Abstract
Here we show that Bmp signaling is necessary and sufficient for the specification of ventral endoderm in Xenopus embryos. Overexpression of Bmp4 in ectoderm induces markers of endoderm, including Sox17β, Mixer and VegT, but cannot induce the expression of the dorsoanterior markers, Xhex and Cerberus. Furthermore, knockdown approaches using overexpression of Bmp antagonists and morpholinos designed against Bmp4, Bmp2 and Bmp7 demonstrate that Bmp signaling is critical for ventral, but not dorsoanterior endoderm formation. This activity is not simply a result of embryonic dorsalization as markers for dorsal endoderm are not expanded. We further show that no endodermal cells of either ventral or dorsal character form when both Wnt and Bmp signals are abolished. Overall, this report strongly suggests that Bmp plays an essential role in ventral endoderm specification.
Keywords: Bmp, endoderm, Xenopus, ventrolateral, TGFβ
Introduction
Endoderm is one of the first cell types to emerge in the embryo and gives rise to a host of critical tissues, including the pancreas, liver and lung. In the frog, endoderm is initially specified by cytoplasmic determinants localized vegetally within the oocyte. The T-box transcription factor VegT plays an essential role in endoderm formation in Xenopus (Zhang et al., 1998; Xanthos et al., 2001). During blastula stages, a gradient of VegT induces different cell fates. High levels of VegT in the vegetal blastomeres induce endodermal cell fates while lower levels of VegT in the overlying cells induce mesodermal cell fates equatorially. Although VegT is thought to trigger these inductive events, zygotically transcribed TGFβ molecules, primarily Nodal-related factors, maintain the expression of VegT, forming a positive feedback loop that ensures both mesodermal and endodermal specification (Kofron et al., 1999; Xanthos et al., 2001). These Nodal signals are necessary and sufficient for both the formation of endoderm and mesoderm and therefore must be interpreted selectively in each tissue to form correct lineage specific cell fates. Sox17β and Mixer are transcription factors expressed within the endoderm and represent such downstream cell fate modulators (Hudson et al., 1997; Henry and Melton, 1998). These factors are involved in defining the boundary between endoderm and mesoderm (Engleka et al., 2001; Kofron et al., 2004). In fact, expression of Sox17 inhibits the ability of Nodal-like molecules to induce mesoderm, thus suggesting that it helps to distinguish the two tissue types (Hudson et al., 1997; Engleka et al., 2001). Mixer, also an endoderm inducer, is thought to antagonize the formation of mesoderm and thus controls widespread mesoderm formation within the embryo (Henry and Melton, 1998; Kofron et al., 2004).
The endoderm is comprised of several different cellular subtypes, which create a dorsal-ventral polarity. Endoderm formed on the dorsal side is not equivalent to that of the ventral side and, in fact, the dorsal endoderm provides a substantial and powerful effect on the formation of the Spemann’s Organizer. Recently, it has been shown that - in addition to its role in dorsal ventral patterning - functional β-catenin is critical for the formation of the dorsal anterior endoderm, and is necessary for the correct expression levels of Sox17β target genes, including Edd and FoxA2 (Jones et al., 1999; Zorn et al., 1999; Sinner et al., 2004). Other molecular signals that specify distinct endodermal domains have yet to be elucidated.
Here we show that Bmp signaling plays an essential role in endoderm formation during Xenopus development; overexpression of Bmp4 induces endodermal fates and a triple knockdown of Bmp2, 4 and 7 selectively inhibit ventral, but not dorsoanterior, endoderm formation. This provides strong evidence that Bmp signaling is involved in the specification of the ventral aspect of this critical germ layer.
Materials and Methods
Transcription Reactions
DNA plasmids were linearized at the appropriate restriction sites and mRNA was transcribed with mMessage machine (Ambion, Austin, TX). The plasmids used are as follows:
| RNA | Vector | Linearization | Reference |
|---|---|---|---|
| Noggin | pCS2+ | NotI | (Smith and Harland, 1992) |
| Bmp4 | pSP64T | EcoRI | (Nishimatsu et al., 1992) |
| mBmp4 | CS107 | AscI | (Marc Dionne, unpublished) |
| Alk3 Q233D | pCS2+ | NotI | (Hsu et al., 1998; Wieser et al., 1995) |
| DN-Alk3 | p64T | EcoRI | (Graff et al., 1994) |
| Chordin | pCS2+ | NotI | (Sasai et al., 1994) |
| β-galactosidase | pCS2 | NotI | (Turner and Weintraub 1994) |
Microinjections
Female frogs were primed for ovulation with human chorionic gonadotropin (Condie and Harland, 1987). Xenopus laevis eggs were collected and fertilized in vitro according to the standard protocol (Sive et al., 2000). We used 1× modified ringers (MR) solution (instead of MBS) for egg collection and 1/3× MR for fertilization. Embryos were transferred into dishes containing 1/3× MR with 2.5% ficoll and injected at the one-cell stage into the animal pole (presumptive ectoderm) for animal cap assays or the marginal zone (presumptive mesoderm) and vegetal pole (presumptive endoderm) for whole mount in situ hybridization assays.
For X. tropicalis, ovulation of adult X. tropicalis (Nasco) and in vitro fertilization of eggs were conducted as described (http://tropicalis.yale.edu). Fertilized eggs were de-jellied in 3% cysteine (pH 8.0) in water for 10–15 minutes before transferring to agarose-coated dishes containing 3% ficoll 400 in 1/9X modified frog ringer’s solution (MR) prior to injection. Miniruby fluorescent dextran (Molecular Probes, Lane and Smith 1999) was used as a tracer for microinjection of MOs, and nuclear-localized beta-galactosidase mRNA (Turner and Weintraub 1994) was used as a lineage tracer for mRNA microinjection. Embryos were raised at 28°C and collected at gastrula stages.
Morpholino sequences
Bmp2: GCCCCTTTCCTTACCTTCATGGTGA
Bmp4: CCAGGAATCATGGTGTCTTGACAGA
Bmp7.1: TGCAGAACATTCATTTCCCTTGGAT
β-catenin: TTTCAACAGTTTCCAAAGAACCAGG
In situ hybridizations
Anti-sense probes were generated from the following plasmids:
| Probe | Vector | Reference |
|---|---|---|
| Xbra | pGEM7 | (Smith et al., 1991) |
| Sox17β | pBSSK | (Hudson et al., 1997) |
| Mixer | pBSKS | (Henry and Melton, 1998) |
| xtMixer | CS107 | Tgas105b05 |
| xtSox17α | pCMVSport6 | Image: 6994003 |
| xtCerberus | CS107 | Tgas016c17 |
| xtHex | CS107 | Tgas075h17 |
| xtMyf5 | CS107 | Tgas04013 |
| xtVent2 | CS107 | Image:4462078 |
| xtBra | CS107 | Tgas023E12 |
| xtDerriere | CS107 | Tgas141f11 |
Xenopus embryos were developed to stage 10.5, fixed in MEMFA and stored in methanol. In situ hybridization analysis was performed as described (Harland, 1991; Sive et al., 2000, Khokha 2002) on whole or bisected embryos.
