Abstract
Nitrate and nitrite are commonly thought of as inert end products of nitric oxide (NO) oxidation, possibly carcinogenic food additives, or well-water contaminants. However, recent studies have shown that nitrate and nitrite play an important role in cardiovascular and gastrointestinal homeostasis through conversion back into NO via a physiological system involving enterosalivary recirculation, bacterial nitrate reductases, and enzyme-catalyzed or acidic reduction of nitrite to NO. The diet is a key source of nitrate in adults; however, infants ingest significantly less nitrate due to low concentrations in breast milk. In the mouth, bacteria convert nitrate to nitrite, which has gastro-protective effects. However, these nitrate-reducing bacteria are relatively inactive in infants. Swallowed nitrite is reduced to NO by acid in the stomach, affecting gastric blood flow, mucus production, and the gastric microbiota. These effects are likely attenuated in the less acidic neonatal stomach. Systemically, nitrite acts as a reservoir of NO bioactivity that can protect against ischemic injury, yet plasma nitrite concentrations are markedly lower in infants than in adults. The physiological importance of the diminished nitrate→nitrite→NO axis in infants and its implications in the etiology and treatment of newborn diseases such as necrotizing enterocolitis and hypoxic/ischemic injury are yet to be determined.
The discovery that nitric oxide (NO) is produced endogenously by NO synthases initiated a paradigm shift from thinking of NO as a toxic gas to the realization that it is a key regulator of vascular homeostasis, amongst many other physiological roles. One of the greatest clinical impacts of this discovery has been the use of inhaled NO gas for the treatment of persistent pulmonary hypertension in newborns, where it has significantly reduced the need for extracorporeal oxygenation (1,2). Initially, it was thought that the effects of inhaled NO would be confined to the lungs, as free NO gas in blood is scavenged by reactions with hemoglobin in only a few milliseconds (3). However, it is now widely reported that inhaled NO has an array of extrapulmonary effects, suggesting that one or more of its metabolites serve as reservoirs of NO bioactivity capable of circulating from the lungs to the peripheral organs. The existence of such an endocrine mediator of NO and its effects would be of great physiological relevance and much recent research has focused on identifying metabolites of NO that may serve in that role.
Nitrate (NO3−) and nitrite (NO2−) are the two major end products of NO metabolism. Historically, these compounds were considered to be relatively inert at physiological concentrations but environmental pollutants that posed potential health risks to humans at high concentrations. However, similar to the turnabout course of our understating of NO in biology, evidence now indicates that while nitrate and nitrite may be toxic at high concentrations, they play an important physiological role as they can be converted back into NO via a system involving enterosalivary recirculation, bacterial nitrate reductases, and enzyme-catalyzed or acidic reduction of nitrite to NO. This review will summarize our current knowledge regarding the bioactivity of nitrate and nitrite in adults and will outline known differences in infants (summarized in Figure 1 and Supplementary Table S1 online).
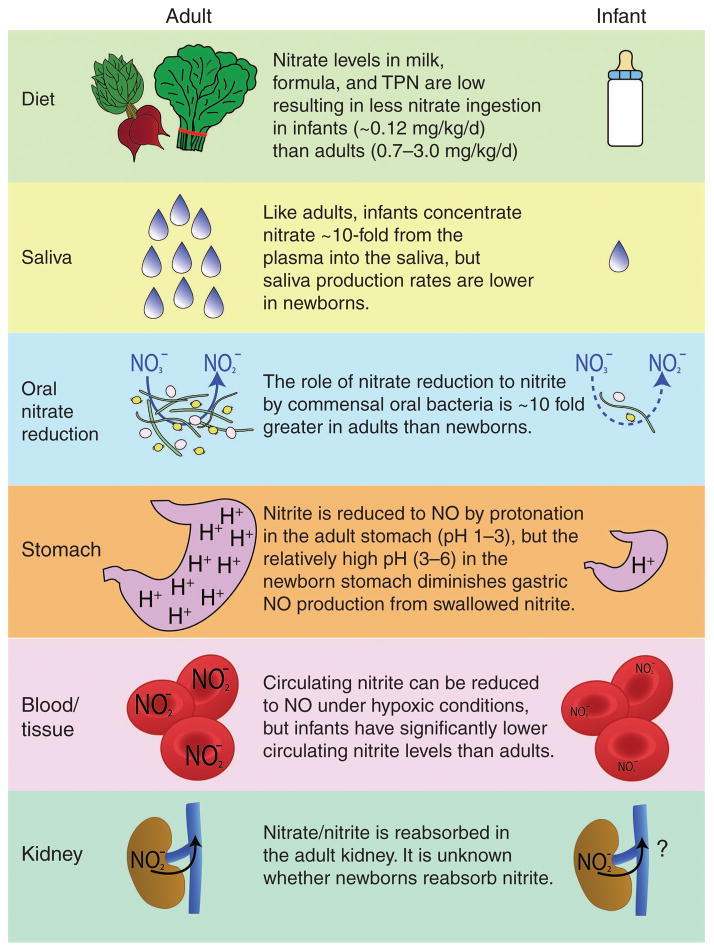
Figure 1.
Schematic summary showing major differences in the supply and handling of nitrite and nitrate by the adult and infant. Multiple deficiencies in the infant lead to diminished nitric oxide (NO) bioactivity in the stomach and lower circulating nitrite concentrations in the blood.
DIET
Dietary Nitrate and Nitrite
Nitrate is the most prevalent nitrogen oxide species in the body. Although some of it is derived as an end product of the oxidation of endogenous NO and nitrite, nitrate concentrations are also heavily influenced by dietary intake. Nitrate itself is inert in mammalian tissues, but it can be reduced to nitrite by symbiotic bacteria that are part of the normal flora in the mouth and gastrointestinal (GI) tract (discussed below). Thus, by the action of these bacteria, dietary nitrate contributes to the body’s pool of nitrite. Vegetables are the most common source of dietary nitrate with particularly high concentrations (>2500 mg/kg) in beets, radishes, celery, and green leafy vegetables such as lettuce, kale, and spinach. Although daily nitrate ingestion can vary significantly dependent upon the types and amount of vegetables eaten, it is estimated that a typical adult ingests approximately 0.7–3.0 mg/kg body weight of nitrate per day (4).
Compared with nitrate, the amount of nitrite ingested in a normal adult diet is relatively small. In fact, it is likely that more of the nitrite in the body is derived from the bacterial reduction of nitrate and oxidation of endogenously-produced NO than from the diet (5,6). The biggest source of nitrite in the diet is cured and processed meats, where it is used as an additive to prevent bacterial growth and enhance the color. A typical adult ingests about 0.1 mg/kg body weight of dietary nitrite daily (7).
