Abstract
Major strides have been made in improving the treatment of medical emergencies associated with malignancies. Nonetheless, metabolic emergencies in cancer patients can often times be life-threatening. Type B lactic acidosis is a rare but potentially fatal paraneoplastic phenomenon that has been described in association with hematologic and solid malignancies and portends a poor prognosis if not rapidly recognized and treated. It is believed that this occurs as a result of cancer cells switching their glucose metabolism from an oxidative oxygen- dependent pathway towards a glycolytic phenotype, also known as the “Warburg effect”. Though rare, it is important to consider this entity in the differential diagnosis of type B lactic acidosis since prompt identification and treatment may help improve outcomes in this otherwise fatal process. We present a case of type B lactic acidosis in a patient with chronic lymphocytic leukemia along with a brief review of the literature.
Keywords: Lactic acidosis, malignancy, hematologic emergency
Introduction
Otto Warburg was a German physiologist awarded a Nobel Prize in 1931 for his pioneering work with respiratory enzymes [1]. Later on, his continued investigations led to the discovery that cancer cells would produce lactate from glucose even under normoxic conditions, a process termed aerobic glycolysis [2]. It is thought that this reprogramming of energy metabolism, also known as the Warburg effect, which at first seems counterintuitive, eventually enables cancer cells to divert their biosynthetic machinery to fuel the assembly line of new cancer cells [3-6]. More recent work has also demonstrated that the “Warburg phenotype” can be switched on/off depending on the microenvironment supply of oxygen [4]. Thus, upregulated tumor metabolism plays a central role in supporting cancer cell proliferation, growth, survival, and tumor progression [3-6].
Lactic acidosis is a medical emergency and in a majority of cases it reflects tissue depleted of oxygen supply due to hypoperfusion, also known as type A lactic acidosis. Type B lactic acidosis on the other hand, occurs under normoxic conditions, with no evidence of organ hypoperfusion, and is usually a result of drug or toxin interference of cellular metabolism or a nutritional deficiency state, such as thiamine deficiency (Table 1) [7-9]. Type B lactic acidosis has also rarely been reported in association with solid and more often with hematological malignancies [7-10]. The mechanism behind this is incompletely understood and probably multifactorial, but it may be partly explained by an enhanced aerobic glycolytic activity in the cancer cell triggered by oncogenic lesions such as expression of hypoxia-inducible factor-1α (HIF1α). These changes may ultimately promote increased glucose uptake by the tumor and diversion from the normal oxidative process towards a glycolytic pathway with generation of lactate [11].
Table 1.
Etiologies of lactic acidosis
| Type A lactic acidosis | Type B lactic acidosis | Type D lactic acidosis |
|---|---|---|
| Hypoperfusion conditions | Diabetes | Short bowel syndrome |
| Massive hemorrhage | Drugs: metformin | Diabetic ketoacidosis |
| Shock | Toxins: ethanol | |
| Septic shock | Malignancy | |
| Trauma | HIV: antiretroviral | |
| Thiamine deficiency |
Case report
The patient was a 67 years old male diagnosed with chronic lymphocytic leukemia with CD38 (dim), ZAP-70 (dim) positivity. He was initially observed without therapy for about two years up until December 2012 when he showed signs of symptomatic and radiologic progression. He was started on a Lenalidomide in the context of a clinical trial with partial hematologic response but was discontinued due to side effects. He remained on observation for 10 months after which clinical and radiological assessment showed marked disease progression with worsening peripheral and intra-abdominal lymphadenopathy, retroperitoneal bulky lymph node conglomerate and worsening splenomegaly. A PET-CT scan showed extensive hyper-metabolic activity in the nodal areas. A biopsy of an enlarging cervical lymph node (SUV 11) did not reveal any histological transformation, but only infiltration by CLL. He was referred to our institution for further management and was offered a rituximab based regimen with Bendamustine. His WBC was 120 k/μL, platelets 30 k/μL and Hb 10 g/dL, LDH was 680 U/L.
