Abstract
Objective
This study is to investigate the role of glucose-regulated protein 78 (GRP78) in the pulmonary microvascular remodeling during hepatopulmonary syndrome (HPS) development.
Methods
The rat models with liver cirrhosis and HPS were induced by multiple pathogenic factors for 4 to 8 wk. The concentrations of alanine transferase (ALT) and endotoxin in plasma were detected in the models, followed by the detection of GRP78 expression. RT-PCR, quantitative real-time PCR and Western blotting were employed to assess the mRNA and protein expression levels of vascular endothelial growth factor (VEGF), respectively. Immunohistochemistry staining was used to examine the expression of a specific vascular marker, factor VIII-related antigen (FVIII-RAg), and several cell proliferation- and apoptosis-related proteins, including CHOP/GADD153, caspase-12, Bcl-2 and nuclear factor (NF)-κB.
Results
The levels of endotoxin and ALT in plasma were gradually increased as the disease progressed, so did GRP78, which were in a positive correlation. The expression levels of VEGF (both mRNA and protein) and FVIII-RAg were significantly elevated in the HPS models, indicating active angiogenesis, which was also positively correlated with GRP78 expression. Furthermore, the expression levels of the pro-apoptotic proteins of CHOP/GADD153 and caspase-12 were dramatically decreased, while the anti-apoptotic proteins of Bcl-2 and NF-κB were significantly elevated, in the HPS models. There were also close correlations between these proteins and GRP78.
Conclusions
Over-expression of GRP78 in lungs may be the critical pathogenic factor for HPS. Through promoting cell proliferation and survival and inhibiting apoptosis, GRP78 may promote the pulmonary microvascular remodeling in HPS pathogenesis. Our results provide a potential therapeutic target for clinical prevention and treatment for HPS and related complications.
Keywords: Liver cirrhosis, Hepatopulmonary syndrome, Glucose-regulated protein 78, Pulmonary microvascular remodeling, Endotoxin
1. Introduction
Hepatopulmonary syndrome (HPS) is a pulmonary microvascular disorder that occurs in the early stage of cirrhosis, which aggravates the primary liver disease and related complications (Ho, 2008). The 78 kD glucose-regulated protein (GRP78) is a marker of endoplasmic reticulum (ER) stress (Ni and Lee, 2007). High expression of GRP78 is closely related to a variety of diseases (Gonzalez-Gronow et al., 2009). The HPS progression is associated with the increased pulmonary microvascular changes (Zhang et al., 2007). The enterogenous endotoxemia associated with cirrhosis is an important trigger for ER stress response. The activation of ER stress response in lung tissues leads to increased expression of GRP78, which may be a key factor in the pathogenesis of HPS (Jia et al., 2011). It is reported that over-expressed GRP78 has proliferation-promoting and apoptosis-inhibiting effects during development of many diseases (Ni et al., 2011; Weng et al., 2011), suggesting that high expression of GRP78 may induce pulmonary microvascular reconstruction in HPS.
In this study, we examined the changes in the expression levels of GRP78 and some apoptosis-related factors in lung tissues to explore the role of GRP78 in pulmonary microvascular remodeling in rat models with multiple pathogenic factors-induced cirrhosis and HPS. Our results provide evidence for clinical prevention and treatment for HPS and related complications.
2. Materials and methods
2.1. Reagents
The rabbit anti-rat polyclonal antibody against GRP78 was purchased from Sigma (St Louis, Missouri, USA). The antibodies against vascular endothelial growth factor (VEGF) and C/EBP homologous protein (CHOP)/DNA damage-induced protein 153 (GADD153) were purchased from Santa Cruz (California, USA). The caspase-12, nuclear factor (NF)-κB, Bcl-2, and factor VIII related antigen (FVIII-RAg) polyclonal antibodies were purchased from Beijing Biosynthesis Biotechnology Co., Ltd. (Beijing, China). Horseradish peroxidase (HRP) conjugated goat anti-rabbit IgG was purchased from ZSGB-BIO (Beijing, China). The mouse anti-rabbit monoclonal antibody against glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and HRP conjugated goat anti-mouse IgG were purchased from Beyotime Institute of Biotechnology (Haimen, Jiangsu, China). The super ECL Plus hypersensitivity luminous fluid and BCA protein assay kit were purchased from Pulilai gene Technology Co., Ltd. (Beijing, China). The Trizol reagent kit and RT-PCR kit were purchased from Gibco (Carlsbad, CA, USA). The TaqDNA polymerase was purchased from Promega Corporation (Madison, WI, USA). Primers for VEGF, GAPDH and 18s RNA were synthesized by Shanghai Biological Engineering Co., Ltd. (Shanghai, China).
