Abstract
OBJECTIVE
To estimate activity and safety of trabectedin 1.5 mg/m2 IV over 24 hours every 3 weeks (1 cycle) in uterine leiomyosarcoma.
METHODS
Patients with chemotherapy naive, advanced, persistent or recurrent uterine leiomyosarcoma, acceptable organ function and PS ≤ 2 were eligible. A two-stage design was utilized. Three responses were required in the first stage to initiate the second stage; the target sample size was 40 for the combined stages. If the true response rate was 10%, the study design provided a 95% chance of correctly classifying the treatment as “inactive.” Conversely, if the true response rate was 30%, then the average probability of correctly classifying the treatment as active would be 90%.
RESULTS
Twenty patients were eligible and evaluable. The median number of cycles was 10 (123 total cycles, range 2–29). The number of patients with partial responses was 2 (10%; 95% confidence interval of 1.2%–31.7%). Response durations were 3.3 and 5.7 months. Ten patients had stable disease (50%). The median progression-free survival (PFS) and overall survival were 5.8 months and greater than 26.1 months (median not reached), respectively. Observed grade 3/4 toxicity included: neutropenia 16/20 (1 infection); thrombocytopenia 3/20; metabolic 3/20; anemia, gastrointestinal and vascular 1/20 each. There were no treatment related deaths nor cases of liver failure.
CONCLUSIONS
Although a second stage of accrual was not indicated based on the overall response rate, the drug was well tolerated.
Keywords: Trabectedin, Uterine Cancer, Leiomyosarcoma
INTRODUCTION
Uterine sarcomas are rare tumors that account for about 3% of uterine cancers [1]. If carcinosarcomas (malignant mixed mesodermal tumors or MMMT) are classified as metaplastic carcinomas, leiomyosarcomas (LMS) are the most common uterine sarcomas. LMS are frequently diagnosed incidentally following routine hysterectomy and are at high risk for distant metastases and recurrence. The rate of recurrence and subsequent death is primarily related to mitotic activity and stage [2]. Cases of advanced or recurrent disease have a poor prognosis with limited treatment options [3]. Although adjuvant radiation therapy improves local control in some series, it has not shown any survival advantage, and systemic therapy remains the foundation of palliative therapy and is often used in the adjuvant setting, in spite of the lack of clear evidence in support of this strategy [4]. Meaningful clinical responses, however, are rare with existing chemotherapeutic regimens and a survival advantage associated with a systemic therapy has never been demonstrated. Thus, the identification of active agents in treating uterine LMS is a high scientific priority and urgent unmet medical need.
The Gynecologic Oncology Group (GOG) performs series of phase II studies among both chemotherapy naive (Protocol 87 series) and previously treated (Protocol 131 series) women with advanced, persistent or recurrent uterine LMS. The current study evaluates trabectedin in the 87 queue. Trabectedin (ET743, Yondelis®, Johnson & Johnson Pharmaceutical Research and Development, L.L.C. and PharmaMar), a tetrahydroisoquinoline alkaloid, is a natural product derived from the marine tunicate Ecteinascidia turbinate and currently produced synthetically (ATC code: L01CX01) [5]. Trabectedin binds covalently to the minor groove of DNA, bending DNA towards the major groove, and disrupts transcription leading to G2-M cell cycle arrest and ultimately apoptosis [6,7]. In 2007, trabectedin obtained marketing authorization from the European Medicines Agency and in many other countries worldwide for the treatment of patients with advanced soft tissue sarcoma after failure of anthracyclines and ifosfamide.. Based on the recently reported results of a large phase III study (OVA-301) comparing pegylated liposomal doxorubicin (PLD) alone with a combination of PLD and trabectedin in patients with recurrent ovarian cancer, in 2009 the European Medicines Agency granted marketing authorization for trabectedin combined with PLD for the treatment of patients with relapsed platinum-sensitive ovarian cancer [8].
We sought to determine the objective response rate of trabectedin as first-line therapy for advanced, unresectable uterine leiomyosarcoma in this phase II study.
