Abstract
One mechanism contributing to immunologic unresponsiveness toward tumors may be presentation of tumor antigens by tolerogenic host APCs. We show that mouse tumor-draining LNs (TDLNs) contained a subset of plasmacytoid DCs (pDCs) that constitutively expressed immunosuppressive levels of the enzyme indoleamine 2,3-dioxygenase (IDO). Despite comprising only 0.5% of LN cells, these pDCs in vitro potently suppressed T cell responses to antigens presented by the pDCs themselves and also, in a dominant fashion, suppressed T cell responses to third-party antigens presented by nonsuppressive APCs. Adoptive transfer of DCs from TDLNs into naive hosts created profound local T cell anergy, specifically toward antigens expressed by the transferred DCs. Anergy was prevented by targeted disruption of the IDO gene in the DCs or by administration of the IDO inhibitor drug 1-methyl-D-tryptophan to recipient mice. Within the population of pDCs, the majority of the functional IDO-mediated suppressor activity segregated with a novel subset of pDCs coexpressing the B-lineage marker CD19. We hypothesize that IDO-mediated suppression by pDCs in TDLNs creates a local microenvironment that is potently suppressive of host antitumor T cell responses.
Introduction
The immune system of tumor-bearing hosts fails to respond protectively to tumor antigens. Functionally, the host is tolerant toward the tumor (1). This is not due to a peculiarity of tumor antigens, since even highly immunogenic viral proteins can become tolerizing when introduced on tumors (2). The molecular mechanisms underlying tumor-induced tolerance are still poorly understood. Contributing factors may include a component of immunologic “ignorance” (3–5), particularly in the early phase of tumor growth, as well as active suppression by regulatory T cells (Treg’s) (6). In addition, it has been proposed that certain host APCs may present tumor antigens in a fashion that leads to tolerance (7–9).
Presentation of tumor antigens by host APCs (indirect presentation, or cross-presentation) is a key step by which naive T cells first become aware of tumor-derived antigens. Cross-presentation can lead to activation of tumor-specific T cells (10, 11), although usually without regression of established tumors, or it can lead to anergy (8, 9). DCs are the key APCs for naive T cells (12, 13), and it is now clear that DCs may be either activating or tolerizing (14). However, the specific role of tolerogenic DCs in tumor immunology is not yet well defined.
In part, this may be because the mechanisms by which DCs create tolerance in T cells are still poorly understood (15). In the current study we focus on the tryptophan-degrading enzyme indoleamine 2,3-dioxygenase (IDO), an immunosuppressive mechanism shared by several different cell types in the immune system (16, 17). Expression of IDO by certain types of human monocyte-derived macrophages and in vitro–derived DCs allows them to inhibit T cell proliferation (18–20). In vivo, endogenous IDO has been implicated in maternal tolerance toward the allogeneic fetus (21); in tolerance to self antigens in NOD mice (22); as a downstream effector mechanism for the tolerance-inducing agent CTLA4–Ig (23); and as a protective negative regulator of experimentally induced autoimmune disorders (24, 25).
Based on this, we and others have hypothesized that IDO may be exploited by tumors as a mechanism of immune evasion (26). Consistent with this possibility, IDO has been shown to be expressed by a variety of primary human tumors and human tumor cell lines (27, 28). Transfection of recombinant IDO into murine tumor cell lines confers the ability to inhibit antigen-specific T cell responses in vitro (29). In vivo, IDO-transfected tumor cells are able to escape immune rejection, even in previously immunized hosts that would normally be fully protected against tumor challenge (27). These data suggest that expression of IDO by malignant cells could contribute to local immunosuppression at the site of tumors.
However, expression of IDO by the tumor cells themselves would not affect cross-presentation by host APCs. To influence this critical regulatory step, IDO would need to be expressed in host DCs in tumor-draining LNs (TDLNs). Consistent with this possibility, we have reported that certain patients display a prominent population of IDO-expressing host-derived mononuclear cells in TDLNs (20, 30). Similar cells are also seen in murine TDLNs (31). However, the immunologic role of these cells is unknown. In the current study we use a murine tumor model to test the hypothesis that IDO-expressing cells in TDLNs represent a population of tolerogenic host DCs.
Results
IDO-expressing cells in human TDLNs correlate with a worse clinical outcome.
The starting point for our murine studies was the clinical observation that a subset of patients with malignant tumors (breast carcinoma, malignant melanoma, and a number of other solid tumors) displayed abnormal accumulation of IDO-expressing cells in the LNs draining the tumors (Figure 1A). A pilot study was performed using archival specimens from 40 patients with malignant melanoma. Figure 1B shows that the presence of abnormal IDO+ cells in the sentinel LN at the time of initial diagnosis correlated with a significantly worse long-term outcome. None of these patients had detectable LN metastases at the time of diagnosis (by H&E and immunohistochemistry), which suggested that the recruitment of IDO+ cells occurred early in the course of the disease. While this small pilot study was not intended to validate IDO as a clinical prognostic marker, the findings were consistent with the possibility that IDO might play a mechanistic role in the biology of certain tumors. This prompted the development of a defined mouse model in which to test this hypothesis.
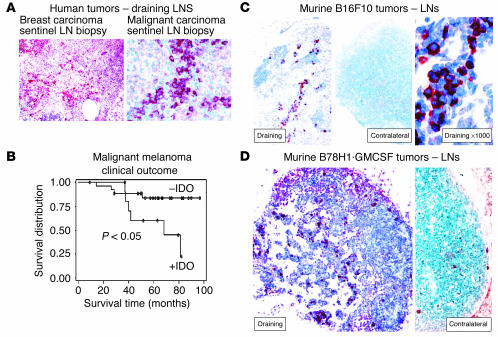
Figure 1.
Expression of IDO in human and murine TDLNs. (A) Sentinel (first draining) LN from patients with breast carcinoma (left, ×100) and malignant melanoma (right, ×400), showing an abnormal infiltration of IDO+ cells (red chromogen). (B) Kaplan-Meier survival plot of 40 patients with malignant melanoma, stratified into those with an abnormal accumulation of IDO+ cells in the sentinel LN (+IDO), versus a normal (negative) pattern. (C) Expression of IDO in murine B16F10 melanoma. Left: Draining inguinal LN from a mouse with a B16F10 tumor, day 12, stained for IDO (red, ×100). Middle: Contralateral inguinal LN from the same animal as at left, stained for IDO (red, ×100). Right: High-power view of IDO+ cells shown in the left panel (×1,000). Controls for staining (anti-IDO antibody neutralized with the immunizing peptide) showed a negative pattern similar to that seen in the contralateral LN (not shown). (D) Draining and contralateral LNs from a mouse with B78H1–GM-CSF tumor, day 12, stained for IDO (red, both ×200).
IDO-expressing cells in murine TDLNs.
Figure 1C shows that IDO+ cells were found in TDLNs of established B16F10 mouse melanomas. The contralateral (non–tumor-draining) LNs from the same animals showed few or no IDO-expressing cells (Figure 1C). Although the IDO+ cells were morphologically similar to the plasmacytoid-appearing cells seen in humans, the absolute number of these cells found in the B16F10 model was much lower than in heavily infiltrated human LNs. Therefore, we tested a subline of B16 (clone B78H1) that had been transfected with the cytokine GM-CSF (32), which markedly increases the number of APCs recruited into TDLNs (33). Figure 1D shows that B78H1·GM–CSF tumors recruited significantly more IDO-expressing cells. As with B16F10 tumors, the IDO+ cells were prominent only in the draining node; few or none were observed in the contralateral nodes from the same animal (Figure 1D, right panel), and in systemic sites such as spleen (not shown).
TDLNs contain a population of suppressive plasmacytoid DCs.
Since the B78H1·GM-CSF tumors yielded the most DCs, these were used for functional studies. Single-cell suspensions were prepared from TDLNs, or from the paired contralateral LNs, and used as stimulator cells in mixed leukocyte reactions (MLRs). Responder cells were T cell receptor–transgenic (TCR-transgenic) CD8+ T cells from BM3 mice (34), which recognize H2Kb as an alloantigen. (This antigen was constitutively expressed by all APCs from the tumor-bearing C57BL/6 mice, and preliminary validation studies confirmed that expression of H2Kb was identical in DCs from TDLNs and normal LNs.) As shown in Figure 2A, TDLN cells appeared to be poor stimulators of BM3 T cells, whereas cells from the contralateral LNs of the same animals were excellent stimulators (comparable to LN cells from non–tumor-bearing mice, not shown).