2.2. Animal grouping and modeling
Male Wistar rats, weighing 200 to 240 g, were provided by the Experimental Animal Center of Shanxi Medical University. These rats were randomly divided into the following six groups: (1) 4-wk control group (fed with standard diet for 4 wk), (2) 4-wk HPS group (fed with the complex diet and injected with CCl4 for 4 wk), (3) 6-wk control group (fed with standard diet for 6 wk), (4) 6-wk HPS group (fed with the complex diet and injected with CCl4 for 6 wk), (5) 8-wk control group (fed with standard diet for 8 wk), and (6) 8-wk HPS group (fed with the complex diet and injected with CCl4 for 8 wk). Standard diet and water were given to animals in control group, and cirrhosis with HPS was induced by multiple pathogenic factors in the model groups (Jia et al., 2011; Zhang et al., 2007). At each time point, blood was collected through aorta from anesthetized animals and centrifuged at 3000 r/min for 15 min under sterile and endotoxin-free conditions. The blood plasma was collected and kept at −80 °C. Parts of the lung tissues were immediately frozen in liquid nitrogen, and the remaining parts of the lung tissues were fixed in 10% neutral formalin for histology analysis.
2.3. Biochemical detection
Plasma samples were determined by the Reitman method for ala-nine transferase (ALT) activity with the kit purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, Jiangsu, China), and endotoxin was detected with the chromogenic substrate limulus amebocyte lysate kit purchased from Xiamen LAL Experimental Factory Co., Ltd. (Xiamen, Fujian, China), according to the manufacturer's instructions.
2.4. Immunohistochemistry
Paraffin-embedded lung tissue was sliced into 4 μm sections. After dewaxing, hydration and antigen retrieval treatment, SP method was performed in accordance with the instructions. The primary antibodies included rabbit anti-rat polyclonal antibodies against GRP78, FVIII-RAg, GADD153, caspase-12, Bcl-2, and NF-κB, respectively. The samples were stained with DAB and hematoxylin, dehydrated and examined with microscopy. The primary antibody was replaced with PBS for negative control. Brown granules were considered as positive staining, which were analyzed using BI-2000 medical image analysis system (Chengdu Taimeng Technology Co., Ltd., Chengdu, Sichuan, China). Ten fields were randomly selected on each slice under an optical microscope, and the percentages of the cells with NF-κB+ cytoplasm or with NF-κB+ nucleus out of the total cells were calculated, respectively.
2.5. RT-PCR
Total RNA was extracted from the lung tissues (100 mg). UV spectrophotometer was used to detect the absorbance at 260 nm and 280 nm (A260 nm and A280 nm), and the ratio represented the RNA purity and concentration. According to the manufacturer's instructions, 50 μg total RNA was used for the reverse transcription and amplification. VEGF primer sequences were as follows, forward: 5′-CTGCTCTCTTGG GTGCACTG-3′; reverse: 5′-CTGCGGATCTTGGACAAACA-3′, and the amplified fragment length was 465 bp. GAPDH was used as an internal reference, and its primer sequences were as follows, forward: 5′-GGTCAT CAACGGGAAACCC-3′; reverse: 5′-TCTGAGTGGCAGTGATGGCA-3′, and the amplified fragment length was 450 bp. The products were separated with 2% agarose gel electrophoresis, and the DNA standard marker (D12000) was used to determine the molecular weight. The target bands and GAPDH were detected by using Quantity One gel analysis system (Bio-Rad, USA).
2.6. Quantitative real-time PCR
Total RNA was extracted from frozen specimens using TRIzol reagent. The 20 μl reverse transcription (RT) reactions were performed using a PrimeScript® RT reagent Kit (Takara, Dalian, China) and incubated for 30 min at 37 °C, 5 s at 85 °C, and then maintained at 4 °C. For real-time PCR, 1 μl diluted RT products were mixed with 10 μl of 2× SYBR® Premix Ex Taq™ (Takara, Dalian, China), 0.6 μl forward and reverse primers (10 μM), and 8.4 μl nuclease-free water in a final volume of 20 μl, according to manufacturer's instructions. The following primers for VEGF were used: forward primer, 5′CGTCTACCAGCGCAGC TATTG3′, and reverse primer, 5′CACACAGGACGGCTTGAAGAT3′. The 18s RNA was used as an internal reference, and the primers for 18s RNA were as follows: forward primer, 5′-CGGCTACCACATCCAAGGAA- 3′, and reverse primer, 5′-GCTGGAATTACCGCGGCT-3′. Quantitative real-time PCR was performed with MyiQ2 two-color real-time PCR detection system (model IQ5; Bio-Rad Laboratories). All reactions were run on the Eppendorf Mastercycler EP Gradient S (Eppendorf, Germany). And, the following protocol was used: 1 cycle at 95 °C for 3 min followed by 40 cycles of 95 °C for 15 s, 58 °C for 20 s, and 70 °C for 30 s. The specificity of the PCR amplification was validated by the presence of a single peak in the melting curve analyses. Each quantitative real-time PCR experiment was repeated three times. Relative expression of genes was calculated using the comparative cycle threshold (CT) (2 -Δ ΔCT) method with 18s RNA as the internal control.
