alphagan P). The percentage of the cumulative amount of brimonidine released after 24 hours from eye drops, gel, and in situ gelling system, respectively, are: 71.97%, 62.12%, and 54.48% for CS-NPs preparations, and 66.44%, 59.47%, and 47.93% for ALG-NPs preparations. Our data demonstrated that the release of brimonidine from all of our preparations was significantly slower than that of the control preparation (P < 0.001), which had a very rapid release pattern with 100% of the drug was released within only 8 hours. The release of brimonidine from all CS-NPs and ALG-NPs preparations was significantly different from each other (P < 0.001, Fig. 3B). Eye drops and preformed gel containing CS-NPs possessed significantly higher brimonidine release rate than those of ALG-NPs (P < 0.01), or the two in situ gelling systems (P < 0.001). Figure 3 also shows that the release patterns of our formulations are free of any burst effect and that they can sustain brimonidine release for up to one day.
Figure 3.
(a) Brimonidine release profiles (percent amount released) from ophthalmic formulations containing brimonidine-loaded CS-NPs or ALG-NPs and Aphagan® P eye drops and (b) statistical comparison among the final formulations. Data are presented as mean ± SD; n = 3.
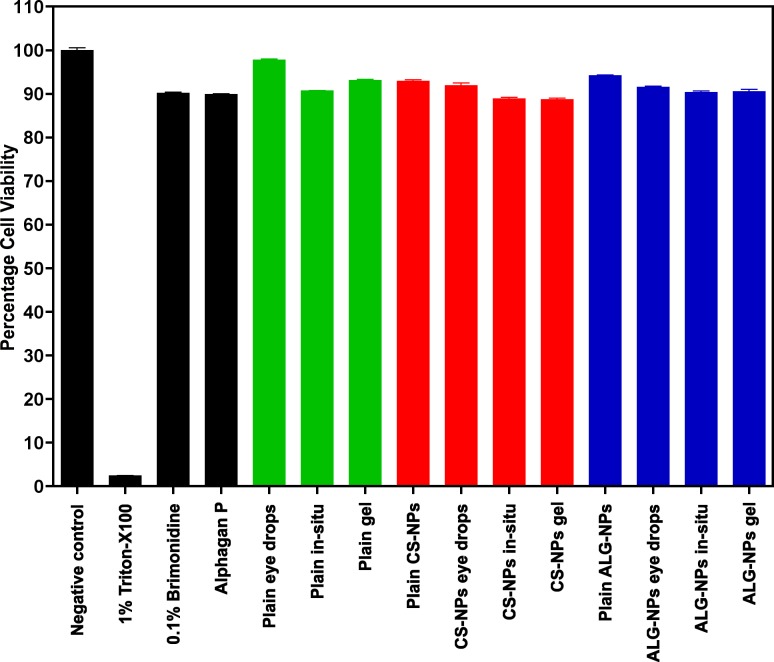
In vitro cell toxicity studies (Fig. 4) demonstrated that percentage cell viability of our ophthalmic formulations ranged from 88.78% to 97.43% and 89.79% for Alphagan P eye drops. These results are not significantly different (P > 0.05) from the control preparation containing 0.1% brimonidine suspension. They are, however, different from the positive control containing 1% of the cytotoxic agent Triton-X 100 (P < 0.001).
Figure 4.
Cytotoxicity histograms of ophthalmic formulations containing brimonidine-loaded CS-NPs or ALG-NPs. Data are presented as mean ± SD; n = 6.