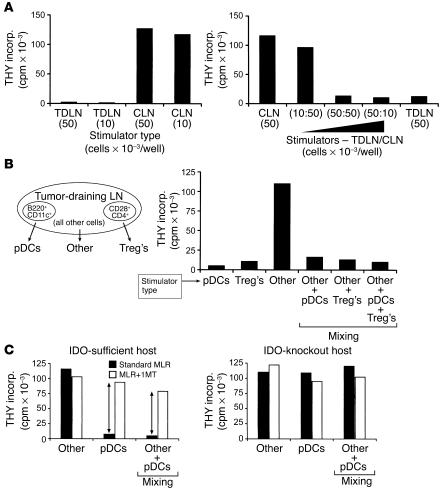
Figure 2.
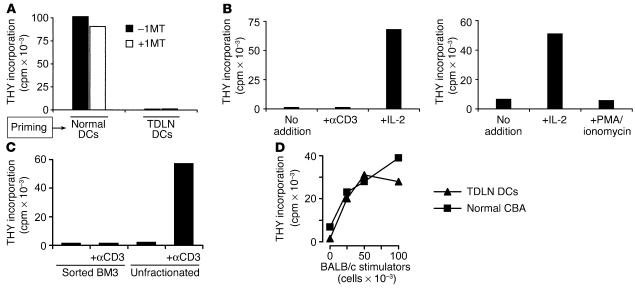
Suppression of T cell responses by TDLN cells. (A) Cells from TDLNs and contralateral LNs (CLN) were harvested from mice with B78H1–GM-CSF tumors (day 14), and used as stimulators in MLRs (stimulator cell number in parentheses; responder BM3 T cells at 5 × 104 per well). Mixing experiments (right) demonstrated dominant suppression by the TDLN cells. (B) TDLN cells were sorted by four-color flow cytometry into pDCs (<1%), Treg’s (2–3%), and all other cells (95–97%), using the markers shown. Each fraction was used as stimulator cells in MLRs, adding the number of cells that would have been present in 5 × 104 of the original TDLN population (thus, the pDCs were added at approximately 500 cells per well). All MLRs received 5 × 104 BM3 responders. For mixing experiments, the sorted fractions were mixed in the same ratio in which they were present in the original unsorted preparation. (C) Suppression by pDCs is mediated by IDO. TDLN cells were sorted as described for B. The pDCs and “other” fraction were used as stimulators in MLRs (BM3 responders), with and without the IDO inhibitor 1MT. Left: TDLN cells taken from a wild-type (IDO-sufficient) host, showing suppression by pDCs, which was blocked by 1MT (arrows), and dominant suppression with mixing. Right: Tumors were grown in IDO-KO hosts, and the IDO-KO TDLN cells were harvested and assayed as at left. THY incorp., thymidine incorporation.
The lack of response to TDLN cells might be due either to inadequate APC function, or to some form of active suppression. Mixing experiments (Figure 2A, right panel) showed that the lack of proliferation was due to a dominant suppressor activity present in the TDLN cells. Sorting experiments (Figure 2B) revealed that one component of this suppression segregated with the CD25+CD4+ (Treg) fraction (as expected, since Treg’s are known to be present in mice with B16 tumors [ref. 35]). However, removing the Treg’s did not abrogate the suppressor activity. Further fractionation revealed the existence of a second, equally potent suppressor activity associated with the CD11c+B220+ fraction, which is the phenotype of murine plasmacytoid DCs (pDCs). This population contained all of the remaining suppressor activity, such that when both the pDCs and the Treg’s were removed, the remaining 95–97% of TDLN cells (which included all the APCs other than pDCs) stimulated excellent proliferation. Mixing experiments showed that suppression mediated by pDCs was dominant over stimulation mediated by all other APCs: i.e., admixture of the <1% fraction of pDCs with the >95% of other, nonsuppressive cells inhibited T cell responses to both populations (Figure 2B).
Immunosuppression by pDCs is mediated by IDO.
To test the role of IDO in this system, MLRs were performed in the presence or absence of the IDO inhibitor 1-methyl-D-trytophan (1MT). Figure 2C shows that 1MT blocked the inhibitory activity of pDCs, converting them into potent stimulators of T cell proliferation. When pDCs and the nonsuppressive “other” fraction were mixed together, 1MT reversed the dominant suppression mediated by pDCs. The effect of 1MT was not due to nonspecific activation of T cells, since the same T cells stimulated by the nonsuppressive “other” fraction showed no effect of 1MT (Figure 2C). Next, tumors were implanted in mice with a targeted disruption of the IDO gene (IDO–KO mice). The pDCs isolated from TDLNs of IDO–KO mice showed no suppressor activity (Figure 2C, right panel), confirming that suppression required an intact IDO gene. Likewise, 1MT had no effect on MLRs stimulated by the IDO-KO DCs, confirming the specificity of 1MT for IDO.
In other experiments (not shown in the interest of space), sorted pDCs from TDLNs pulsed with SIINFEKL peptide inhibited proliferation of OVA-specific CD8+ OT-I T cells (36) by more than 90% compared with nonsuppressive APC controls. Just as with BM3 T cells, inhibition was fully reversed by 1MT, there was no inhibition by the B220– (nonplasmacytoid) fraction of DCs from the same TDLNs, and there was no inhibition by B220+ pDCs when they were derived from normal LNs (C57BL/6 mice without tumors).
IDO can suppress third-party T cells.
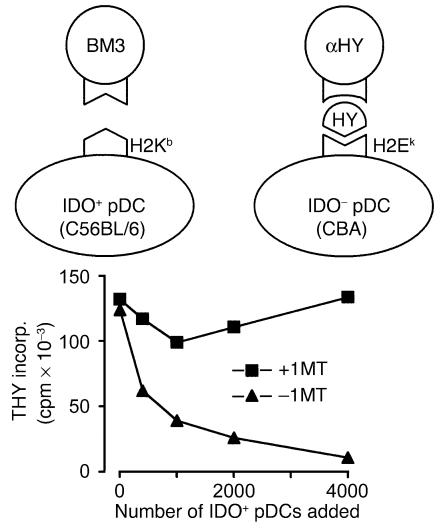
The mixing experiments in Figure 2C suggested that IDO could inhibit T cell responses even to antigens presented by neighboring, nonsuppressive APCs. To test this hypothesis, we performed mixing experiments using a defined population of third-party T cells that could only see antigen presented by nonsuppressive (IDO–) APCs. IDO+ pDCs and responder BM3 cells, as in the preceding experiments, were mixed with third-party TCR-transgenic CD4+ T cells (CBA background) recognizing an HY-derived peptide presented by H2Ek. APCs for these cells were nonsuppressive CD11c+ DCs from normal CBA spleen. (This experiment is shown schematically in Figure 3.) When the two sets of APC/responder pairs were mixed together, the IDO-expressing pDCs dominantly suppressed the HY-specific T cell proliferation, in a progressive dose-dependent fashion (Figure 3). Suppression was mediated by IDO, as shown by its reversal with 1MT.
Figure 3.
Dominant third-party suppression mediated by IDO+ pDCs. Two independent pairs of APCs and responder T cells were used to test the effect of IDO on third-party T cell responses. IDO+ pDCs from TDLNs presented H2Kb antigen to BM3 T cells, while IDO– DCs (sorted CD11c+ cells from normal CBA spleen) presented antigen to TCR-transgenic CD4+ T cells (recognizing a peptide from HY, restricted on H2Ek). In both cases, the APC/T cell ratio was 1:25. Graded numbers of the pDC+BM3 pair were added to 1 × 105 cells of the DC+αHY pair, and the total proliferation was measured after 72 hours. The number of IDO+ pDCs added is shown on the x axis (with 4,000 cells reflecting a 1:1 ratio between the two pairs of MLRs). Duplicate sets of wells received either 1MT (squares) or no 1MT (triangles).
Adoptive transfer of DCs from TDLNs creates immunologic unresponsiveness in vivo.
We next asked whether the DCs isolated from TDLNs were able to create immunologic unresponsiveness in new hosts without tumors. CD11c+ DCs were isolated from TDLNs of H2Kb-positive hosts and adoptively transferred to naive, allogeneic CBA hosts (H2Kb-negative). Recipients were preloaded with a cohort of H2Kb-specific BM3 T cells, so that defined antigen-specific responses could be followed. We chose an alloantigen for these studies (rather than a cross-presented tumor antigen) because the alloantigen was constitutively expressed on all DCs, thus ensuring that it would not be lost after adoptive transfer. To test whether the IDO+ DCs would dominantly suppress all antigen-specific T cell responses (even to H2Kb presented by nonsuppressive APCs in the transferred population, as predicted by Figure 3), we transferred all of the CD11c+ DCs from TDLNs, not just the IDO+ pDC fraction.