2.7. Western blotting
The homogenate of lung tissues was prepared with sonication. Then the BCA protein assay kit was used to quantify the protein amount. Proteins were separated on 10% SDS-PAGE gel electrophoresis and then transferred onto the nitrocellulose membrane. After the samples were blocked at 4 °C overnight, rabbit anti-rat polyclonal antibody against VEGF (1:800 dilution) was added. After washing, HRP conjugated goat anti-rabbit IgG (1:800 dilution) was added for incubation at room temperature for a further 1 h. After another round of washing, ECL hypersensitivity luminescence was added. The samples were then exposed to X-ray and developed. GAPDH was used as an internal reference. The sample bands were then scanned and analyzed with an image analysis system to determine the protein expression levels.
2.8. Statistical analysis
Data were expressed as means ± SD. SPSS 13.0 software was applied for statistical analysis. Single-factor analysis of variance was used to perform the multiple sample mean comparison. LSD-t test was used to make pair-wise comparisons. The linear correlation method was used for the correlation analysis. P < 0.05 was considered statistically significant.
3. Results
3.1. GRP78 levels in lung tissues are increased as HPS progresses
To characterize the biochemical indexes of the HPS models, the levels of endotoxin and ALT in plasma were first investigated. As shown in Table 1, both levels of endotoxin and ALT in plasma were gradually increased as HPS progressed, and the levels in the HPS groups were all higher than those in the corresponding control groups. The dynamically increased levels of endotoxin and ALT in plasma indicated the progression of hepatic dysfunction in these rat HPS models.
Table 1.
Levels of endotoxin and ALT in plasma in control and HPS groups.
| Endotoxin (EU/ml)
|
ALT (IU/l)
|
|||
|---|---|---|---|---|
| Control (n = 6) | HPS (n = 11) | Control (n = 6) | HPS (n = 11) | |
| 4-wk | 0.039 ± 0.014 | 0.068 ± 0.035 | 209.25 ± 5.234 | 220.92 ± 1.697 |
| 6-wk | 0.040 ± 0.021 | 0.273 ± 0.169*Δ | 207.05 ± 1.835 | 311.04 ± 2.758*Δ |
| 8-wk | 0.042 ± 0.032 | 0.642 ± 0.427*Δ# | 199.90 ± 2.459 | 253.66 ± 3.765*Δ# |
Note:
, P < 0.05 vs. normal control.
, P < 0.05 vs. 4-wk HPS group.
, P < 0.05 vs. 6-wk HPS group.
We next investigated the expression levels of GRP78 in lung tissues in these HPS models. Immunohistochemistry showed that GRP78 was expressed in the cytoplasm and membrane, but not in the nucleus, and the staining intensity was increased as the disease progressed (Fig. 1A). The statistical analysis indicated that, in HPS model groups, the expression levels of GRP78 were significantly elevated than those in corresponding control groups, and GRP78 was higher in the 8-wk HPS group than the 4-wk HPS group (Table 2; P < 0.05). The correlation analysis indicated that the GRP78 protein expression levels in lung tissues and the levels of endotoxin in plasma were positively correlated (r = 0.833; P < 0.01). These results indicate that the expression levels of GRP78 are increased in lung tissues of the HPS models.
Fig. 1.
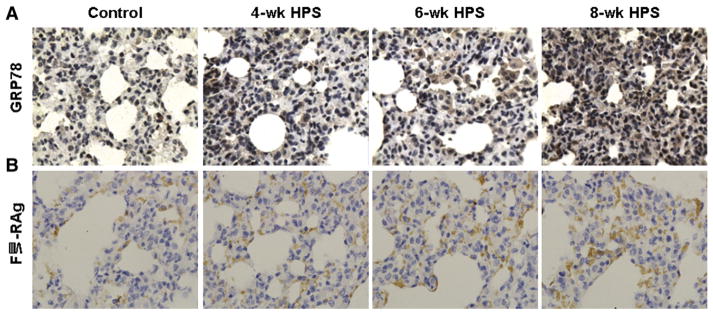
The expressions of GRP78 and FVIII-RAg in lung tissues in HPS models. The expressions of GRP78 (A) and FVIII-RAg (B) were detected with immunohistochemistry (×400). Rabbit anti-rat polyclonal primary antibodies against GRP78 and FVIII-RAg, respectively, were used.
Table 2.