In Vivo Evaluation of the Formulations
Upon application of all formulations that we synthesized, all mice were calm and showed no signs of ocular discomfort, such as redness, irritation, burning, stinging, or tearing. In contrast, the control preparation (Alphagan P eye drops) elicited immediate mild eye redness and lacrimation that lasted for 3 to 5 minutes after instillation of the eye drops. These transient side effects may be attributed to the high local drug concentration on the eye surface.17
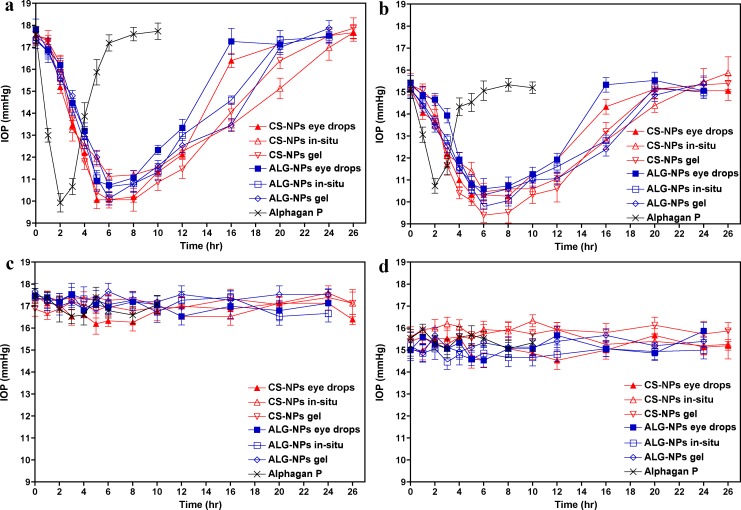
The mean IOP profiles of treated and control eyes after instillation of the formulations or Alphagan P eye drops into the right eyes of BXD29 and BXD96 mice are shown in Figure 5 and the relevant pharmacodynamic data are listed in Tables 4 and 5, respectively. The IOP profiles in Figures 5A and 5B confirmed that all our ophthalmic formulations have the ability to sustain the IOP lowering effect of brimonidine when compared to the control eye drops. As shown in Tables 4 and 5, there were no significant differences between the Imax values of our formulations and the control preparation for both mice strains, as the formulations decreased the IOP to nearly the same level (P > 0.05). There was, however, a significant difference between the Imax values of CS-NPs gel and ALG-NPs eye drops for BXD96 mice (P < 0.05, Table 5).
Figure 5.
Twenty-four hour IOP profiles (a and b) after a single application of ophthalmic formulation containing brimonidine-loaded NPs in one eye or (c and d) untreated control eye of mice. IOP profiles of the treated eyes of mice with spontaneously elevated IOP are shown in panel a while mice with normal IOP are shown in panel b. IOP profiles of the untreated control eyes of mice with spontaneously elevated IOP and mice with normal IOP are shown in c and d, respectively. Data are presented as mean ± SEM; n = 5.
Table 4.
Pharmacodynamic Parameters After Administration of Ophthalmic Formulations Containing Brimonidine-Loaded CS-NPs, ALG-NPs or Alphagan P Eye Drops to Mice With Spontaneously Elevated IOP (BXD29)
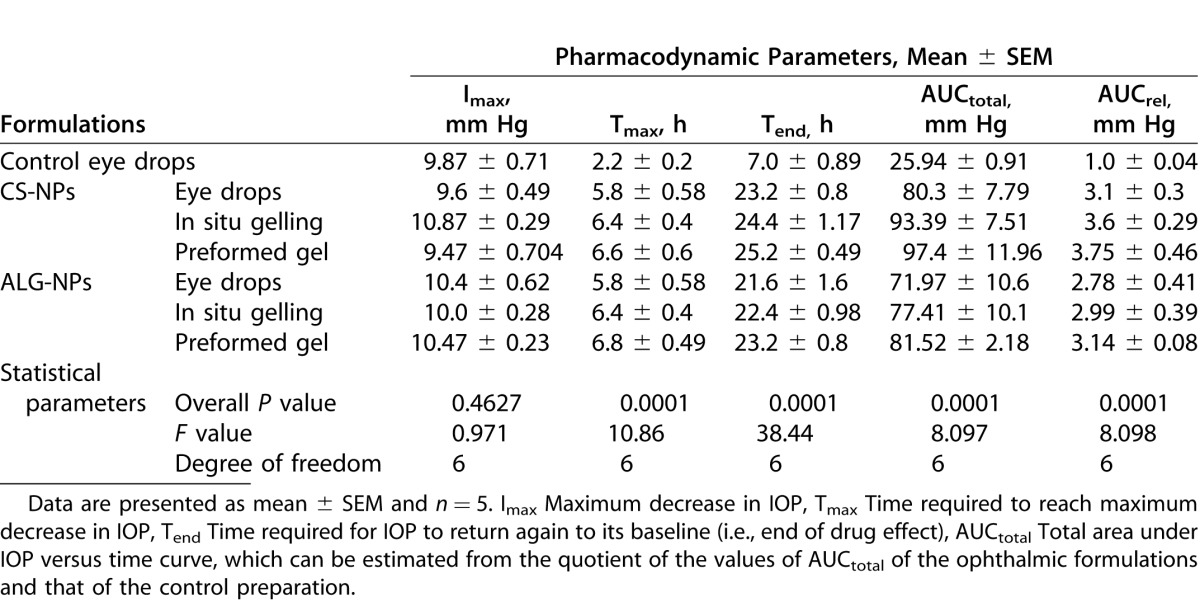
Table 5.