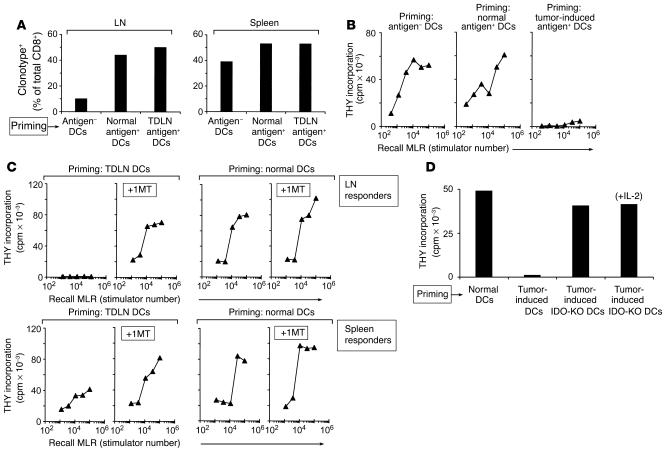
Ten days after adoptive transfer, T cells were isolated from recipients. Figure 4A shows that the transferred DCs induced selective accumulation of antigen-specific BM3 T cells in the LNs draining the site of injection (inguinal LNs). This accumulation was comparable whether the transferred DCs were derived from TDLNs or from normal C57BL/6 LNs, but it did not occur when DCs were derived from antigen-negative CBA mice. In all groups, the number of BM3 T cells in host spleens was similar, indicating that the initial systemic loading of BM3 was comparable.
Figure 4.
Adoptive transfer of DCs from TDLNs creates immunosuppression in new hosts. (A) Recruitment of BM3 T cells to draining LNs. CD11c+ DCs were purified from TDLNs and injected subcutaneously into CBA mice; recipients had previously received 4 × 107 BM3 splenocytes intravenously (CBA+BM3 hosts). Control recipients received normal CD11c+ DCs from antigen-postive C57BL/6 mice without tumors (Normal antigen+ DCs); or normal DCs from antigen-negative CBA mice (Antigen– DCs). After 10 days, LNs draining the site of DC injection (left) and spleens (right) were harvested. BM3 cells were enumerated by FACS using anti-clonotypic antibody (expressed as a percentage of the total CD8+ T cells). Each bar represents four pooled nodes. (B) Functional unresponsiveness of T cells primed with DCs from TDLNs. CBA+BM3 mice were primed as described above, and LN cells were used as responders in recall MLRs (1 × 105 responder cells with a titration of irradiated C57BL/6 splenocyte stimulators). (C) CBA+BM3 mice were primed for 10 days with DCs from TDLNs (left) or normal C57BL/6 LNs (right). Half of each group received 1MT (5 mg/d) via subcutaneous pellet as described in Methods, from the time of adoptive transfer until the end of the experiment; the other half received vehicle alone. Recall MLRs were performed as above. (D) Creation of unresponsiveness required functional IDO in the transferred DCs. Tumors were grown in IDO-KO mice, and TDLN DCs were isolated and used to prime CBA+BM3 mice, as in the preceding panels. Control recipients received TDLN DCs from wild-type hosts, or normal DCs from non–tumor-bearing mice. Just as above, normal DCs did not create unresponsiveness in recall MLRs, and IDO-sufficient TDLN DCs created complete unresponsiveness. The IDO-KO DCs, even though from TDLNs, did not create unresponsiveness; and responses were not further enhanced by addition of exogenous IL-2 to the recall MLR (last bar), which argues against any component of partial or cryptic anergy.
Inguinal LN cells from recipient mice were assayed for responsiveness in MLRs against H2Kb-positive targets (irradiated C57BL/6 splenocytes). Figure 4B shows that T cells from mice receiving TDLN DCs displayed profound hyporesponsiveness to recall antigen, despite the fact that there were ample BM3 T cells present in the LNs (Figure 4A). In contrast, T cells from LNs of animals receiving normal C57BL/6 DCs displayed a brisk MLR response. Mice receiving antigen-negative DCs also responded well in MLRs, showing that the large cohort of pre-positioned BM3 cells allowed a vigorous ex vivo response, without the need for previous priming. Thus, the lack of response in the mice receiving TDLN DCs represented the active elimination of a preexisting state of responsiveness, not a failure of priming.
Creation of immunologic unresponsiveness is IDO-dependent.
To test whether this acquired unresponsiveness was caused by IDO, recipient mice were treated with the IDO inhibitor 1MT, beginning at the time of adoptive transfer. After 10 days, T cells were harvested and tested for response to antigen. Figure 4C shows that administration of 1MT in vivo prevented the induction of T cell unresponsiveness by TDLN DCs. In contrast, 1MT had no effect on mice receiving normal DCs (right panels). In all experiments, flow cytometry confirmed that LNs from all groups contained comparable numbers of BM3 T cells (not shown). In the same experiments, we tested the response of systemic T cells from a remote site (spleen). Figure 4C (lower panels) shows that splenic T cells from animals primed with TDLN DCs displayed modestly reduced responses compared with controls, and that this was prevented by 1MT administration; however, splenic T cells did not show the complete unresponsiveness of cells from draining LNs.
The profound unresponsiveness created in draining LNs following adoptive transfer was prevented by coadministration of 1MT (and was thus IDO-dependent), but it was not clear whether the relevant IDO was expressed by the adoptively transferred DCs, or by some host cell. To test this, B78H1·GM–CSF tumors were grown in IDO-KO mice, and then the IDO-KO TDLN DCs were harvested and transferred into CBA+BM3 hosts, exactly as in the preceding experiments. Figure 4D shows that IDO-deficient DCs from TDLNs did not create unresponsiveness in the recipient T cells, despite the fact that the tumor, the CBA recipients, and the BM3 T cells were all wild-type for IDO. Thus, the relevant site of expression for IDO was in the transferred DCs. Further, the only mechanistic intervention required to render TDLN DCs nonsuppressive was the removal of IDO (either by knockout or by 1MT).
IDO+ DCs create antigen-specific anergy in draining LNs.
The profound T cell unresponsiveness created following adoptive transfer was not due simply to carry-over of the original IDO+ DCs, as shown by the fact that T cell proliferation was not restored by addition of 1MT to the recall MLRs (Figure 5A). (This was in contrast to the effect of 1MT administered in vivo, during the afferent priming phase, where IDO was absolutely required; compare Figure 4C.) To test whether this unresponsiveness reflected antigen-specific anergy, LN cells were harvested 10 days after priming with TDLN DCs, and the BM3 T cells were sorted to greater than 95% purity based on expression of clonotypic TCR. Figure 5B shows that purified BM3 T cells were anergic to stimulation by irradiated C57BL/6 spleen cells and were also refractory to mitogenic anti-CD3 antibody, or to stimulation with PMA plus ionomycin (which bypasses the need for TCR signaling). In all of these models, however, proliferation was restored by the addition of recombinant IL-2 to the MLRs. The unresponsive state of BM3 T cells was thus consistent with classical descriptions of clonal anergy (37) and was also consistent with tumor-induced anergy observed in other systems (38).
Figure 5.
Antigen-specific anergy induced by adoptive transfer of TDLN DCs. (A) T cell unresponsiveness following adoptive transfer was not due to carry-over of IDO-mediated suppression, as shown by the lack of effect of 1MT when added to the recall MLRs. Priming of CBA+BM3 recipients was with TDLN DCs or normal C57BL/6 DCs, as in the previous figures. (B) Anergic BM3 cells are rescued by exogenous IL-2. CBA+BM3 recipients were primed with TDLN DCs, and BM3 T cells were sorted from draining LNs based on clonotypic TCR expression versus CD8. Recall MLRs were performed using these purified BM3 responders and irradiated C57BL/6 spleen cell stimulators, with or without the addition of mitogenic anti-CD3 antibody, recombinant IL-2, or PMA/ionomycin to the MLRs, as shown. (C) CBA+BM3 hosts were primed with TDLN DCs, and then anergic BM3 T cells (clonotype-positive, CD8+) were sorted and tested for responsiveness to irradiated C57BL/6 spleen cells, with or without anti-CD3 antibody. In contrast, the unfractionated host CD8+ population from the same LN showed good response to mitogen. (D) Response to third-party BALB/c antigens (irradiated BALB/c splenocytes, 5-day MLR) was intact in CBA+BM3 recipients primed with TDLN DCs, compared with normal CBA control mice.
To test whether anergy was created in an antigen-specific fashion, we asked whether the other host CD8+ T cells in the same LNs retained responsiveness. (For these experiments we used the unfractionated CD8+ cells from the same LNs as the anergic BM3 cells, which would include all the host CD8+ T cells that had not recognized antigen on the transferred DCs.) Figure 5C shows that the unfractionated host CD8+ T cells remained fully responsive to mitogenic anti-CD3 antibody, despite anergy of the purified BM3 cells from the same node. Likewise, the response of LN cells to irrelevant (BALB/c) antigens was comparable between hosts receiving TDLN DCs and normal, untreated CBA mice (Figure 5D).