Ectoderm and Vegetal pole explants
Synthetic mRNA was injected into the animal poles of one-cell embryos, developed to stage 8 and transferred to 1% agarose coated dishes containing 3/4× NAM (Peng, 1991). Ectodermal tissue was excised using an eyebrow knife and hair loop and cultured in 3/4× NAM until intact sibling embryos reached stage 10.5 or 18. Uninjected embryos were developed to stage 8 and transferred to 1% agarose coated dishes containing 3/4× NAM. For vegetal explants, endodermal tissue was excised and cultured as described for ectodermal explants above.
RT-PCR Analysis
RNA was isolated and RT-PCR was performed as previously described (Wilson and Melton, 1994). For new primers, PCR products were analyzed after 30 cycles of amplification. The following primer sequences were used:
| Gene | Primer Sequence | Reference |
|---|---|---|
| Sox17β | F-AACTCCCACCAGCAGGCTACTTTG | (Myers et al., 2004) |
| R-TGTCAATGTCACTCTCCAGATGTCC | ||
| Mixer | F-CACCAGCCCAGCACTTAACC | (Henry and Melton, 1998) |
| R-CAATGTCACATCAACTGAAG | ||
| VegT | F-CAAGTAAATGTGAGAAACCGTG | (Zhang et al., 1998) |
| R-CAAATACACACACATTTCCCGA | ||
| Xbra | F-GGATCGTTATCACCTCTG | (Wilson and Melton, 1994) |
| R-GTGTAGTCTGTAGCAGCA | ||
| ODC | F-CAGCTAGCTGTGGTGTGG | (Agius et al., 2000) |
| R-CAACATGGAAACTCACACC | ||
| Cerberus | F-GCTTGCAAAACCTTGCCCTT | (Bouwmeester et al., 1996) |
| R-GTGATGGAACAGAGATCTTG | ||
| Xhex | F-AACAGCGCATCTAATGGGAC | (Newman et al., 1997) |
| R-CCTTTCCGCTTGTGCAGAGG | ||
| Mix.1 | F-GCAGATGCCAGTTCAGCCAAT | (Wilson and Melton, 1994) |
| R-TTTGTCCATAGGTTCCGCCCTG | ||
| Bmp2 | F-AGCCAGCCGAGCAAATACAGTG | |
| R-GGTGTCCAATAGTCTCACAACAGGG | ||
| Bmp4 | F-AGCCCCGTTGTCATAGAAACTTC | |
| R-AGTGTGCGTGTGGTGATTCCAG | ||
| Bmp7 | F-TTTATCCAGAGGAGGTTGCGAGGC | |
| R-TGTCAGCGTCATTGAGGAAGTTG | ||
| Alk3 | F-TTCTCCAAAAGCCCTGTCACGG | |
| R-CAATAATGGTAGCCCTGAGCCAC | ||
| Xhox-3 | F-ATATGATGAGCCACGCAGCAG | (Suzuki et al., 1997) |
| R-CAGATGCTGCAGCTCTTTGGC | ||
| Msx-1 | F-GCTAAAAATGGCTGCTAA | |
| R-AGG TGG GCT GTG TAA AGT |
Results
Bmp4 induces ventral endodermal fates
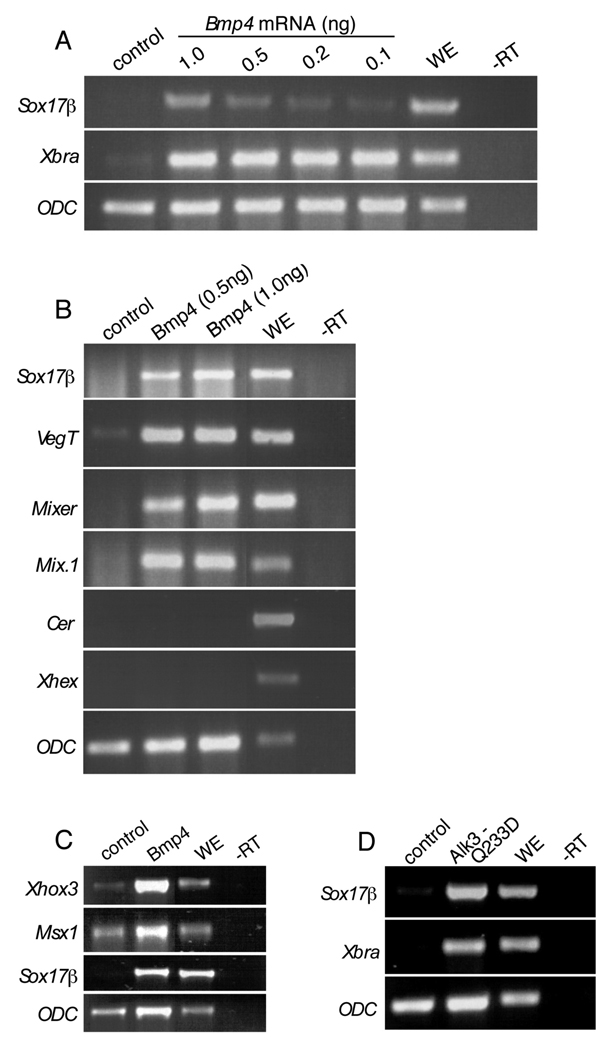
The Bmp family plays critical roles in the establishment of dorsal/ventral patterning and induction of neural tissue in the vertebrate embryo. We hypothesized that Bmp4 may contribute to to the specification of endodermal sub-types. To test this theory, we overexpressed Bmp4 and assayed for its ability to induce endoderm in ectodermal explants. Indeed, Bmp4 can induce Sox17β, VegT, Mixer, and Mix.1 (Fig. 1A–B), as well as inducing its known downstream targets Xhox3 and Msx1 (Fig. 1C; Hemmati-Brivanlou and Thomsen, 1995; Suzuki et al., 1997). Explants exposed to Bmp4 protein also display Sox17β induction (data not shown). Although Bmp4 can induce markers of a general endodermal character, it cannot induce the dorsoanterior markers, Cerberus and Xhex, suggesting that Bmp4-mediated endoderm induction specifies more ventrolateral endodermal fates and thus discriminates this Bmp-like activity from that of Nodal, which can induce the broad range of fates (Jones et al., 1995; Osada and Wright, 1999) (Fig. 1B). To verify that the observed induction of Sox17β is truly a result of Bmp signaling and not the consequence of a promiscuous ligand activating the Nodal pathway, we overexpressed the activated Bmp receptor, Alk3 Q233D (Wieser et al., 1995). Like Bmp4, Alk3 Q233D can induce Sox17β and Xbra in ectoderm (Fig. 1D). This result confirms that Bmp signaling is sufficient to induce endodermal gene expression.
Figure 1. Bmp4 and Alk3Q233D induce endodermal markers.