Pharmacodynamic Parameters After Administration of Ophthalmic Formulations Containing Brimonidine-Loaded CS-NPs, ALG-NPs or Alphagan P Eye Drops to Mice With Normal IOP (BXD96)
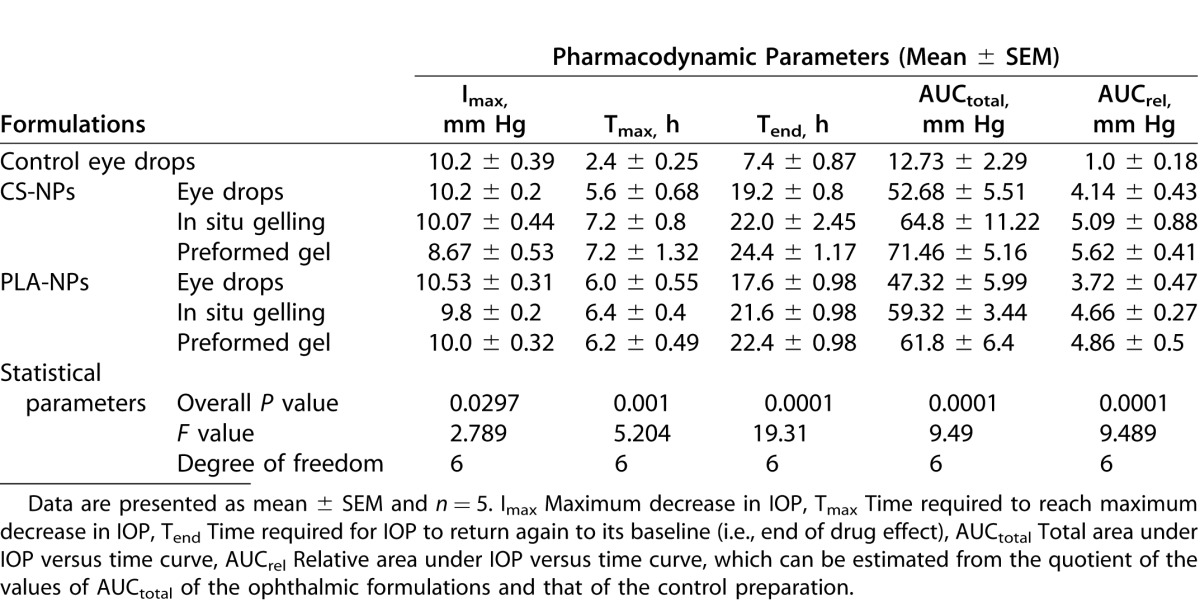
Upon comparing the Tmax values, it is evident that our formulations possessed significantly delayed Tmax values (i.e., 5.6–7.2 hours) compared to the control eye drops (i.e., 2.2–2.4 hours; P < 0.01 for BXD96 and P < 0.001 for BXD29). In contrast, there were no significant differences between the Tmax values of our formulations (P > 0.05). Regarding the Tend values, our data demonstrated that our formulations significantly extended the duration of the IOP lowering effect of brimonidine in both mice strains, as their Tend values ranged from 17.2 to 25.2 hours compared to 7 to 7.4 hours for the control eye drops (P < 0.001). There were no significant differences between the Tend values of our formulations (P > 0.05), with the exception of CS-NPs gel versus ALG-NPs eye drops for BXD96 mice (P < 0.05, Table 5). Also the pharmacodynamic parameters in Table 5 demonstrated that AUCtotal and AUCrel of our formulations are highly significantly different from those of the control eye drops for both mice strains at (P < 0.001). In contrast, for CS-NPs and ALG-NPs eye drops significance was P < 0.01, and there was no significant difference between the remainder of our formulations (P > 0.05).
Discussion
Our aim in this investigation was to develop long-acting, sustained release, topical ophthalmic formulations for the chronic management of glaucoma. This was accomplished by synthesizing NP preparations from two natural bioadhesive and biodegradable polymers (CS and ALG) and incorporating each into three different bioadhesive topical ophthalmic vehicles. All of our prepared formulations achieved an improved release of brimonidine and an extended IOP-lowering effect compared to Alphagan P eye drops. Our success is due to the bioadhesive nature of our NPs and vehicles, along with the sustained release of brimonidine from the formulations. In our previous research22 we prepared NPs-based ophthalmic formulations in which the NPs were made from synthetic polymers (i.e., polycaprolactone [PCL], polylactide [PLA], and polylactic-co-glycolic acid [PLGA]). Advantageously, the current work is superior to our previous study in several points. Firstly, ALG- and CS-based NPs are less toxic as they prepared from natural polymers. Secondly, the current formulations possess a dual bioadhesive effect because NPs and formulation vehicles are bioadhesive. In contrast, in our previous work, only the formulations vehicles were bioadhesive. This improved bioadhesiveness resulted in a more extended IOP-lowering effect that lasted for up to 26 hours in the current work compared to only 16 hours for our previous work. Finally, the hydrophilicity of the natural polymers resulted in higher in vitro release rates for the current NPs than our previous work that contained NPs prepared from hydrophobic synthetic polymers.