IDO-mediated suppression preferentially segregates with a subset of CD19+ DCs.
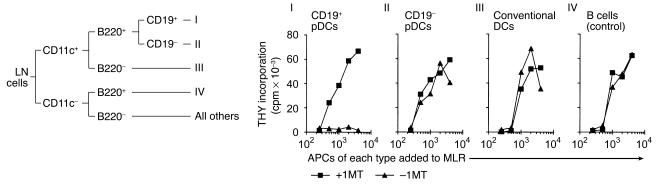
The experiments shown in Figure 2B suggested that IDO-mediated suppression segregated with the plasmacytoid (B220+) DC fraction. To better define the specific cell type responsible for suppression, we performed further phenotyping studies. These revealed that the pDCs from TDLNs were heterogeneous in expression of a number of markers, but in particular CD19. To determine whether this heterogeneity corresponded to functional differences, pDCs from TDLN cells were sorted into the CD19+ and CD19– fractions, plus the conventional B220–CD11c+ DCs, as depicted schematically in Figure 6. B cells were also isolated, for use as IDO-negative controls. In preliminary studies, both the CD19+ and the CD19– pDCs were found to express IDO by immunohistochemistry and Western blot, and some of the B220– DCs were IDO+ as well. However, it has been shown that IDO protein may be detectable without functional IDO-mediated immunosuppression (39), so it was important to perform functional studies of each subset. When each sorted population was used as APCs in MLRs, virtually all of the functional IDO-mediated suppression was found to segregate with the CD19+ fraction of pDCs. When these cells were removed, the remaining DCs showed little suppression (Figure 6, groups II and III).
Figure 6.
IDO-mediated suppressor activity segregates with a CD19+ subset of pDCs. Cells from TDLNs were sorted into five populations based on expression of CD11c, B220, and CD19, as shown in the schematic at the top. The three CD11c+ DC fractions (groups I, II, and III) or B cells (group IV) were used as stimulators in MLRs (2 × 105 BM3 responder cells with a titration of stimulator cells), with and without 1MT. Only the CD19+ pDCs (fraction I) showed significant IDO-mediated suppression.
Despite thus comprising virtually all of the IDO-mediated suppressor activity, CD19+ pDCs represented only a small fraction of total LN cells. In 13 experiments, analyzing two to six pooled TDLNs each, the total CD11c+ DC fraction was found to constitute 1–1.5% of cells; of these, the CD19+ pDCs comprised 31% ± 15%. Thus, the potent IDO-induced suppression seen in TDLNs was mediated by only 0.3–0.5% of total LN cells. The fact that CD19+ pDCs mediated such potent suppression, whereas CD19+ B cells mediated none, effectively ruled out the possibility that the CD19+ pDCs were simply contaminating B cells.
CD19+ DCs show a phenotype of pDCs.
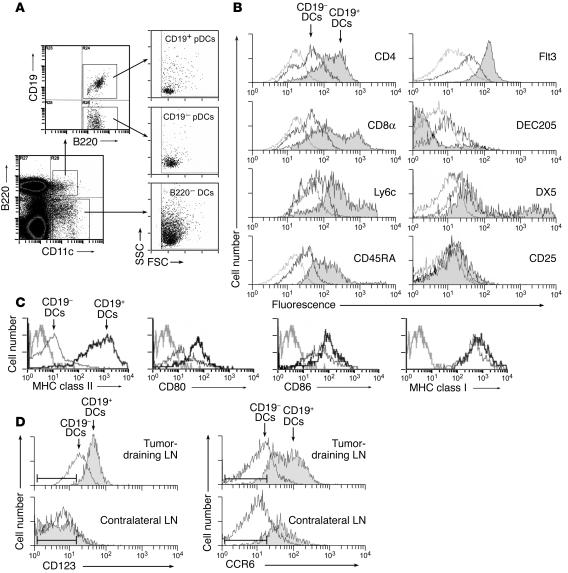
Figure 7A shows three-color staining for expression of B220 versus CD11c versus CD19 in a representative TDLN preparation. The B220+CD11c+ (pDC) cells were strongly bimodal with respect to CD19 expression, as shown in the upper dot plot. When the CD19+ and CD19– subsets of pDCs were gated separately, both were seen on forward scatter versus side scatter to be similar in properties to large lymphocytes (consistent with pDCs). In contrast, the B220–CD11c+ (conventional) DCs were larger cells with greater side-scatter properties. Figure 7B shows immunophenotyping of the CD11c+ cells from a TDLN preparation, gated separately on the CD19+ cells, versus all other CD19– DCs for comparison. Many of the CD19+ DCs expressed CD4 and/or CD8α, both of which can be found on pDCs; this helped to further distinguish the CD19+ DCs from B cells. Many of the CD19+ DCs also expressed the markers Ly6c and CD45RA, which are associated with murine pDCs (40, 41). The CD19+ DCs uniformly expressed the receptor tyrosine kinase Flt3 (CD135), which is not expressed on mature B cells (42). Expression of DEC205 was low to absent, and DX5 (expressed on the recently described tolerogenic NK-DCs [ref. 43]) was found on a subset of the CD19+ fraction. CD25 (which was included in some experiments as a marker of T cell activation) was negative on both the CD19+ and CD19– fractions, as expected. Expression of CD19 mRNA in sorted CD19+ pDCs was confirmed by quantitative real-time PCR, as was expression of the obligate upstream transcription factor for CD19, pax5 (44, 45) (see Supplemental Figure 1; supplemental material available at http://www.jci.org/cgi/content/full/114/2/280/DC1).
Figure 7.
The CD19+ DC subset displays a phenotype consistent with pDCs. (A) Forward and side-scatter characteristics of B220+ DCs versus B220+ DCs, with the latter further gated into CD19+ and CD19– subsets. (B) TDLNs stained by four-color flow cytometry for CD11c versus CD19 versus the various markers shown. Overlay histograms show the CD19+ (filled trace) and CD19– subsets of CD11c+ cells. Isotype-matched controls (gated on CD11c+ cells) are shown in gray. Each histogram is representative of 4–12 experiments with each marker. (C) TDLN DCs were stained for markers of DC maturity, and analyzed as above. (D) Expression of cell-surface markers CD123 and CCR6 on the CD19+ and CD19– DC subsets. Cells were analyzed from TDLNs and from contralateral LNs of the same animals, as shown. The range for the isotype-matched negative control antibody (± 3 SD) is shown by the bars in each histogram.
Figure 7C shows that CD19+ pDCs from TDLNs uniformly expressed high levels of MHC class II, as well as costimulatory molecules CD80 and CD86, suggestive of a mature phenotype. These markers were expressed at levels equal to or greater than the levels expressed by conventional CD19– DCs from the same LN. (In TDLNs, the CD19– subset of DCs showed significant variability in the number of mature versus immature DCs; but in every experiment all of the CD19+ pDCs were invariably mature.)
In other experiments (data not shown), CD19+ pDCs of the same phenotype (CD11c+, CD19+, B220+, Ly6c+, CD45RA+) were also found in normal LNs from non–tumor-bearing hosts, as well as in the contralateral LNs of mice with B78H1·GM-CSF tumors. In the normal LNs they comprised 18% ± 6% of total DCs, compared with 31% ± 15% of total DCs in TDLNs of B78H1·GM-CSF tumors, and 19% ± 2% of total DCs in TDLNs of B16F10 tumors. Thus, while there were was perhaps a modest increase in the number of CD19+ pDCs in TDLNs, they were also found in normal lymphoid tissue. The key difference was that CD19+ pDCs from normal tissues did not express functional IDO and were not constitutively suppressive.
In addition to expressing markers of murine pDCs, the CD19+ pDCs in TDLNs also expressed two markers previously observed in human IDO+ APCs. We have reported that CD123 (IL-3R α chain) and the chemokine receptor CCR6 segregate closely with IDO expression in human monocyte-derived DCs and monocyte-derived macrophages (20, 46). In murine TDLNs, we found that expression of CD123 preferentially associated with the CD19+ subset of DCs in TDLNs (Figure 7D). In contrast, the majority of CD19– DCs were low or negative for these markers, as were all of the DCs (with or without CD19) from contralateral (non–tumor-draining) LNs. A similar pattern was observed for CCR6, with preferential expression on the CD19+ subset, and higher expression in TDLNs compared with contralateral LNs.
Discussion
In the current study we identify a population of immunoregulatory DCs in TDLNs that are capable of mediating active immunosuppression in vitro, and of creating profound local T cell anergy in vivo. Previous studies of DCs isolated from tumors or TDLNs have observed a marked defect in their ability to stimulate T cells (47–50), which has usually been attributed to immaturity. However, it can be difficult to distinguish immaturity from more active forms of suppression (15). The key advance embodied in the current study is the application of specific experimental models (IDO inhibitors, IDO-KO mice, in vivo adoptive transfer) to unambiguously demonstrate the presence of active, dominant, IDO-mediated suppression in TDLNs.