MATERIALS AND METHODS
Eligibility
This study was an open-label prospective phase II trial of single agent trabectedin (GOG protocol 87M). Eligible subjects included women with measurable advanced, persistent or recurrent uterine LMS who were not previously exposed to chemotherapy and/or biological therapy, with documented disease progression. Histological confirmation of the original primary tumor was performed by central review of the GOG Pathology Committee.”. Patients were required to have measurable disease with at least 1 “target lesion” used to assess response according to the Response Evaluation Criteria in Solid Tumors (RECIST) [9]. Lesions within a previously irradiated field were designated as “non-target” lesions unless progression was documented or a biopsy was obtained to confirm persistence at least 90 days following completion of radiation therapy. Patients ≥ 18 years of age with a GOG performance status of 0, 1, or 2 were eligible. Adequate bone marrow function (platelet count greater than or equal to 100,000/mcl, absolute neutrophil count (ANC) count greater than or equal to 1,500/mcl, equivalent to Common Terminology Criteria for Adverse Events (CTCAE) v3.0 [10] grade 1, and Hgb >9), renal function (serum creatinine less than or equal to 1.5 x institutional upper limit normal (ULN), CTCAE v3.0 grade 1), hepatic function (bilirubin less than or equal to ULN. SGOT ≤ 2.5 × ULN. Alkaline phosphatase ≤ 1.5 × ULN), and neurologic function (neuropathy {sensory and motor} less than or equal to CTCAE v3.0 grade 1) were required as well as a serum CPK less than or equal to ULN. All patients signed a locally approved informed consent and authorization permitting release of personal health information. Patients of childbearing potential had a negative serum pregnancy test prior to the study entry and were practicing an effective form of contraception.
Drug Administration
Trabectedin 1.5 mg/m2 was diluted to 500 ml and administered IV over 24 hours every 3 weeks (one cycle) using a central line and an infusion pump. All subjects were pretreated with 10 mg dexamethasone (IV) 30 minutes before the trabectedin infusion. A maximum body surface area of 2.0 m2 as per the GOG Chemotherapy Procedures Manual was used for dose calculations. Antiemetics were at the discretion of the investigator. Patients remained on study until refusal, evidence of disease progression or unacceptable toxicity.
Dose Modifications
A dose reduction (1 level = 1.2 mg/m2, 2 levels =1.0 mg/m2), was required in the event of febrile neutropenia, neutropenia with sepsis, documented grade 4 neutropenia persisting ≥ 7 days, grade 4 thrombocytopenia, grade 2 (or greater) peripheral neuropathy, grade 3 (or greater) gastrointestinal toxicity, as well as other non-hematologic toxicities with an impact on organ function of grade 2 (or greater). Grade 3 transaminase elevations, initial grade 2 alkaline phosphatase of non-osseous origin > ULN or recurrent grade 1 elevations, and conjugated bilirubin >ULN at any time all required dose reduction of 1 level. Retreatment was only allowed if the ANC was ≥ 1500 cells/mcl (CTCAE v3.0 grade 1) and the platelet count was ≥ 100,000/mcl (CTCAE v3.0 grade 0) and all other toxicity had recovered to grade 1 or less. Therapy was delayed for a maximum of 3 weeks until these values were achieved. Patients who failed to recover adequate counts or who had persistent non-hematologic toxicity > grade 1 longer than 3 weeks were to be removed from study. No more than 2 dose reductions were permitted throughout the course of the study. Those experiencing grade 4 transaminase toxicity and/or grade 2 or greater alkaline phosphatase or bilirubin toxicity were to be removed from study. Patients were not allowed to receive prophylactic growth factors unless they experience recurrent neutropenic complications after treatment modifications specified above.