Our NPs were consistently small and ranged from 107 nm (plain CS-NPs) to 158 nm (brimonidine-loaded Alg-NPs). Although there was an increase in the mean particle size of our optimized NPs after incorporation of brimonidine, it still is in the reasonable range as it is known that NPs less than 200 nm are considered acceptable for passive drug targeting.28,29 The effect brimonidine had on the zeta potential of the NPs may be due to its basic nature, which may increase the positive charge of CS-NPs, while it decreased the negative one of ALG-NPs. Transmission electron microscopy images confirmed the particle size data obtained by the zetasizer and demonstrated that NPs have a distinct shape which is spherical with a solid dense polymer core surrounded by evenly distributed coat that may be comprised of the emulsifiers. Importantly, drug incorporation did not affect the shape of the NPs.
Among the characteristics of our optimal formulations were the neutral pH, low toxicity, the suitable viscosity and flow behavior, and the homogeneous drug contents. The pH of tears is 7.4 and due to its natural buffering capacity, the eye can tolerate ophthalmic formulations within a wide pH range (3.5 to 8.5).30 Our formulations possessed ideal pH values that can be easily tolerated by the eye without any irritation or discomfort. In addition, cell toxicity curves (Fig. 4) showed that all tested formulations were nontoxic. These highly favorable results may be due to the fact that all the polymers used for preparation of the vehicles31 or the NPs1,8 possessed an excellent biocompatibility.
Regarding the flow behavior of our formulations, the eye drops possessed Newtonian flow behavior, which may be due to their HPMC content,32 In contrast, the non-Newtonian pseudoplastic flow behaviors of our in situ gelling systems and preformed gels may be due to their poloxamer 18833 and MC34 contents, respectively. A pseudoplastic behavior is preferred for topical ophthalmic preparations, so that the ophthalmic formulation does not interfere with the pseudoplastic properties of the precorneal tear film and, therefore, it does not cause patient discomfort during eye blinking. The ocular shear rate is very low (0.03 s−1) during the interblinking rest periods, while it is very high during blinking (4250–28,500 s−1).3 During the interblinking periods, our formulations had higher viscosities that prevent their drainage from the eye, while during blinking, they had very low viscosities that did not cause patient discomfort. The ability of the in situ gelling systems to undergo sol-gel transition by increasing the temperature of measurement indicated that it would be converted to a viscous gel upon instillation into the eye, which in turn improved its ocular surface contact time. Lastly, among the characteristic features of our formulations is the homogeneity of drug contents, which are complied with the United States Pharmacopeial Convention (USP) official requirements as it was less than ±3% of the originally incorporated brimonidine-loaded NPs.30
In our study, brimonidine free-base was used rather than brimonidine tartrate salt to achieve higher encapsulation efficiency. Because the salt has higher water solubility (34 mg/mL), it may be lost in the aqueous phase during emulsification step.16 On the other hand, brimonidine base is poorly water soluble, which preferentially partitioned in the organic phase. Consequently, only a small amount of brimonidine was lost in the aqueous phase. Also, the use of acetone, which is a highly diffusible and volatile solvent, increased the brimonidine encapsulation efficiency by rapidly evaporating leaving brimonidine within NPs away from the aqueous phase.35
The release profiles of brimonidine from our formulations confirmed that it was possible to prepare sustained release topical ophthalmic formulations containing drug-loaded bioadhesive NPs. All formulations possessed sustained drug release rate that was free of any burst release that may cause a toxic effect. The absence of burst release may be due to two factors. The first is that the NPs are not directly suspended in the dissolution medium. Rather, they are dispersed in dosage form vehicles that allowed the drug to be dispersed in the vehicles after release from the NPs, which in turn also sustained its release from the vehicle to the release medium. The second factor is the presence of a coat around the NPs that was confirmed by TEM data. The coating shields the surface of the NPs surface and prevents the drug from associating with it which would be available for burst effect. There are many studies that induced a coat around NPs using various materials to decrease or prevent the burst release.36–38 The smaller particle size of CS-NPs may be the cause that CS-NPs preparations possessed higher release rates than those of ALG-NPs, as the smaller the size, the higher the surface area available for drug release and, thus, the higher the release rate.39