An important attribute of IDO-mediated suppression was its fundamentally local nature. First, the IDO+ cells themselves were localized, being found in TDLNs but not in other LNs from the same animal. Second, adoptive-transfer studies demonstrated that complete anergy was created in the local draining LNs, even though T cells at remote sites retained reactivity. From the perspective of the tumor, we hypothesize that local anergy may be all that is required. A number of studies have shown that tumors can be locally tolerated despite the systemic presence of competent, tumor-specific T cells (5, 11, 51–54). Such tumors grow unchecked, demonstrating that immunosuppression does not have to be systemic in order to be effective. If all the LNs draining all the sites of tumor create local anergy, then the tumor will be de facto tolerated.
In this regard, it becomes relevant that IDO-mediated suppression was dominant over other, nonsuppressive APCs. This occurred even when antigen presentation was known to be restricted solely to IDO– APCs (compare Figure 3). Quantitatively, even a few percent of IDO-expressing pDCs was sufficient to suppress all T cell responses in vitro. Consistent with this, in our adoptive-transfer experiments the majority of transferred DCs were nonsuppressive (as shown by the fractionation experiments in Figure 6), yet all of the BM3 cells in the recipient LNs were rendered anergic. Anergy was antigen-specific (i.e., other host T cells remained responsive to mitogen, and to antigens not expressed on the transferred DCs). The antigen-specific nature of this anergy would not be incompatible with participation of a third-party (bystander) mode of suppression. As described in other systems (55, 56), induction of anergy may require both an antigen-nonspecific suppressive signal, and simultaneous signaling via the TCR (conferring antigen specificity). In our system, we postulate that anergy is induced in any T cell that encounters antigen (regardless of the APC) in a milieu that is rendered immunosuppressive by IDO. Thus, we hypothesize that a small population of IDO+ pDCs is able to redefine the entire TDLN as a tolerogenic milieu, even for tumor antigens being cross-presented by other, normally immunogenic APCs.
The downstream molecular mechanisms by which IDO is able to suppress neighboring T cells remains to be elucidated. The existence of some such mechanism has been inferred from previous reports showing that even a small minority of IDO-expressing DCs can dominantly suppress T cell responses in vivo (57–59). Conceptually, the suppressive effects of IDO fall into two categories: (a) those mediated by depletion of tryptophan, which include its antimicrobial (60, 61) and antiviral (62, 63) effects, and inhibition of T cell proliferation in some models (18, 25, 64); and (b) effects mediated by toxic downstream metabolites of tryptophan, which include CD4+ T cell apoptosis (65) and inhibition of T cell proliferation in other models (66, 67). In the case of third-party suppression, where inhibition must occur at a distance, a role for soluble factors such as tryptophan metabolites would seem intuitively likely, but experiments using the defined four-party system described in Figure 3 should help elucidate these mechanisms.
IDO-mediated suppressor activity in TDLNs segregated with a novel population of pDCs expressing CD19, a marker of the B cell lineage (68, 69). It is known that early CD19+ pro-B cells can give rise to DCs in vitro (70, 71), and recent analyses of both human and murine pDCs have suggested a B-lymphoid origin for a subset of pDCs (72, 73). Despite these observations, however, most previous studies have failed to appreciate the CD19+ subset of pDCs; and where they have been described (74), they were excluded on the assumption that they were contaminating B cells. Previously, we have reported that pDCs constitute one of the “IDO-competent” subsets of DCs, able to upregulate IDO (by immunohistochemistry) in response to CTLA4-Ig treatment (58). In that earlier study we, like most investigators (40, 75), excluded CD19+ cells from our sorted pDC preparation (which we defined as CD11c+B220+CD19–). We did not realize that — although the CD19– pDCs did indeed express IDO by immunohistochemistry, as reported — we were excluding a functionally important subset of the pDCs. (In that study, suppressor activity was measured only in vivo, not on the sorted subsets.) In the current study, the development of micro-scale MLR assays allowed us to follow the functional IDO-mediated suppressor activity (not just protein expression), and hence we were able to identify the novel CD19+ pDC subset. It is not surprising that some subsets of DCs expressed IDO protein (by immunohistochemistry or Western blot) without significant suppressor activity, since this is well described in the literature (39). Thus, the conclusion of our earlier report, that pDCs are one of the IDO-competent DC subsets, remains unchanged; but we would now expand the definition of pDCs to include the functionally important CD19+ subset.
With the exception of their B-lineage attributes, the CD19+ pDCs expressed markers consistent with murine pDCs. Some DCs can display high autofluorescence and nonspecific binding, which may complicate analysis by FACS, so we were careful to ensure that our characterization of the CD19+ pDCs also rested on functional studies, not just on marker analysis. We show that expression of CD19 and pax5 mRNA segregated with surface CD19 staining (see supplemental material), and that the CD19+ pDCs contained essentially all of the IDO-mediated suppressor activity. Thus, by functional criteria, the CD19+ pDCs represented a distinct and important subset of DCs in TDLNs.
The CD19+ pDCs were not restricted to TDLNs. They were also found in normal LNs and spleen, but in these sites they did not constitutively express IDO and were not spontaneously suppressive. We have previously shown that administration of recombinant CTLA4-Ig in vivo upregulates IDO, preferentially affecting the B220+ and CD8α+ subsets of DCs (58). By analogy, we hypothesize that some factor in the TDLNs “pre-activates” the CD19+ pDCs for constitutive IDO-mediated suppression. This might be a microenvironmental signal (e.g., a local cytokine); or, as recently described (59), it might be a population of IDO-inducing Treg’s. Elucidating these upstream inducing factors will be important in understanding how tumors have evolved to exploit the IDO mechanism.
The CD19+ subset of pDCs in TDLNs expressed CD123 and CCR6, which we have previously described as segregating preferentially with IDO-expressing human monocyte-derived DCs and monocyte-derived macrophages (20, 46). CCR6 has been described on at least a subset of murine pDCs (41), but (unlike the case in humans) CD123 has been reported to be negative on murine pDCs. However, CD123 expression may have been missed, since we found that there was essentially no CD123 expression on pDCs from normal (non–tumor-draining) LNs (see Figure 7D). Even when pDCs were isolated from TDLNs, it was only the CD19+ pDCs that expressed significant levels of CD123 (and these cells are usually excluded from analysis). Thus, in mice as in humans, there may be a preferential association of CD123 and CCR6 as markers of IDO-expressing APCs. Whether the converse will prove true, that the human IDO+ DCs in TDLNs resemble their murine CD19+ counterparts, remains to be determined. Others have shown that some human pDCs do indeed appear to arise from the B cell lineage (72), and we observe B cell markers such as CD20 on at least a subset of IDO-expressing cells in TDLNs (see Supplemental Figure 2). The key point, however, is that IDO-expressing human APCs (whatever the details of their immunophenotype) are found in human TDLNs, and they appear to correlate with poor clinical outcome in the patient population studied (Figure 1B).
The current study demonstrates that IDO-expressing DCs can be a potent endogenous immunosuppressive mechanism in tumor-bearing mice. We know that IDO is not the only way that tumors evade the immune system, because IDO-KO mice are still susceptible to tumors, and tumors do not spontaneously regress in mice treated with 1MT alone (31). This is hardly surprising, however, since tumors deprived of any single protective mechanism will rapidly evolve escape variants (76). The salient point from a clinical perspective is that all human tumors will have arisen in IDO-sufficient hosts; therefore some tumors may have evolved to rely upon this mechanism for protection. The ability to acutely deprive these tumors of the protective IDO mechanism, by administering IDO-inhibitor drugs such as 1MT, may provide a therapeutic window in which to break tolerance to tumor antigens.
Methods
Clinical materials.
Samples from 40 patients with malignant melanoma were randomly selected from the Moffitt Cancer Center archives, based on the following inclusion criteria: radiographically mapped sentinel LN at the time of diagnosis; no metastases to the sentinel node by light microscopy and immunohistochemistry; and no further therapy given following initial surgical resection. Sentinel LNs were stained for IDO by immunohistochemistry, expression was graded by two pathologists as previously described (30), and a consensus score was obtained. Patients were stratified into normal (grade 0) versus abnormal (grade 1+ or higher), and compared by Kaplan-Meier survival analysis. Sentinel LN biopsies from patients with breast cancer and melanoma were selected from the archives of the Medical College of Georgia. Studies using human subjects were approved by the respective Institutional Review Boards.
Mouse tumor models.