(A) Embryos were injected with doses of Bmp4 mRNA ranging from 0.1 ng to 1.0 ng. Ectoderm was explanted from these embryos, harvested at stage 10.5 and analyzed by RTPCR for the expression of Sox17β and Xbra. (B) Embryos were injected with 0.5 or 1.0 ng Bmp4. Ectoderm was explanted and harvested at stage 10.5. RT-PCR was performed using primers for Sox17β, VegT, Mixer, Mix.1, Cerberus (Cer). (C) Embryos were injected with 0.5ng of Bmp4 and explants were performed as in (A), but with primers for Xhex or Xhox3, Msx1, and Sox17β. (D) Embryos were injected with 0.5 ng Alk3 Q233D and explants were performed as in (A). ODC was used as a loading control for all experiments (A – D). WE, whole embryo; -RT, minus reverse transcriptase.
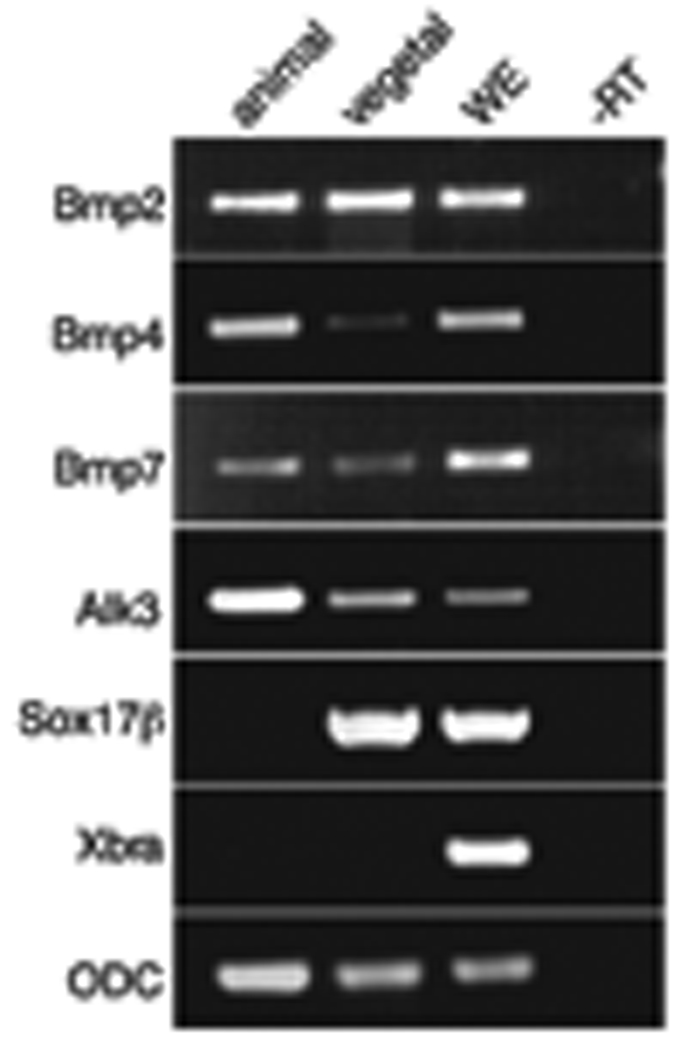
If Bmp signaling is of physiological importance for endoderm formation, then at least the mediating receptor and perhaps the cognate ligand should be expressed within this domain. Therefore, to deduce whether components of Bmp signaling are present within the endoderm, we performed RT-PCR analysis on vegetal explants for Bmp2, Bmp4, Bmp7 and Alk3. This analysis revealed that all four molecules are expressed vegetally as well as in the animal pole, suggesting that they could be involved in the formation of endoderm (Fig. 2). This evidence supports the work of others who detected robust Bmp activity in the ventral vegetal endodermal cells at stage 10 using an antibody against phosphorylated Smad1 (Faure et al., 2000; Kurata et al., 2001).
Figure 2. Bmp2, Bmp4, Bmp7 and Alk3 are expressed in vegetal cells.
Animal and vegetal hemispheres were explanted at stage 8.0, cultured and harvested at stage 10.5. RT-PCR analysis was performed using primers for Bmp2, Bmp4, Bmp7, Alk3, Sox17β and Xbra. cDNA amounts were normalized. ODC was used as a loading control. WE, whole embryo; -RT, minus reverse transcriptase.
Bmp antagonists disrupt early endoderm formation
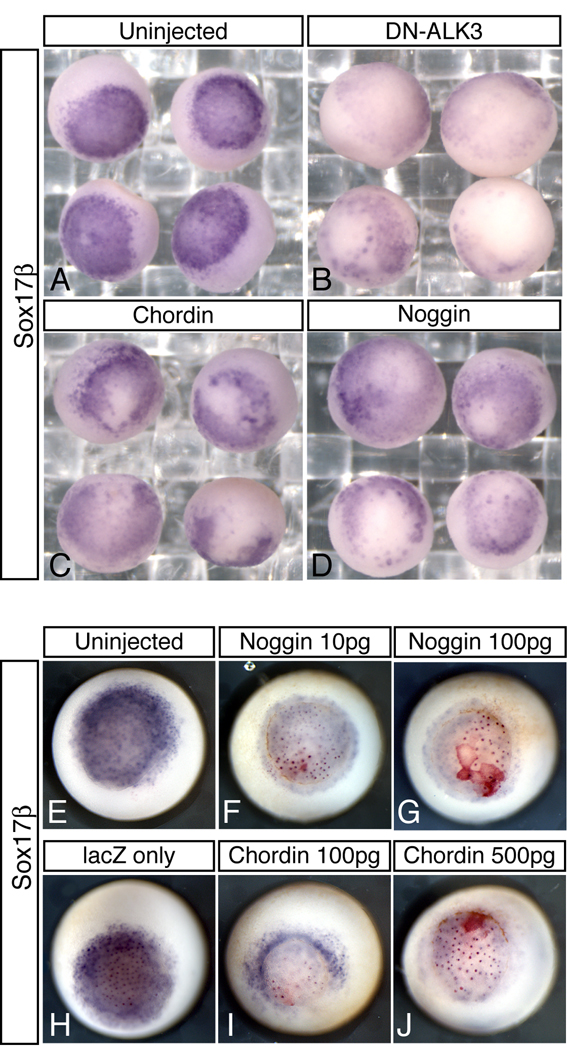
We have shown that Bmp signaling is sufficient for endoderm induction and that components of this pathway are present within endogenous endoderm. Therefore, we wanted to examine whether Bmp signaling is necessary for endoderm formation in Xenopus. Toward this goal, we overexpressed the Bmp antagonists, Chordin and Noggin, and dominant negative Alk3 (DN-Alk3) in the vegetal hemisphere and assayed for effects on Sox17β expression during gastrulation. Embryos containing any sign of cell death were discarded and not used in the analysis. As shown in Fig. 3, we observed disrupted Sox17β expression in 67% of embryos injected with Chordin (n=36) and 48% of embryos injected with Noggin (n=27) versus 11% of water injected control embryos (n=27). In a duplicate experiment, Chordin affected 52% (n=50; Fig. 3C), Noggin affected 68% (n=62; Fig. 3D), compared to 15% in controls (n=61; Fig. 3A). In a separate experiment, we overexpressed DN-Alk3 in the endoderm and observed disrupted Sox17β expression in 75% of injected embryos (n=55) compared to 15% of control embryos (n=42) (Fig. 3B). The more lineage-specific inhibition of Sox17β observed in this sample may reflect the difference between cell-autonomous (DN-Alk3) and secreted (Chordin and Noggin) Bmp antagonists. To verify that the reduction of Sox17β expression observed in antagonist-injected embryos was not due to cell death, we repeated these experiments using a range of mRNA doses, and using nuclear lacZ as a tracer for microinjection (Figure 3E–J). While only 5.9% of embryos injected vegetally with lacZ alone showed reduced Sox17β expression (n=17, Figure3H), embryos coinjected with lacZ and low or high doses of Bmp antagonists had broadly reduced Sox17β expression while retaining clear nuclear β-gal staining, confirming a non cell-autonomous effect on Sox17β. Specifically, we found that Sox17β expression was reduced in 77.8% of embryos injected with 10pg of Noggin mRNA (n=27, Fig 3F), 85.2% of embryos injected with 100pg of Noggin (n=27, Fig 3G), 82.1% of embryos injected with 100pg Chordin mRNA (n=28, Fig 3I), and 84% of embryos injected with 500pg of Chordin mRNA (n=25, Fig 3J). The degree of expression lost was variable with slightly different targeting, as might be expected for the effects of secreted antagonists. These data strongly suggest that Bmp signaling has an endogenous function during early endoderm formation.