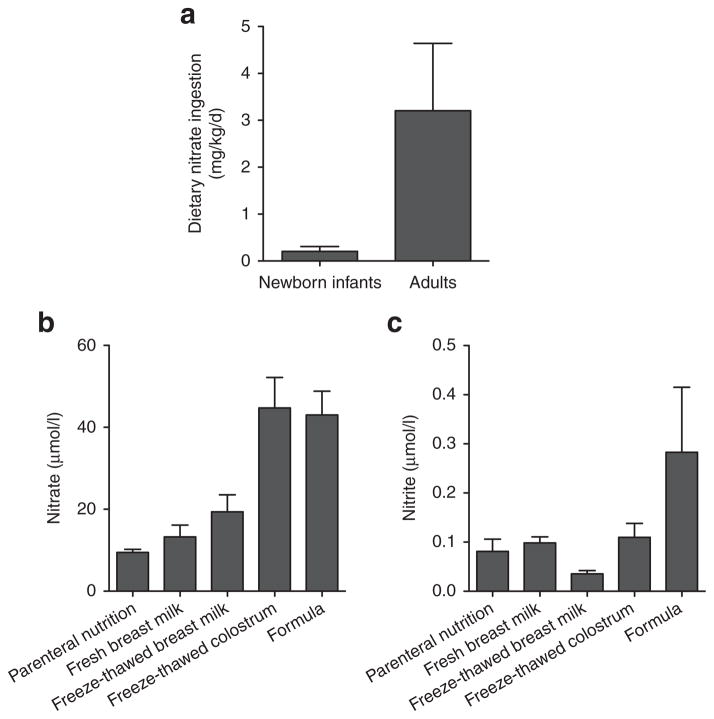
Although there are some discrepancies in the reported concentrations of nitrate and nitrite in breast milk and artificial milk (perhaps due to differing assay methodologies), we and others have recently shown that newborn infants ingest markedly lower amounts of nitrate and nitrite than adults on a per kg body weight basis. This is true regardless of whether they are receiving breast milk, artificial milk, or parenteral nutrition (8–10). Based on a breast milk intake of 150ml/kg/day and our measurements of nitrate and nitrite concentrations (13 and 0.13 μmol/l, respectively), we have estimated that infants ingest approximately 0.12ml/kg/day of nitrate and 0.0007 ml/kg/day of nitrite from fresh breast milk, which equates to only 5% and 0.6% of the nitrate and nitrite intake of adults (10). A comparison of the average dietary nitrate intake in newborn infants and adults is shown in Figure 2.
Figure 2.
Dietary nitrate and nitrite levels for newborns and adults. (a) Daily dietary nitrate ingestion, normalized for body weight, is shown for newborns and adults, based on a mean (±SEM) of reported concentrations in breast milk and formula (for newborns) (8–10) and a typical adult diet (4). (b,c) Nitrate and nitrite concentrations in total parenteral nutrition (TPN), fresh and freeze-thawed breast milk, freeze-thawed colostrum, and a convenience sample of artificial milk formulas. (Figure adapted from Jones et al., 2014 (10).)
We have also shown that nitrite is oxidized to nitrate in breast milk by an enzyme normally present in milk, lactoperoxidase, leading to even lower levels in milk that has been allowed to sit at room temperature or which has been freeze-thawed (10). Breast milk nitrite concentrations also fall during the first few weeks of life, with the highest levels found in colostrum and decreasing to nearly undetectable amounts in milk collected after the third week postpartum (9,10). The levels of nitrate and nitrite in artificial milk vary widely across a range that extends above and below concentrations measured in breast milk, averaging 43 and 0.3 μmol/l, respectively (10) (Figure 2). The recently increased use of nutritional additives for caloric and protein enhancement raises the possibility of an additional source of dietary nitrate and nitrite, although to our knowledge concentrations in these additives have not yet been reported. Whether the newborn deficiency of dietary nitrite and nitrate serves an important physiological role, or whether supplementation of breast milk with these anions would be beneficial or problematic remains to be studied.
Until recently, a majority of research related to dietary nitrate and nitrite was in the context of toxicology. It has been known since 1945 that unusually high nitrate concentrations in vegetables and drinking water, often due to contamination with fertilizer, can cause cyanosis due to oxidation of hemoglobin to methemoglobin by nitrite derived from bacterial nitrate reductases, a problem often referred to as “blue baby syndrome.” Newborn infants are particularly susceptible to this problem as they have ~25% lower methemoglobin reductase activity than adults (11). It is also proposed that dietary nitrate and nitrite are associated with GI cancer due to the formation of carcinogenic N-nitroso compounds. Although a definitive causal link between dietary nitrate and cancer has not been identified (12), the associations between the intake of nitrite-treated meats and gastric cancer are more established (13). This is consistent with evidence that nitrite added to meats as a preservative can be converted to harmful N-nitrosamines in the meat itself before ingestion or in the body after it has been ingested (14). In an effort to protect against toxicity, the Environmental Protection Agency has set limits on inorganic nitrate and nitrite levels in drinking water and the World Health Organization has put forward acceptable daily intakes for nitrate at 3.7 mg/kg of body weight and for nitrite at 0.06mg/kg of body weight. These levels are easily exceeded, however, with a high vegetable diet, and some have called for a resetting of these limits based on recent advances in our understanding of the roles of dietary nitrate and nitrite (9). The upper limits of toxicity of dietary nitrate in newborns have been investigated. Phillips et al. found that up to 21 mg/kg of nitrate per day was well tolerated by seven newborn infants, with six infants showing no increase in methemoglobin and the other one only a slight increase, and not enough to produce detectable cyanosis. Likewise, no symptoms of cyanosis occurred even when 100 mg nitrate kg/day was given to an infant for 8 d (15).
In contrast to the evidence of the toxic effects of nitrate and nitrite, the data increasingly indicate that a diet rich in nitrate is beneficial to overall cardiovascular health. In adults, raising dietary nitrate intake has been shown to improve exercise tolerance (13,16,17), decrease blood pressure (5,18), inhibit platelet aggregation (5), decrease risk of cardiovascular disease (19), and improve vascular compliance (20) (see Weitzberg and Lundberg, 2013 for a comprehensive review) (21). In addition, nitrite supplementation ameliorates microvascular inflammation and endothelial dysfunction in mice fed a high-cholesterol diet (22). Dietary nitrate also appears to have beneficial effects in the GI tract of adult rats, where it has been shown to protect against nonsteroidal anti-inflammatory drug (NSAID)-induced ulcers (23). Weighing the beneficial effects of increasing dietary nitrate and nitrite against the potential risks of methemoglobinemia and carcinogenicity is the focus of ongoing studies.
SALIVA
In addition to dietary intake of nitrate and nitrite, the levels of these anions in swallowed saliva also have a significant impact on the amount of nitrate and nitrite that is ingested. As discussed in this section, this appears to be another point of significant difference between adults and newborns, thus compounding the effects of low dietary nitrate and nitrite ingestion in newborns.
Bacterial Conversion of Salivary Nitrate to Nitrite
Fasting nitrate concentrations average about 200 μmol/l in the saliva of adults but can reach as high as 10 mmol/l after a nitrate-rich meal (24). These concentrations are approximately 10-fold higher than the concentrations measured in plasma due to active transport of nitrate from the blood into the saliva by the salivary glands. The transport of nitrate has been suggested to be mediated by the enzyme sialin via an adenosine 59-triphosphate-dependent electrogenic NO3−/H+ transport mechanism in the salivary acinar cells (25). The nitrate concentration in the saliva of newborns is approximately 200 μmol/l, similar to that of adults (26). As in adults, this concentration is many-fold higher than in blood (16–40 μmol/l). Thus, the active transport mechanisms in the salivary glands of newborn infants are present with a concentrating power comparable with that of adults (27,28). That the body expends energy to actively concentrate nitrate into the saliva suggests that nitrate is not just an inert end product of NO metabolism, but has potential bioactivity in the body.