Study Endpoints
Tumor assessments were made every other cycle according to RECIST [9]. Disease progression was defined as any of the following: At least a 20% increase in the sum of longest dimension (LD) of the target lesions taking as reference the smallest sum LD recorded since study entry; in the case where the only target lesion was a solitary pelvic mass measured by physical exam which is not radiographically measurable, a 50% increase in the LD is required taking as reference the smallest LD recorded since study entry; the appearance of 1 or more new lesions; death due to disease without prior objective documentation of progression; global deterioration in health status attributable to the disease requiring a change in therapy without objective evidence of progression; unequivocal progression of existing non-target lesions. Partial response was defined as at least a 30% decrease in the sum of LD of all target measurable lesions taking as reference the baseline sum of LD. Documentation by 2 disease assessments at least 4 weeks apart was required. Complete response (CR) was defined as disappearance of all target and non-target lesions and no evidence of new lesions. Stable disease was any condition not meeting the above criteria. Side effects were graded according to the CTCAE v3.0 [10]. Survival was the observed length of life from entry into the study to death or the date of last contact.
Statistical Design
Based upon prior GOG studies in this population, it was determined that a response rate of at least 30% would be clinically interesting. Therefore, it was desired to test the hypothesis that the true response rate was <5% (H0) versus the alternative hypothesis that the true rate was >30% (H1). This study employed an optimal but flexible two-stage design with an early stopping rule intended to limit patient accrual to inactive treatments [11]. (Table 1) During the first stage, the targeted accrual was 17 eligible and evaluable patients; however, accrual was permitted to range between 12–19 patients due to administrative coordination. If more than 1 out of 12–14 or more than 2 out of 15–19 patients responded and medical judgment indicated, accrual to the second stage was to be initiated. Otherwise, the study would be stopped and treatment regimen classified as uninteresting. If opened to the second stage, the overall study accrual would be to 40 eligible and evaluable patients, but was permitted to range from 36 to 43 for administrative reasons. If more than 6 out of 36–39 or more than 7 out of 40–43 patients responded, then the regimen would be considered worthy of additional investigation. If the true probability of responding is only 10%, the study design provides a 95% chance of correctly classifying the treatment as inactive. Conversely, if the true response rate is 30%, then the average probability of correctly classifying the treatment as active is 90%.
Table 1.
Targeted accrual and decision guidelines by stage of accrual
| Stage of Accrual | Targeted Cumulative Accrual | Limits of Actual Accrual | Max Number of Responses to Reject H1 (conclude response rate <10%) |
|---|---|---|---|
| 1 | 17 | 12–19 | 1/(12–14), 2/(15–19) |
| 2 | 40 | 36–43 | 6/(36 to 39), 7/(40 to 43) |
RESULTS
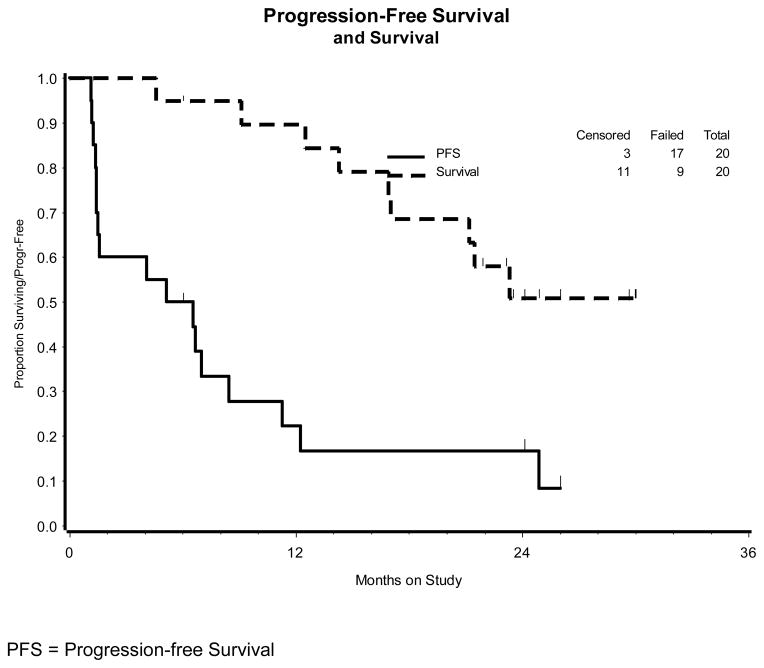
