Abstract
Objectives
To project the cost-effectiveness of population-based echo screening to prevent rheumatic heart disease (RHD) consequences.
Background
RHD is a leading cause of cardiovascular mortality and morbidity during adolescence and young adulthood in low- and middle-per capita income settings. Echocardiography-based screening approaches can dramatically expand the number of children identified at risk of progressive RHD. Cost-effectiveness analysis can inform public health agencies and payers about the net economic benefit of such large-scale population-based screening.
Methods
A Markov model was constructed comparing a no-screen to echo screen approach. The echo screen program was modeled as a 2-staged screen of a cohort of 11-year-old children with initial short screening performed by dedicated technicians and follow-up complete echo by cardiologists. Penicillin RHD prophylaxis was modeled to only reduce rheumatic fever recurrence-related exacerbation. Quality-adjusted life years (QALYs) and societal costs (in 2010 Australian dollars) associated with each approach were estimated. One-way, two-way and probabilistic sensitivity analyses were performed on RHD prevalence and transition probabilities; echocardiography test characteristics; and societal level costs including supplies, transportation, and labor.
Results
The incremental costs and QALYs of the screen compared to no screen strategy were −$432 (95% CI = −$1357 to $575) and 0.007 (95% CI = −0.0101 to 0.0237), respectively. The joint probability that the screen was both less costly and more effective exceeded 80%. Sensitivity analyses suggested screen strategy dominance depends mostly on the probability of transitioning out of sub-clinical RHD.
Conclusion
Two-stage echo RHD screening and secondary prophylaxis may achieve modestly improved outcomes at lower cost compared to clinical detection and deserves closer attention from health policy stakeholders.
Keywords: Cost-benefit analysis, Echocardiography, Pediatrics, Rheumatic heart disease, Valves
Introduction
Rheumatic heart disease (RHD) occupies an unusual space within global health. RHD is a chronic disease of the heart valves with debilitating consequences including congestive heart failure, stroke and arrhythmia during the prime of life. RHD impacts functional status, quality-of-life, earning potential, fertility and ultimately causes mortality in more than 250,000 persons per year1,2. However, as RHD is a consequence of an aberrant immune response to group A streptococcal infection, a scalable intervention analogous to infectious disease mitigation campaigns appears to be a rational method of preventing chronic RHD and complications. Antibiotic prophylaxis in affected individuals with monthly intramuscular benzathine penicillin prevents the progression of RHD3–5. A challenge to secondary prophylaxis regimes is intervening at an early stage, since affected persons often present with advanced disease6.
Recent reports document the feasibility of population-based echocardiography screening to identify early, clinically latent valvular damage consistent with RHD7–13. These studies suggest a greater prevalence of valvular RHD damage than suspected by clinical exam alone, leading to uncertainty about proper utilization of echo and provision of prophylaxis. To aid in echo utilization, a recent multi-center World Heart Federation expert panel promulgated standardized guidelines to rigorously identify true RHD14. The expert panel identified determining the cost-effectiveness of echo-based screening as a research priority that can form the rational basis for scaling up such screening. We respond to this evidence gap by constructing a Markov cohort model to evaluate the cost-effectiveness of echo-based RHD screening. Fixing the effect of improved screening solely as increasing the number of eligible persons on prophylaxis to prevent rheumatic fever recurrence, with no effect on later progression or complications, we estimated the cost-effectiveness of a single echo-based RHD screening at age 11 years of age as a function of RHD prevalence, a range of health and non-health related costs, and the projected improvement in health status over time. The robustness of our findings was tested using one way, two way and probabilistic sensitivity analyses.
Methods
Model
Markov cohort models can be a transparent and efficient way to compare interventions with respect to health outcomes and consequent costs among cohorts of patients over time. The models consist of mutually exclusive disease states and allow given proportions of individuals to transition between these states over time. Costs and utilities are assigned to each state and aggregated. We formulated a Markov model on a yearly time cycle and compared societal costs and quality-adjusted life years (QALYs) between (a) routine clinical exam detection (current practice) vs (b) a population-based rapid echocardiography single screening followed by full echo in screen positive patients. The screening occurs in a hypothetical cohort of 11 year olds followed for 40 yearly cycles following that screen (longer evaluations did not change our conclusions). We chose 11 year olds as the age roughly around which published echo screening investigators were centered7–9,11,12,15. Our target population was the Northern Territory, Australia, a location with highly endemic RHD and reliable cost and census data in which we identified 3663 11-year-olds. However, in sensitivity analyses we also aimed to test the model across a range of costs and transition probabilities which can reflect situations beyond the selected population, as detailed below.
Initial distributions across health states were derived from recently published studies. We used a weighted average approach (fixed effect meta-analysis) to combine incidence rates when more than one study was available and then converted these rates to annual probabilities. In transitions where only cross-sectional data were available, we calculated age-specific incidence rates using a constant rate approach after excluding those prevalent cases calculated to have occurred during a previous age stratum. We assumed screening echo was highly sensitive for RHD, but the estimates that we used do account for some false negatives as children with echo visible RHD have recovered with no echo evidence of RHD11,12. Test characteristics for screening echo and prevalence data were extracted from the literature. We began by assuming full delivery and compliance for secondary prophylaxis, and then modeled relaxation of this assumption. Since no actual patients were involved, the Boston Children’s Hospital Committee on Clinical Investigation declined review.
State definitions, care, and costs
We allocated apparently well children into seven simplified health states (Figure 1). Dead is the costless, absorbing state available from all other states and relevant causes of death, including penicillin anaphylaxis. These state names reflect current clinical consensus.
Figure 1.
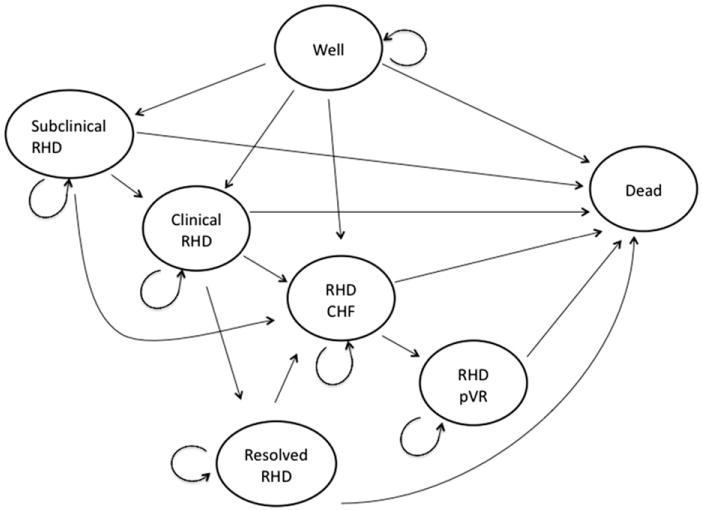
Model diagram of relevant health states.