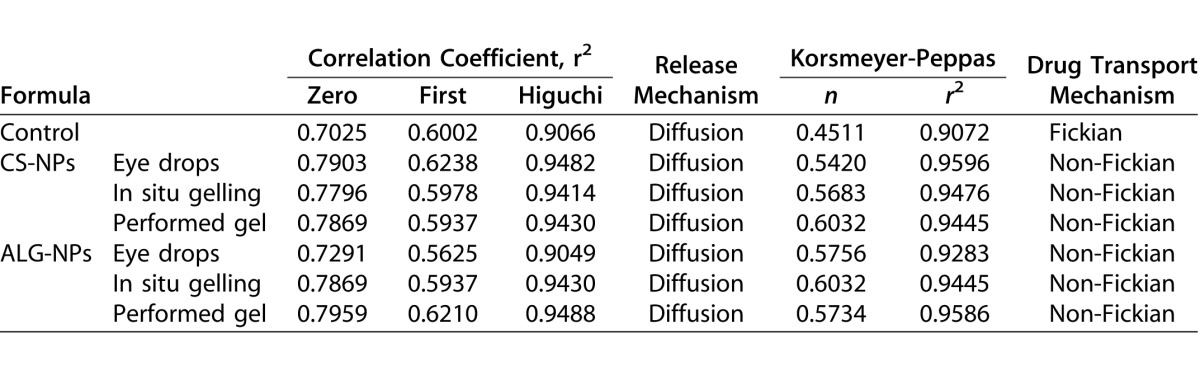
To determine release kinetic model of brimonidine from our formulations, the release data were analyzed according to zero, first, and Higuchi models. Table 6 shows that all formulations followed the Higuchi model, as it is the model of the highest correlation coefficient.40 The Korsmeyer-Peppas semi-empirical model was used for further in depth analysis,41 where the value of the release exponent for all formulations were located between 0.45 and 0.89, which indicated that they exhibited a non-Fickian or anomalous diffusion. Collectively, the drug release from the formulations occurred by a mixture of both, diffusion from NPs and erosion of NPs polymers, while the (n) value of the control preparation was 0.4511, which suggested a Fickian diffusion or pure diffusion mechanism.42
Table 6.
In Vitro Release Kinetics of Brimonidine
In our in vivo evaluations, BXD mice strains we used were carefully selected. Our criteria included lack of mutations in Tyrp1 and Gpnmb that cause pigmentary dispersion glaucoma in the D2 parent strain.43–45 We also wanted to test our formulations on one strain with spontaneously high IOP and a second strain with normal IOP levels.22 Strain BXD29 was selected as a strain with elevated IOP (plausible model of primary open angle glaucoma). Strain BXD96 was selected as a strain with a normal IOP level (plausible model of normotensive glaucoma). Our formulations sustained the IOP lowering effect of brimonidine and improved its bioavailability compared to control eye drops as evidenced by the high Tend, AUCtotal, Tmax, and AUCrel of our formulations. Our formulations reduced IOP up to 25 hours compared 7 hours for the control preparation. These data suggested that the dosing frequency could be reduced to once daily instead of 2 or 3 times daily.
This improvement in brimonidine bioavailability may be due to several factors, such as the small particle size and bioadhesiveness. As previously mentioned, our NPs possessed a particle size, which is small enough to be readily absorbed and/or allow them to pass through biological membranes and undergo passive drug targeting.28,29 Also, our NPs are made from bioadhesive polymers, which can easily adhere to the eye surface and act as drug reservoir that resists removal by tears so that the drug can be continuously released for a longer duration,7,8,11,13 Our results showed that CS-NPs formulations are superior to ALG-NPs formulations. This superiority may be due to the chitosan penetration enhancing properties10 and the positive charge carried by CS-NPs, which may increase its mucoadhesion by interacting with the negative charges carried by epithelial cells of the cornea and conjunctiva. These factors may contribute to a prolonged residence time and improved drug absorption.11
The dosage form also had an important role in improving the drug contact time with eye surface. The eye drops and the in situ gelling system containing HPMC and the preformed gel consisted of MC. Both HPMC and MC are bioadhesive cellulose derivatives, which increased the contact time of the formulations with eye surface and so improved its bioavailability.46 The results showed that the bioavailability of gel is better than in situ gelling system than eye drops. This order of bioavailability may be due to the difference in the viscosity of the formulations after application into the eye surface as the highest the viscosity the longer the contact time with the eye surface.47 Also the use of brimonidine free-base, which is a nonionized form that is readily absorbed through cornea and conjunctiva by the passive diffusion, while the control preparation containing brimonidine tartrate salt, which is the ionized form, and its absorption depends mainly on the pH of the eye surface.48
Our outcomes indicated that the combination of increased drug loading due to the selection of the free base drug form, sustained release behavior, and long retention time of the formulation are essential criteria to achieving a high bioavailability, sustained drug release, and prolonged IOP reduction into the eye after topical application of an ophthalmic formulation. These characteristics combine to support the generation of a once daily topical formulation, which may increase patient compliance and reduce visual field loss secondary to uncontrolled IOP.