All studies were performed under protocols approved by our institutional animal-use committee. Tumors were implanted in female C57BL/6 mice (The Jackson Laboratory, Bar Harbor, Maine, USA), 8–12 weeks of age, or in mice with a targeted disruption of the IDO gene (58). IDO-KO mice were self-mated C57BL/6 × 129 background, and C57BL/6 tumors grew in these mice; since both strains are H2b haplotype, all mice were obligate homozygotes for the target H2Kb antigen used in some experiments. Mice were implanted with tumors in the anteriomedial thigh, using either 4 × 104 B16F10 cells (American Type Culture Collection, Bethesda, Maryland, USA) or 1 × 106 B78H1·GM-CSF cells (32) (gift of H. Levitsky). The B78H1·GM-CSF cells were originally designed as vaccine adjuvants (77), since they recruit large numbers of APCs to the tumor and TDLN (33). However, while GM-CSF–transfected tumor cells are immunogenic if irradiated prior to injection, live GM-CSF–expressing tumors paradoxically create systemic tolerance to tumor antigens (78). Thus, B78H1·GM-CSF offered an excellent model for our studies, because it recruited many DCs (including IDO+ DCs) yet failed to provoke a protective immune response. Although this model was intentionally biased toward recruitment of IDO-expressing cells, it was a biologically relevant model system, since similar cells were found in nontransfected tumors (B16F10 and LLC), and many human tumors and TDLNs spontaneously express GM-CSF (78–80). FACS analysis showed that the pDCs recruited by B78H1·GM-CSF tumors were phenotypically identical to those recruited by B16F10 tumors but could be recovered in larger quantities. Tumors were used 10–12 days after implantation (5–7 mm diameter), well prior to any metastasis to spleen or draining LNs (which occurred at day 20–30).
In some experiments, mice received 1MT (NSC 721782; National Cancer Institute, Rockville, Maryland, USA) as a continuous subcutaneous infusion, using implantable copolymer pellets as previously described (21). Control mice received vehicle pellets alone.
Immunohistochemistry.
Immunohistochemistry was performed on human materials as previously described (20). Formalin-fixed paraffin-embedded mouse tissues were treated with proteinase K antigen retrieval prior to staining with rabbit anti–mouse IDO polyclonal antibody (58). The IDO epitope is not stable under conditions of prolonged storage in thin sections, so staining was performed within 24 hours of sectioning. Negative controls for human and mouse staining were the anti-IDO antibody neutralized with a 50-fold molar excess of the immunizing peptide. Multiple immunohistochemical studies of established B16F10 and B78H1·GM-CSF tumors showed that neither tumor expressed IDO in the tumor cells themselves. Consistent with this, the studies presented herein using IDO-deficient hosts formally showed that the relevant IDO expression was in the host-derived APCs, not in the tumor cell lines.
Flow cytometry and cell sorting.
Single-cell suspensions of LNs were obtained by teasing and disaggregation through a 40-μm mesh; spleen cells were obtained by ground-glass homogenization and hypotonic lysis of erythrocytes. Cells were stained by four-color immunofluorescence, using CD11c versus B220 versus CD19 versus a panel of other markers. Fc binding was blocked using a commercial anti–CD16/CD32 cocktail (BD Biosciences — Pharmingen, San Diego, California, USA). Acquisition and sorting were performed using pulse-processing doublet discrimination on a MoFlo cell sorter (Cytomation Inc., Ft. Collins, Colorado, USA). (Analysis was also performed on a four-color FACSCalibur [BD Biosciences — Immunocytometry Systems, San Jose, California, USA] with similar results.) Antibodies against the following antigens were from BD Biosciences — Pharmingen: CD11c (clone HL3), B220 (clone RA3-6B2), CD19 (clone 1D3), CD4 (clone H129.19), CD8α (clone 53.6.7), Ly6c (clone AL-21), CD45RA (clone HI100), MHC class II (anti-H2Ab, clone 25-9-17), CD80 (clone 16-10A1), CD86 (clone GL1), H2Kb (clone AF6-88.5), CD123 (clone 5B11), CD135/Flt3 (clone A2F10.1), CD49b/DX5 (clone DX5), CD25 (clone PC61). DEC205-FITC (clone NLDC-145) was obtained from Serotec Inc. (Raleigh, North Carolina, USA). Anti–mouse CCR6 (clone 140706) was from R&D Systems Inc. (Minneapolis, Minnesota, USA). Anti-clonotypic antibody Ti98 against the BM3 TCR was biotinylated and used as previously described (34). All antibodies were used with isotype-matched negative controls; each isotype control was gated on the specific DC population of interest.
T cell activation in MLR.
TCR-transgenic BM3 responder T cells (CBA background, anti-H2Kb [ref. 34]) were prepared from spleen by nylon-wool enrichment. Stimulator cells (either sorted DCs or unfractionated TDLN cells) were mixed with 1 × 105 BM3 responder cells at the ratios shown in each figure, and cultured in 200 μl medium (10% FCS in RPMI-1640, with antibiotics and 50 μM 2-mercaptoethanol). Anti-HY TCR-transgenic mice (CD4+, clone A1, CBA background) have been described previously (81). These T cells recognize the cognate peptide REEALHQFRSGRKPI in the context of H2Ek (82). CD11c+ DCs were sorted from spleens of normal female CBA mice, pulsed for 90 minutes with 100 μM cognate peptide, washed thoroughly, and used as APCs in MLRs.
All MLR assays were performed in quadruplicate. After 3 days, proliferation was measured by 4-hour thymidine incorporation assay. All MLRs were performed in V-bottom culture wells (Nalge Nunc International, Rochester, New York, USA), as previously described (20), in order to ensure the close cell-cell contact required for optimal sensitivity to IDO-mediated suppression. Stimulator DCs were not irradiated, because preliminary validation studies showed that irradiation significantly altered the viability and functional attributes of IDO+ pDCs. MLRs were thus “two-way” reactions; however, the small number of sorted DCs used as stimulators contributed negligible proliferation compared with the large population of TCR-transgenic responder cells. Since the relevant readout was dominant suppression of all T cell proliferation, the two-way MLR design presented no problem in interpretation. Where indicated, replicate groups of wells received 200 μM 1MT (Sigma-Aldrich) or 250 μM L-tryptophan (Sigma-Aldrich). (Stock solution of 20 mM 1MT was dissolved in 0.1N NaOH, then adjusted to pH 7.4.)
Adoptive-transfer studies.
Recipient CBA mice (The Jackson Laboratory) were prepared by intravenous injection of 4 × 107 congenic BM3 splenocytes, to generate “CBA+BM3” hosts. Adoptively transferred BM3 T cells are stable in CBA hosts for over 100 days (34). CD11c+ DCs were isolated from TDLNs, or from normal LNs of non–tumor-bearing C57BL/6 and CBA mice, using a Cytomation Inc. MoFlo high-speed cell sorter. Two aliquots of 5 × 104 DCs each were injected subcutaneously into each recipient, bilaterally into the anteriomedial thigh (analogous to the position of the original tumor). After 10–12 days, recipient mice were euthanized, and the inguinal LNs (draining the sites of injection) were removed for analysis. Spleens were also harvested, as a representative distant site.
Supplementary Material
Acknowledgments
We thank Joyce Wilson and Jingping Sun for expert technical assistance, Jeanene Pihkala and the Medical College of Georgia Flow Cytometry Core Facility for cell sorting, and Alan Cantor for statistical analysis. This work was supported by NIH grants R01 CA103220 and R01 CA096651 (to D.H. Munn), R01 HD41187 (to A.L. Mellor), and the Carlos and Marguerite Mason Trust.
Footnotes
Nonstandard abbreviations used: indoleamine 2,3-dioxygenase (IDO); 1-methyl-d-tryptophan (1MT); mixed leukocyte reaction (MLR); plasmacytoid DC (pDC); regulatory T cell (Treg); T cell receptor (TCR); tumor-draining LN (TDLN).