Outcome
The patient received a dose of Rituximab but Bendamustine was initially held due to thrombocytopenia. He returned to the hospital 5 days later with laboratorial evidence of tumor lysis syndrome (uric acid 13 mg/dl) accompanied by acute kidney injury (creatinine 1.5 mg/dL) and hyperkalemia. He received Rasburicase 6 mg intravenously and was aggressively hydrated with rapid improvement of his creatinine and uric acid to normal levels. He had complains of mild respiratory distress at admission and had a persistent tachypnea and tachycardia. Further workup revealed a marked anion gap metabolic acidosis. Lactic acid level was 16 mmol/L and pH 7.13. Chest x-ray was unrevealing. There were no signs of hypoperfusion or sepsis to explain his metabolic acidosis. There was also no history of diabetes or use of metformin or another potential drug that could have caused lactic acidosis. Blood cultures and urine cultures were negative. A repeat CT scan of chest/abdomen/pelvis to assess his disease status showed massive splenomegaly and bulky retroperitoneal abdominal lymphadenopathy with central necrosis. No liver involvement was appreciated in the computed tomography scan, but liver function tests (LFTs) were altered with a cholestatic pattern. His LDH steadily increased up to 11,000 U/L in a matter of 6 days after his Rituximab treatment. His WBC count went up to 151 k/μL. Refractory acidemia ensued and he was started on bicarbonate drip with partial correction of his acidosis. A concern was raised for transformation to acute prolymphocytic leukemia and leukostasis and he was initiated on leukapheresis. Despite these measures, his lactic acid levels continued to climb up to 24 U/L. His renal function and eletrolytes remained mostly normal after being treated with rasburicase. LFTS continued to worsen and he developed laboratory evidence of disseminated intravascular coagulation as well as severe hypoglycemia needing treatment with continuous infusion of 10% dextrose. A few hours after initiating leukapheresis his hemodynamics status deteriorated with hypotension refractory to vasopressors. Patient and his family changed his goals of care to comfort measures only and he expired a few hours later. No necropsy was performed.
Discussion
Hematological malignancies, including lymphomas and leukemias affect over 130,000 individuals annually in the United States alone [12]. Most of these malignancies are highly treatable and potentially curable. One of the challenges in treating hematological malignancies is their obvious intrinsic relationship with the blood/lymphatic system, which can lead to unavoidable remote and systemic consequences by the disease itself or as a result of its treatment. Type B lactic acidosis is a rare but potentially life threatening emergency that has been described mostly in hematological malignancies, though a few cases involving solid tumors have been documented as well [8-10].
The hypothesized mechanism behind type B lactic acidosis in hematological malignancies may be explained by the full expression of the “Warburg effect” [2]. Also termed aerobic glycolysis, the “Warburg” effect is an increasingly recognized cancer cell hallmark, since its initial description in 1920 [1]. It is well known that many solid and hematological cancer cells are avid utilizers of glucose via upregulation of key glucose transporters and hexokinases [3,5,11]. As cancer cells grow, they constantly outpace their blood supply, and are exposed to a range of oxygen concentration in their microenvironment [3,5]. The environmental pressure in the tumor microenvironment eventually selects the clones that have adapted their metabolic machinery to an upregulated glycolytic phenotype. These changes result in an inefficient production of ATP, with the generation of 2 ATP molecules only, as opposed to the 36 ATP molecules generated during a normal oxidative process (Figure 1). As a consequence, an excessive amount of lactic acid and H+ are produced and accumulated locally, creating an acidic microenvironment. Local hypoxic environment and acidosis promotes expression of survival genes, invasion, enhanced metastatic potential, clonal evolution and radio-resistance. Ultimately, this counterintuitive glycolytic phenotype enables the fittest cancer clone to survive, compete and thrive in a harsh environment [3-6,11].
Figure 1.
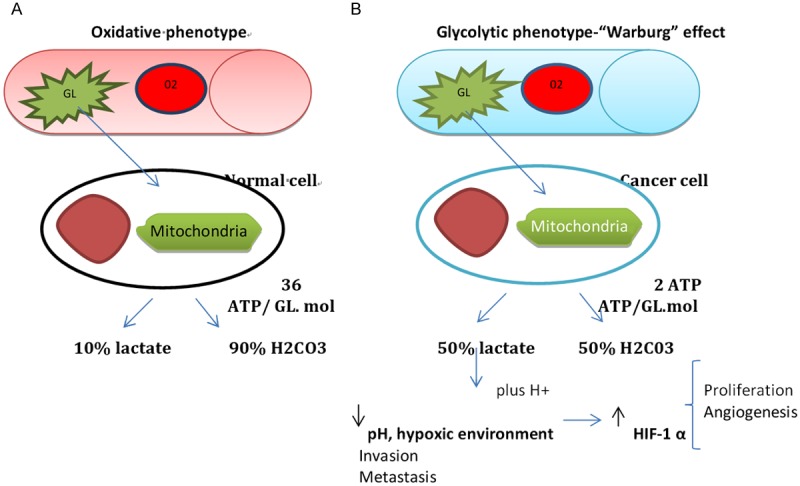
A. Oxydative pathway utilized by normal cell, largely dependent on oxygen concentration. B. Malignant phenotype with acquired glycolytic pathway, regardless of oxygen concentration. Gl-glucose, O2-RBC carrying oxygen, Mol-molecule, HIF-1- Hypoxia inducible factor 1.