The study opened June 2007 and was closed to patient entry after the first stage of enrollment in November 2008. A second stage of enrollment was not indicated. Twenty patients were enrolled and all were evaluable as of January 2011. Patient characteristics are outlined in Table 2. The median age was 60 (range 34–82). The median number of cycles was 10 (range 2–29). The number of patients with partial responses was 2 (10%; 95% confidence interval of 1.2%–31.7%). Response durations were 3.3 and 5.7 months. Ten patients had stable disease (50%). The median progression-free survival (PFS) was 5.8 months, while the median overall survival (OS) was 26.1+ months (several observations are censored). (Figure 1). Adverse events are summarized in Table 3. The numbers of subjects with grade 3/4 toxicity included: Neutropenia 16/20 (1 infection); thrombocytopenia 3/20; metabolic 3/20; anemia, gastrointestinal and vascular 1/20 each. No grade 4 toxicity was seen except that related to the neutropenia (n=5). The median WBC for the 18 patients experiencing leukopenia was 1770/mcl (range 900–2900/mcl). Four subjects discontinued therapy due to toxicity (after 3, 3, 5, 7, and 8 courses) and 1 patient withdrew consent prior to progression. The progression-free interval for these 5 patients was 17.2, 24.1+, 26.0+, 31.1+ and 33.6 months. There were no treatment related deaths nor cases of liver failure.
Table 2.
Patient Characteristics (N = 20)
| Characteristic | Number of Cases |
|---|---|
| Age | |
| <40 | 1 |
| 40–49 | 3 |
| 50–59 | 5 |
| 60–69 | 7 |
| 70–79 | 3 |
| >79 | 1 |
| Performance Status | |
| 0 | 14 |
| 1 | 6 |
| Race | |
| White | 15 |
| Black | 4 |
| American Indian | 1 |
| Prior Chemotherapy | 0 |
| Prior Radiotherapy | 7 |
| Courses | |
| 1 | 0 |
| 2 | 8 |
| 3 | 2 |
| 4 | 0 |
| 5 | 1 |
| 6 | 2 |
| 7 | 1 |
| 8 | 3 |
| >8 | 3 |
Figure 1.
Kaplan-Meier plots demonstrating overall and progression-free survival (PFS) for the 20 patients in the study population (GOG 87M)
Table 3.
Adverse Events (N =20)
| Grade | |||||
|---|---|---|---|---|---|
| Adverse Event | 0 | 1 | 2 | 3 | 4 |
| Leukopenia | 2 | 0 | 7 | 10 | 1 |
| Thrombocytopenia | 13 | 4 | 0 | 3 | 0 |
| Neutropenia | 2 | 0 | 2 | 11 | 5 |
| Anemia | 3 | 10 | 6 | 1 | 0 |
| Other hematologic | 18 | 0 | 2 | 0 | 0 |
| Coagulation | 19 | 1 | 0 | 0 | 0 |
| Hemorrhage | 18 | 2 | 0 | 0 | 0 |
| Nausea | 3 | 11 | 6 | 0 | 0 |
| Vomitting | 7 | 10 | 3 | 0 | 0 |
| Bilirubin | 19 | 1 | 0 | 0 | 0 |
| ALT | 9 | 4 | 5 | 2 | 0 |
| Alkaline phosphatase | 19 | 1 | 0 | 0 | 0 |
| Dermatologic | 14 | 4 | 2 | 0 | 0 |
| Infection | 17 | 0 | 2 | 1 | 0 |
| Pulmonary | 15 | 4 | 1 | 0 | 0 |
| Metabolic | 8 | 5 | 4 | 3 | 0 |
| Lymphatics | 16 | 4 | 0 | 0 | 0 |
| Pain | 8 | 10 | 2 | 0 | 0 |
| Constitutional | 17 | 3 | 0 | 0 | 0 |
| Fatigue | 5 | 8 | 7 | 0 | 0 |
| Musculoskeletal | 15 | 4 | 1 | 0 | 0 |
| Neurotoxicity | 16 | 4 | 0 | 0 | 0 |
| Peripheral neuropathy | 18 | 2 | 0 | 0 | 0 |
| Renal | 19 | 1 | 0 | 0 | 0 |
| Ocular | 16 | 3 | 1 | 0 | 0 |
| Vascular | 19 | 0 | 0 | 1 | 0 |
| Flu-like syndrome | 19 | 0 | 1 | 0 | 0 |
DISCUSSION
The chemotherapeutic agent with the highest objective response rate (ORR) in treating advanced, persistent and recurrent LMS is gemcitabine. Even among previously treated patients (GOG protocol 131E), single agent gemcitabine demonstrated and ORR of 20.5% (9/44), with 1 confirmed CR [12]. By comparison, paclitaxel had an ORR of 8.4% (4/48) in a similarly designed phase II study (GOG protocol131C) [13]. When docetaxel was added to gemcitabine (GOG protocol 131G), the ORR increased to 27.1% (13/48) [14]. Better results with this combination have also been seen in untreated patients (GOG protocol 87L) with an ORR of 35.5% (15/42) [15]. By comparison, PLD showed an ORR of 15.6% (5/32) in this untreated population [16].