Although nitrate itself appears to be inert in mammalian tissues, it is made physiologically relevant after reduction to nitrite by bacteria residing in the crypts of the dorsal posterior surface of the tongue. These bacteria utilize nitrate as the terminal electron acceptor in the respiratory chain, rather than oxygen and reduce about 20% of salivary nitrate in adults (13,29). A true symbiotic relationship between these bacteria and the human host exists as humans lack the requisite enzymes to bring about this conversion independently but provide nitrate to the bacteria that then perform nitrate reduction via respiration. As discussed below, these nitrate-reducing bacteria are critical to the beneficial effects of dietary nitrate.
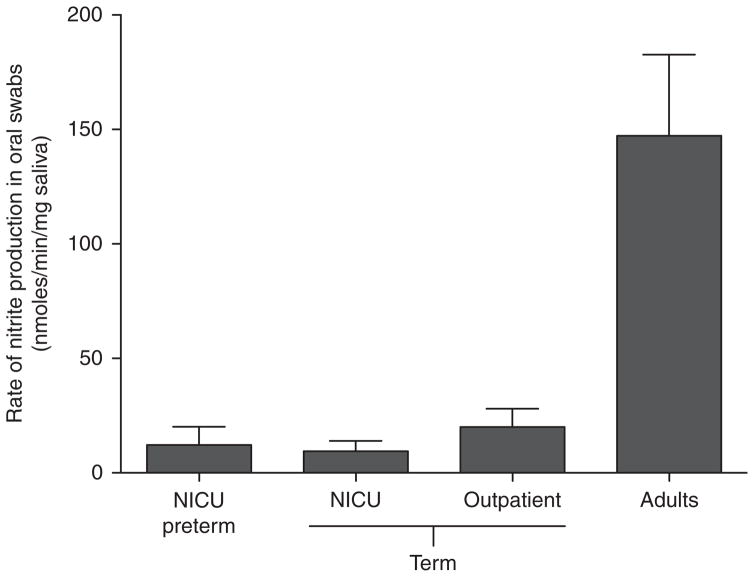
The primary bacteria that mediate nitrate reduction in the mouth are obligate anaerobes of the Veillonella species and facultative anaerobes of the Actinomyces, Rothia, and Staphylococcus species, all of which possess nitrate reductase enzymes that allow them to respire nitrate and rapidly produce nitrite (30). Veillonella and Actinomyces species have been found in saliva collected from infants in the first 2 mo of life and appear to be some of the first bacteria to colonize the mouths of newborns (26,31–33). Despite the presence of these bacteria, oral nitrate reductase activity is markedly lower in newborn infants when compared with adults, as shown in Figure 3. It is unknown whether this difference comes from insufficient numbers of bacteria, whether the bacteria do not possess sufficient nitrate-reducing capacity, or whether the mouth of newborns lack some cofactor for nitrate reduction or some other necessary element.
Figure 3.
Nitrate-reducing activity, normalized for saliva weight, in swab samples collected from the mouths of preterm (born at <35 wk gestation) and term (born at >36 wk) infants in the neonatal intensive care unit (NICU) or from healthy infants in an outpatient clinic between 2 and 6 wk after birth, or from normal healthy adults. Mammalian cells lack the enzymes required for nitrate reduction, but bacteria dwelling in crypts of the tongue bring about the reaction. Note that the rate in infants is ~10% of that in adults. (Figure adapted from Kanady et al., 2012 (26).)
By measuring the nitrate-reducing capacity of bacteria in oral swabs collected from infants, we have shown that there is essentially no detectable nitrite production from nitrate in the first 5 d of life. While there is measurable nitrate-reducing capacity in swabs (normalized to the saliva content in the swab) collected from infants at 2–8wk of age, the rate of nitrite production is only ~10% of that of adults (26), as illustrated in Figure 3. Notably, infants also produce relatively small volumes of saliva during the first few weeks of life, which may attenuate bacterial growth and may also result in less swallowed salivary nitrite compared with the adult (26). The developmental time point at which the nitrate-reducing capacity of the infant mouth becomes comparable to the adult is unknown.
The diminished bacterial nitrate-reducing capacity in infants may be of physiological relevance because salivary nitrite impacts both GI and cardiovascular function in adults. Blockade of salivary nitrate secretion by ligation of the sub-mandibular gland duct in rats results in decreased gastric nitrate, nitrite, and NO concentrations and exacerbates stress-induced gastric ulcers (34). The severity of gastric ulcers in these rats is reduced upon supplemental nitrate treatment (34). These gastroprotective effects appear to be mediated through the action of increased salivary nitrite, as nitrite-rich saliva results in increased gastric mucosal blood flow, a thicker mucus layer, and attenuation of the inflammatory response associated with NSAID administration in rats (23,35,36). These effects of nitrite on the stomach are likely due to its conversion to NO, as discussed below.
In addition to the effects in the GI tract, increasing dietary nitrate, and concomitant increases in salivary nitrite, have been shown to decrease arterial blood pressure, protect against ischemia–reperfusion (I/R)-induced endothelial dysfunction, and decrease platelet aggregation (5,18). The importance of salivary nitrite production by oral bacteria is again highlighted by the finding that if subjects refrain from swallowing saliva after a dietary nitrate-load or are given antibacterial mouthwash to decrease bacterial nitrate-reducing activity, the hypotensive effects of nitrate are attenuated and there is no inhibition of platelet aggregation (5,37). Increasing dietary nitrate also leads to increased circulating nitrite concentrations (5), which is associated with a host of beneficial effects ranging from improved exercise tolerance to protection against I/R injury (discussed below).
Thus, considering the beneficial GI and cardiovascular effects of dietary nitrate and subsequent salivary nitrite production by oral nitrate-reducing bacteria in adults, the lack of the critical bacterial nitrate reduction in infants is noteworthy and deserves investigation. Moreover, the lack of bacterial nitrate-reducing activity in infants will compound the already low levels of nitrate and nitrite in their diet, ultimately leading to significantly lower nitrite delivery to the infant stomach. The potential benefit of adding a mother’s oral bacteria to an infant’s mouth is untested.
GI TRACT
Intragastric Conversion of Nitrite to NO
In 1994, two independent studies showed that NO was generated from nitrite in the stomach of human adults (38,39). These studies showed a novel nitric oxide synthase (NOS)-independent in-vivo mechanism by which NO could be generated from nitrite. Since then, there has been great interest in nitrite as not merely an inert NO metabolite but as a physiologically relevant source of NO bioactivity. The chemical reaction by which NO is generated in the acidic stomach of adults involves protonation of nitrite to form nitrous acid (pKa 3.3), which rapidly decomposes to several highly reactive nitrogen oxides, including NO free radical, NO2, N2O3, and peroxynitrite (38,40). In the stomach, these nitrogen oxides can form new stable products through nitration and nitrosylation of amines, amides, thiols, and fatty acids. These products have wide-ranging bioactivities, which include modulation of inflammatory signaling pathways, inhibition of platelet aggregation, vasodilation, mucus production, and bacterial colonization, among many other functions (40–43).
Via gastric conversion to NO, ingested nitrite has been shown to kill many different enteropathogens including Salmonella, Shigella, Helicobacter pylori, Escherichia coli, Yersinia enerocolitica, Clostridium difficile, and Candida albicans, establishing nitrite as a key player in host defense (44–47). In addition to acting as a bactericidal agent, NO plays a key role in host defense in the GI tract by stimulating mucus and fluid secretion, regulating the epithelial barrier, mediating vascular smooth muscle tone, diminishing leukocyte adherence to the endothelium, modulating mucosal repair, and influencing the release of inflammatory mediators (21,48). While it is now apparent that nitrite-derived NO plays many protective roles in the stomach and GI tract, it is important to note that nitrite in the stomach (via conversion to nitrous acid and other nitrogen oxides) can also act as a nitrosating agent, converting ingested amines into their carcinogenic N-nitroso derivatives (13). However, while the nitrosating ability of acidified nitrite is clear, there is still no direct evidence that increased ingestion of nitrate, and subsequent conversion to salivary nitrite and gastric NO, causes increased risk of gastric cancer (29).