Conclusions
This study describes the preparation and evaluation of new topical ophthalmic sustained release brimonidine dosage forms containing brimonidine-loaded CS- or ALG-NPs prepared by a spontaneous emulsification solvent diffusion method. The optimized NP formulations had the desirable particle sizes, zeta potential, and surface morphology. The prepared formulations possessed pH and viscosity values that are compatible with the eye and have uniform drug contents that comply with official requirements. In vitro release data of ophthalmic formulations showed a sustained release that is free from any burst effect with the formulations following a Higuchi non-Fickian diffusion mechanism. In vitro cytotoxicity results revealed that all the prepared formulations were nontoxic. An in vivo study revealed that the IOP-lowering effect of our formulation lasted for more than 25 hours after a single topical application. This suggests that once daily application for complete management of glaucoma is possible, which should result in improved patient compliance. We concluded that our investigation has determined the optimal formulations for sustained delivery of brimonidine for the management of glaucoma.
Acknowledgments
Supported by The Egyptian Government Joint Supervision Program; The University of Tennessee Research Foundation and an Unrestricted Grant from Research to Prevent Blindness, New York, NY.
Disclosure: M.M. Ibrahim, None; A.-E.H. Abd-Elgawad, None; O.A.-E. Soliman, None; M.M. Jablonski, None.
References
- 1. De S,, Robinson D. Polymer relationships during preparation of chitosan-alginate and poly-l-lysine-alginate nanospheres. J Control Release. 2003; 89: 101–112. [DOI] [PubMed] [Google Scholar]
- 2. Alonso MJ. Nanomedicines for overcoming biological barriers. Biomed Pharmacother. 2004; 58: 168–172. [DOI] [PubMed] [Google Scholar]
- 3. Bourlais CL,, Acar L,, Zia H,, Sado PA,, Needham T,, Leverge R. Ophthalmic drug delivery systems--recent advances. Prog Retin Eye Res. 1998; 17: 33–58. [DOI] [PubMed] [Google Scholar]
- 4. Smolin G,, Okumoto M,, Feiler S,, Condon D. Idoxuridine-liposome therapy for herpes simplex keratitis. Am J Ophthalmol. 1981; 91: 220–225. [DOI] [PubMed] [Google Scholar]
- 5. Losa C,, Calvo P,, Castro E,, Vila-Jato JL,, Alonso MJ. Improvement of ocular penetration of amikacin sulphate by association to poly(butylcyanoacrylate) nanoparticles. J Pharm Pharmacol. 1991; 43: 548–552. [DOI] [PubMed] [Google Scholar]
- 6. Losa C,, Marchal-Heussler L,, Orallo F, Vila Jato JL, Alonso MJ. Design of new formulations for topical ocular administration: polymeric nanocapsules containing metipranolol. Pharm Res. 1993; 10: 80–87. [DOI] [PubMed] [Google Scholar]
- 7. Motwani SK,, Chopra S,, Talegaonkar S,, Kohli K,, Ahmad FJ,, Khar RK. Chitosan-sodium alginate nanoparticles as submicroscopic reservoirs for ocular delivery: formulation optimisation and in vitro characterisation. Eur J Pharm Biopharm. 2008; 68: 513–525. [DOI] [PubMed] [Google Scholar]
- 8. Alonso MJ,, Sanchez A. The potential of chitosan in ocular drug delivery. J Pharm Pharmacol. 2003; 55: 1451–1463. [DOI] [PubMed] [Google Scholar]
- 9. Rabea EI,, Badawy ME,, Stevens CV,, Smagghe G,, Steurbaut W. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules. 2003; 4: 1457–1465. [DOI] [PubMed] [Google Scholar]
- 10. De la Fuente M,, Ravina M,, Paolicelli P,, Sanchez A,, Seijo B,, Alonso MJ. Chitosan-based nanostructures: a delivery platform for ocular therapeutics. Adv Drug Deliv Rev. 2010; 62: 100–117. [DOI] [PubMed] [Google Scholar]
- 11. De Campos AM,, Sanchez A,, Alonso MJ. Chitosan nanoparticles: a new vehicle for the improvement of the delivery of drugs to the ocular surface. Application to cyclosporin A. Int J Pharm. 2001; 224: 159–168. [DOI] [PubMed] [Google Scholar]