However, in spite of cancer cells generating lactate byproducts in the tumor microenvironment, systemic effects are rarely significant. So, why is it that in certain malignancies, the clinical course is complicated by systemic lactic acidosis? Concomitant nutritional deficiency, such as thiamine deficiency, decreased lactate clearance caused by concomitant liver and/or renal impairment or cancer cells in high proliferative mode may help amplify the process and at least partly explain this association. Indeed, many of the cases reported in the literature showed some evidence of renal or liver impairment [13-15]. In addition, thiamine deficiency, a critical cofactor for pyruvate dehydrogenase, is frequently observed in malignancy and may further divert the glucose away from the Krebs cycle into the production of lactate.
Type B lactic acidosis has been reported with a range of hematological malignancies such as Hodgkin lymphoma, Non-Hodgkin lymphoma (NHL), acute and chronic leukemias and hystiocytosis. A collection of case reports in the literature by Ruiz et al indicated that lactate levels ranged from 4-46 mmol/L in the cases reported, despite no signs of systemic hypoperfusion, signaling a different non-hypoxia related etiologic mechanism. Intravenous bicarbonate infusion was used in the majority of the cases to attenuate the systemic effects of acidemia. In addition, implementation of renal replacement therapy and thiamine replacement was also used in some patients including ours. Hypoglycemia, likely related to high glucose consumption by the tumor, was a conspicuous feature in our case and several others reported [13-15].
Type B lactic acidosis portends a grim prognosis. In a literature review, out of 29 cases reported, 22 patients died within 2 days to 5 weeks range since its detection. A total of seven patients survived after initiation of chemotherapy with normalization of their lactic acid [14].
Looking retrospectively in our case it seems that our patient suffered a steady and relentless progression of his CLL since diagnosis. Lenalidomide therapy probably slowed the pace of his progression but it did not hold it for very long. His PET-CT scan showed avid SUV uptake in the neck and abdomen, denoting a high metabolic activity of his tumor and raising suspicion for transformation. Nonetheless, an excisional biopsy of the highly metabolic neck lymph node did not confirm Ritcher’s transformation. Additionally, despite noticing an increased population of pro-lymphocytes in the peripheral blood at the time of plasmapheresis, no conclusion could be drawn to confirm pro-lymphocytic transformation. Also, he had little to no response to rituximab and in retrospect, it would probably have been better to give him the bendamustine early regardless of severe thrombocytopenia to see if it would have attenuated the malignant cells sufficiently to stop the process of progressive acidosis.
Altogether, despite the presence in this patient of trisomy 12, a favorable prognostic finding, the poor prognostic features of ZAP-70 and CD38 expression on his tumor probably weighted heavily on the outcome. This was reflected by a rather aggressive clinical course with a demonstration of a large tumor burden and bulky retroperitoneal disease. Interestingly, a necrotic center noted on his bulky lymphadenopathy on the CT scan probably reflects the hypoxic environment in the tumor. This was also reflected by his skyrocketing LDH in a few days. This was further complicated by progressive liver impairment, a critical organ for lactate clearance. Thus, an exaggerated “Warburg effect” (high tumor burden), co-substantiated by development of persistent hypoglycemia (tumor consumption), along with worsening liver impairment (decreased lactate clearance) were key determining factors to cause the perfect storm in this patient.
Outlook
Despite its rarity, the case reported above highlights the importance of an accurate and prompt diagnosis of type B lactic acidosis followed by an appropriate interventional approach. As depicted above, tumor-related Type B L.A seems to be the end result of interplay of several factors. Hence, directing actions at several fronts simultaneously may be a more successful strategy. Optimization of L.A clearance through renal replacement therapy, correction of nutritional deficiencies (e.g. with thiamine replacement) and L.A buffering with bicarbonate infusion seem to be appropriate supportive measures. Finally, urgent and aggressive treatment of the underlying malignancy should be a crucial element of the overall strategy without which the rest of the measures are unlikely to be of much benefit.