Well or sub-clinical RHD
Well indicates a child with neither clinically evident nor echo-visible RHD. Sub-clinical RHD exists when a child has echo visible lesions consistent with RHD, but no murmur nor clinical signs of RHD. As reliable data on transition from sub-clinical to other forms of RHD do not currently exist, we assumed the minimum transition probability must be no lower than acute rheumatic fever (ARF) without cardiac involvement progressing to RHD in natural history studies and then widely varied this assumption in sensitivity analyses. The essential difference between No screen and Screen scenarios was the presence and transition of the Sub-clinical RHD group to more severe health states. All other transitions across health states occurred with the same probability between the two scenarios.
Well children underwent local general practice (GP) check-ups on a yearly basis, transportation to and from a local provider for that check-up and 1 day lost wages at minimum wage to their caregiver or themselves for attending the GP visit. Sub-clinical RHD accrued the same costs each year as the Well group, since without an echo there is no clinical distinction from Well.
Clinical or resolved RHD
Clinical RHD exists when a child has echo evidence of RHD and an RHD murmur but no other clinical signs of RHD. Resolved RHD indicates a child with previously detected Clinical RHD who at a subsequent cycle no longer has a murmur. RHD CHF (congestive heart failure), RHD pVR (post-valve replacement) and Sub-clinical RHD patients cannot enter the resolved RHD state.
Clinical RHD entails the same yearly costs as Well, plus costs of secondary prophylaxis until age 25. Contemporaneous on-site mixing of penicillin was modeled in lieu of a cold-chain supported pre-mixed regime. The modeled regime included two 600 mg vials per month, diluent, syringe, needle, 0.5 mL of 2% lidocaine solution to minimize muscle discomfort, salary for a level 4 nurse to mix and administer prophylaxis, and the transport cost of the local health clinic nurse to each child at an average distance of 25 km each way. We assume the single prophylaxis person can engage 10 children per day given travel time, with sensitivity analyses varying this efficiency. In the Resolved state, the same costs accrue. For both states, no RHD specific follow-up occurs due to the lack of clinical symptoms.
RHD CHF or RHD pVR
RHD CHF indicates a child with exertional shortness of breath or peripheral edema and echo evidence of RHD. RHD pVR indicates the health state after valve replacement surgery among children who have been RHD CHF for at least 1 year prior. We focused on replacement alone as the destination intervention in resource-poor circumstances where repeated interventions are infeasible. Individuals in the RHD CHF state are only allowed to stay in RHD CHF, obtain valve replacement surgery or die, as we are unaware of data indicating secondary prophylaxis altering the natural history of RHD once CHF is present. Similarly, RHD pVR is allowed only to remain in state or die. Progression from Clinical RHD directly to RHD pVR was not allowed to ensure a symptomatic state was a necessary precursor to surgery.
Beyond the costs of Clinical RHD, we assume lifelong prophylaxis, an average of a GP visit every 3 months for symptom management with attendant costs, an annual trip to a cardiologist with echocardiography with attendant costs, and we assume a productivity loss of 50% of one person’s yearly wages due to debilitation, either on the part of the affected person or a parent caring for a child. For CHF symptom management we assigned a regimen 20 mg of enalapril and 40 mg of furosemide per day. For those in RHD pVR, ongoing costs included all costs from RHD CHF and an average of 5 mg of Coumadin daily and monthly international normalized ratio assessment. RHD pVR also incurred the one-time cost of valve replacement, including the valve, operating theater with staff and consumables, 2 days of recovery in intensive care, 10 days of recovery in an inpatient ward, 1 day of rehabilitation training, another 14 days of recovery at home, lost wages for the full month, and transport to the referral center.
Interventions costs
The echo screening costs were decomposed into the per child average cost of the echo machine, screening staff salary at the level of a registered nurse, one time echo training for the screener, screen staff transport costs from a referral center, and cardiologist salary of 24 days per year for quality control regime of screening echoes. For all children positive in step 1 of screening, step 2 full echocardiography confirmation and transport to referral site for cardiology visit are included. We assume 18 children screened per day in the initial screen stage and modeled more and less children screened per day in sensitivity analysis. The costs related to screening were divided over the entire cohort of screened children.
Transport costs were based on the assumption that the average person lives 250 km from a referral center and 25 km from a primary health center. The price of petrol was assumed at $1.5 per liter, with an average fuel economy of 100 km with 13.8 L16,17. All costs are denominated in 2010 Australian dollars and adjusted for inflation using the Australian Consumer Price Index18. Costs and QALYs were discounted by 3.5% yearly.
Outcomes
Outcomes are measured in QALYs and net healthcare costs as accumulated in the No screen and Screen scenarios separately. We borrowed utilities from echo diagnosed congestive heart failure as follows: Well at 0.9; Sub-clinical RHD, Clinical RHD, Resolved RHD at 0.75; RHD CHF, RHD pVR at 0.58; and Death at 019. The same utility was assigned for clinically equivalent states of sub-clinical and clinical RHD as they only differ by virtue of the presence of murmur and prophylaxis administration. While children may favor Sub-clinical RHD in order to avoid prophylaxis injection disutility, we did not have reliable data on this disutility.