Figure 3. Bmp antagonists disrupt early endoderm formation.
One-cell embryos were injected vegetally with mRNA encoding the following Bmp Antagonists: (B) DN-Alk3 (0.5 ng), (C) Chordin (0.5 ng), and (D) Noggin (0.5 ng) and assayed by in situ hybridization with a probe for Sox17β at stage 10.5. (A) is a control injected with water. In F-J, 2-cell stage embryos were injected vegetally with 200pg of lacZ mRNA, and mRNA for Chordin or Noggin as indicated. Embryos were fixed at stage 11, stained for lacZ using red gal substrate and analyzed for Sox17β expression.
Bmps are necessary for endoderm formation in Xenopus
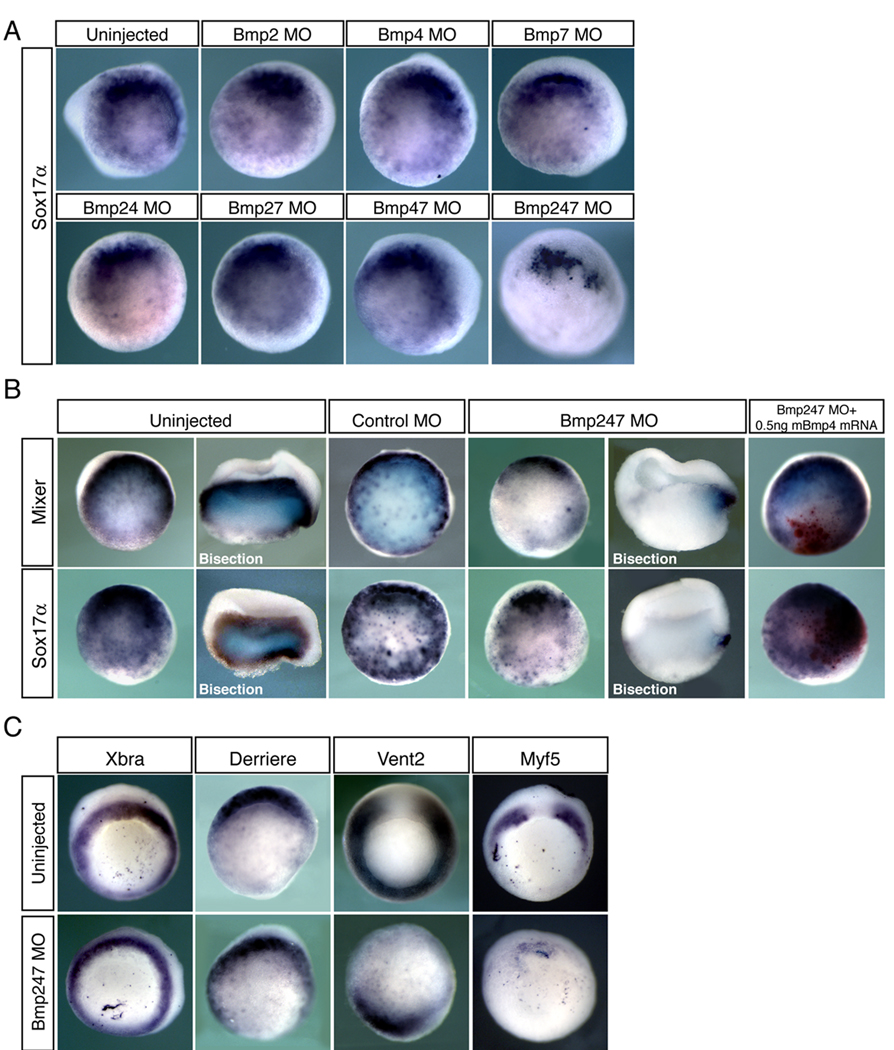
Although Bmp antagonists are thought to specifically affect Bmp signaling during development, it remains plausible that they may also inhibit other pathways. Therefore, to define the specific role of Bmp signaling in Xenopus endoderm specification, we used morpholino oligos (MO) to knock down Bmp2, Bmp4, and Bmp7, together or in various combinations (Fig. 4A, B). We injected X. tropicalis embryos with 10ng of each MO per blastomere at the 2-cell stage. While we observed little to no reduction in the expression of Sox17α in either the single or double knockdowns, a triple knock down of Bmp2, Bmp4 and Bmp7 dramatically decreased the expression of Sox17α (92.1%, n=38, sum of four experiments) and Mixer (86.8%, n=38 sum of four experiments) (Fig. 4A,B). Loss of endodermal cell fates could be rescued by co-injection of mouse Bmp4 mRNA (Fig. 4B). Interestingly, embryos injected with all three Bmp MOs retain the most dorsal expression of Sox17α (65.8% n=38, sum of four experiments) and Mixer (71.1%, n=38 sum of four experiments) despite the substantial reduction of their ventral endoderm expression (Fig. 4B, Fig. 5). This was especially clear when Bmp morphants were bisected along the dorsal-ventral axis, which revealed strong staining in the dorsal lip but not ventrolaterally. Consistent with this, Cerberus and XHex, two markers of dorsoanterior endoderm, retained strong expression in embryos depleted of all three Bmps (Cerberus: 100%, n=30; Xhex: 89.5%, n=19; Fig.5), further suggesting that Bmp signaling is necessary for ventrolateral, but not dorsoanterior, endoderm specification. This result is consistent with our previous findings that expression of Bmp4 itself is not sufficient to induce dorsoanterior endoderm. Furthermore, embryos lacking Bmp2, 4 and 7 still contain mesoderm as judged by staining for Xbra, and still have intact Smad2 signaling as judged by staining for the Vg-1 related gene Derriere. However, patterning within the mesoderm appears to be affected since Myf5 and Vent2 are missing or largely reduced (Fig. 4C). The reduction of expression of these markers is consistent with our expectation for embryos depleted of Bmps, since Vent2 is a direct target of Bmp signaling and Myf5 is known to be sensitive to manipulation of Bmp levels (Dosch et al. 1997; Sinner et al. 2004) and supports the established role of Bmp in dorsal ventral mesodermal patterning.