Levels of GRP78 and FVIII-RAg in lung tissues in control and HPS groups.
| GRP78
|
FVIII-RAg
|
|||
|---|---|---|---|---|
| Control (n = 6) | HPS (n = 11) | Control (n = 6) | HPS (n = 11) | |
| 4-wk | 0.9551 ± 0.1231 | 1.526 ± 0.1618* | 0.0137 ± 0.0072 | 0.0141 ± 0.0061* |
| 6-wk | 1.0250 ± 0.2452 | 1.728 ± 0.4161* | 0.0145 ± 0.0039 | 0.0167 ± 0.0051*Δ |
| 8-wk | 1.2213 ± 0.2864 | 1.8636 ± 0.3184*Δ | 0.0138 ± 0.0075 | 0.0354 ± 0.0113*Δ# |
Note:
, P < 0.05 vs. normal control.
, P < 0.05 vs. 4-wk HPS group.
, P < 0.05 vs. 6-wk HPS group.
3.2. GRP78 expression levels are positively correlated with the pulmonary microvascular remodeling in the HPS models
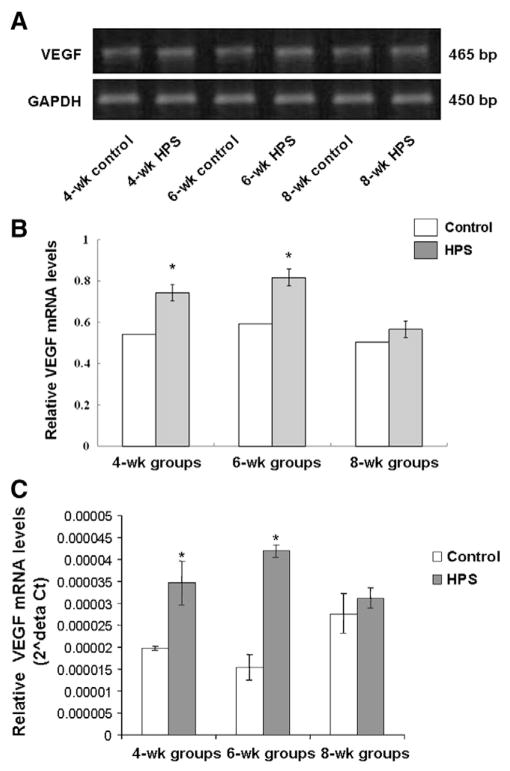
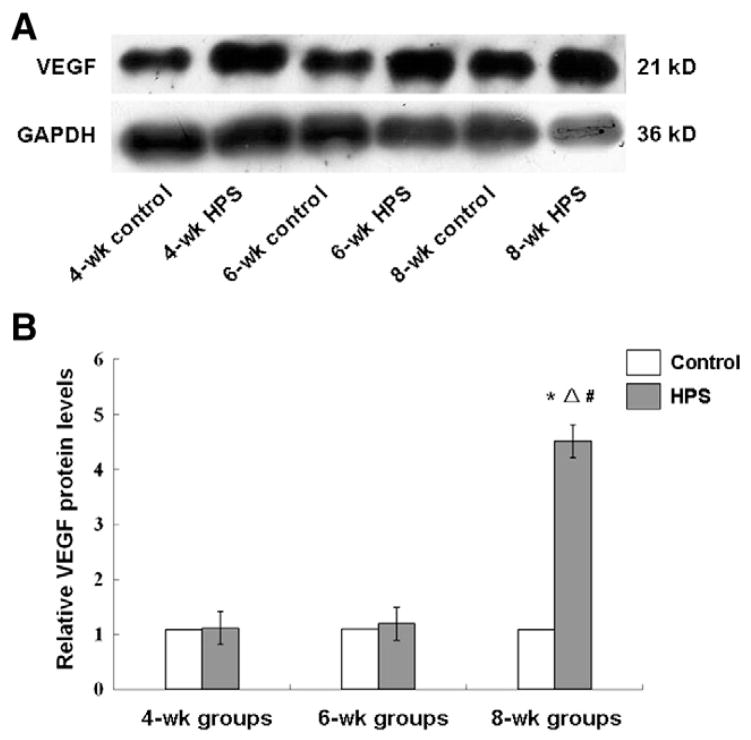
To determine the pulmonary microvascular remodeling in rat HPS models, the expression levels of FVIII-RAg and VEGF in lung tissues were investigated, respectively. FVIII-RAg is a specific vascular marker for the density of blood vessels. Immunohistochemistry revealed the expression pattern of FVIII-RAg in the cytoplasm and membrane, but not in the nucleus, and that the intensity was increased along with the HPS development (Fig. 1B). The statistical analysis suggested that the FVIII-RAg levels in the HPS groups were significantly higher than those in the corresponding control groups (P < 0.05), and in the HPS groups, compared with the 4-wk HPS group, the FVIII-RAg level was significantly increased in the 6-wk HPS group (P < 0.05). The FVIII-RAg level was further elevated in the 8-wk HPS group than that in the 4-wk and 6-wk HPS groups (P < 0.05) (Table 2). In addition, since VEGF is involved in the vascular reconstruction under physiological and pathological conditions, we also measured the mRNA and protein expression levels of VEGF in lung tissues. The RT-PCR results indicated that VEGF mRNA expression levels were significantly elevated in the 4-wk and the 6-wk HPS group, compared with the corresponding control groups (both P < 0.05) (Fig. 2A and B). This result was further verified by quantitative real-time PCR analysis (Fig. 2C). In the Western blotting detection, the protein expression levels of VEGF gradually increased along with the disease progression, with the VEGF level significantly higher in the 8-wk HPS group than the control groups, the 4-wk and the 6-wk HPS group (Fig. 3; P < 0.05). The above results indicate active angiogenesis in lung tissues, i.e., pulmonary microvascular remodeling, in these rat HPS models.