Statistical analysis
Incremental cost-effectiveness ratios were constructed by dividing the differences in per patient costs and QALYs between the Screen vs No screen scenarios. The base case adopted a societal perspective; in sub-analyses we adopted a healthcare system perspective by including direct health costs alone and excluding transport and wage loss. In oneway sensitivity analyses, single cost, disease prevalence, test characteristic, or transition probability was varied one at a time. We also performed one-way sensitivity analyses where the range of values was extended to 90% of each point transition probability and cost estimate. This collection of sensitivity analyses allowed us, for example, to relax the full delivery assumption by modeling ranges around the transition probability from treated RHD to downstream consequences, or vary the number of children screened by varying the costs accrued per screened child, or account for very low cost echo equipment by varying the echo machine cost per child. We also conducted select sensitivity analyses, where two variables were varied simultaneously with ranges derived from weighted averages in the literature or reasonable estimated ranges as explicitly specified. Probabilistic sensitivity analysis (PSA) was performed via 5000 Monte Carlo simulations of the model using beta distributions around the transition probabilities for Clinical RHD to RHD CHF, Clinical RHD to Resolved RHD, Clinical RHD to Dead, RHD CHF to Dead, Subclinical RHD to Clinical RHD, RHD pVR to Dead, and RHD CHF to RHD pVR; as well as the probabilities for echo screen specificity, Sub-clinical RHD prevalence, and Clinical RHD prevalence. Utilities were also incorporated in the PSA assuming a uniform distribution with lower and upper bounds of ±5% of the base value. Finally, we conducted sensitivity analyses using a discount rate of 0–6%. Sensitivity analyses when adopting the healthcare system perspective were repeated, and had no impact on our conclusions. TreeAge Pro 2013 (Williamstown, MA) was used for all modeling. Willingness-to-pay (WTP) threshold was placed at $64,000 based on the societal willingness to pay for an additional QALY elicited from a sample of the Australian population.20 The authors had full access to the data and the corresponding author takes final responsibility for the decision to submit for publication.
Results
The initial distributions of both cohorts across health states were as follows: Well (98.15%), Sub-clinical RHD (1.71%), Clinical RHD (0.14%), resolved RHD (0%), RHD with congestive heart failure (RHD CHF, 0%), RHD post-valve replacement (RHD pVR, 0%), and Dead (0%), as compiled from echo screening data (Table 1).
Table 1.
Initial state distributions and Echo screen results.
| Webb et al.13 | Marijon et al.8 | Reeves et al.9 | Beaton et al.7 | Bhaya et al.12 | Saxena et al.11 | Weighted probability±SE | |
|---|---|---|---|---|---|---|---|
| n | 1142 | 2170 | 362 | 4869 | 1059 | 6270 | |
| Sub-clinical RHD | 55 | 61 | 16 | 49 | 53 | 123 | 0.017,095,96±0.001,026,014 |
| Clinical RHD | 4 | 5 | 4 | 23 | 1 | 5 | 0.001,404,44±0.000,297,071 |
| Echo Screen positive | 95 | 124 | 99 | 130 | 0.037,015,469±0.002,022,628 | ||
| True Positive Screen | 62% | 53% | 20% | 55% | 45.605,483,6±2.212,980,6% | ||
| False Positive Screen | 38% | 47% | 80% | 45% | 54.394,516,4±2.212,980,6% |
The proportion of Screen positive children with actual RHD was estimated at 45.60 ± 2.21%. Fixed and age-specific transition probabilities are given in Table 2. The estimated annual costs by state are given in Table 3. As can be expected, the RHD CHF and the RHD pVR states accumulated the largest per patient costs ($16,757 and 16,054, respectively). The bulk of these costs were driven by productivity losses. It is also worth noting the relatively high cost associated with prophylaxis, which occurred at a greater rate in the Screen and is, therefore, of potential interest to decision-makers. Table 4 presents the underlying costs that were used to estimate total annual costs per state, as well as a breakdown of all intervention costs.
Table 2.
Annual transition probabilities.
| Transition states | Age | Yearly probabilitya |
|---|---|---|
| Subclinical RHD to RHD CHF26–28 | 0.025,824,67±0.008,261,654 | |
| Clinical RHD/Resolved RHD to CHF* | 0.012,912,34±0.008,259,597 | |
| Clinical RHD to Resolved RHD on Prophylaxis29–34 | 0.087,325,441±0.015,435,476 | |
| RHD CHF to Dead32,35 | 0.061,010,739±0.014,796,559 | |
| Sub-clinical RHD to Well11,12 | 0.100,289,612±0.063,021,044 | |
| Sub-clinical RHD to Clinical RHD28,35 | 0.028,248,41±0.010,411,038 | |
| RHD surgery perioperative mortality36 | 0.012,345,679 | |
| RHD pVR to Dead36 | 0.028,358,342±0.006,432,767 | |
| RHD CHF to RHD pVR23,37 | 0.128,468,899±0.0813 | |
| Prophylaxis mortality38 | 0.001,480,202 | |
| Well to Clinical RHD39 | 5–9 | 0.000,599 |
| 10–14 | 0.000,884 | |
| 15–19 | 0.002,123 | |
| 20–24 | 0.003,112 | |
| 25–29 | 0.005,067 | |
| 30–34 | 0.002,711 | |
| 35–39 | 0.003,534 | |
| 40–44 | 0.003,433 | |
| 45–49 | 0.002,993 | |
| Well to Sub-clinical RHD11 | 5–10 | 0.002,532 |
| 11–15 | 0.002,907 | |
| 15+ | 0.000,634# | |
| Well to RHD CHF40 | 5–9 | 3.722,67 × 10−5 |
| 10–14 | 4.553,66 × 10−5 | |
| 15–19 | 2.130,59 × 10−5 | |
| 20–24 | 1.356,54 × 10−5 | |
| 25–29 | 7.947,68 × 10−6 | |
| 30–34 | 9.319,79 × 10−7 | |
| 35–39 | 2.252,28 × 10−6 | |
| 40–44 | 3.365,47 × 10−6 | |
| 45–49 | 4.038,57 × 10−6 | |
| Well to Dead41 | 5–9 | 0.000,199,96 |
| 10–14 | 0.000,399,84 | |
| 15–19 | 0.000,499,75 | |
| 20–24 | 0.001,198,561 | |
| 25–29 | 0.000,999,001 | |
| 30–34 | 0.001,298,311 | |
| 35–39 | 0.003,189,787 | |
| 40–44 | 0.002,991,022 | |
| 45–49 | 0.004,677,996 | |
| Clinical RHD to Dead40 | 5–14 | 0 |
| 15–19 | 1.448,14 × 10−6 | |
| 20–24 | 3.519,65 × 10−6 | |
| 25–29 | 7.315,82 × 10−7 | |
| 30–34 | 6.606,37 × 10−7 | |
| 35–39 | 4.064,95 × 10−6 | |
| 40–44 | 2.597,26 × 10−6 | |
| 45–49 | 2.735,38 × 10−6 | |
| Acute Rheumatic Fever to Dead40 | 5–9 | 1.999,96 × 10−5 |
| 10–19 | 3.399,88 × 10−5 |
Estimated as a weighted average ± standard error.