Figure 4. Bmp signaling is essential for endoderm formation in Xenopus.
X. tropicalis embryos were injected at the two-cell stage with 10ng per blastomere of each MO indicated, then analyzed for expression of Sox17α (A, B) or Mixer (B) at stage 10.5. Miniruby fluorescent dextran was used as a tracer for MO injection. In (B), a subset of MO-injected embryos were re-injected in one ventral blastomere at the 4-cell stage with 0.5ng of mouse Bmp4. Nuclear β-galactosidase (red) was used as a tracer. (C) Triple Bmp morphant embryos were also analyzed for expression of Xbra, Derriere, Myf5 and Vent2.
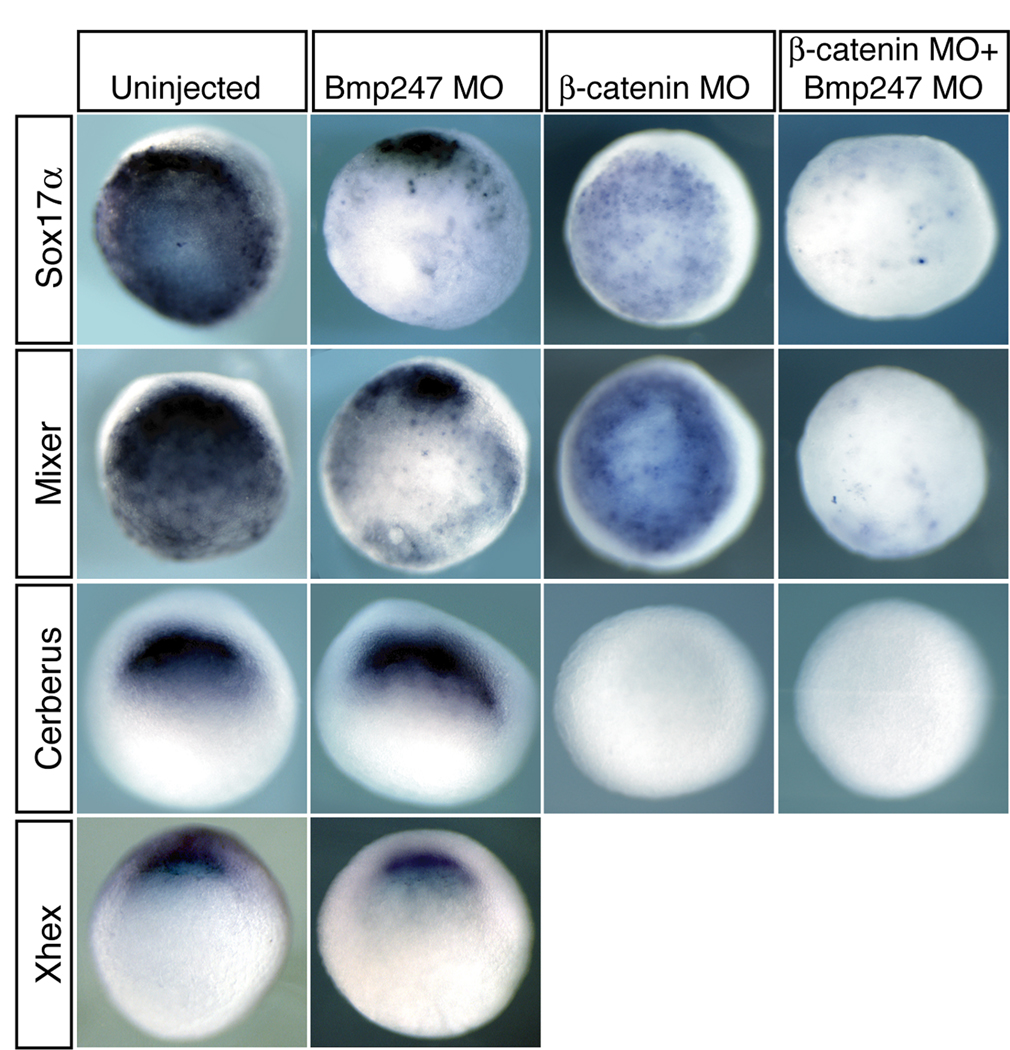
Figure 5. Combination of Wnt and Bmp signaling is required for endoderm formation.
X. tropicalis embryos were injected with 10ng per blastomere of each MO indicated at the 2-cell stage, then analyzed at stage 10.5 for expression of the general endoderm markers Sox17α and Mixer, or the dorsoanterior endoderm markers Cerberus and Xhex.
Since Wnt signaling is a critical component of dorsal ventral patterning, which is known to set up the anterior endodermal domain (Zorn et al., 1999; Jones et al., 1999, Sinner et al., 2004), we sought to determine whether knocking down all Bmp and Wnt signaling would eliminate the formation of all endodermal tissues. Knockdown of Wnt signaling with a β-catenin MO (Heasman, 2000; Khokha, 2002) reduces the expression of dorsoanterior endoderm marked by loss of Cerberus expression (12/12=100%) but did not appear to affect general endoderm specification (Fig. 5). However, a quadruple knockdown of Bmp2, 4, 7 and β-catenin abolishes all endoderm (Fig. 5). In embryos depleted of all four gene products, expression of Mixer and Sox17α was either absent (Mixer: 42.9%, n=14; Sox17α: 10%, n=20), or very weakly expressed in only a few cells (Mixer: 57.1%; Sox17α: 90%), while Cerberus expression was absent in all injected embryos (n=12). Thus the combined effect of Bmp and Wnt signaling is essential for all aspects of endoderm in Xenopus embryos.
Discussion
In this report we show that Bmp signaling is critical for the specification of ventrolateral endoderm in Xenopus. While Bmp signaling has been implicated in having many critical roles in developmental processes, including neural induction and dorsal ventral patterning, its affect on endoderm has not been widely studied (Jones et al., 1992; Suzuki et al., 1994; Hawley et al., 1995; Schmidt et al., 1995; Harland and Gerhart, 1997; Dale and Jones, 1999; Shivdasani, 2002). It has been known for many years that Bmp4 is expressed within the ectodermal domain and that high concentrations of Bmp4 or the correct formation of heterodimers between Bmp4 and Bmp7 can induce mesoderm (Suzuki et al., 1997; Dale and Jones, 1999) . We now show that this is also true for endoderm. Although Bmp4 is expressed within the ectoderm during gastrulation stages, this does not mean that it is capable of inducing mesoendodermal derivatives within these cells. It is possible that the complete signaling network is not normally activated within these cells in the endogenous stage, which occurs only with addition of high levels of exogenous protein. The main focus of this paper however is to provide genetic evidence for the involvement of endogenous bmp signaling within the embryoinic mileu and thus here we show both that bmp signaling is necessary and sufficient for endodermal specification.