Fig. 2.
The mRNA expression levels of VEGF in HPS models. VEGF mRNA levels in lung tissues were detected by RT-PCR and quantitative real-time PCR, respectively. (A) The mRNA levels of VEGF in lung tissues as detected with RT-PCR. GAPDH was used as an internal control. (B) Quantitative results of RT-PCR showing VEGF mRNA expression levels in lung tissues. GAPDH was used as an internal control. (C) Quantitative real-time PCR results of VEGF mRNA expression levels. The 18s RNA was used as an internal control. *, P < 0.05 vs. normal control.
Fig. 3.
The protein expression levels of VEGF in HPS models. (A) The protein levels of VEGF in lungs were detected with Western blotting. (B) Statistical analysis of VEGF protein expression levels in HPS models. *, P < 0.05 vs. normal control; Δ, P < 0.05 vs. the 4-wk HPS group; #, P < 0.05 vs. the 6-wk HPS group.
We next aimed to find out whether there was a relationship between the elevated GRP78 expression levels and the pulmonary microvascular remodeling in HPS. The correlation analysis suggested that the expression levels of GRP78 protein were positively correlated with the VEGF expression levels (r = 0.775; P < 0.01) and FVIII-RAg expression levels (r = 0.7824; P < 0.01), respectively, in lung tissues. These results show that GRP78 expression levels are closely associated with the pulmonary microvascular reconstruction, therefore implying the crucial role of GRP78 in the development of hepatopulmonary syndrome.
3.3. GRP78 is closely linked with the expression levels of cell proliferation-and apoptosis-related proteins in the HPS models
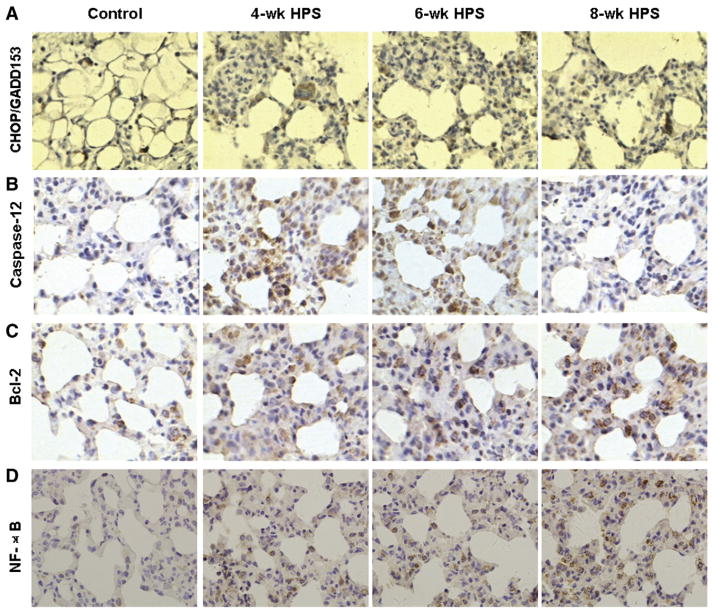
The angiogenesis process is obviously related with cell proliferation and death. To investigate the possible mechanism through which GRP78 may promote the pulmonary microvascular reconstruction, the relationship between the GRP78 expression and the expression levels of cell proliferation- and apoptosis-related proteins in HPS was assessed. Immunohistochemistry indicated that the apoptosis-related proteins, CHOP/GADD153 and caspase-12 were expressed in the cytoplasm, and the protein staining intensities decreased with the disease progression (Fig. 4A, B). The statistical analysis indicated that the expression levels of CHOP/GADD153 and caspase-12 were dramatically decreased in the HPS groups, compared with the corresponding control groups, and exhibited a declining trend in these HPS groups as the disease progressed (Table 3). In contrast, the staining intensities of the anti-apoptotic factors, Bcl-2 and NF-κB were increased with the disease progression (Fig. 4C, D). The Bcl-2 expression levels in the HPS groups were all higher than those in corresponding control groups (Table 4). For NF-κB, which was expressed in both cytoplasm and nucleus, the elevated expression levels were also observed in the HPS groups, compared with the corresponding control groups, and the expression levels increased even more as HPS progressed, especially in the nucleus (Table 4).