Assuming 50% reduction from preprophylaxis era.
Assuming 25% of 5–10 year old stratum.
Table 3.
Costs by health state per person per year in 2010 Australian dollars.
| Component cost | Total state cost | |
|---|---|---|
| Well or Subclinical RHD | 152 | |
| GP visit | 22 | |
| Transport to GP | 10 | |
| Lost wages | 120 | |
| Clinical RHD or Resolved RHD | 680 | |
| GP visit | 22 | |
| Transport to GP | 10 | |
| Lost wages | 120 | |
| Penicillin | 103 | |
| Syringe, needles, sterile diluent | 6 | |
| Lignocaine 2% | 4 | |
| Prophylaxis staff salary | 405 | |
| Prophylaxis transport | 124 | |
| RHD CHF | 16,054 | |
| GP visit | 87 | |
| Transport to GP | 41 | |
| Cardiologist visit | 60 | |
| Transport to Cardiologist at referral center | 52 | |
| Penicillin | 103 | |
| Syringe, needles, sterile diluent | 6 | |
| Lignocaine 2% | 4 | |
| Prophylaxis staff salary | 405 | |
| Prophylaxis transport | 124 | |
| Enalapril 20mg | 204 | |
| Frusemide 40mg | 30 | |
| Lost wages | 15000 | |
| RHD pVR | 16,757 | |
| GP visit | 87 | |
| Transport to GP | 41 | |
| Cardiologist visit | 60 | |
| Transport to Cardiologist at referral center | 52 | |
| Penicillin | 103 | |
| Syringe, needles, sterile diluent | 56 | |
| Lignocaine 2% | 4 | |
| Prophylaxis staff salary | 405 | |
| Prophylaxis transport | 124 | |
| Enalapril 20mg | 204 | |
| Frusemide 40mg | 30 | |
| Coumarin 5mg | 102 | |
| INR monitoring | 600 | |
| Lost wages | 15,000 |
Table 4.
Cost inputs per person per event in 2010 Australian dollars.
| Component cost | Total cost | |
|---|---|---|
| One time events | ||
| Echo screening | 146.21 | |
| Screening Echo machine** | 13.65 | |
| Screening staff salary42 | 10.81 | |
| Screener training (one-time)43 | 0.96 | |
| Screener transport (roundtrip)16,17 | 10.36 | |
| Cardiologist overread of echo#44 | 3.93 | |
| Cardiologist visit and echo for Screen Positive44 | 59.93 | |
| Transport to Cardiologist at referral center (roundtrip)16,17 | 103.50 | |
| Valve Replacement | 23,657.49 | |
| Mitral Value** | 960.83 | |
| Operation Theater45 | 9,755.18a | |
| ICU – 2days45 | 5,052.73 | |
| Hospital – 10 days45 | 3700 | |
| Rehabilitation – 1 day45 | 537 | |
| Transport to referral center (roundtrip)16,17 | 103.50 | |
| 30 days of lost wages46 | 3,600 | |
| Repeated costs | ||
| Daily minimum wage46 | 120 | |
| General practitioner visit47 | 21.66 | |
| Transport for general practitioner or prophylaxis (one trip)16,17 | 10.36 | |
| Transport to referral center (roundtrip)16,17 | 103.50 | |
| Penicillin- 600mg vials × 248 | 8.58 | |
| Syringe, needles, sterile diluent | 0.47 | |
| Lignocaine 2% 5cc vial- 0.5cc used per injection49 | 0.74 | |
| Prophylaxis staff salary (per injection)42 | 33.74 | |
| Enalapril 20mg tablet50 | 0.56 | |
| Frusemide 40mg tablet51 | 0.083 | |
| Coumarin 5mg table52 | 0.28 | |
| Monthly INR monitoring53 | 50 |
Personal communication.
Operation theater Includes room, consumables, staff salaries.
After running the Markov model over 40 yearly cycles, the No screen strategy accrued roughly 19.15 QALYs, while Screen accrued 19.16 QALYs (Table 5). Considering total costs, Screen was associated with lower cost compared to No screen, making Screen the dominant strategy. From a healthcare system perspective, however, Screen was associated with higher cost than No screen, with an ICER of $3571 per QALY.
Table 5.
Average per person costs (2010 Australian dollars) and QALYs.
| Screen | No screen | Difference (95% CI) | Cost/QALY (Screen vs No screen) | |
|---|---|---|---|---|
| Point estimates | ||||
| Total societal cost | $7749 | $8181 | −$432 (−$1357 to 575) | Dominates |
| Health care costs only | $1799 | $1774 | $25 (−$117 to 176) | $3571 |
| Quality adjusted life years | 19.16 | 19.15 | 0.007 (−0.010 to 0.024) | |
| PSA comparing Screen vs No screen | ||||
| % Dominant | 81.0% | |||
| % Lower costs and QALYs | 1.2% | |||
| % Dominated | 16.6% | |||
| % ICER>64,000/QALY | 1.1% | |||
| % ICER≤64,000/QALY | 0.1% |
PSA, Probabilistic sensitivity analyses, 5000 simulations; QALY, Quality-adjusted life year; ICER, Incremental cost-effectiveness ratio.