- 12. Rajaonarivony M,, Vauthier C,, Couarraze G,, Puisieux F,, Couvreur P. Development of a new drug carrier made from alginate. J Pharm Sci. 1993; 82: 912–917. [DOI] [PubMed] [Google Scholar]
- 13. Sechoy O,, Tissie G,, Sebastian C,, Maurin F,, Driot JY,, Trinquand C. A new long acting ophthalmic formulation of carteolol containing alginic acid. Int J Pharm. 2000; 207: 109–116. [DOI] [PubMed] [Google Scholar]
- 14. Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996; 80: 389–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gao H,, Qiao X,, Cantor LB,, WuDunn D. Up-regulation of brain-derived neurotrophic factor expression by brimonidine in rat retinal ganglion cells. Arch Ophthalmol. 2002; 120: 797–803. [DOI] [PubMed] [Google Scholar]
- 16. Holden CA,, Tyagi P,, Thakur A,, et al. Polyamidoamine dendrimer hydrogel for enhanced delivery of antiglaucoma drugs. Nanomedicine. 2012; 8: 776–783. [DOI] [PubMed] [Google Scholar]
- 17. Aburahma MH,, Mahmoud AA. Biodegradable ocular inserts for sustained delivery of brimonidine tartarate: preparation and in vitro/in vivo evaluation. AAPS PharmSciTech. 2011; 12: 1335–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Greenfield DS,, Liebmann JM,, Ritch R. Brimonidine: a new alpha2-adrenoreceptor agonist for glaucoma treatment. J Glaucoma. 1997; 6: 250–258. [PubMed] [Google Scholar]
- 19. Yoles E,, Wheeler LA,, Schwartz M. Alpha2-adrenoreceptor agonists are neuroprotective in a rat model of optic nerve degeneration. Invest Ophthalmol Vis Sci. 1999; 40: 65–73. [PubMed] [Google Scholar]
- 20. Bhagav P,, Upadhyay H,, Chandran S. Brimonidine tartrate-eudragit long-acting nanoparticles: formulation optimization, in vitro and in vivo evaluation. AAPS PharmSciTech. 2011; 12: 1087–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Singh KH,, Shinde UA. Chitosan nanoparticles for controlled delivery of brimonidine tartrate to the ocular membrane. Die Pharmazie. 2011; 66: 594–599. [PubMed] [Google Scholar]
- 22. Ibrahim MM,, Abd-Elgawad AE,, Soliman OA,, Jablonski MM. Novel topical ophthalmic formulations for management of glaucoma. Pharm Res. 2013; 30: 2818–2831. [DOI] [PubMed] [Google Scholar]
- 23. Niwa T,, Takeuchi H,, Hino T,, Kunou N,, Kawashima Y. Preparations of biodegradable nanospheres of water-soluble and insoluble drugs with D L-lactide/glycolide copolymer by a novel spontaneous emulsification solvent diffusion method, and the drug release behavior. J Control Release. 1993; 25: 89–98. [Google Scholar]
- 24. Ibrahim MM,, Abd-Elgawad AE,, Soliman OA,, Jablonski MM. Nanoparticle-based topical ophthalmic formulations for sustained celecoxib release. J Pharm Sci. 2013; 102: 1036–1053. [DOI] [PubMed] [Google Scholar]
- 25. Ibrahim MM,, Abd-Elgawad AH,, Soliman OA,, Jablonski MM. Natural bioadhesive biodegradable nanoparticles-based topical ophthalmic formulations for sustained celecoxib release: in vitro study. J Pharm Tech Drug Res. 2013; 2: 7. [DOI] [PubMed] [Google Scholar]
- 26. Kuzma JW,, Bohnenblust SE. Basic Statistics for the Health Sciences. New York, NY: McGraw-Hill Education; 2005. [Google Scholar]
- 27. Zhang Y,, Zhuo RX. Synthesis, characterization, and in vitro 5-Fu release behavior of poly(2,2-dimethyltrimethylene carbonate)-poly(ethylene glycol)-poly(2,2-dimethyltrimethylene carbonate) nanoparticles. J Biomed Mat Res A. 2006; 76: 674–680. [DOI] [PubMed] [Google Scholar]
- 28. Seijo B,, Fattal E,, Roblot-Treupel L,, Couvreur P. Design of nanoparticles of less than 50 nm diameter: preparation, characterization and drug loading. Int J Pharm. 1990; 62: 1–7. [Google Scholar]
- 29. Scholes P,, Coombes A,, Illum L,, Daviz S,, Vert M,, Davies M. The preparation of sub-200 nm poly (lactide-co-glycolide) microspheres for site-specific drug delivery. J Control Release. 1993; 25: 145–153. [Google Scholar]