NO generation from nitrite in the stomach is highly pH dependent and is effectively attenuated with proton pump inhibitors (39). Consequently, the protective effects of swallowed nitrite appear to be highly dependent upon gastric acidity as increasing the pH above a value of 4 effectively prevents nitrite-induced increases in blood flow (35) and reduction in pathogenic bacteria (38,47) and blocks nitrite’s hypotensive effects (49).
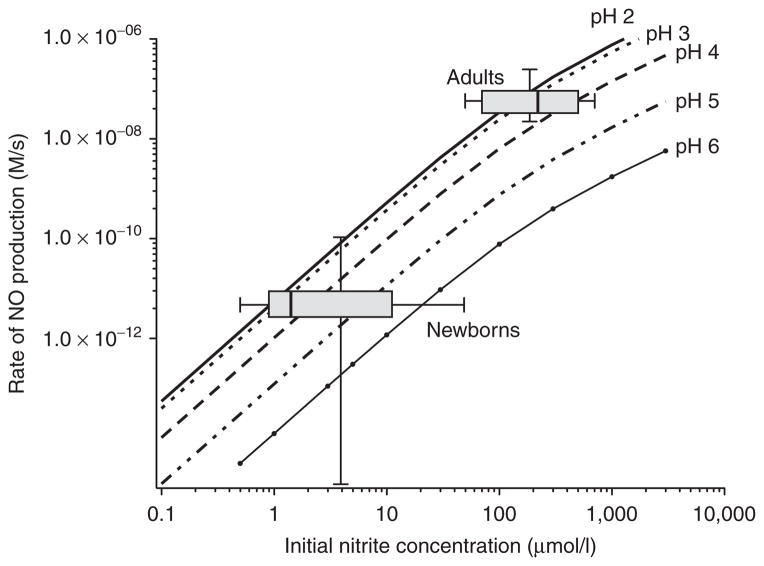
The pH dependence of nitrite-derived gastric NO is of particular interest in the newborn, as the newborn stomach has a relatively high pH compared with the adult stomach (50–52). Figure 4 illustrates the effect of pH on the rate of NO production from nitrite using previously calculated rate constants (53). As shown in Figure 4, the less acidic environment of the newborn stomach would attenuate the generation of NO from nitrite delivered to the stomach, compounding the already low nitrite ingestion from the saliva and diet. High gastric pH in newborns has been associated with an increased risk of necrotizing enterocolitis (NEC) (54,55), whereas low gastric pH protects against bacterial translocation across the gut wall in neonatal rabbit pups (56). Considering that acidified nitrite kills bacteria, improves mucus secretion and mucosal blood flow, and is protective against I/R injury (discussed below), it is worth speculating that enhancing NO generation from nitrite would be protective against NEC.
Figure 4.
Nomogram showing the rate of nitric oxide (NO) generation in gastric fluid for various pH and nitrite concentrations. The adult and newborn ranges of nitrite concentrations are shown as box and whisker (min to max) plots of measurements of saliva (26) and placed at reported typical ranges of gastric pH (
 pH 2;
pH 2;
 pH 3;
pH 3;
 pH 4;
pH 4;
 pH 5;
pH 5;
 pH 6) for adults and newborns (50). The infant rate is estimated to be about 100-fold slower than the adult rate due to less acidity and lower nitrite levels in the newborn stomach. Curves were constructed using rate constants and equations given by Zweier et al. (53).
pH 6) for adults and newborns (50). The infant rate is estimated to be about 100-fold slower than the adult rate due to less acidity and lower nitrite levels in the newborn stomach. Curves were constructed using rate constants and equations given by Zweier et al. (53).
Intestine
In contrast to the NOS-independent generation of NO in the stomach, NO generation in the colon appears to be mediated by NOS-dependent mechanisms, as demonstrated by the finding that rats treated with the NOS inhibitor NG-nitro-L-arginine methyl ester have significantly less NO generation in the colon while NO generation in the stomach is unaffected (57). In rats, NO concentrations in the stomach (>4000 ppb) are orders of magnitude higher than in the small intestine (<20 ppb), cecum (~200 ppb), or colon (<25 ppb) (57). Interestingly, germ-free rats have markedly lower NO generation in all areas of the GI tract, including the stomach, indicating an important role for bacteria in NO production throughout the GI tract. Germ-free rats provide a useful comparison to newborn infants, as diminished gastric NO in germ-free rats is thought to be due to the lack of oral nitrate-reducing bacteria since gastric NO production is dependent on substrate (ingested nitrite) availability (57). In the cecum, NO is in part formed via reduction of nitrate and nitrite by strains of Lactobacilli and Bifidobacteria (58) and stimulation of the mucosal NOS enzymes by GI bacteria (57). NO generation in the intestine, either by Lactobacilli farciminis or by administration of an NO-donor, has been demonstrated to have anti-inflammatory effects in an animal model of colitis (59), highlighting the potential protective effects of increased NO generation in the intestine.
Given the beneficial effects of NO derived from ingested nitrite on the GI microbiota, blood flow, and mucus production (described above), it is interesting to speculate about nitrite’s role in the context of NEC. NEC is the most common GI disease to a*ict premature infants. It is a disease characterized by intestinal barrier failure (60) most likely subsequent to an ischemic insult. The main factors contributing to the regulation of GI blood flow in the preterm infant are poorly defined, and it is not known whether a deficiency in NO contributes to the dysregulation of GI blood flow that is thought to precede NEC. Translocation of bacteria across the compromised GI wall leads to activation of an inflammatory response characterized by pronounced up-regulation of NO production by inducible NOS. In this inflammatory stage of the disease, overproduction of NO by inducible NOS results in toxic levels of peroxynitrite, further damaging the integrity of the gut wall by inducing enterocyte apoptosis and necrosis, or by disrupting tight junctions and gap junctions that normally maintain epithelial monolayer integrity (61,62). Thus, a vicious cycle characteristic of severe NEC is created by bacterial invasion, immune activation, uncontrolled inflammation with production of reactive oxygen species and nitrogen species, vasoconstriction followed by I/R injury, gut barrier failure, intestinal necrosis, sepsis, and shock (63). It is reasonable to hypothesize that NO plays a dichotomous role in NEC, with deficient levels of NO contributing to an increased vascular resistance during the initiating ischemic event, and subsequent overproduction of NO during the inflammatory stage of the disease leading to propagation of the injury. Interestingly, Yazji et al. have recently reported that nitrite/nitrate-deficient formula predisposes newborn mice to NEC, and that both the incidence and severity of NEC was ameliorated by nitrite/nitrate supplementation to the formula to achieve levels comparable with that of breast milk (64). However, the clinical relevance of this finding is uncertain given the fact that nitrite and nitrate concentrations of many commercially available formulas are already higher than those found in breast milk (10). Whether manipulation of the decreased levels of ingested and circulating nitrite in the preterm infant would prevent or alter the course of NEC is an area worthy of study.