Conclusion
Based on available data, mostly consisting of case reports and small series, type B acidic acidosis represents a life-threatening oncologic emergency and carries a very poor prognosis. Thus, physicians must entertain the diagnosis of type B lactic acidosis in any patient with hematological malignancies who develop unexplained anion gap acidosis with a normal blood pressure or deterioration of their respiratory status without an obvious cause. An urgent and aggressive strategy upfront to simultaneously treat the underlying malignancy as well as the metabolic derangements (with early use of renal replacement therapy) may reduce the mortality and improve outcomes. Managing the severe metabolic disorder however is challenging with current modalities and improvements in this area would be a significant advance by allowing more time for controlling the underlying disease.
The growing appreciaronment through the glycolytic pathway seems to promote growth, invasion and metastasis. Thus, targeting the lactate pathway seems to be a reasonable strategy. Inhibitors of lactatetion that tumor metabolism is an important feature for cancer cell evolution, has led researchers in a quest to target this pathway. Local generation of lactate in the tumor microenvi dehydrogenase A (LDHA) and/or the monocarboxylate transporters (MCTs) have been shown to have anti-tumor effects in the pre-clinical setting and have advanced their way into human clinical trials. Further exploitation of tumor metabolism pathways may not only hold promise for future approaches against cancer but may also allow for better control of lactic acidosis in patients such as ours by targeted blockade of the responsible pathways rather than inefficient attempts to mitigate the downstream effects such as is done with most current modalities [11].
References
- 1. http://www.nobelprize.org/nobel_prizes/medicine/laureates/1931/
- 2.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Weiberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Xie J, Wu H, Dai C, Pan Q, Ding Z, Hu D, Ji B, Luo Y, Hu X. Beyond Warburg effect - dual metabolic nature of cancer cells. Sci Rep. 2014;4:4927. doi: 10.1038/srep04927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gillies RJ, Gatenby RA. Adaptive landscapes and emergent phenotypes: why do cancers have high glycolysis? J Bioenerg Biomembr. 2007;39:251–7. doi: 10.1007/s10863-007-9085-y. [DOI] [PubMed] [Google Scholar]
- 6.Bonuccelli G, Tsirigos A, Whitaker-Menezes D, Pavlides S, Pestell RG, Chiavarina B, Frank PG, Flomenberg N, Howell A, Martinez-Outschoorn UE, Sotgia F, Lisanti MP. Ketones and lactate “fuel” tumor growth and metastasis: Evidence that epithelial cancer cells use oxidative mitochondrial metabolism. Cell Cycle. 2010;9:3506–14. doi: 10.4161/cc.9.17.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spinazzé S, Schrijvers D. Metabolic emergencies. Crit Rev Oncol Hematol. 2006;58:79–89. doi: 10.1016/j.critrevonc.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Kraut JA, Madias NE. Lactic Acidosis. N Engl J Med. 2014;371:2309–2319. doi: 10.1056/NEJMra1309483. [DOI] [PubMed] [Google Scholar]
- 9.Andersen LW, Mackenhauer J, Roberts JC, Berg KM, Cocchi MN, Donnino MW. Etiology and therapeutic approach to elevated lactate levels. Mayo Clin Proc. 2013;88:1127–40. doi: 10.1016/j.mayocp.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Groot R, Sprenger RA, Imholz AL, Gerding MN. Type B lactic acidosis in solid malignancies. Neth J Med. 2011;69:120–3. [PubMed] [Google Scholar]
- 11.Doherty JR, Cleveland JL. Targeting lactate metabolism for cancer therapeutics. J Clin Invest. 2013;123:3685–92. doi: 10.1172/JCI69741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 13.Yun S, Walker CN, Vincelette ND, Anwer F. Acute renal failure and type B lactic acidosis as first manifestation of extranodal T-cell lymphoblastic lymphoma. BMJ Case Rep. 2014;9:1–4. doi: 10.1136/bcr-2014-205044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan FH, Carl D, Lyckholm LJ. Severe lactic acidosis in a patient with B-cell lymphoma: a case report and review of the literature. Case Rep Med. 2009;2009:1–6. doi: 10.1155/2009/534561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedenberg AS, Brandoff DE, Schiffman FJ. Type B lactic acidosis as a severe metabolic complication in lymphoma and leukemia: a case series from a single institution and literature review. Medicine. 2007;86:225–32. doi: 10.1097/MD.0b013e318125759a. [DOI] [PubMed] [Google Scholar]
- 16.Ruiz JP, Singh AK, Hart P. Type B lactic acidosis secondary to malignancy: case report, review of published cases, insights into pathogenesis, and prospects for therapy. ScientificWorldJournal. 2011;11:1316–24. doi: 10.1100/tsw.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]