- 30. USP29-NF24. Pharmaceutical dosage forms, ophthalmic preparation and uniformity of dosage units chapters. : The United States Pharmacopeia 29th, The National Formulary 24th. Rockville, MD: United States Pharmacopeial Convention, Inc.; 2005. [Google Scholar]
- 31. Vandervoort J,, Ludwig A. Biocompatible stabilizers in the preparation of PLGA nanoparticles: a factorial design study. Int J Pharm. 2002; 238: 77–92. [DOI] [PubMed] [Google Scholar]
- 32. Wu H,, Liu Z,, Peng J,, et al. Design and evaluation of baicalin-containing in situ pH-triggered gelling system for sustained ophthalmic drug delivery. Int J Pharm. 2011; 410: 31–40. [DOI] [PubMed] [Google Scholar]
- 33. Hammer ME,, Burch TG. Viscous corneal protection by sodium hyaluronate chondroitin sulfate, and methylcellulose. Invest Ophthalmol Vis Sci. 1984; 25: 1329–1332. [PubMed] [Google Scholar]
- 34. Lin HR,, Sung KC. Carbopol/pluronic phase change solutions for ophthalmic drug delivery. J Control Release. 2000; 69: 379–388. [DOI] [PubMed] [Google Scholar]
- 35. Kim TH,, Jeong YI,, Jin SG,, et al. Preparation of polylactide-co-glycolide nanoparticles incorporating celecoxib and their antitumor activity against brain tumor cells. Int J Nanomed. 2011; 6: 2621–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huang YY,, Chung TW,, Tzeng TW. A method using biodegradable polylactides/polyethylene glycol for drug release with reduced initial burst. Int J Pharm. 1999; 182: 93–100. [DOI] [PubMed] [Google Scholar]
- 37. Garcia-Fuentes M,, Torres D,, Alonso MJ. New surface-modified lipid nanoparticles as delivery vehicles for salmon calcitonin. Int J Pharm. 2005; 296: 122–132. [DOI] [PubMed] [Google Scholar]
- 38. Budhian A,, Siegel SJ,, Winey KI. Controlling the in vitro release profiles for a system of haloperidol-loaded PLGA nanoparticles. Int J Pharm. 2008; 346: 151–159. [DOI] [PubMed] [Google Scholar]
- 39. Leroux JC,, Allémann E,, De Jaeghere F,, Doelker E,, Gurny R. Biodegradable nanoparticles from sustained release formulations to improved site specific drug delivery. J Control Release. 1996; 39: 339–350. [Google Scholar]
- 40. Tamizharasi S,, Rathi JC,, Rathi V. Formulation and evaluation of pentoxifylline-loaded poly(epsilon-caprolactone) microspheres. Indian J Pharm Sci. 2008; 70: 333–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Korsmeyer RW,, Gurny R,, Doelker E,, Buri P,, Peppas NA. Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm. 1983; 15: 25–35. [DOI] [PubMed] [Google Scholar]
- 42. Dash S,, Murthy PN,, Nath L,, Chowdhury P. Kinetic modeling on drug release from controlled drug delivery systems. Acta PolonPharm. 2010; 67: 217–223. [PubMed] [Google Scholar]
- 43. Hong Lu LL,, Watson ER,, Williams RW,, Geisert EE,, Jablonski MM,, Lu L. Complex interactions of Tyrp1 in the eye. MolVis. 2011; 17: 2455–2468. [PMC free article] [PubMed] [Google Scholar]
- 44. Lu H,, Wang XS,, Pullen M,, et al. Genetic dissection of the Gpnmb network in the eye. Invest Ophthalmol Vis Sci. 2011; 52: 4132–4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Swaminathan SLH,, Williams R,, Lu L,, Jablonski MM. Genetic modulation of the iris transillumination defect: a systems genetics analysis using the expanded family of BXD glaucoma strains. Pigment Cell Melanoma Res. 2013; 26: 487–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhou M,, Donovan MD. Intranasal mucociliary clearance of putative bioadhesive polymer gels. Int J Pharm. 1996; 135: 115–125. [Google Scholar]
- 47. Cohen S,, Lobel E,, Trevgoda A,, Peled Y. A novel in situ-forming ophthalmic drug delivery system from alginates undergoing gelation in the eye. J Control Release, 1997; 44: 201–208. [Google Scholar]
- 48. Cantor LB. Brimonidine in the treatment of glaucoma and ocular hypertension. Ther Clin Risk Manag. 2006; 2: 337–346. [DOI] [PMC free article] [PubMed] [Google Scholar]