Sensitivity analyses
One-way sensitivity analyses varying all costs between 33–300% of the point estimate showed that Screen remains the dominant strategy (Table 6). Two-way sensitivity analyses simultaneously varying the Sub-clinical RHD prevalence from 1 to 50/1000 against a false positive echo screening prevalence from 0–70% demonstrated Screen was the superior strategy [not shown]. In PSA using 5000 random draws from the parameters’ distributions, Screen had more QALYs with lower cost in 81% of the iterations. It had both lower cost and QALYs in 1.2%, was more effective but had an ICER exceeding the $64,000 per QALY willingness-to-pay threshold in 1.1%, and was dominated in 16.6% of the iterations (Figure 2). Cost-effectiveness acceptability curves (not shown) suggest that the probability of Screen being cost-effective exceeded 80% across a wide range of WTP thresholds. In analyses varying the Sub-clinical RHD exit transitions, the model was sensitive to the probability of transitioning from Sub-clinical to RHD CHF. Screen was cost-effective when the transition was higher than 1.53% and was the dominant strategy above 1.65%. Varying utilities and limiting to direct health costs did not alter Screen dominance. The discount rate had little impact on the results; when it was varied from 0–6%, savings associated with screening were $954 and $324, respectively; the gain in QALYs was 0.02 and 0.004, respectively.
Table 6.
One-way sensitivity analyses for ICER of Screen vs No screen.
| Cost inputs | Range | Low ICER | High ICER |
|---|---|---|---|
| Daily Wage | 39.6–360 | −187,329 | −17,451 |
| Short Term Prophylaxis Staff Salary | 133–1214 | −65,385 | −44,242 |
| Operating Room | 3219–29,265 | −67,021 | −57,754 |
| Short Term Prophylaxis Penicillin | 33–308 | −61,429 | −56,052 |
| Echo Machine | 4.5–40 | −61,351 | −56,282 |
| Intensive Care Unit Day | 813–7398 | −63,588 | −58,904 |
| Lifelong Prophylaxis Staff Salary | 133–1214 | −63,509 | −58,930 |
| INR (International Normalized Ratio) | 198–1800 | −63,132 | −59,057 |
| Echo Screen Staff Salary | 3.6–32 | −61,087 | −57,072 |
| Transport to Screening | 3–31 | −61,045 | −57,197 |
| Inpatient Ward Day | 122–1110 | −62,712 | −59,197 |
| Enalapril | 67–613 | −61,811 | −59,499 |
| Cardiology Quality Control | 1.3–11 | −60,445 | −58,986 |
| Lifelong Prophylaxis Penicillin | 33–308 | −60,951 | −59,787 |
| Heart Valve | 317–2882 | −60,763 | −59,850 |
| General Practitioner Visit | 7.1–64 | −60,614 | −59,900 |
| Coumarin | 33–306 | −60,599 | −59,905 |
| Transport for Short Term Prophylaxis | 41–372 | −60,215 | −59,674 |
| Screen Staff Training | 0.31–2.8 | −60,169 | −59,812 |
| Frusemide | 9.9–90 | −60,336 | −59,993 |
| Transport to General Practitioner | 3–31 | −60,335 | −59,994 |
| Transport to Referral Hospital | 34–310 | −60,154 | −59,855 |
| Short Term Prophylaxis Paraphernalia | 1.9–16 | −60,153 | −59,859 |
| Short Term Prophylaxis Lignocaine | 1.4–13.3 | −60,137 | −59,906 |
| Cardiology Visit | 19–179 | −60,123 | −59,950 |
| Transport for Lifelong Prophylaxis | 41–372 | −60,167 | −60,050 |
| Lifelong Prophylaxis Paraphernalia | 1.9–16 | −60,127 | −60,063 |
| Lifelong Prophylaxis Lignocaine | 1.4–13.2 | −60,117 | −60,067 |
ICER, Incremental cost-effectiveness ratio.
Figure 2.
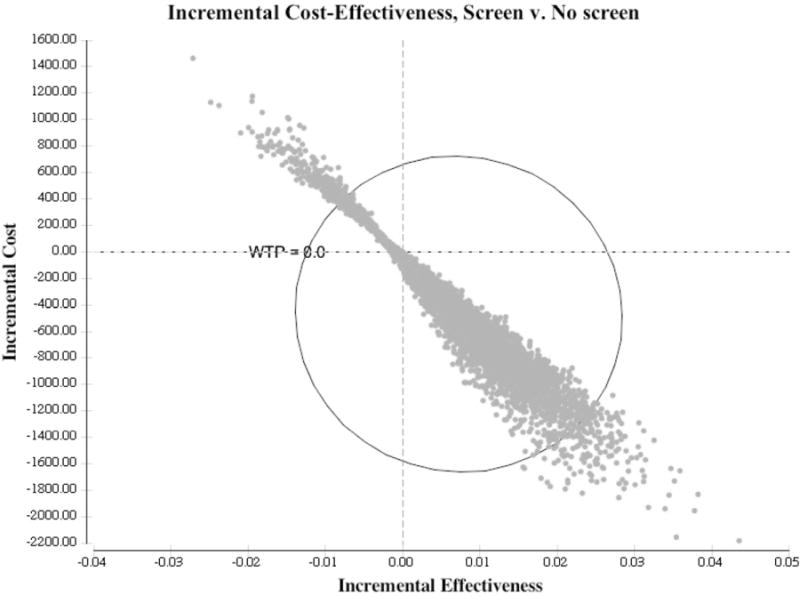
Cost-effectiveness plane for incremental change of Screen strategy over No screen. Incremental benefit is along the x-axis and incremental cost in on the y-axis. Toward the right indicates more QALYs and toward the top indicates higher cost.
Discussion
Echocardiographic screening detects several-fold more children with RHD-type changes than clinical exam alone7–9,11–13,21. Juxtaposed against this usefulness is the absence of sophisticated, expensive medical equipment and personnel in many of the places most affected by RHD. In such places public health decision-makers are faced with the dilemma of whether to invest in such additional health resources as they lack information about long-term savings and net societal benefits resulting from improved health. Acknowledging the current controversy inherent in expanding the definition of RHD to include echo only cases, we engaged in a Markov modeling scenario to test the cost-effectiveness of echo-based RHD screening vs clinical detection. Screen was associated with modestly improved outcomes and appeared to be cost-effective below the $64,000 per QALY threshold. This result remained robust to widely ranging assumptions about underlying costs and health utilities. Given the uncertainty of transition probabilities and costs, since many places with endemic RHD are precisely where data capture is poorest, PSA was performed, and again Screen was deemed the dominant strategy in the vast majority of simulations. The PSA did show some combinations of transition probabilities where Screen was not cost-effective, indicating that the cost-effectiveness may vary by the transition probabilities. Specifically, while Screen superiority was robust across a range of hypothetical Sub-clinical to Clinical RHD transition values, it was highly sensitive to the Sub-clinical RHD to RHD CHF transition probability. However, overall the wide variety of sensitivity analyses performed using wide ranges of model inputs suggests the model inferences are robust across a range of possible scenarios.