CIRCULATION
Circulating Nitrite
Shortly after the discovery that NO is generated from nitrite in the acidic environment of the stomach, Zweier et al. showed that NO could also be generated from nitrite in ischemic heart tissue (65). Nitrite reduction to NO in hypoxic tissues appears to be mediated either by acidic disproportionation (similar to the mechanism in the acidic stomach) or by the activity of metal-containing proteins with nitrite-reducing activity. These proteins include the heme-associated globins in their deoxygenated state such as deoxygenated myoglobin, hemoglobin, cytoglobin, and neuroglobin as well as mitochondrial enzymes like complex III; molybdenum metalloenzymes such as xanthine oxidoreductase (66); cytochrome P450 enzymes; and endothelial NOS (see the review by Kim-Shapiro and Gladwin (67)). While the initial report by Zweier et al. suggested that nitrite reduction to NO in acidic tissues exacerbates post-ischemic injury (65), nitrite has since been consistently shown in experimental animals to be protective against I/R injury in the heart, brain, liver, and kidney (29,68). The mechanism by which nitrite confers protection against I/R injury is not well understood, but is thought to involve reduction to NO which modulates the function of the mitochondria, leading to more efficient oxygen utilization, decreased reactive oxygen species formation, and the inhibition of apoptotic signaling (69). The therapeutic potential of nitrite against I/R injury in newborns has not yet been studied. Considering that neonates are particularly at risk for hypoxic and ischemic insults, further research is needed to address the therapeutic and preventative potential for nitrite supplementation in the neonatal population.
While nitrite supplementation has yet to be studied in neonatal populations, it has been shown that treatment of persistent pulmonary hypertension of the newborn with inhaled NO (iNO) increases nitrite levels in the blood at least twofold (27,70). Although the resulting nitrite concentrations reached only ~300 nmol/l after iNO administration, similar increases in circulating nitrite concentrations have been shown to protect mice against hepatic infarct (71), increase blood flow in the human forearm (72), and decrease systolic blood pressure in adults (5,18,73). Thus, increases in circulating nitrite levels resulting from iNO treatment may be enough to cause significant systemic effects (27). Indeed, there are reports demonstrating protective effects of iNO therapy in a mouse model of myocardial infarction (74), adult human liver transplant patients (75), and in children following cardiopulmonary bypass (76). Whether the protective effects of iNO are due to elevations in circulating nitrite remains to be determined.
Given that nitrite could theoretically improve an infant’s ability to withstand ischemic stress, it is important to discuss the mounting evidence that normal newborn infants appear to have numerous mechanisms in place that decrease systemic nitrite levels during the first few weeks of life. As shown in Figure 1, these mechanisms include low dietary nitrite and nitrate intake, the lack of bacterial nitrate reduction in the mouth, and the relatively high pH in the stomach. It has now become evident that plasma nitrite concentrations fall at birth and remain lower than adult levels for the first few weeks of life. We have recently found (data not published) that circulating nitrite concentrations decrease markedly after birth, falling from approximately 0.18 ± 0.02 μmol/l in umbilical cord plasma to 0.08 ± 0.02 μmol/l in plasma collected from term infants on their first day of life. Interestingly, plasma collected from preterm infants has even lower nitrite concentrations (0.04 ± 0.01 μmol/l). Plasma nitrite concentrations are significantly lower in infants than those measured in adults, which typically range from 0.05 to 0.30 μmol/l (44). These findings are consistent with previous reports that adult plasma nitrite levels are significantly higher than those of newborn infants (27), but are similar to those in umbilical cord blood (77). The relevance of the dramatic fall in circulating nitrite levels immediately after birth is uncertain, but may be an important part of the circulatory changes that occur at birth.
There are many factors that contribute to plasma nitrite concentrations. In adults, a majority of plasma nitrite is derived from the oxidation of NO produced by endothelial NOS (6). This oxidation depends on enzyme-catalyzed reactions in the plasma (78), which are significantly attenuated in the newborn (79). In addition, endothelial NOS activity may be diminished in newborns due to low levels of L-arginine (80) and increased levels of asymmetric dimethylarginine, the endogenous inhibitor of NOS (81–83). Furthermore, the rapid increase in tissue PO2 that occurs at birth may result in increased superoxide levels, particularly in preterm infants who are likely to have low antioxidant defenses (84–87). This superoxide can rapidly scavenge NO to produce peroxynitrite instead of nitrite. Another potential cause of relatively low plasma nitrite in infants could be the lack of significant oral bacterial nitrate reduction, as discussed above and illustrated in Figure 3. We have recently shown that adults given antibacterial mouth rinse have significantly reduced plasma nitrite concentrations (26), highlighting the importance of the oral nitrate-reducing bacteria to the amount of circulating nitrite. Thus, with the confluence of all of these factors, it appears that nitrite bioavailability is diminished in the newborn by a system of concerted mechanisms, as evidenced by the sharp fall in nitrite concentrations at birth. The physiological relevance of this decrease remains to be elucidated and should be more fully understood before efforts are made to study the potentially therapeutic use of nitrite in this patient population.
Summary
Before birth, nitrite concentrations in fetal blood are similar to those in maternal blood due to rapid passive exchange of the anions across the placenta. Within hours of birth, however, the nitrite concentration in the newborn falls sharply in association with increases in blood pressure, increases in pulmonary blood flow, and many other adaptations to increasing oxygen tensions. In the early weeks of life, nitrate and nitrite levels remain low for several reasons. There is limited ingestion of nitrate and nitrite because their concentrations are low in milk and formula. There is little reduction of nitrate to the physiologically active nitrite by oral bacteria. Finally, there is little generation of NO in the newborn stomach because the pH is high. The net result is that the recirculation of nitrate and nitrite as bioactive sources of NO is markedly lower in the newborn than in the adult.
In recent decades, the many serious concerns that nitrite in the diet would cause cancer and methemoglobinemia have lessened and been replaced by new findings of cardiovascular benefits. In the newborn period, there arises the prospect of protecting the GI tract from bacterial invasion by supplementation with nitrite, thereby increasing NO bioactivity and its protective actions. However, careful investigation must be done weighing the risks against the benefits before supplementation with nitrite can be undertaken safely in newborn infants.
Supplementary Material
Acknowledgments
STATEMENT OF FINANCIAL SUPPORT
The research was supported by intramural funding from the John Mace Pediatric Research Grant Fund of the Loma Linda University School of Medicine, Department of Pediatrics, Loma Linda, CA, and the National Institutes of Health, Bethesda, MD, grant HL095973 (awarded to Arlin B. Blood).
We gratefully acknowledge the assistance of Shannon Bragg and Nathan Jones in preparing the manuscript.
Footnotes
Disclosure: The authors have no financial ties to the products in the review or any potential or perceived conflicts of interest to disclose.
