Abstract
Background
Although naming deficits are well documented in aphasia, on-line measures of naming processes have been little investigated. The use of on-line measures may offer further insight into the nature of aphasic naming deficits that would otherwise be difficult to interpret when using off-line measures.
Aims
The temporal activation of semantic and phonological processes was tracked in older normal control and aphasic individuals using a picture–word interference paradigm. The purpose of the study was to examine how word interference results can augment and/or corroborate standard language testing in the aphasic group, as well as to examine temporal patterns of activation in the aphasic group when compared to a normal control group.
Methods & Procedures
A total of 20 older normal individuals and 11 aphasic individuals participated. Detailed measures of each aphasic individual's language and naming skills were obtained. A visual picture–word interference paradigm was used in which the words bore either a semantic, phonological, or no relationship to 25 pictures. These competitor words were presented at stimulus onset asynchronies of −300 ms, +300 ms, and 0 ms.
Outcomes & Results
Analyses of naming RTs in both groups revealed significant early semantic interference effects, mid-semantic interference effects, and mid-phonological facilitation effects. A matched control-aphasic group comparison revealed no differences in the temporal activation of effects during the course of naming. Partial support for this RT pattern was found in the aphasic naming error pattern. The aphasic group also demonstrated greater SIEs and PFEs compared to the matched control group, which indicated disruptions of the phonological processing stage. Analyses of behavioural performances of the aphasic group corroborated this finding.
Conclusions
The aphasic naming RTs results were unexpected given the results from the priming literature, which has supported the idea of slowed or reduced patterns of activation in aphasic individuals. However, analyses of naming RTs also confirmed the behavioural finding of a disruption surrounding phonological processes; thus, the analyses of naming latencies offers another potential means of pinpointing breakdowns of lexical access in individuals with aphasia.
Keywords: Aphasia, Picture–word interference paradigm, Naming, Spoken word production
Of all the symptoms associated with aphasia, none is more pervasive than the inability to name (Goodglass & Wingfield, 1997; Kohn & Goodglass, 1985). The elucidation of the source of aphasic naming errors has been approached in different ways. Current spoken word production models emphasise the identification of processing levels necessary for lexical access and retrieval of single word production. Common to these models is the inclusion of at least two processing levels: a semantic processing level and phonological processing level(s). The semantic processing level governs operations involved in lexical selection of an intended concept (and perhaps its grammatical properties, known as lemmas), while phonological processing level(s) govern operations involved in its phonological specification (e.g., Caramazza, 1997; Dell, 1986; Dell & O'Seaghdha, 1992; Foygel & Dell, 2000; Levelt, 1992; Levelt, Roelofs, & Meyer, 1999; Peterson & Savoy, 1998; Roelofs, 1992, 1997; Starreveld & LaHeij, 1996).
Within this framework, aphasic naming errors have been localised to each of these processing levels. Semantic paraphasias, or substitutions in which the word is semantically related to the target word, can be a result of a breakdown in any of the component processes leading up to naming. Individuals whose semantic paraphasic errors arise as a result of a breakdown at the semantic system usually present with a comparable semantic comprehension impairment, a sensitivity to such variables as concreteness/imageability, a susceptibility to being miscued when provided with incorrect category coordinate cues (e.g., the picture, tiger, coupled with the phonemic cue, [l] for lion, results in the naming response, lion), a co-occurrence of errors across all modalities of input and output coupled with comparable error rates across tasks, and consistent item-specific errors across tasks (Butterworth, Howard, & McLoughlin 1984; Caramazza & Hillis, 1990, 1991; Gainotti, Miceli, Caltagirone, Silveri, & Masullo, 1981; Hillis & Caramazza, 1995; Hillis, Rapp, Romani, & Caramazza, 1990; Howard & Gatehouse, 2006; Howard & Orchard-Lisle, 1984; Martín, Serrano, & Iglesias, 1999; Miceli, Benvengnu, Capasso, & Caramazza, 1997; Nickels & Howard, 1994). There have also been case reports of individuals who produce semantic paraphasias, but whose errors are thought to have a post-semantic origin. Individuals who fit this performance profile demonstrate relatively intact semantic/comprehension skills in conjunction with semantic naming errors on tasks requiring oral or written production (Caramazza & Hillis, 1990, 1991; Franklin, Howard, & Patterson, 1995; Hillis & Caramazza, 1995; Kay & Ellis, 1987). In cases such as these it has been argued that semantic errors occurred because target phonological representation had become inaccessible, allowing the most highly activated, semantically related, phonological representation to be produced instead.