Previous investigators from RHD endemic sites have suggested echo screening is a low cost tool and perhaps a cost-effective tool3,9. The present results are consonant with these previous conclusions and are bolstered by the Markov modeling techniques detailed herein, including detailed delineation of costs, utilities, and pooled transition probabilities and the inclusion of a societal perspective with inclusion of non-healthcare costs. Alternatively, other experts have promoted primary prophylaxis as the superior approach22–24. This work does not examine a scenario preventing primary group A streptococcal pharyngitis progression to RHD as secondary prophylaxis is currently the most widely accepted approach. We leave the resolution of the controversial primary vs secondary prophylaxis debate to other investigators7,8,11,14,25. Likewise, experts are debating the severity of echo only RHD that triggers secondary prophylaxis, leading to wide ranges of RHD affected children depending of the selected threshold. As no current consensus exists, published data was used to develop a pooled estimate of Sub-clinical RHD prevalence and then that pooled estimate was widely varied to simulate various thresholds. The uncertainty about which RHD severity to initiate prophylaxis can affect prophylaxis effectiveness. We modeled a change in RHD progression and prognosis assuming Sub-clinical RHD and Clinical RHD are similar with some variation around progression rates for Sub-clinical RHD. More data is needed to document the actual health effects of prophylaxis of Sub-clinical RHD to validate the great hopes in this area. Nevertheless, using the best available data, the results of this model can be applied by policymakers based on the particular RHD prevalence in their locality deserving prophylaxis, wherever that threshold is eventually set. We solicit other investigators and stakeholders to utilize our model with local probabilities and costs by contacting the corresponding author.
Limitations
This model entails a variety of limitations. First, some parameters are gleaned from single sources or are assigned based on conservative assumptions. These assumptions were systematically explored through multiple sensitivity analyses and the key results remained largely unaffected. While our conclusions remained unchanged within the utility ranges used in sensitivity analyses, more data on relevant inputs, including the health utilities of various RHD states, would be most welcome. Second, this model is theoretical. It does not, for instance, account for sex differences or health system issues. Third, much of the natural history data is more than 40 years old. Careful follow-up of Sub-clinical patients in the current milieu where the indications for prophylaxis are unclear may be appropriate, since more persons prophylaxed will likely appear more clinically effective in preventing RHD cases. Repeated screening of the same cohort was not modeled as conditional probabilities cannot be interpolated from currently available data. Similarly, we acknowledge time dependency of transitions between an attack of rheumatic fever and progression to RHD, but the existing literature limits our ability to reliably model this dependency on a population basis. We also limit this model to RHD and do not include effects of detecting other structural heart diseases. Finally, applying the inference from this study to any specific set of local circumstances must be done cautiously, since under a few conditions echo screening was not cost-effective.
Conclusions
In a Markov model of idealized population-based RHD screening and secondary prophylaxis, 2-stage echocardiography is an attractive strategy compared to clinical screening. However, attention to local Sub-clinical RHD disease prevalence and transitions are warranted. Combined with population education about the precursors and signs of ARF and RHD, widespread availability and delivery of secondary prophylaxis, rising standards of living, and widely scalable echo screening across populations, the burden of RHD can be reduced. RHD echo screening appears to be a reasonable health investment and deserves closer attention from health policy stakeholders.
Acknowledgments
The authors acknowledge Dr Rosemary Wyber (Harvard School of Public Health) for her excellent assistance.
Declaration of funding: This work was supported by a National Heart, Lung, and Blood Institute Career Development Award (K23) HL111335.
Footnotes
Declaration of financial/other relationships
JPZ and MS have disclosed that they have no significant relationships with or financial interests in any commercial companies related to this study or article.
Contributor Information
Justin P. Zachariah, Department of Cardiology, Boston Children’s Hospital, Boston, MA, USA Department of Pediatrics, Harvard Medical School, Boston, MA, USA.
Mihail Samnaliev, Clinical Research Program, Boston Children’s Hospital, Boston, MA, USA.
References
- 1.Marijon E, Mirabel M, Celermajer DS, et al. Rheumatic heart disease. Lancet. 2012;379:953–64. doi: 10.1016/S0140-6736(11)61171-9. [DOI] [PubMed] [Google Scholar]
- 2.Carapetis JR, Steer AC, Mulholland EK, et al. The global burden of group a streptococcal diseases. Lancet Infect Dis. 2005;5:685–94. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- 3.Manji RA, Witt J, Tappia PS, et al. Cost-effectiveness analysis of rheumatic heart disease prevention strategies. Expert Rev Pharmacoecon Outcomes Res. 2013;13:715–24. doi: 10.1586/14737167.2013.852470. [DOI] [PubMed] [Google Scholar]
- 4.Nordet P, Lopez R, Duenas A, et al. Prevention and control of rheumatic fever and rheumatic heart disease: The cuban experience (1986–1996–2002) Cardiovasc J Afr. 2008;19:135–40. [PMC free article] [PubMed] [Google Scholar]