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at http://www.nature.com/pr
References
- 1.Clark RH, Kueser TJ, Walker MW, et al. Low-dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. Clinical Inhaled Nitric Oxide Research Group. N Engl J Med. 2000;342:469–74. doi: 10.1056/NEJM200002173420704. [DOI] [PubMed] [Google Scholar]
- 2.Neonatal Inhaled Nitric Oxide Study Group. Inhaled nitric oxide in full-term and nearly full-term infants with hypoxic respiratory failure. N Engl J Med. 1997;336:597–604. doi: 10.1056/NEJM199702273360901. [DOI] [PubMed] [Google Scholar]
- 3.Eich RF, Li T, Lemon DD, et al. Mechanism of NO-induced oxidation of myoglobin and hemoglobin. Biochemistry. 1996;35:6976–83. doi: 10.1021/bi960442g. [DOI] [PubMed] [Google Scholar]
- 4.Mensinga TT, Speijers GJ, Meulenbelt J. Health implications of exposure to environmental nitrogenous compounds. Toxicol Rev. 2003;22:41–51. doi: 10.2165/00139709-200322010-00005. [DOI] [PubMed] [Google Scholar]
- 5.Webb AJ, Patel N, Loukogeorgakis S, et al. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51:784–90. doi: 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleinbongard P, Dejam A, Lauer T, et al. Plasma nitriter effects constitutive nitric oxide synthase activity in mammals. Free Radic Biol Med. 2003;35:790–6. doi: 10.1016/s0891-5849(03)00406-4. [DOI] [PubMed] [Google Scholar]
- 7.Pennington JAT. Dietary exposure models for nitrates and nitrites. Food Control. 1998;9:385–95. [Google Scholar]
- 8.Ohta N, Tsukahara H, Ohshima Y, et al. Nitric oxide metabolites and adrenomedullin in human breast milk. Early Hum Dev. 2004;78:61–5. doi: 10.1016/j.earlhumdev.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Hord NG, Ghannam JS, Garg HK, Berens PD, Bryan NS. Nitrate and nitrite content of human, formula, bovine, and soy milks: implications for dietary nitrite and nitrate recommendations. Breastfeed Med. 2011;6:393–9. doi: 10.1089/bfm.2010.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones JA, Ninnis JR, Hopper AO, et al. Nitrite and nitrate concentrations and metabolism in breast milk, infant formula, and parenteral nutrition. JPEN. 2014;38:856–66. doi: 10.1177/0148607113496118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Power GG, Bragg SL, Oshiro BT, Dejam A, Hunter CJ, Blood AB. A novel method of measuring reduction of nitrite-induced methemoglobin applied to fetal and adult blood of humans and sheep. J Appl Physiol (1985) 2007;103:1359–65. doi: 10.1152/japplphysiol.00443.2007. [DOI] [PubMed] [Google Scholar]
- 12.Gangolli SD, van den Brandt PA, Feron VJ, et al. Nitrate, nitrite and N-nitroso compounds. Eur J Pharmacol. 1994;292:1–38. doi: 10.1016/0926-6917(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 13.Bryan NS, van Grinsven H. The role of nitrate in human health. In: Donald S, editor. Advances in Agronomy. Vol. 119. San Diego, CA: Elsevier; 2013. pp. 153–82. [Google Scholar]
- 14.Jakszyn P, Agudo A, Berenguer A, et al. Intake and food sources of nitrites and N-nitrosodimethylamine in Spain. Public Health Nutr. 2006;9:785–91. doi: 10.1079/phn2005884. [DOI] [PubMed] [Google Scholar]
- 15.Phillips WE. Naturally occurring nitrate and nitrite in foods in relation to infant methaemoglobinaemia. Food Cosmet Toxicol. 1971;9:219–28. doi: 10.1016/0015-6264(71)90307-5. [DOI] [PubMed] [Google Scholar]
- 16.Bailey SJ, Winyard P, Vanhatalo A, et al. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J Appl Physiol (1985) 2009;107:1144–55. doi: 10.1152/japplphysiol.00722.2009. [DOI] [PubMed] [Google Scholar]
- 17.Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol (Oxf) 2007;191:59–66. doi: 10.1111/j.1748-1716.2007.01713.x. [DOI] [PubMed] [Google Scholar]
- 18.Larsen FJ, Ekblom B, Sahlin K, Lundberg JO, Weitzberg E. Effects of dietary nitrate on blood pressure in healthy volunteers. N Engl J Med. 2006;355:2792–3. doi: 10.1056/NEJMc062800. [DOI] [PubMed] [Google Scholar]
- 19.Zand J, Lanza F, Garg HK, Bryan NS. All-natural nitrite and nitrate containing dietary supplement promotes nitric oxide production and reduces triglycerides in humans. Nutr Res. 2011;31:262–9. doi: 10.1016/j.nutres.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 20.Bahra M, Kapil V, Pearl V, Ghosh S, Ahluwalia A. Inorganic nitrate ingestion improves vascular compliance but does not alter flow-mediated dilatation in healthy volunteers. Nitric Oxide. 2012;26:197–202. doi: 10.1016/j.niox.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weitzberg E, Lundberg JO. Novel aspects of dietary nitrate and human health. Annu Rev Nutr. 2013;33:129–59. doi: 10.1146/annurev-nutr-071812-161159. [DOI] [PubMed] [Google Scholar]
- 22.Stokes KY, Dugas TR, Tang Y, Garg H, Guidry E, Bryan NS. Dietary nitrite prevents hypercholesterolemic microvascular inflammation and reverses endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2009;296:H1281–8. doi: 10.1152/ajpheart.01291.2008. [DOI] [PubMed] [Google Scholar]
- 23.Jansson EA, Petersson J, Reinders C, et al. Protection from nonsteroidal anti-inflammatory drug (NSAID)-induced gastric ulcers by dietary nitrate. Free Radic Biol Med. 2007;42:510–8. doi: 10.1016/j.freeradbiomed.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 24.Lundberg JO, Govoni M. Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic Biol Med. 2004;37:395–400. doi: 10.1016/j.freeradbiomed.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 25.Qin L, Liu X, Sun Q, et al. Sialin (SLC17A5) functions as a nitrate transporter in the plasma membrane. Proc Natl Acad Sci USA. 2012;109:13434–9. doi: 10.1073/pnas.1116633109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanady JA, Aruni AW, Ninnis JR, et al. Nitrate reductase activity of bacteria in saliva of term and preterm infants. Nitric Oxide. 2012;27:193–200. doi: 10.1016/j.niox.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ibrahim YI, Ninnis JR, Hopper AO, et al. Inhaled nitric oxide therapy increases blood nitrite, nitrate, and s-nitrosohemoglobin concentrations in infants with pulmonary hypertension. J Pediatr. 2012;160:245–51. doi: 10.1016/j.jpeds.2011.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biban P, Zangardi T, Baraldi E, Dussini N, Chiandetti L, Zacchello F. Mixed exhaled nitric oxide and plasma nitrites and nitrates in newborn infants. Life Sci. 2001;68:2789–97. doi: 10.1016/s0024-3205(01)01086-4. [DOI] [PubMed] [Google Scholar]
- 29.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate–nitrite–nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–67. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 30.Doel JJ, Benjamin N, Hector MP, Rogers M, Allaker RP. Evaluation of bacterial nitrate reduction in the human oral cavity. Eur J Oral Sci. 2005;113:14–9. doi: 10.1111/j.1600-0722.2004.00184.x. [DOI] [PubMed] [Google Scholar]