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Smyth MJ, Godfrey DI, Trapani JA. A fresh look at tumor immunosurveillance and immunotherapy. Nat. Immunol. 2001;2:293–299. doi: 10.1038/86297. [DOI] [PubMed] [Google Scholar]
- 2.Staveley-O’Carroll K, et al. Induction of antigen-specific T cell anergy: an early event in the course of tumor progression. Proc. Natl. Acad. Sci. U. S. A. 1998;95:1178–1183. doi: 10.1073/pnas.95.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ochsenbein AF, et al. Roles of tumour localization, second signals and cross priming in cytotoxic T-cell induction. Nature. 2001;411:1058–1064. doi: 10.1038/35082583. [DOI] [PubMed] [Google Scholar]
- 4.Spiotto MT, et al. Increasing tumor antigen expression overcomes “ignorance” to solid tumors via crosspresentation by bone marrow-derived stromal cells. Immunity. 2002;17:737–747. doi: 10.1016/s1074-7613(02)00480-6. [DOI] [PubMed] [Google Scholar]
- 5.Wick M, et al. Antigenic cancer cells grow progressively in immune hosts without evidence for T cell exhaustion or systemic anergy. J. Exp. Med. 1997;186:229–238. doi: 10.1084/jem.186.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallimore A, Sakaguchi S. Regulation of tumour immunity by CD25+ T cells. Immunology. 2002;107:5–9. doi: 10.1046/j.1365-2567.2002.01471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pardoll D. T cells and tumours. Nature. 2001;411:1010–1012. doi: 10.1038/35082676. [DOI] [PubMed] [Google Scholar]
- 8.Sotomayor EM, et al. Cross-presentation of tumor antigens by bone marrow-derived antigen-presenting cells is the dominant mechanism in the induction of T-cell tolerance during B-cell lymphoma progression. Blood. 2001;98:1070–1077. doi: 10.1182/blood.v98.4.1070. [DOI] [PubMed] [Google Scholar]
- 9.Cuenca A, et al. Extra-lymphatic solid tumor growth is not immunologically ignored and results in early induction of antigen-specific T-cell anergy: dominant role of cross-tolerance to tumor antigens. Cancer Res. 2003;63:9007–9015. [PubMed] [Google Scholar]
- 10.Yu P, Spiotto MT, Lee Y, Schreiber H, Fu YX. Complementary role of CD4+ T cells and secondary lymphoid tissues for cross-presentation of tumor antigen to CD8+ T cells. J. Exp. Med. 2003;197:985–995. doi: 10.1084/jem.20021804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen LT, et al. Tumor growth enhances cross-presentation leading to limited T cell activation without tolerance. J. Exp. Med. 2002;195:423–435. doi: 10.1084/jem.20010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spiotto MT, Fu YX, Schreiber H. Tumor immunity meets autoimmunity: antigen levels and dendritic cell maturation. Curr. Opin. Immunol. 2003;15:725–730. doi: 10.1016/j.coi.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 13.Melief CJ. Mini-review. Regulation of cytotoxic T lymphocyte responses by dendritic cells: peaceful coexistence of cross-priming and direct priming? Eur. J. Immunol. 2003;33:2645–2654. doi: 10.1002/eji.200324341. [DOI] [PubMed] [Google Scholar]
- 14.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu. Rev. Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 15.Moser M. Dendritic cells in immunity and tolerance: do they display opposite functions? Immunity. 2003;19:5–8. doi: 10.1016/s1074-7613(03)00182-1. [DOI] [PubMed] [Google Scholar]
- 16.Mellor AL, Munn DH. Tryptophan catabolism and regulation of adaptive immunity. J. Immunol. 2003;170:5809–5813. doi: 10.4049/jimmunol.170.12.5809. [DOI] [PubMed] [Google Scholar]
- 17.Grohmann U, Fallarino F, Puccetti P. Tolerance, DCs and tryptophan: much ado about IDO. Trends Immunol. 2003;24:242–248. doi: 10.1016/s1471-4906(03)00072-3. [DOI] [PubMed] [Google Scholar]
- 18.Munn DH, et al. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J. Exp. Med. 1999;189:1363–1372. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwu P, et al. Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J. Immunol. 2000;164:3596–3599. doi: 10.4049/jimmunol.164.7.3596. [DOI] [PubMed] [Google Scholar]
- 20.Munn DH, et al. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297:1867–1870. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- 21.Munn DH, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 22.Grohmann U, et al. A defect in tryptophan catabolism impairs tolerance in nonobese diabetic mice. J. Exp. Med. 2003;198:153–160. doi: 10.1084/jem.20030633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grohmann U, et al. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat. Immunol. 2002;3:985–1109. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 24.Gurtner GJ, Newberry RD, Schloemann SR, McDonald KG, Stenson WF. Inhibition of indoleamine 2,3-dioxygenase augments trinitrobenzene sulfonic acid colitis in mice. Gastroenterology. 2003;125:1762–1773. doi: 10.1053/j.gastro.2003.08.031. [DOI] [PubMed] [Google Scholar]
- 25.Sakurai K, Zou J, Tschetter J, Ward J, Shearer G. Effect of indoleamine 2,3-dioxygenase on induction of experimental autoimmune encephalomyelitis. J. Neuroimmunol. 2002;129:186–196. doi: 10.1016/s0165-5728(02)00176-5. [DOI] [PubMed] [Google Scholar]
- 26.Munn DH, Mellor AL. IDO and tolerance to tumors. Trends Mol. Med. 2004;10:15–18. doi: 10.1016/j.molmed.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Uyttenhove C, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat. Med. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 28.Taylor MW, Feng G. Relationship between interferon-γ, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991;5:2516–2522. [PubMed] [Google Scholar]
- 29.Mellor AL, Keskin DB, Johnson T, Chandler P, Munn DH. Cells expressing indoleamine 2,3 dioxygenase inhibit T cell responses. J. Immunol. 2002;168:3771–3776. doi: 10.4049/jimmunol.168.8.3771. [DOI] [PubMed] [Google Scholar]
- 30.Lee JR, et al. Pattern of recruitment of immunoregulatory antigen presenting cells in malignant melanoma. Lab. Invest. 2003;83:1457–1466. doi: 10.1097/01.lab.0000090158.68852.d1. [DOI] [PubMed] [Google Scholar]
- 31.Friberg M, et al. Indoleamine 2,3-dioxygenase contributes to tumor cell evasion of T cell-mediated rejection. Int. J. Cancer. 2002;101:151–155. doi: 10.1002/ijc.10645. [DOI] [PubMed] [Google Scholar]
- 32.Huang AY, et al. Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science. 1994;264:961–965. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]
- 33.Dranoff G, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc. Natl. Acad. Sci. U. S. A. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tarazona R, et al. Effects of different antigenic microenvironments on the course of CD8+ T cell responses in vivo. Int. Immunol. 1996;8:351–358. doi: 10.1093/intimm/8.3.351. [DOI] [PubMed] [Google Scholar]
- 35.Sutmuller RPM, et al. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25+ regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoractive cytotoxic T lymphocyte responses. J. Exp. Med. 2001;194:823–832. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hogquist KA, et al. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz RH. Models of T cell anergy: is there a common molecular mechanism? J. Exp. Med. 1996;184:1–8. doi: 10.1084/jem.184.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shrikant P, Khoruts A, Mescher MF. CTLA-4 blockade reverses CD8+ T cell tolerance to tumor by a CD4+ T cell- and IL-2-dependent mechanism. Immunity. 1999;11:483–493. doi: 10.1016/s1074-7613(00)80123-5. [DOI] [PubMed] [Google Scholar]
- 39.Fallarino F, et al. Functional expression of indoleamine 2,3-dioxygenase by murine CD8alpha(+) dendritic cells. Int. Immunol. 2002;14:65–68. doi: 10.1093/intimm/14.1.65. [DOI] [PubMed] [Google Scholar]
- 40.O’Keeffe M, et al. Mouse plasmacytoid cells: long-lived cells, heterogeneous in surface phenotype and function, that differentiate into CD8(+) dendritic cells only after microbial stimulus. J. Exp. Med. 2002;196:1307–1319. doi: 10.1084/jem.20021031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin P, et al. Characterization of a new subpopulation of mouse CD8alpha+ B220+ dendritic cells endowed with type 1 interferon production capacity and tolerogenic potential. Blood. 2002;100:383–390. doi: 10.1182/blood.v100.2.383. [DOI] [PubMed] [Google Scholar]
- 42.Karsunky H, Merad M, Cozzio A, Weissman IL, Manz MG. Flt3 ligand regulates dendritic cell development from Flt3+ lymphoid and myeloid-committed progenitors to Flt3+ dendritic cells in vivo. J. Exp. Med. 2003;198:305–313. doi: 10.1084/jem.20030323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Homann D, et al. CD40L blockade prevents autoimmune diabetes by induction of bitypic NK/DC regulatory cells. Immunity. 2002;16:403–415. doi: 10.1016/s1074-7613(02)00290-x. [DOI] [PubMed] [Google Scholar]