- 5.Bach JF, Chalons S, Forier E, et al. 10-year educational programme aimed at rheumatic fever in two french caribbean islands. Lancet. 1996;347:644–8. doi: 10.1016/s0140-6736(96)91202-7. [DOI] [PubMed] [Google Scholar]
- 6.Zhang W, Mondo C, Okello E, et al. Presenting features of newly diagnosed rheumatic heart disease patients in mulago hospital: a pilot study. Cardiovasc J Afr. 2013;24:28–33. doi: 10.5830/CVJA-2012-076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beaton A, Okello E, Lwabi P, et al. Echocardiography screening for rheumatic heart disease in ugandan schoolchildren. Circulation. 2012;125:3127–32. doi: 10.1161/CIRCULATIONAHA.112.092312. [DOI] [PubMed] [Google Scholar]
- 8.Marijon E, Ou P, Celermajer DS, et al. Prevalence of rheumatic heart disease detected by echocardiographic screening. N Engl J Med. 2007;357:470–6. doi: 10.1056/NEJMoa065085. [DOI] [PubMed] [Google Scholar]
- 9.Reeves BM, Kado J, Brook M. High prevalence of rheumatic heart disease in fiji detected by echocardiography screening. J Paediatr Child Health. 2011;47:473–8. doi: 10.1111/j.1440-1754.2010.01997.x. [DOI] [PubMed] [Google Scholar]
- 10.Carapetis JR. Rheumatic heart disease in developing countries. N Engl J Med. 2007;357:439–41. doi: 10.1056/NEJMp078039. [DOI] [PubMed] [Google Scholar]
- 11.Saxena A, Ramakrishnan S, Roy A, et al. Prevalence and outcome of sub-clinical rheumatic heart disease in india: the rheumatic (rheumatic heart echo utilisation and monitoring actuarial trends in indian children) study. Heart. 2011;97:2018–22. doi: 10.1136/heartjnl-2011-300792. [DOI] [PubMed] [Google Scholar]
- 12.Bhaya M, Beniwal R, Panwar S, et al. Two years of follow-up validates the echocardiographic criteria for the diagnosis and screening of rheumatic heart disease in asymptomatic populations. Echocardiography. 2011;28:929–33. doi: 10.1111/j.1540-8175.2011.01487.x. [DOI] [PubMed] [Google Scholar]
- 13.Webb RH, Wilson NJ, Lennon DR, et al. Optimising echocardiographic screening for rheumatic heart disease in new zealand: not all valve disease is rheumatic. Cardiol Young. 2011;21:436–43. doi: 10.1017/S1047951111000266. [DOI] [PubMed] [Google Scholar]
- 14.Remenyi B, Wilson N, Steer A, et al. World heart federation criteria for echocardiographic diagnosis of rheumatic heart disease–an evidence-based guideline. Nat Rev Cardiol. 2012;9:297–309. doi: 10.1038/nrcardio.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Webb R, Wilson NJ, Lennon D. Rheumatic heart disease detected by echocardiographic screening. N Engl J Med. 2007;357:2088. doi: 10.1056/NEJMc072555. author reply 2088–9. [DOI] [PubMed] [Google Scholar]
- 16.Northern Territory Government of Australia. Consumer affairs fuel price watch. Casuarina Northern Territory, Australia: 2013. http://www.Consumeraffairs.Nt.Gov.Au/pages/nt-fuel-watch.Aspx. Accessed 2013. [Google Scholar]
- 17.Australian Bureau of Statistics. 4710.0 – housing and infrastructure in aboriginal and torres strait islander communities. Canberra, Australia: 2007. http://www.Abs.Gov.Au/ausstats/abs@.Nsf/latestproducts/4710.0main%20features42006?Opendocument&tabname=summary&prodno=4710.0&issue=2006&num=&view=. Accessed 2013. [Google Scholar]
- 18.National Information and Referral Service. 6401.0 – consumer price index, Australia. Canberra, Australia: 2013. http://www.Abs.Gov.Au/ausstats/abs@.Nsf/mf/6401.0. Accessed 2013. [Google Scholar]
- 19.Miller G, Randolph S, Forkner E, Smith B, Galbreath AD. Long-term cost-effectiveness of disease management in systolic heart failure. Med Decis Making. 2009;29:325–33. doi: 10.1177/0272989X08327494. [DOI] [PubMed] [Google Scholar]
- 20.Shiroiwa T, Sung YK, Fukuda T, et al. International survey on willingness-to-pay (wtp) for one additional qaly gained: What is the threshold of cost effectiveness? Health Econ. 2010;19:422–37. doi: 10.1002/hec.1481. [DOI] [PubMed] [Google Scholar]
- 21.Roberts KV, Brown AD, Maguire GP, et al. Utility of auscultatory screening for detecting rheumatic heart disease in high-risk children in australia’s northern territory. Med J Aust. 2013;199:196–9. doi: 10.5694/mja13.10520. [DOI] [PubMed] [Google Scholar]
- 22.Irlam JH, Mayosi BM, Engel ME, et al. A cost-effective strategy for primary prevention of acute rheumatic fever and rheumatic heart disease in children with pharyngitis. S Afr Med J. 2013;103:894–5. doi: 10.7196/samj.7244. [DOI] [PubMed] [Google Scholar]
- 23.Soudarssanane MB, Karthigeyan M, Mahalakshmy T, et al. Rheumatic fever and rheumatic heart disease: Primary prevention is the cost effective option. Indian J Pediatr. 2007;74:567–70. doi: 10.1007/s12098-007-0094-y. [DOI] [PubMed] [Google Scholar]
- 24.Irlam J, Mayosi BM, Engel M, et al. Primary prevention of acute rheumatic fever and rheumatic heart disease with penicillin in south african children with pharyngitis: a cost-effectiveness analysis. Circ Cardiovasc Qual Outcomes. 2013;6:343–51. doi: 10.1161/CIRCOUTCOMES.111.000032. [DOI] [PubMed] [Google Scholar]
- 25.Grimaldi A, Ammirati E, Mirabel M, et al. Challenges of using ultrasounds for subclinical rheumatic heart disease screening. Int J Cardiol. 2013;167:3061. doi: 10.1016/j.ijcard.2012.11.083. [DOI] [PubMed] [Google Scholar]
- 26.Jones TD, Bland EF. Rheumatic fever and heart disease, completed 10-year observations on 1000 patients. Tr A Am Phys. 1942;57:8–12. [Google Scholar]
- 27.Grant RT. After histories for ten years of a thousand men suffering from heart disease. Heart. 1933;16:275. [Google Scholar]
- 28.Ash R. The first ten years of rheumatic infection in childhood. Am Heart J. 1948;36:89–97. doi: 10.1016/0002-8703(48)90548-1. [DOI] [PubMed] [Google Scholar]