- 31.Könönen E, Kanervo A, Takala A, Asikainen S, Jousimies-Somer H. Establishment of oral anaerobes during the first year of life. J Dent Res. 1999;78:1634–9. doi: 10.1177/00220345990780100801. [DOI] [PubMed] [Google Scholar]
- 32.Sarkonen N, Könönen E, Summanen P, Kanervo A, Takala A, Jousimies-Somer H. Oral colonization with Actinomyces species in infants by two years of age. J Dent Res. 2000;79:864–7. doi: 10.1177/00220345000790031301. [DOI] [PubMed] [Google Scholar]
- 33.Pearce C, Bowden GH, Evans M, et al. Identification of pioneer viridans streptococci in the oral cavity of human neonates. J Med Microbiol. 1995;42:67–72. doi: 10.1099/00222615-42-1-67. [DOI] [PubMed] [Google Scholar]
- 34.Jin L, Qin L, Xia D, et al. Active secretion and protective effect of salivary nitrate against stress in human volunteers and rats. Free Radic Biol Med. 2013;57:61–7. doi: 10.1016/j.freeradbiomed.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Björne H, Petersson J, Phillipson M, Weitzberg E, Holm L, Lundberg JO. Nitrite in saliva increases gastric mucosal blood flow and mucus thickness. J Clin Invest. 2004;113:106–14. doi: 10.1172/JCI200419019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petersson J, Phillipson M, Jansson EA, Patzak A, Lundberg JO, Holm L. Dietary nitrate increases gastric mucosal blood flow and mucosal defense. Am J Physiol Gastrointest Liver Physiol. 2007;292:G718–24. doi: 10.1152/ajpgi.00435.2006. [DOI] [PubMed] [Google Scholar]
- 37.Petersson J, Carlström M, Schreiber O, et al. Gastroprotective and blood pressure lowering effects of dietary nitrate are abolished by an antiseptic mouthwash. Free Radic Biol Med. 2009;46:1068–75. doi: 10.1016/j.freeradbiomed.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 38.Benjamin N, O’Driscoll F, Dougall H, et al. Stomach NO synthesis. Nature. 1994;368:502. doi: 10.1038/368502a0. [DOI] [PubMed] [Google Scholar]
- 39.Lundberg JO, Weitzberg E, Lundberg JM, Alving K. Intragastric nitric oxide production in humans: measurements in expelled air. Gut. 1994;35:1543–6. doi: 10.1136/gut.35.11.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rocha BS, Gago B, Barbosa RM, Lundberg JO, Radi R, Laranjinha J. Intra-gastric nitration by dietary nitrite: implications for modulation of protein and lipid signaling. Free Radic Biol Med. 2012;52:693–8. doi: 10.1016/j.freeradbiomed.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 41.Baker PR, Lin Y, Schopfer FJ, et al. Fatty acid transduction of nitric oxide signaling: multiple nitrated unsaturated fatty acid derivatives exist in human blood and urine and serve as endogenous peroxisome proliferator-activated receptor ligands. J Biol Chem. 2005;280:42464–75. doi: 10.1074/jbc.M504212200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coles B, Bloodsworth A, Clark SR, et al. Nitrolinoleate inhibits superoxide generation, degranulation, and integrin expression by human neutrophils: novel antiinflammatory properties of nitric oxide-derived reactive species in vascular cells. Circ Res. 2002;91:375–81. doi: 10.1161/01.res.0000032114.68919.ef. [DOI] [PubMed] [Google Scholar]
- 43.Trostchansky A, Bonilla L, González-Perilli L, Rubbo H. Nitro-fatty acids: formation, redox signaling, and therapeutic potential. Antioxid Redox Signal. 2013;19:1257–65. doi: 10.1089/ars.2012.5023. [DOI] [PubMed] [Google Scholar]
- 44.Lundberg JO, Weitzberg E. NO-synthase independent NO generation in mammals. Biochem Biophys Res Commun. 2010;396:39–45. doi: 10.1016/j.bbrc.2010.02.136. [DOI] [PubMed] [Google Scholar]
- 45.Fite A, Dykhuizen R, Litterick A, Golden M, Leifert C. Effects of ascorbic acid, glutathione, thiocyanate, and iodide on antimicrobial activity of acidified nitrite. Antimicrob Agents Chemother. 2004;48:655–8. doi: 10.1128/AAC.48.2.655-658.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dykhuizen RS, Frazer R, Duncan C, et al. Antimicrobial effect of acidified nitrite on gut pathogens: importance of dietary nitrate in host defense. Antimicrob Agents Chemother. 1996;40:1422–5. doi: 10.1128/aac.40.6.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cunningham R, Mustoe E, Spiller L, Lewis S, Benjamin N. Acidified nitrite: a host defence against colonization with C. dificile spores? J Hosp Infect. 2014;86:155–7. doi: 10.1016/j.jhin.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 48.Wallace JL, Miller MJ. Nitric oxide in mucosal defense: a little goes a long way. Gastroenterology. 2000;119:512–20. doi: 10.1053/gast.2000.9304. [DOI] [PubMed] [Google Scholar]
- 49.Amaral JH, Montenegro MF, Pinheiro LC, et al. TEMPOL enhances the antihypertensive effects of sodium nitrite by mechanisms facilitating nitrite-derived gastric nitric oxide formation. Free Radic Biol Med. 2013;65:446–55. doi: 10.1016/j.freeradbiomed.2013.07.032. [DOI] [PubMed] [Google Scholar]
- 50.Harries JT, Fraser AJ. The acidity of the gastric contents of premature babies during the first fourteen days of life. Biol Neonat. 1968;12:186–93. doi: 10.1159/000240105. [DOI] [PubMed] [Google Scholar]
- 51.Ward RM, Kearns GL. Proton pump inhibitors in pediatrics: mechanism of action, pharmacokinetics, pharmacogenetics, and pharmacodynamics. Paediatr Drugs. 2013;15:119–31. doi: 10.1007/s40272-013-0012-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miclat NN, Hodgkinson R, Marx GF. Neonatal gastric pH. Anesth Analg. 1978;57:98–101. doi: 10.1213/00000539-197801000-00018. [DOI] [PubMed] [Google Scholar]
- 53.Samouilov A, Kuppusamy P, Zweier JL. Evaluation of the magnitude and rate of nitric oxide production from nitrite in biological systems. Arch Biochem Biophys. 1998;357:1–7. doi: 10.1006/abbi.1998.0785. [DOI] [PubMed] [Google Scholar]
- 54.More K, Athalye-Jape G, Rao S, Patole S. Association of inhibitors of gastric acid secretion and higher incidence of necrotizing enterocolitis in preterm very low-birth-weight infants. Am J Perinatol. 2013;30:849–56. doi: 10.1055/s-0033-1333671. [DOI] [PubMed] [Google Scholar]
- 55.Terrin G, Passariello A, De Curtis M, et al. Ranitidine is associated with infections, necrotizing enterocolitis, and fatal outcome in newborns. Pediatrics. 2012;129:e40–5. doi: 10.1542/peds.2011-0796. [DOI] [PubMed] [Google Scholar]
- 56.Dinsmore JE, Jackson RJ, Smith SD. The protective role of gastric acidity in neonatal bacterial translocation. J Pediatr Surg. 1997;32:1014–6. doi: 10.1016/s0022-3468(97)90389-4. [DOI] [PubMed] [Google Scholar]
- 57.Sobko T, Reinders C, Norin E, Midtvedt T, Gustafsson LE, Lundberg JO. Gastrointestinal nitric oxide generation in germ-free and conventional rats. Am J Physiol Gastrointest Liver Physiol. 2004;287:G993–7. doi: 10.1152/ajpgi.00203.2004. [DOI] [PubMed] [Google Scholar]