Trabectedin is another agent with documented activity in LMS. For example, a recent subset analysis of 5 phase II soft tissue sarcoma trials, showed that trabectedin had an ORR of 17.7% among 62 pretreated patients with uterine LMS [17]. This drug is not currently approved for use in the United States, and it has not been prospectively studied in a population of uterine LMS alone. It is approved in many other countries though for use in soft tissue sarcomas after failure of anthracyclines and ifosfamide. Interestingly, Tewari et al reported an objective response in recurrent LMS after failing both anthracycline treatment as well as combination gemcitabine and docetaxel [18]. Many patients with recurrent LMS receive multiple lines of therapy and the optimal sequencing of these drugs has not been defined.
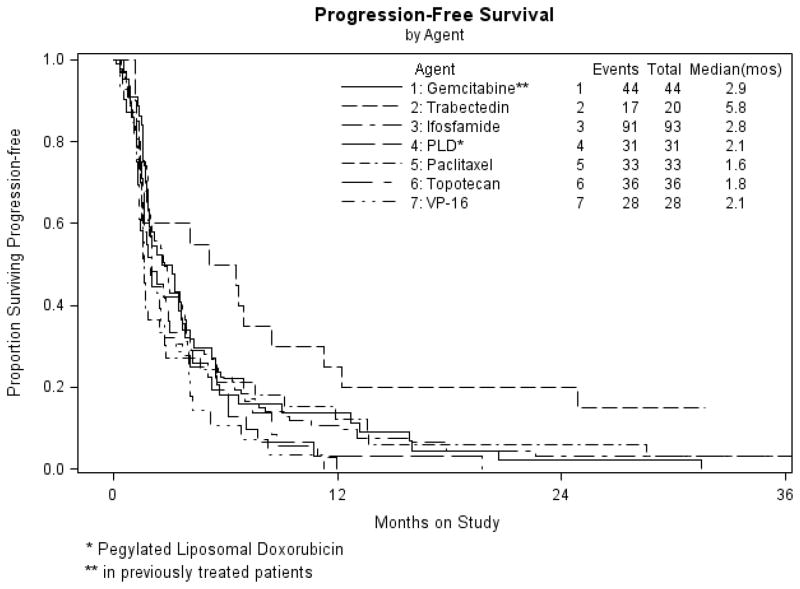
The current trial confirms the modest activity of trabectedin as first-line therapy in advanced, persistent or recurrent LMS. No unusual toxicities were demonstrated, and more than half the subjects remained progression free and without treatment-ending toxicity for more than 10 cycles (> 6 months). One patient received 29 doses. With such a long and well tolerated treatment duration, including a rate of stable disease of 50%, one wonders if PFS rather than ORR, would have been a better metric to assess activity. The ORR of 10% (2/20) did not meet the prescribed ORR of 15% (3/20) to justify enrollment to a second stage of accrual. However, GOG studies investigating biologic agents in uterine LMS as well as ovarian cancer often use a dual primary endpoint of either objective response or PFS at 6 months [19]. Figure 2 compares the PFS of the current trial to other single agents in the GOG 87 protocol series [16, 20–23] A clinically relevant delay in progression associated with the use of trabectedin can be seen.