- 29.Thomas GT. Five-year follow-up on patients with rheumatic fever treated by bed rest, steroids, or salicylate. Br Med J. 1961;1:1635–9. doi: 10.1136/bmj.1.5240.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tompkins DG, Boxerbaum B, Liebman J. Long-term prognosis of rheumatic fever patients receiving regular intramuscular benzathine penicillin. Circulation. 1972;45:543–51. doi: 10.1161/01.cir.45.3.543. [DOI] [PubMed] [Google Scholar]
- 31.Sanyal SK, Berry AM, Duggal S, et al. Sequelae of the initial attack of acute rheumatic fever in children from north india. A prospective 5-year follow-up study. Circulation. 1982;65:375–9. doi: 10.1161/01.cir.65.2.375. [DOI] [PubMed] [Google Scholar]
- 32.Rheumatic Fever Working Party of the Medical Research Council of Great Britain and the Subcommittee of Principal Investigators of the American Council on Rheumatic Fever and Congenital Heart Disease, American Heart Association. The natural history of rheumatic fever and rheumatic heart disease. Ten-year report of a cooperative clinical trial of acth, cortisone, and aspirin. Circulation. 1965;32:457–76. doi: 10.1161/01.cir.32.3.457. [DOI] [PubMed] [Google Scholar]
- 33.Feinstein AR, Wood HF, Spagnuolo M, et al. Rheumatic fever in children and adolescents. A long-term epidemiologic study of subsequent prophylaxis, streptococcal infections, and clinical sequelae. Vii. Cardiac changes and sequelae. Ann Intern Med. 1964;60(Suppl 5):87–123. [PubMed] [Google Scholar]
- 34.Majeed HA, Batnager S, Yousof AM, et al. Acute rheumatic fever and the evolution of rheumatic heart disease: a prospective 12 year follow-up report. J Clin Epidemiol. 1992;45:871–5. doi: 10.1016/0895-4356(92)90070-4. [DOI] [PubMed] [Google Scholar]
- 35.Bland EF, Duckett Jones T. Rheumatic fever and rheumatic heart disease; a twenty year report on 1000 patients followed since childhood. Circulation. 1951;4:836–43. doi: 10.1161/01.cir.4.6.836. [DOI] [PubMed] [Google Scholar]
- 36.Carapetis JR, Powers JR, Currie BJ, et al. Outcomes of cardiac valve replacement for rheumatic heart disease in aboriginal australians. Asia Pacific Heart J. 1999;8:138–47. [Google Scholar]
- 37.Grover A, Dhawan A, Iyengar SD, et al. Epidemiology of rheumatic fever and rheumatic heart disease in a rural community in northern india. Bull World Health Organ. 1993;71:59–66. [PMC free article] [PubMed] [Google Scholar]
- 38.International Rheumatic Fever Study Group. Allergic reactions to long-term benzathine penicillin prophylaxis for rheumatic fever. International rheumatic fever study group. Lancet. 1991;337:1308–10. [PubMed] [Google Scholar]
- 39.Carapetis JR, Currie BJ, Mathews JD. Cumulative incidence of rheumatic fever in an endemic region: A guide to the susceptibility of the population? Epidemiol Infect. 2000;124:239–44. doi: 10.1017/s0950268800003514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carapetis JR, Currie BJ. Mortality due to acute rheumatic fever and rheumatic heart disease in the northern territory: a preventable cause of death in aboriginal people. Aust N Z J Public Health. 1999;23:159–63. doi: 10.1111/j.1467-842x.1999.tb01227.x. [DOI] [PubMed] [Google Scholar]
- 41.Australian Bureau of Statistics; 3302 table 2: death rates, summary, states and territories-2002 to 2012. Canberra, Australia: 2010. http://www.Abs.Gov.Au/ausstats/abs@.Nsf/detailspage/3302.02012?Opendocument. Accessed 2013. [Google Scholar]
- 42.Northern Territory Government: 2013. Department of health. Nursing salary structure. Casuarina, NT: http://www.Nursing.Nt.Gov.Au/information/career-structure-salary. Accessed 2013. [Google Scholar]
- 43.Australian Institute of Ultrasound. Echocardiography fast track training. Broadbeach Waters, Australia: 2013. http://www.Aiu.Edu.Au/echocardiographyfasttrack.Php. Accessed 2013. [Google Scholar]
- 44.Northern Territory Public Sector. Medical officers ntps enterprise agreement 2011–2013. Casuarina Northern Territory, Australia: 2013. http://www.Ocpe.Nt.Gov.Au/__data/assets/pdf_file/0019/53380/mo-2010-2013-ea.Pdf. Accessed 2013. [Google Scholar]
- 45.Kam JK, Cooray SD, Smith JA, et al. A cost-analysis study of robotic versus conventional mitral valve repair. Heart Lung Circ. 2010;19:413–18. doi: 10.1016/j.hlc.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 46.Australian Bureau of Statistics. 5673.0.55.003 – regional wage and salary earner statistics, Australia. Canberra, Australia: http://www.Abs.Gov.Au/ausstats/abs@.Nsf/lookup/5673.0.55.003main+features12005-06?Opendocument#paralink2. Accessed 2013. [Google Scholar]
- 47.Australia GPR. General practice registrars australia: salary structure. Melbourne, Australia: 2013. http://www.Gpra.Org.Au/benchmarking-survey. Accessed 2012. [Google Scholar]
- 48.Australian Government Department of Health. Pencillin. Melbourne, Australia: 2012. http://www.Pbs.Gov.Au/medicine/item/1775k-3398w-3486l. Accessed 2013. [Google Scholar]
- 49.Australian Government Department of Health. Lignocaine. Canberra, Australia: 2013. http://www.Pbs.Gov.Au/medicine/item/2875h-3474w-5142p. Accessed 2013. [Google Scholar]
- 50.Australian Government Department Of Health. Enalapril 2013. Canberra, Australia: 2013. http://www.Pbs.Gov.Au/medicine/item/1369c. Accessed 2013. [Google Scholar]
- 51.Australian Government Department of Health. Frusemide. Canberra, Australia: 2013. http://www.Pbs.Gov.Au/medicine/item/2412y. Accessed 2013. [Google Scholar]
- 52.Australian Government Department of Health. Coumarin. Canberra, Australia: 2013. http://www.Pbs.Gov.Au/medicine/item/2211j. Accessed 2013. [Google Scholar]
- 53.Chambers S, Chadda S, Plumb JM. How much does international normalized ratio monitoring cost during oral anticoagulation with a vitamin k antagonist? A systematic review. Int J Lab Hematol. 2010;32:427–42. doi: 10.1111/j.1751-553X.2009.01205.x. [DOI] [PubMed] [Google Scholar]