- 58.Sobko T, Reinders CI, Jansson E, Norin E, Midtvedt T, Lundberg JO. Gastrointestinal bacteria generate nitric oxide from nitrate and nitrite. Nitric Oxide. 2005;13:272–8. doi: 10.1016/j.niox.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 59.Lamine F, Fioramonti J, Bueno L, et al. Nitric oxide released by Lactobacillus farciminis improves TNBS-induced colitis in rats. Scand J Gastroenterol. 2004;39:37–45. doi: 10.1080/00365520310007152. [DOI] [PubMed] [Google Scholar]
- 60.Hackam DJ, Upperman JS, Grishin A, Ford HR. Disordered enterocyte signaling and intestinal barrier dysfunction in the pathogenesis of necrotizing enterocolitis. Semin Pediatr Surg. 2005;14:49–57. doi: 10.1053/j.sempedsurg.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 61.Chokshi NK, Guner YS, Hunter CJ, Upperman JS, Grishin A, Ford HR. The role of nitric oxide in intestinal epithelial injury and restitution in neonatal necrotizing enterocolitis. Semin Perinatol. 2008;32:92–9. doi: 10.1053/j.semperi.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Watkins DJ, Besner GE. The role of the intestinal microcirculation in necrotizing enterocolitis. Semin Pediatr Surg. 2013;22:83–7. doi: 10.1053/j.sempedsurg.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.De Plaen IG. Inflammatory signaling in necrotizing enterocolitis. Clin Perinatol. 2013;40:109–24. doi: 10.1016/j.clp.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yazji I, Sodhi CP, Lee EK, et al. Endothelial TLR4 activation impairs intestinal microcirculatory perfusion in necrotizing enterocolitis via eNOS–NO–nitrite signaling. Proc Natl Acad Sci USA. 2013;110:9451–6. doi: 10.1073/pnas.1219997110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zweier JL, Wang P, Samouilov A, Kuppusamy P. Enzyme-independent formation of nitric oxide in biological tissues. Nat Med. 1995;1:804–9. doi: 10.1038/nm0895-804. [DOI] [PubMed] [Google Scholar]
- 66.Li H, Samouilov A, Liu X, Zweier JL. Characterization of the magnitude and kinetics of xanthine oxidase-catalyzed nitrite reduction. Evaluation of its role in nitric oxide generation in anoxic tissues. J Biol Chem. 2001;276:24482–9. doi: 10.1074/jbc.M011648200. [DOI] [PubMed] [Google Scholar]
- 67.Kim-Shapiro DB, Gladwin MT. Mechanisms of nitrite bioactivation. Nitric Oxide. 2014;38:58–68. doi: 10.1016/j.niox.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dezfulian C, Raat N, Shiva S, Gladwin MT. Role of the anion nitrite in ischemia–reperfusion cytoprotection and therapeutics. Cardiovasc Res. 2007;75:327–38. doi: 10.1016/j.cardiores.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Murillo D, Kamga C, Mo L, Shiva S. Nitrite as a mediator of ischemic preconditioning and cytoprotection. Nitric Oxide. 2011;25:70–80. doi: 10.1016/j.niox.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Posencheg MA, Gow AJ, Truog WE, et al. Inhaled nitric oxide in premature infants: effect on tracheal aspirate and plasma nitric oxide metabolites. J Perinatol. 2010;30:275–80. doi: 10.1038/jp.2009.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Duranski MR, Greer JJ, Dejam A, et al. Cytoprotective effects of nitrite during in vivo ischemia–reperfusion of the heart and liver. J Clin Invest. 2005;115:1232–40. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dejam A, Hunter CJ, Tremonti C, et al. Nitrite infusion in humans and nonhuman primates: endocrine effects, pharmacokinetics, and tolerance formation. Circulation. 2007;116:1821–31. doi: 10.1161/CIRCULATIONAHA.107.712133. [DOI] [PubMed] [Google Scholar]
- 73.Vanhatalo A, Bailey SJ, Blackwell JR, et al. Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1121–31. doi: 10.1152/ajpregu.00206.2010. [DOI] [PubMed] [Google Scholar]
- 74.Nagasaka Y, Fernandez BO, Garcia-Saura MF, et al. Brief periods of nitric oxide inhalation protect against myocardial ischemia–reperfusion injury. Anesthesiology. 2008;109:675–82. doi: 10.1097/ALN.0b013e318186316e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lang JD, Jr, Teng X, Chumley P, et al. Inhaled NO accelerates restoration of liver function in adults following orthotopic liver transplantation. J Clin Invest. 2007;117:2583–91. doi: 10.1172/JCI31892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Checchia PA, Bronicki RA, Muenzer JT, et al. Nitric oxide delivery during cardiopulmonary bypass reduces postoperative morbidity in children—a randomized trial. J Thorac Cardiovasc Surg. 2013;146:530–6. doi: 10.1016/j.jtcvs.2012.09.100. [DOI] [PubMed] [Google Scholar]
- 77.Tsukahara H, Sekine K, Miura M, et al. Vasoactive and natriuretic mediators in umbilical cord blood: a report of our observation and review of the literature. Early Hum Dev. 2002;69:57–64. doi: 10.1016/s0378-3782(02)00038-5. [DOI] [PubMed] [Google Scholar]
- 78.Shiva S, Wang X, Ringwood LA, et al. Ceruloplasmin is a NO oxidase and nitrite synthase that determines endocrine NO homeostasis. Nat Chem Biol. 2006;2:486–93. doi: 10.1038/nchembio813. [DOI] [PubMed] [Google Scholar]
- 79.Vrancken K, Schroeder HJ, Longo LD, Power GG, Blood AB. Role of ceruloplasmin in nitric oxide metabolism in plasma of humans and sheep: a comparison of adults and fetuses. Am J Physiol Regul Integr Comp Physiol. 2013;305:R1401–10. doi: 10.1152/ajpregu.00266.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu G, Jaeger LA, Bazer FW, Rhoads JM. Arginine deficiency in preterm infants: biochemical mechanisms and nutritional implications. J Nutr Biochem. 2004;15:442–51. doi: 10.1016/j.jnutbio.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 81.Maeda T, Yoshimura T, Okamura H. Asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, in maternal and fetal circulation. J Soc Gynecol Investig. 2003;10:2–4. [PubMed] [Google Scholar]
- 82.Braekke K, Ueland PM, Harsem NK, Staff AC. Asymmetric dimethylarginine in the maternal and fetal circulation in preeclampsia. Pediatr Res. 2009;66:411–5. doi: 10.1203/PDR.0b013e3181b33392. [DOI] [PubMed] [Google Scholar]
- 83.Vida G, Sulyok E, Ertl T, Martens-Lobenhoffer J, Bode-Boger SM. Plasma asymmetric dimethylarginine concentration during the perinatal period. Neonatology. 2007;92:8–13. doi: 10.1159/000098411. [DOI] [PubMed] [Google Scholar]
- 84.Brüne B. The intimate relation between nitric oxide and superoxide in apoptosis and cell survival. Antioxid Redox Signal. 2005;7:497–507. doi: 10.1089/ars.2005.7.497. [DOI] [PubMed] [Google Scholar]
- 85.Thomas DD, Ridnour LA, Espey MG, et al. Superoxide fluxes limit nitric oxide-induced signaling. J Biol Chem. 2006;281:25984–93. doi: 10.1074/jbc.M602242200. [DOI] [PubMed] [Google Scholar]
- 86.Nassi N, Ponziani V, Becatti M, Galvan P, Donzelli G. Anti-oxidant enzymes and related elements in term and preterm newborns. Pediatr Int. 2009;51:183–7. doi: 10.1111/j.1442-200X.2008.02662.x. [DOI] [PubMed] [Google Scholar]
- 87.Saugstad OD. Oxygen toxicity in the neonatal period. Acta Paediatr Scand. 1990;79:881–92. doi: 10.1111/j.1651-2227.1990.tb11348.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.