Figure 2.
Kaplan-Meier plots demonstrating progression-free survival (PFS) for the 20 patients in the study population (GOG 87M) compared to other single agent studies in the GOG protocol 87 series studying cytotoxic agents
Though this drug did not meet the required response criteria, the duration and rate of stable disease is interesting. Trabectedin was well tolerated.
RESEARCH HIGHLIGHTS.
Trabectedin 1.5 mg/m2 IV over 24 hours every 3 weeks did not generate enough objective responses among women with chemotherapy naive, advanced, persistent or recurrent uterine leiomyosarcoma to justify a second stage of accrual.
However, fifty percent of treated subjects remained progression-free for at least 6 months..
Acknowledgments
This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group Administrative Office (CA 27469), the Gynecologic Oncology Group Statistical and Data Center (CA 37517).
Footnotes
The following Gynecologic Oncology Group member institutions participated in this study: Abington Memorial Hospital, Walter Reed Army Medical Center, Northwestern Memorial Hospital, University of Washington, University of Cincinnati, University of North Carolina School of Medicine, Rush-Presbyterian-St. Luke’s Medical Center, University of New Mexico, Memorial Sloan-Kettering Cancer Center, University of Oklahoma, The Hospital of Central Connecticut, and Georgia Core.
CONFLICT OF INTEREST
Dr. Bradley Monk has an honoraria and research funding from Johnson & Johnson. All other co-authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.D’Angelo E, Prat J. Uterine sarcomas: a review. Gynecol Oncol. 2010;116:131–9. doi: 10.1016/j.ygyno.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 2.Major FJ, Blessing JA, Silverberg SG, Morrow CP, Creasman WT, Currie JL, et al. Prognostic factors in early stage uterine sarcoma. Cancer. 1993;71:1702–9. doi: 10.1002/cncr.2820710440. [DOI] [PubMed] [Google Scholar]
- 3.Kanjeekal S, Chambers A, Fung MF, Verma S. Systemic therapy for advanced uterine sarcoma: a systematic review of the literature. Gynecol Oncol. 2005;97:624–37. doi: 10.1016/j.ygyno.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 4.Mahdavi A, Monk BJ, Ragazzo J, Hunter MI, Lentz SE, Vasilev SA, et al. Pelvic radiation improves local control after hysterectomy for uterine leiomyosarcoma: a 20-year experience. Int J Gynecol Cancer. 2009;19:1080–4. doi: 10.1111/IGC.0b013e3181acae50. [DOI] [PubMed] [Google Scholar]
- 5.Cuevas C, Francesch A. Development of Yondelis (trabectedin, ET-743). A semisynthetic process solves the supply problem. Nat Prod Rep. 2009;26:322–37. doi: 10.1039/b808331m. [DOI] [PubMed] [Google Scholar]
- 6.Carter NJ, Keam SJ. Trabectedin: a review of its use in soft tissue sarcoma and ovarian cancer. Drugs. 70:355–76. doi: 10.2165/11202860-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 7.D’Incalci M, Galmarini CM. A review of trabectedin (ET-743): a unique mechanism of action. Mol Cancer Ther. 2010;9:2157–63. doi: 10.1158/1535-7163.MCT-10-0263. [DOI] [PubMed] [Google Scholar]
- 8.Monk BJ, Herzog TJ, Kaye SB, Krasner CN, Vermorken JB, Muggia FM, et al. Trabectedin plus pegylated liposomal Doxorubicin in recurrent ovarian cancer. J Clin Oncol. 2010;28:3107–14. doi: 10.1200/JCO.2009.25.4037. [DOI] [PubMed] [Google Scholar]