Fig. 4.
The expressions of cell proliferation- and apoptosis-related proteins in lung tissues in HPS models. The expressions of CHOP/GADD153 (A), caspase-12 (B), Bcl-2 (C), and NF-κB (D) were detected with immunohistochemistry (×400). Rabbit anti-rat polyclonal primary antibodies against GADD153, caspase-12, Bcl-2, and NF-κB, respectively, were used.
Table 3.
Levels of CHOP/GADD153 and caspase-12 in lung tissues in control and HPS groups.
| CHOP/GADD153
|
Caspase-12
|
|||
|---|---|---|---|---|
| Control (n = 6) | HPS (n = 11) | Control (n = 6) | HPS (n = 11) | |
| 4-wk | 0.0158 ± 0.0074 | 0.0136 ± 0.0095* | 0.0220 ± 0.0069 | 0.0207 ± 0.0122 |
| 6-wk | 0.0168 ± 0.0042 | 0.0131 ± 0.0089* | 0.0233 ± 0.0034 | 0.0126 ± 0.0080*Δ |
| 8-wk | 0.0170 ± 0.0060 | 0.0057 ± 0.0047*Δ# | 0.0244 ± 0.0100 | 0.0108 ± 0.0063*Δ |
Note:
, P < 0.05 vs. normal control.
, P < 0.05 vs. 4-wk HPS group.
, P < 0.05 vs. 6-wk HPS group.
Table 4.
Levels of Bcl-2 and NF-κB in lung tissues in control and HPS groups.
| Bcl-2
|
NF-κB (cytoplasm)
|
NF-κB (nucleus)
|
||||
|---|---|---|---|---|---|---|
| Control (n = 6) | HPS (n = 11) | Control (n = 6) | HPS (n = 11) | Control (n = 6) | HPS (n = 11) | |
| 4-wk | 0.0072 ± 0.0047 | 0.0131 ± 0.0060* | 0.0429 ± 0.0189 | 0.0639 ± 0.0057* | 0.0125 ± 0.0037 | 0.0499 ± 0.0074* |
| 6-wk | 0.0079 ± 0.0050 | 0.0137 ± 0.0111* | 0.0397 ± 0.0161 | 0.0909 ± 0.0062*Δ | 0.0189 ± 0.0066 | 0.1093 ± 0.01219*Δ |
| 8-wk | 0.0104 ± 0.0081 | 0.0145 ± 0.0100* | 0.0389 ± 0.0082 | 0.1340 ± 0.0210*Δ# | 0.0201 ± 0.0081 | 0.1489 ± 0.0284*Δ# |
Note:
, P < 0.05 vs. normal control.
, P < 0.05 vs. 4-wk HPS group.
, P < 0.05 vs. 6-wk HPS group.
We next examined the relationship between the expression levels of GRP78 and these cell proliferation- and apoptosis-related proteins. Correlation analysis indicated that GRP78 expression was negatively correlated with CHOP/GADD153 (r = −0.6921; P < 0.01) and caspase-12 (r = −0.7012; P < 0.01) expression levels, respectively. All these results suggest that GRP78 is closely related with the cell proliferation and apoptosis in lung tissues, which would be the underlying mechanism through which GRP78 may promote the pulmonary micro-vascular remodeling process.
4. Discussion
The ER is one of the most important intracellular organelles. Endo-toxin (Jia et al., 2011), hypoxia and Ca2+ are important triggers to activate ER stress response pathways, which would 1) reduce protein translation and further prevent accumulation of unfolded proteins; 2) up-regulate the molecular chaperones of GRP78 and GRP94, and various factors that benefit protein folding, restore the ER protein homeosta-sis environment, and clear misfolded proteins through the ubiquitin–proteasome system at late stage; 3) activate immune and anti-apoptotic mediator NF-κB; and 4) activate apoptotic pathway to clear damaged cells when the ER function is severely impaired. The apoptotic pathway involves the transcription of CHOP/GADD153, the stimulation of c-JUN NH2-terminal kinase (JNK) pathway and caspase-12 (Oyadomari and Mori, 2004). Under various pathological conditions, depending on the degree of the ER stress and the balance between anti- and pro-apoptotic signals, cell survival or death is ultimately determined (Puskás et al., 2010). In this study, the observed changes of the ER stress-related signaling molecules of GRP78, caspase-12, GADD153, and NF-κB in the lung tissues of HPS animals further demonstrate that ER stress may play a key role in the disease pathogenesis.