- 44.Nutt SL, Morrison AM, Dorfler P, Rolink A, Busslinger M. Identification of BSAP (Pax-5) target genes in early B-cell development by loss- and gain-of-function experiments. EMBO J. 1998;17:2319–2333. doi: 10.1093/emboj/17.8.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mikkola I, Heavey B, Horcher M, Busslinger M. Reversion of B cell commitment upon loss of Pax5 expression. Science. 2002;297:110–113. doi: 10.1126/science.1067518. [DOI] [PubMed] [Google Scholar]
- 46.Munn DH, Sharma MD, Mellor AL. Ligation of B7-1/B7-2 by human CD4+ T cells triggers indoleamine 2,3-dioxygenase activity in dendritic cells. J. Immunol. 2004;172:4100–4110. doi: 10.4049/jimmunol.172.7.4100. [DOI] [PubMed] [Google Scholar]
- 47.Vicari AP, et al. Reversal of tumor-induced dendritic cell paralysis by CpG immunostimulatory oligonucleotide and anti-interleukin 10 receptor antibody. J. Exp. Med. 2002;196:541–549. doi: 10.1084/jem.20020732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Almand B, et al. Clinical significance of defective dendritic cell differentiation in cancer. Clin. Cancer Res. 2000;6:1755–1766. [PubMed] [Google Scholar]
- 49.Yang L, et al. Cancer-associated immunodeficiency and dendritic cell abnormalities mediated by the prostaglandin EP2 receptor. J. Clin. Invest. 2003;111:727–735. doi:10.1172/JCI200316492. doi: 10.1172/JCI16492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Furumoto K, Soares L, Engleman EG, Merad M. Induction of potent antitumor immunity by in situ targeting of intratumoral DCs. J. Clin. Invest. 2004;113:774–783. doi:10.1172/JCI200419762. doi: 10.1172/JCI19762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hanson HL, et al. Eradication of established tumors by CD8+ T cell adoptive immunotherapy. Immunity. 2000;13:265–276. doi: 10.1016/s1074-7613(00)00026-1. [DOI] [PubMed] [Google Scholar]
- 52.Nelson DJ, et al. Tumor progression despite efficient tumor antigen cross-presentation and effective “arming” of tumor antigen-specific CTL. J. Immunol. 2001;166:5557–5566. doi: 10.4049/jimmunol.166.9.5557. [DOI] [PubMed] [Google Scholar]
- 53.Speiser DE, et al. Self antigens expressed by solid tumors do not efficiently stimulate naive or activated T cells: implications for immunotherapy. J. Exp. Med. 1997;186:645–653. doi: 10.1084/jem.186.5.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Overwijk WW, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J. Exp. Med. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.DeSilva DR, Urdahl KB, Jenkins MK. Clonal anergy is induced in vitro by T cell receptor occupancy in the absence of proliferation. J. Immunol. 1991;147:3261–3267. [PubMed] [Google Scholar]
- 56.Powell JD, Lerner CG, Schwartz RH. Inhibition of cell cycle progression by rapamycin induces T cell clonal anergy even in the presence of costimulation. J. Immunol. 1999;162:2775–2784. [PubMed] [Google Scholar]
- 57.Grohmann U, et al. IFN-gamma inhibits presentation of a tumor/self peptide by CD8 alpha- dendritic cells via potentiation of the CD8 alpha+ subset. J. Immunol. 2000;165:1357–1363. doi: 10.4049/jimmunol.165.3.1357. [DOI] [PubMed] [Google Scholar]
- 58.Mellor AL, et al. Cutting edge: induced indoleamine 2,3 dioxygenase expression in dendritic cell subsets suppresses T cell clonal expansion. J. Immunol. 2003;171:1652–1655. doi: 10.4049/jimmunol.171.4.1652. [DOI] [PubMed] [Google Scholar]
- 59.Fallarino F, et al. Modulation of tryptophan catabolism by regulatory T cells. Nat. Immunol. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 60.Pfefferkorn ER. Interferon γ blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc. Natl. Acad. Sci. U. S. A. 1984;81:908–912. doi: 10.1073/pnas.81.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gupta SL, et al. Antiparasitic and antiproliferative effects of indoleamine 2,3-dioxygenase enzyme expression in human fibroblasts. Infect. Immun. 1994;62:2277–2284. doi: 10.1128/iai.62.6.2277-2284.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Adams O, et al. Role of indoleamine-2,3-dioxygenase in alpha/beta and gamma interferon-mediated antiviral effects against herpes simplex virus infections. J. Virol. 2004;78:2632–2636. doi: 10.1128/JVI.78.5.2632-2636.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bodaghi B, et al. Role of IFN-gamma-induced indoleamine 2,3 dioxygenase and inducible nitric oxide synthase in the replication of human cytomegalovirus in retinal pigment epithelial cells. J. Immunol. 1999;162:957–964. [PubMed] [Google Scholar]
- 64.Bubnoff D, et al. Fc-gamma RI induces the tryptophan degradation pathway involved in regulating T cell responses. J. Immunol. 2002;169:1810–1816. doi: 10.4049/jimmunol.169.4.1810. [DOI] [PubMed] [Google Scholar]
- 65.Fallarino F, et al. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002;9:1069–1077. doi: 10.1038/sj.cdd.4401073. [DOI] [PubMed] [Google Scholar]
- 66.Frumento G, et al. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J. Exp. Med. 2002;196:459–468. doi: 10.1084/jem.20020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Terness P, et al. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J. Exp. Med. 2002;196:447–457. doi: 10.1084/jem.20020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fearon DT, Carroll MC. Regulation of B lymphocyte responses to foreign and self-antigens by the CD19/CD21 complex. Annu. Rev. Immunol. 2000;18:393–422. doi: 10.1146/annurev.immunol.18.1.393. [DOI] [PubMed] [Google Scholar]
- 69.Nutt SL, Heavey B, Rolink AG, Busslinger M. Commitment to the B-lymphoid lineage depends on the transcription factor Pax5. Nature. 1999;401:556–562. doi: 10.1038/44076. [DOI] [PubMed] [Google Scholar]
- 70.Bjorck P, Kincade PW. CD19+ pro-B cells can give rise to dendritic cells in vitro. J. Immunol. 1998;161:5795–5799. [PubMed] [Google Scholar]
- 71.Izon D, et al. A common pathway for dendritic cell and early B cell development. J. Immunol. 2001;167:1387–1392. doi: 10.4049/jimmunol.167.3.1387. [DOI] [PubMed] [Google Scholar]
- 72.Rissoan MC, et al. Subtractive hybridization reveals the expression of immunoglobulin-like transcript 7, Eph-B1, granzyme B, and 3 novel transcripts in human plasmacytoid dendritic cells. Blood. 2002;100:3295–3303. doi: 10.1182/blood-2002-02-0638. [DOI] [PubMed] [Google Scholar]
- 73.Corcoran L, et al. The lymphoid past of mouse plasmacytoid cells and thymic dendritic cells. J. Immunol. 2003;170:4926–4932. doi: 10.4049/jimmunol.170.10.4926. [DOI] [PubMed] [Google Scholar]
- 74.Iwasaki A, Kelsall BL. Freshly isolated Peyer’s patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J. Exp. Med. 1999;190:229–239. doi: 10.1084/jem.190.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Asselin-Paturel C, et al. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat. Immunol. 2001;2:1144–1150. doi: 10.1038/ni736. [DOI] [PubMed] [Google Scholar]
- 76.Khong HT, Restifo NP. Natural selection of tumor variants in the generation of “tumor escape” phenotypes. Nat. Immunol. 2002;3:999–1005. doi: 10.1038/ni1102-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Borrello I, Sotomayor EM, Cooke S, Levitsky HI. A universal granulocyte-macrophage colony-stimulating factor-producing bystander cell line for use in the formulation of autologous tumor cell-based vaccines. Hum. Gene Ther. 1999;10:1983–1991. doi: 10.1089/10430349950017347. [DOI] [PubMed] [Google Scholar]
- 78.Bronte V, et al. Unopposed production of granulocyte-macrophage colony-stimulating factor by tumors inhibits CD8+ T cell responses by dysregulating antigen-presenting cell maturation. J. Immunol. 1999;162:5728–5737. [PMC free article] [PubMed] [Google Scholar]
- 79.Mattei S, et al. Expression of cytokine/growth factors and their receptors in human melanoma and melanocytes. Int. J. Cancer. 1994;56:853–857. doi: 10.1002/ijc.2910560617. [DOI] [PubMed] [Google Scholar]
- 80.Leong SP, Peng M, Zhou YM, Vaquerano JE, Chang JW. Cytokine profiles of sentinel lymph nodes draining the primary melanoma. Ann. Surg. Oncol. 2002;9:82–87. doi: 10.1245/aso.2002.9.1.82. [DOI] [PubMed] [Google Scholar]
- 81.Zelenika D, et al. Rejection of H-Y disparate skin grafts by monospecific CD4+ Th1 and Th2 cells: no requirement for CD8+ T cells or B cells. J. Immunol. 1998;161:1868–1874. [PubMed] [Google Scholar]
- 82.Scott D, et al. Dendritic cells permit identification of genes encoding MHC class II-restricted epitopes of transplantation antigens. Immunity. 2000;12:711–720. doi: 10.1016/s1074-7613(00)80221-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.