Several lines of evidence over the past ten years lend support to our finding that Bmp signaling plays a role in endoderm formation. Perhaps the most striking of these is fact that Bmp activated phosphorylated Smad1 is present throughout the vegetal cells in Xenopus after MBT as early as stage 9 (Kurata et al., 2001). Phosphorylated Smad2, the downstream modulator of Nodal-like TGFβ signals, is not detected throughout the vegetal cells until stage 9.5, although some expression is observed in the dorsal marginal region at stage 9 (Faure et al., 2000; Kurata et al., 2001). Furthermore, during gastrulation phospho-Smad1 is detected strongly in the ventral vegetal cells and excluded from the dorsal zone, consistent with the proposed role of Bmp4 signaling in ventral but not dorsoanterior endoderm specification (Faure et al., 2000; Kurata et al., 2001). Genetics in the mouse and Zebrafish also support the involvement of Bmp signaling in the formation and patterning of endoderm. Mouse embryos having mutations in Bmp4, its type I receptor, BmpRIA, and its type II receptor, BmpRII, all arrest at gastrulation, failing to form mesoderm and subsequent tissues (Mishina et al., 1995; Winnier et al., 1995; Beppu et al., 2000). This inhibits studying the role of these molecules in the direct formation of endoderm, although it does demonstrate the importance of a role for Bmp signaling during gastrulation. Studies on BmpRIA mutant chimeric mice demonstrate that embryos develop defects in endoderm morphogenesis with an improperly formed and patterned endoderm (Davis et al., 2004). Similar effects have been shown with Bmp2b embryonic mutations in Zebrafish (Tiso et al., 2002). In these mutant embryos, endoderm is formed, but patterning is severely affected. Multiple Bmps and signaling components exist in all vertebrates, making single mutations difficult to interpret in light of the complexity of signaling cascades. While there are clearly endodermal abnormalities in these examples, genetic redundancy may account for the varying degrees of disruption.
Additional evidence for the involvement of Bmp signaling in endoderm formation emerges from the collection of known transcriptional targets for Bmp. Vent1 and Vent2, both known Bmp targets, are expressed within the ventral posterior endoderm in Xenopus, consistent with a role in mediating Bmp signaling within this domain (Sinner et al., 2004). Vent2 was also localized to the endoderm of Zebrafish embryos at tailbud stage (Costa et al., 2003). Furthermore, Mix.1 is a transcription factor known to be involved in early endoderm formation activation and is dependent upon Bmp4 signaling (Mead et al., 1996). We are currently investigating the roles these molecules may play to mediate Bmp role in the formation of ventrolateral endoderm.
While the bulk of literature supports our finding, several reports contradict our results (Sasai et al., 1996; Poulain et al., 2006). Sasai et al., showed that ectoderm expressing Noggin and Chordin led to the induction of the endoderm marker, Edd, and the pancreas marker, Xlhbox8, during late neurula stages (Sasai et al., 1996). This would suggest that inhibition, not activation, of Bmp signaling leads to endoderm formation. While this appears contradictory to our results, this may be attributable to the significantly different stages analyzed in the two reports and thus may reflect the induction of distinct tissue types by Bmp antagonists during embryogenesis. Poulain et al., further suggest that Bmp signaling inhibits endoderm specification in Zebrafish. This result is in direct contrast to ours as they overexpress Bmp2, 4 and 7 vegetally and observe local reduction in Sox17β expression during gastrulation (Poulain et al., 2006). Further work is required to understand the basis of these discrepancies.
In this paper, we propose a model for endoderm specification which appears to be consistent with our results and others. Initially, VegT plays an essential role in specifying endodermal and mesodermal cell fates as depletion leads to a substantial reduction in these two germ layers (Zhang et al., 1998). These signals are actively maintained by Nodal signaling after MBT (Kofron et al., 1999; Xanthos et al., 2001). Cells receiving the VegT signal are then acted upon either by Bmp or β-catenin signals, which are ventrally or dorsally located, respectively. Both Bmp and β-catenin signals are essential for further endodermal specification. β-catenin signaling is activated on the dorsal side of the embryo and results in dorsoanterior endoderm expression marked most clearly by Cerberus expression. Knockdown of β-catenin results in a dramatic reduction in Cerberus expression (Jones et al., 1999; Zorn et al., 1999; Sinner et al., 2004). On the ventral side, Bmp4 overexpression can induce Mixer and Sox17β in naïve ectoderm but not Cerberus. Consistent with this observation, depletion of Bmps strongly affects ventrolateral endodermal fates, but preserves the dorsal endodermal cells, which express Mixer, Sox17α and Cerberus. We propose that this maintenance of dorsal expression is due to the remaining β-catenin mediated activation of dorsal Wnt signaling. The autonomy of these two endoderm-inducing pathways, the Bmp-mediated and the Wnt-mediated, account for our observation that dorsal endoderm is preserved in Bmp morphants, but not necessarily expanded. This is in contrast to mesodermal patterning, where manipulation of Bmp signaling directly changes the proportion of dorsal versus ventral mesodermal fates but not the induction of mesoderm, as we see from the preservation of Xbra but reduction in Vent2 and Myf5 expression in Bmp morphants. Overall, our results, coupled with many reports in the literature, lend a tremendous amount of support for the involvement of Bmp signaling in endoderm formation and point to a mechanism by which Bmp and Wnt signaling act to specify distinct regions within the endoderm.
Acknowledgments
We thank Eric Chiao, Dale Frank and Elena Casey for valuable discussions and comments. We also thank Heather McCullough and Jeff Leonard for assisting with experiments. Special thanks to Eddy DeRobertis, Richard Harland, Janet Heasman, Hugh Woodland, Kunxin Luo, Doug Melton, Joan Massague and Jim Smith for contributing plasmids. CS107 mBMP4 construct was generated by Marc Dionne. This manuscript was funded by the generosity of the Donald E and Delia B. Baxter Foundation and from the National Institutes of Health (R01 HD41557).