Pulmonary microvascular remodeling is another pivotal process in the pathogenesis of HPS. FVIII-RAg is a specific vascular marker whose content directly reflects the density of blood vessels. VEGF is another common factor for vascular remodeling under various physiological and pathological conditions, which can not only specifically promote vascular endothelial cell proliferation and angiogenesis, but also change the extracellular matrix. It has been shown that GRP78 can promote angiogenesis directly, or indirectly, through the VEGF pathway (Raiter et al., 2010; Yoo et al., 2012). In this study, we found that the expression levels of FVIII-RAg and VEGF in lung tissues gradually increased as the disease progressed, and the increased GRP78 were positively correlated with the elevated FVIII-Rag and VEGF, respectively. These results suggested a probable key role of GRP78 for causing the lung microvascular remodeling in HPS pathogenesis. Interestingly, VEGF mRNA level was significantly increased in the 4-wk and 6-wk HPS groups, but not the 8-wk HPS group. On the contrary, VEGF protein level was significantly increased in the 8-wk HPS group, but not the 4-wk and 6-wk HPS groups. We suppose that this discrepancy in VEGF mRNA level and protein level might be caused by mRNA degradation or the delay in mRNA translation. Further studies are needed to clarify this discrepancy.
The interaction of proliferation and apoptosis of the vascular endothelial cells is an important mechanism for vascular angiogenesis. GRP78 can exert different effects on cell growth, depending on specific conditions (Gonzalez-Gronow et al., 2009; Kudo, 2010). Under physiological conditions, GRP78 is expressed in cytoplasm to promote cell apoptosis and inhibit vascular proliferation (Ni and Lee, 2007). On the other hand, under pathological conditions, GRP78 is abnormally over-expressed in cytoplasm and translocated onto the cell membrane to work as a receptor, stimulating the cell proliferation and the subsequent angiogenesis (Ni and Lee, 2007; Ni et al., 2011; Weng et al., 2011). The proliferation-stimulating effect of GRP78 is acted through its binding with a variety of extracellular ligands (such as activated α2-macroglobulin and Kringle 5) or endothelial cell surface anchor proteins (such as Cripto proteins from epidermal growth factor family) (Ni et al., 2011). GRP78 promotes the cell proliferation by activating NF-κB and through pathways of anti-apoptotic Bcl-2 pathway, MAPK signaling pathway, and PI3 kinase pathway (Misra et al., 2006). The apoptosis-suppressing effect of GRP78 is associated with its ATP domain binding with caspase-7 and caspase-12, preventing their release (Fu et al., 2008; Rao et al., 2002). The activating transcription factor 6 (ATF6) or protein kinases R-like ER kinase (PERK) pathway is down-regulated to reduce CHOP/GADD153 production and antagonize the pro-apoptotic members from Bcl-2 family (Gorbatyuk et al., 2010). Besides, Bcl-2 and other family members with anti-apoptotic abilities can down-regulate CHOP/GADD153 to further exert anti-apoptotic effects (Gorbatyuk et al., 2001; Su et al., 2002). In the present study, we found that in HPS development, GRP78 expression gradually increased, and GRP78 expression was also significantly associated with the decreased expression of caspase-12 and CHOP/GADD153. And, the anti-apoptotic protein Bcl-2 expression was gradually increased and NF-κB was activated during HPS. It seemed that the high expression of GRP78 enhanced cell proliferation through the VEGF pathway, prevented caspase-12 release from the ER, and inhibited CHOP-induced apoptosis to promote lung microvascular reconstruction.
In conclusion, our results showed that because of the specific proliferation-promoting and apoptosis-inhibiting effect of GRP78, the protein may promote pulmonary microvascular remodeling and result in ventilation/perfusion imbalance. This might be one of the important mechanisms for HPS, other primary liver diseases, and related complications.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81070339), the International Science and Technology Cooperation Project of Shanxi (No. 2010081068), the fund from Key Laboratory of Cellular Physiology co-established by Shanxi Province and Ministry of Education in Shanxi Medical University (No. 2010-09), the Shanxi Science and Technology Innovation Projects of Universities (No. 2010120), and the Shanxi returned Students funded projects (No. 211-091). Cheng Ji is supported by US NIH grants: R01AA018846 and R01AA018612.
Abbreviations
- GRP78
glucose-regulated protein 78
- HPS
hepatopulmonary syndrome
- ALT
alanine transferase
- VEGF
vascular endothelial growth factor
- FVIII-RAg
factor VIII-related antigen
- NF-κB
nuclear factor-κB
- ER
endoplasmic reticulum
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HRP
horseradish peroxidase
Footnotes
Conflict of interest
All authors declare no financial competing interests.