- 9.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 10.Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–81. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 11.Chen TT, Ng TH. Optimal flexible designs in phase II clinical trials. Stat Med. 1998;17:2301–12. doi: 10.1002/(sici)1097-0258(19981030)17:20<2301::aid-sim927>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 12.Look KY, Sandler A, Blessing JA, Lucci JA, Rose PG. Phase II trial of gemcitabine as second-line chemotherapy of uterine leiomyosarcoma: a Gynecologic Oncology Group (GOG) study. Gynecol Oncol. 2004;92:644–7. doi: 10.1016/j.ygyno.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 13.Gallup DG, Blessing JA, Andersen W, Morgan MA Gynecologic Oncology Group Study. Evaluation of paclitaxel in previously treated leiomyosarcoma of the uterus: a Gynecologic Oncology Group study. Gynecol Oncol. 2003;89:48–51. doi: 10.1016/s0090-8258(02)00136-1. [DOI] [PubMed] [Google Scholar]
- 14.Hensley ML, Blessing JA, Degeest K, Abulafia O, Rose PG, Homesley HD. Fixed-dose rate gemcitabine plus docetaxel as second-line therapy for metastatic uterine leiomyosarcoma: a Gynecologic Oncology Group phase II study. Gynecol Oncol. 2008;109:323–8. doi: 10.1016/j.ygyno.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hensley ML, Blessing JA, Mannel R, Rose PG. Fixed-dose rate gemcitabine plus docetaxel as first-line therapy for metastatic uterine leiomyosarcoma: a Gynecologic Oncology Group phase II trial. Gynecol Oncol. 2008;109:329–34. doi: 10.1016/j.ygyno.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sutton G, Blessing J, Hanjani P, Kramer P Gynecologic Oncology Group. Phase II evaluation of liposomal doxorubicin (Doxil) in recurrent or advanced leiomyosarcoma of the uterus: a Gynecologic Oncology Group study. Gynecol Oncol. 2005;96:749–52. doi: 10.1016/j.ygyno.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 17.Judson IR, Blay J, Chawla SP, Radford JA, Le Cesne A, Verweij J, et al. Trabectedin (Tr) in the treatment of advanced uterine leiomyosarcomas (U-LMS): Results of a pooled analysis of five single-agent phase II studies using the recommended dose. J Clin Oncol (Meeting Abstracts) 2010 May;28(15_suppl):10028. [Google Scholar]
- 18.Tewari D, Saffari B, Cowan C, Wallick AC, Koontz MZ, Monk BJ. Activity of trabectedin (ET-743, Yondelis) in metastatic uterine leiomyosarcoma. Gynecol Oncol. 2006;102:421–4. doi: 10.1016/j.ygyno.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 19.Rose PG, Tian C, Bookman MA. Assessment of tumor response as a surrogate endpoint of survival in recurrent/platinum-resistant ovarian carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2010;117:324–9. doi: 10.1016/j.ygyno.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 20.Sutton GP, Blessing JA, Barrett RJ, McGehee R. Phase II trial of ifosfamide and mesna in leiomyosarcoma of the uterus: a Gynecologic Oncology Group study. Am J Obstet Gynecol. 1992;166:556–9. doi: 10.1016/0002-9378(92)91671-v. [DOI] [PubMed] [Google Scholar]
- 21.Sutton G, Blessing JA, Ball H. Phase II trial of paclitaxel in leiomyosarcoma of the uterus: a gynecologic oncology group study. Gynecol Oncol. 1999;74:346–9. doi: 10.1006/gyno.1999.5463. [DOI] [PubMed] [Google Scholar]
- 22.Miller DS, Blessing JA, Kilgore LC, Mannel R, Van Le L. Phase II trial of topotecan in patients with advanced, persistent, or recurrent uterine leiomyosarcomas: a Gynecologic Oncology Group Study. Am J Clin Oncol. 2000;23:355–7. doi: 10.1097/00000421-200008000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Thigpen T, Blessing JA, Yordan E, Valea F, Vaccarello L. Phase II trial of etoposide in leiomyosarcoma of the uterus: a Gynecologic Oncology Group study. Gynecol Oncol. 1996;63:120–2. doi: 10.1006/gyno.1996.0289. [DOI] [PubMed] [Google Scholar]