References
- Agius E, Oelgeschlager M, Wessely O, Kemp C, De Robertis EM. Endodermal Nodal-related signals and mesoderm induction in Xenopus. Development. 2000;127:1173–1183. doi: 10.1242/dev.127.6.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beppu H, Kawabata M, Hamamoto T, Chytil A, Minowa O, Noda T, Miyazono K. BMP type II receptor is required for gastrulation and early development of mouse embryos. Dev Biol. 2000;221:249–258. doi: 10.1006/dbio.2000.9670. [DOI] [PubMed] [Google Scholar]
- Bouwmeester T, Kim S, Sasai Y, Lu B, De Robertis EM. Cerberus is a head-inducing secreted factor expressed in the anterior endoderm of Spemann's organizer. Nature. 1996;382:595–601. doi: 10.1038/382595a0. [DOI] [PubMed] [Google Scholar]
- Condie BG, Harland RM. Posterior expression of a homeobox gene in early Xenopus embryos. Development. 1987;101:93–105. [PubMed] [Google Scholar]
- Costa RM, Mason J, Lee M, Amaya E, Zorn AM. Novel gene expression domains reveal early patterning of the Xenopus endoderm. Gene Expr Patterns. 2003;3:509–519. doi: 10.1016/s1567-133x(03)00086-3. [DOI] [PubMed] [Google Scholar]
- Dale L, Jones CM. BMP signalling in early Xenopus development. Bioessays. 1999;21:751–760. doi: 10.1002/(SICI)1521-1878(199909)21:9<751::AID-BIES6>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Davis S, Miura S, Hill C, Mishina Y, Klingensmith J. BMP receptor IA is required in the mammalian embryo for endodermal morphogenesis and ectodermal patterning. Dev Biol. 2004;270:47–63. doi: 10.1016/j.ydbio.2004.01.048. [DOI] [PubMed] [Google Scholar]
- De Robertis EM, Kuroda H. Dorsal-ventral patterning and neural induction in Xenopus embryos. Annu Rev Cell Dev Biol. 2004;20:285–308. doi: 10.1146/annurev.cellbio.20.011403.154124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosch R, Gawantka V, Delius H, Blumenstock C, Niehrs C. Bmp-4 acts as a morphogen in dorsoventral mesoderm patterning in Xenopus. Development. 1997;124:2325–2334. doi: 10.1242/dev.124.12.2325. [DOI] [PubMed] [Google Scholar]
- Engleka MJ, Craig EJ, Kessler DS. VegT activation of Sox17 at the midblastula transition alters the response to nodal signals in the vegetal endoderm domain. Dev Biol. 2001;237:159–172. doi: 10.1006/dbio.2001.0366. [DOI] [PubMed] [Google Scholar]
- Faure S, Lee MA, Keller T, ten Dijke P, Whitman M. Endogenous patterns of TGFbeta superfamily signaling during early Xenopus development. Development. 2000;127:2917–2931. doi: 10.1242/dev.127.13.2917. [DOI] [PubMed] [Google Scholar]
- Graff JM, Thies RS, Song JJ, Celeste AJ, Melton DA. Studies with a Xenopus BMP receptor suggest that ventral mesoderm-inducing signals override dorsal signals in vivo. Cell. 1994;79:169–179. doi: 10.1016/0092-8674(94)90409-x. [DOI] [PubMed] [Google Scholar]
- Harland RM. In situ hybridization: an improved whole-mount method for Xenopus embryos. Methods Cell Biol. 1991;36:685–695. doi: 10.1016/s0091-679x(08)60307-6. [DOI] [PubMed] [Google Scholar]
- Harland R, Gerhart J. Formation and function of Spemann's organizer. Annu Rev Cell Dev Biol. 1997;13:611–667. doi: 10.1146/annurev.cellbio.13.1.611. [DOI] [PubMed] [Google Scholar]
- Hawley SH, Wunnenberg-Stapleton K, Hashimoto C, Laurent MN, Watabe T, Blumberg BW, Cho KW. Disruption of BMP signals in embryonic Xenopus ectoderm leads to direct neural induction. Genes Dev. 1995;9:2923–2935. doi: 10.1101/gad.9.23.2923. [DOI] [PubMed] [Google Scholar]
- Heasman J, Kofron M, Wylie C. Beta-catenin signaling activity dissected in the early Xenopus embryo: a novel antisense approach. Developmental Biology. 2000;222(1):124–134. doi: 10.1006/dbio.2000.9720. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A, Thomsen GH. Ventral mesodermal patterning in Xenopus embryos: expression patterns and activities of BMP-2 and BMP-4. Dev Genet. 1995;17:78–89. doi: 10.1002/dvg.1020170109. [DOI] [PubMed] [Google Scholar]
- Henry GL, Melton DA. Mixer, a homeobox gene required for endoderm development. Science. 1998;281:91–96. doi: 10.1126/science.281.5373.91. [DOI] [PubMed] [Google Scholar]
- Hsu DR, Economides AN, Wang X, Eimon PM, Harland RM. The Xenopus dorsalizing factor Gremlin identifies a novel family of secreted proteins that antagonize BMP activities. Mol Cell. 1998;1:673–683. doi: 10.1016/s1097-2765(00)80067-2. [DOI] [PubMed] [Google Scholar]
- Hudson C, Clements D, Friday RV, Stott D, Woodland HR. Xsox17alpha and -beta mediate endoderm formation in Xenopus. Cell. 1997;91:397–405. doi: 10.1016/s0092-8674(00)80423-7. [DOI] [PubMed] [Google Scholar]
- Jones CM, Lyons KM, Lapan PM, Wright CV, Hogan BL. DVR-4 (bone morphogenetic protein-4) as a posterior-ventralizing factor in Xenopus mesoderm induction. Development. 1992;115:639–647. doi: 10.1242/dev.115.2.639. [DOI] [PubMed] [Google Scholar]
- Jones CM, Kuehn MR, Hogan BL, Smith JC, Wright CVE. Nodal-related signals induce axial mesoderm and dorsalize mesoderm during gastrulation. Development. 1995;121:3651–3662. doi: 10.1242/dev.121.11.3651. [DOI] [PubMed] [Google Scholar]
- Jones MJ, Broadbent J, Thomas PQ, Smith JC, Beddington RSP. An anterior signaling centre in Xenopus revealed by the homeobox gene XHex. Current Biology. 1999;9:946–954. doi: 10.1016/s0960-9822(99)80421-7. [DOI] [PubMed] [Google Scholar]
- Khokha MK, Chung C, Bustamante EL, Gaw LW, Trott KA, Yeh J, Lim N, Lin JC, Traverner N, Amaya E, Papalopulu N, Smith JC, Zorn AM, Harland RM, Grammer TC. Techniques and probes for the study of Xenopus tropicalis development. Developmental Dynamics. 2002;225(4):499–510. doi: 10.1002/dvdy.10184. [DOI] [PubMed] [Google Scholar]
- Kofron M, Demel T, Xanthos J, Lohr J, Sun B, Sive H, Osada S, Wright C, Wylie C, Heasman J. Mesoderm induction in Xenopus is a zygotic event regulated by maternal VegT via TGFbeta growth factors. Development. 1999;126:5759–5770. doi: 10.1242/dev.126.24.5759. [DOI] [PubMed] [Google Scholar]
- Kofron M, Wylie C, Heasman J. The role of Mixer in patterning the early Xenopus embryo. Development. 2004;131:2431–2441. doi: 10.1242/dev.01132. [DOI] [PubMed] [Google Scholar]