References
- Fu HY, Minamino T, Tsukamoto O, Sawada T, Asai M, Kato H, Asano Y, Fujita M, Takashima S, Hori M, Kitakaze M. Overexpression of endoplasmic reticulum-resident chaperone attenuates cardiomyocyte death induced by protea-some inhibition. Cardiovascular Research. 2008;79:600–610. doi: 10.1093/cvr/cvn128. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gronow M, Selim MA, Papalas J, Pizzo SV. GRP78: a multifunctional receptor on the cell surface. Antioxidants and Redox Signaling. 2009;11:2299–2306. doi: 10.1089/ARS.2009.2568. [DOI] [PubMed] [Google Scholar]
- Gorbatyuk MS, Knox T, LaVail MM, Gorbatyuk OS, Noorwez SM, Hauswirth WW, Lin JH, Muzyczka N, Lewin AS. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl-2 and perturbing the cellular redox state. Molecular and Cellular Biology. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbatyuk MS, Knox T, LaVail MM, Gorbatyuk OS, Noorwez SM, Hauswirth WW, Lin JH, Muzyczka N, Lewin AS. Restoration of visual function in P23H rhodopsin transgenic rats by gene delivery of BiP/Grp78. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:5961–5966. doi: 10.1073/pnas.0911991107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho V. Current concepts in the management of hepatopulmonary syndrome. Vascular Health and Risk Management. 2008;4:1035–1041. doi: 10.2147/vhrm.s3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia JT, Zhang HY, Tian XX. The role of glucose-regulated protein 78 in the HPS induced by complex risk factors. Chinese Journal of Pathophysiology. 2011;27:1580–1585. [Google Scholar]
- Kudo T. Therapeutic strategies for Alzheimer disease based on endoplasmic reticulum stress. Nihon Shinkei Seishin Yakurigaku Zasshi. 2010;30:163–168. [PubMed] [Google Scholar]
- Misra UK, Deedwania R, Pizzo SV. Activation and cross-talk between Akt, NF-kappaB, and unfolded protein response signaling in 1-LN prostate cancer cells consequent to ligation of cell surface-associated GRP78. Journal of Biological Chemistry. 2006;281:13694–13707. doi: 10.1074/jbc.M511694200. [DOI] [PubMed] [Google Scholar]
- Ni M, Lee AS. ER chaperones in mammalian development and human diseases. FEBS Letters. 2007;58:3641–3651. doi: 10.1016/j.febslet.2007.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni M, Zhang Y, Lee AS. Beyond the endoplasmic reticulum: atypical GRP78 in cell viability, signalling and therapeutic targeting. Biochemical Journal. 2011;434:181–188. doi: 10.1042/BJ20101569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death and Differentiation. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- Puskás LG, Fehér LZ, Vizler C, Ayaydin F, Rásó E, Molnár E, Magyary I, Kanizsai I, Gyuris M, Madácsi R, Fábián G, Farkas K, Hegyi P, Baska F, Ozsvári B, Kitajka K. Polyunsaturated fatty acids synergize with lipid droplet binding thalidomide analogs to induce oxidative stress in cancer cells. Lipids in Health and Disease. 2010;9:56. doi: 10.1186/1476-511X-9-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiter A, Weiss C, Bechor Z, Ben-Dor I, Battler A, Kaplan B, Hardy B. Activation of GRP78 on endothelial cell membranes by an ADAM15-derived peptide induces angiogenesis. Journal of Vascular Research. 2010;47:399–411. doi: 10.1159/000281580. [DOI] [PubMed] [Google Scholar]
- Rao RV, Peel A, Logvinova A, del Rio G, Hermel E, Yokota T, Goldsmith PC, Ellerby LM, Ellerby HM, Bredesen DE. Coupling endoplasmic reticulum stress to the cell death program: role of the ER chaperone GRP78. FEBS Letters. 2002;514:122–128. doi: 10.1016/s0014-5793(02)02289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su HL, Liao CL, Lin YL. Japanese encephalitis virus infection initiates endoplasmic reticulum stress and an unfolded protein response. Journal of Virology. 2002;76:4162–4171. doi: 10.1128/JVI.76.9.4162-4171.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng WC, Lee WT, Hsu WM, Chang BE, Lee H. Role of glucose-regulated protein 78 in embryonic development and neurological disorders. Journal of the Formosan Medical Association. 2011;110:428–437. doi: 10.1016/S0929-6646(11)60064-8. [DOI] [PubMed] [Google Scholar]
- Yoo SA, You S, Yoon HJ, Kim DH, Kim HS, Lee K, Ahn JH, Hwang D, Lee AS, Kim KJ, Park YJ, Cho CS, Kim WU. A novel pathogenic role of the ER chaperone GRP78/BiP in rheumatoid arthritis. Journal of Experimental Medicine. 2012;209:871–886. doi: 10.1084/jem.20111783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HY, Han de W, Su AR, Zhang LT, Zhao ZF, Ji JQ, Li BH, Ji C. Intestinal endotoxemia plays a central role in development of hepatopulmonary syndrome in a cirrhotic rat model induced by multiple pathogenic factors. World Journal of Gastroenterology. 2007;13:6385–6395. doi: 10.3748/wjg.v13.i47.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]