- Kurata T, Nakabayashi J, Yamamoto TS, Mochii M, Ueno N. Visualization of endogenous BMP signaling during Xenopus development. Differentiation. 2001;67:33–40. doi: 10.1046/j.1432-0436.2001.067001033.x. [DOI] [PubMed] [Google Scholar]
- Lane MC, Smith WC. The origins of primitive blood in Xenopus: implications for axial patterning. Development. 1999;126(3):423–434. doi: 10.1242/dev.126.3.423. [DOI] [PubMed] [Google Scholar]
- Mead PE, Brivanlou IH, Kelley CM, Zon LI. BMP-4-responsive regulation of dorsal-ventral patterning by the homeobox protein Mix.1. Nature. 1996;382:357–360. doi: 10.1038/382357a0. [DOI] [PubMed] [Google Scholar]
- Mishina Y, Suzuki A, Ueno N, Behringer RR. Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes Dev. 1995;9:3027–3037. doi: 10.1101/gad.9.24.3027. [DOI] [PubMed] [Google Scholar]
- Myers AP, Corson LB, Rossant J, Baker JC. Characterization of mouse Rsk4 as an inhibitor of fibroblast growth factor-RAS-extracellular signal-regulated kinase signaling. Mol Cell Biol. 2004;24:4255–4266. doi: 10.1128/MCB.24.10.4255-4266.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman CS, Chia F, Krieg PA. The XHex homeobox gene is expressed during development of the vascular endothelium: overexpression leads to an increase in vascular endothelial cell number. Mech Dev. 1997;66:83–93. doi: 10.1016/s0925-4773(97)00092-0. [DOI] [PubMed] [Google Scholar]
- Nishimatsu S, Suzuki A, Shoda A, Murakami K, Ueno N. Genes for bone morphogenetic proteins are differentially transcribed in early amphibian embryos. Biochem Biophys Res Commun. 1992;186:1487–1495. doi: 10.1016/s0006-291x(05)81574-8. [DOI] [PubMed] [Google Scholar]
- Osada SI, Wright CV. Xenopus nodal-related signaling is essential for mesendodermal patterning during early embryogenesis. Development. 1999;126:3229–3240. doi: 10.1242/dev.126.14.3229. [DOI] [PubMed] [Google Scholar]
- Peng HB. Xenopus laevis: Practical uses in cell and molecular biology. Solutions and protocols. Methods Cell Biol. 1991;36:657–662. [PubMed] [Google Scholar]
- Poulain M, Furthauer M, Thisse B, Thisse C, LePage T. Zebrafish endoderm formation is regulated by combinatorial Nodal, FGF, and BMP signaling. Development. 2006;133:2189–2200. doi: 10.1242/dev.02387. [DOI] [PubMed] [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H, Geissert D, Gont LK, De Robertis EM. Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell. 1994;79:779–790. doi: 10.1016/0092-8674(94)90068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai Y, Lu B, Piccolo S, De Robertis EM. Endoderm induction by the organizer-secreted factors chordin and noggin in Xenopus animal caps. Embo J. 1996;15:4547–4555. [PMC free article] [PubMed] [Google Scholar]
- Schmidt JE, Suzuki A, Ueno N, Kimelman D. Localized BMP-4 mediates dorsal/ventral patterning in the early Xenopus embryo. Dev Biol. 1995;169:37–50. doi: 10.1006/dbio.1995.1124. [DOI] [PubMed] [Google Scholar]
- Shivdasani RA. Molecular regulation of vertebrate early endoderm development. Dev Biol. 2002;249:191–203. doi: 10.1006/dbio.2002.0765. [DOI] [PubMed] [Google Scholar]
- Sinner D, Rankin S, Lee M, Zorn A. Sox17 and β-catenin cooperate to regulate the transcription of endodermal genes. Development. 2004;131:3069–3080. doi: 10.1242/dev.01176. [DOI] [PubMed] [Google Scholar]
- Sive HL, Grainger RM, Harland RM. Early development of Xenopus laevis: A laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2000. [Google Scholar]
- Smith JC, Price BM, Green JB, Weigel D, Herrmann BG. Expression of a Xenopus homolog of Brachyury (T) is an immediate-early response to mesoderm induction. Cell. 1991;67:79–87. doi: 10.1016/0092-8674(91)90573-h. [DOI] [PubMed] [Google Scholar]
- Smith WC, Harland RM. Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell. 1992;70:829–840. doi: 10.1016/0092-8674(92)90316-5. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Thies RS, Yamaji N, Song JJ, Wozney JM, Murakami K, Ueno N. A truncated bone morphogenetic protein receptor affects dorsal-ventral patterning in the early Xenopus embryo. Proc Natl Acad Sci U S A. 1994;91:10255–10259. doi: 10.1073/pnas.91.22.10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Ueno N, Hemmati-Brivanlou A. Xenopus msx1 mediates epidermal induction and neural inhibition by BMP4. Development. 1997;124:3037–3044. doi: 10.1242/dev.124.16.3037. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Kaneko E, Maeda J, Ueno N. Mesoderm induction by Bmp-4 and -7 heterodimers. BBRC. 1997;232(1):153–156. doi: 10.1006/bbrc.1997.6219. [DOI] [PubMed] [Google Scholar]
- Tiso N, Filippi A, Pauls S, Bortolussi M, Argenton F. BMP signalling regulates anteroposterior endoderm patterning in zebrafish. Mech Dev. 2002;118:29–37. doi: 10.1016/s0925-4773(02)00252-6. [DOI] [PubMed] [Google Scholar]
- Turner DL, Weintraub H. Expression of achaete-scute homolog 3 in Xenopus embryos converts ectodermal cells to a neural fate. Genes and Development. 1994;8(12):1434–1447. doi: 10.1101/gad.8.12.1434. [DOI] [PubMed] [Google Scholar]
- Wieser R, Wrana JL, Massague J. GS domain mutations that constitutively activate TGF beta R-I, the downstream signaling component in the TGF-beta receptor complex. Embo Journal. 1995;14:2199–2208. doi: 10.1002/j.1460-2075.1995.tb07214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson PA, Melton DA. Mesodermal patterning by an inducer gradient depends on secondary cell-cell communication. Curr Biol. 1994;4:676–686. doi: 10.1016/s0960-9822(00)00152-4. [DOI] [PubMed] [Google Scholar]
- Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- Xanthos JB, Kofron M, Wylie C, Heasman J. Maternal VegT is the initiator of a molecular network specifying endoderm in Xenopus laevis. Development. 2001;128:167–180. doi: 10.1242/dev.128.2.167. [DOI] [PubMed] [Google Scholar]
- Zhang J, Houston DW, King ML, Payne C, Wylie C, Heasman J. The role of maternal VegT in establishing the primary germ layers in Xenopus embryos. Cell. 1998;94:515–524. doi: 10.1016/s0092-8674(00)81592-5. [DOI] [PubMed] [Google Scholar]
- Zorn AM, Butler K, Gurdon JB. Anterior endomesoderm specification in Xenopus by Wnt/beta-catenin and TGF-beta signalling pathways. Dev Biol. 1999;209:282–297. doi: 10.1006/dbio.1999.9257. [DOI] [PubMed] [Google Scholar]