Phonological-based paraphasias, or word substitutions that are phonologically related to the target, are another commonly produced aphasic naming error. Individuals whose phonological-based naming errors are due to breakdowns at the lexical-phonological processing level typically demonstrate a range of auditory/reading comprehension abilities, naming deficits, better repetition than naming abilities, and fluent output characterised by the production of phonemic paraphasias and neologisms. Naming errors associated with impairments at this level tend to be more remotely related to the target or the errors contain phonological information related to previous words. Errors can also show lexicality and frequency effects (Best, 1996; Caramazza, Berndt, & Basili, 1983; Ellis, Miller, & Sin, 1983; Goldrick & Rapp, 2007; Kay & Ellis, 1987; Kohn & Smith, 1994; Martín et al., 1999; Miller & Ellis, 1987; Wilshire, 2002; Wilshire & McCarthy, 1996; Wilshire & Saffran, 2005). Individuals whose phonological naming errors are due to a breakdown of the post-lexical phonological processing level or phonological output buffer level also demonstrate a range of auditory/reading comprehension abilities, fluent but paraphasic output characterised by phonemic paraphasias and neologisms, as well as comparable production of nonwords and real words, and deficits across single word production tasks (oral reading, repetition, naming). Naming errors most strongly associated with this level tend to have a high proportion of the target word's phonemes or consist of minor distortions, and reflect word length effects (Bub, Black, & Howell, 1987; Caramazza, Miceli, & Villa, 1986; Goldrick & Rapp, 2007; Howard & Franklin, 1987, 1993; Nickels, 1995; Nickels, Howard, & Best, 1997; Shallice, Rumiati, & Zadini, 2000; Wilshire, 2002; Wilshire & McCarthy, 1996; Wilshire & Saffran, 2005).
THE VISUAL PICTURE–WORD INTERFERENCE PARADIGM
The wealth of empirical evidence discussed in the previous section has typically been obtained using traditional off-line, qualitative measures. However, these findings reflect more strategic, rather than automatic processes, which can make it difficult to tease apart how each of the various processing stage has contributed to the naming process. The use of an on-line measure, such as the picture–word interference paradigm (henceforth, PWIP) in individuals with aphasia may overcome some of these difficulties since the paradigm provides a direct, automatic, time-constrained measure of the processes that are activated during naming. The PWIP, an adaptation of the Stroop task (Stroop, 1935), involves the presentation of visual or auditory competitor words with the pictures to be named. Participants are required to name the picture while ignoring the competitor word that is typically superimposed on the picture. The type of competitor can be manipulated as well as the timing relation between presentation of the competitor and the target. Naming is slowed if the competitor is semantically (categorically) related to the picture (e.g., cat – RABBIT)1 than if the competitor word is unrelated to the picture (e.g., pencil – RABBIT). This effect is known as the semantic interference effect, or SIE (Lupker, 1979; Rosinski, 1977; Rosinski, Golinkoff, & Kukish, 1975; Smith & Magee, 1980; Underwood, 1976). Conversely, naming is facilitated if the competitor is phonologically/orthographically related (e.g., radish – RABBIT) relative to when there is no relationship between the competitor word and picture name (e.g., pencil – RABBIT). This effect is known as the phonological facilitation effect, or PFE (Lupker, 1982; Rayner & Posnansky, 1978; Rayner & Springer, 1986). The timing of competitor word presentation, relative to the presentation of the picture, or the stimulus onset asynchrony (SOA), can be manipulated, allowing the effects of the competitor word on naming to be tracked during lexical access and retrieval. The SIE can be elicited in the SOA range of −100 ms to +100 ms (i.e., the word is presented 100 ms before picture presentation to 100 ms after picture presentation) while the PFE emerges from SOA = −200 ms to +100 ms, (i.e., the word is presented 200 ms before picture presentation to 100 ms after picture presentation) (Damian & Martin, 1999; Glaser & Düngelhoff, 1984; La Heij, Dirkx, & Kramer, 1990; Lupker, 1979, 1982; Rayner & Springer, 1986; Rosinski, 1977; Starreveld & LaHeij, 1995, 1996; Underwood, 1976).
The interpretation of SIE effects remains controversial. It is assumed that lexical concept representations sharing similar semantic properties will be more closely connected to one another, while lexical concept representations sharing minimal or no semantic properties will have minimal or no connections (Roelofs, 1992). The presentation of a semantically related competitor word activates the target picture name's representation because of the semantic similarities that exist between the lexical concepts. During the course of naming, the competitor word's representation will receive more activation than the target picture name's representation because the visual presentation of the word provides additional activation of its corresponding semantic properties. The SIE arises as a result of the extra time needed to resolve the competition processes in favour to the targeted picture name. Empirical support for this competitive lexical selection view has come from numerous studies that have reported a SIE only when the tasks involved actual production of the target (Bloem, van den Boogaard, & La Heij, 2004; Damian & Bowers, 2003; Damian & Martin, 1999; La Heij, 1988; Levelt et al., 1999; Roelofs, 1992; Schriefers, Meyer, & Levelt, 1990; Starreveld & La Heij, 1995, 1996), rather than, for example, categorising the picture name or recalling if the picture had been presented (Damian & Bowers, 2003; Glaser & Glaser, 1989; Schriefers et al., 1990).
However there have been recent challenges to this view. Some researchers contend that the lexical competition hypothesis cannot satisfactorily explain certain word interference findings, most notably the appearance of semantic facilitation effects across a variety of paradigms when semantically related word–picture pairs are used (Finkbeiner & Caramazza, 2006; Janssen, Schrim, Mahon, & Caramazza, 2008; Mahon, Costa, Peterson, Vargas, & Caramazza, 2007). To account for these findings, Mahon, Caramazza, and colleagues have proposed the response exclusion hypothesis, which localises the SIE at the post-lexical processing stage. Since only one response can be produced at a given time, there can only be a single-channel output buffer. General semantic properties of the corresponding concepts are also available (e.g., semantic category) at the post-lexical processing level. The SIE arises when semantically related competitor words are available for production before the target picture name. Since only one response can be made, the inappropriate word reading response must be removed from the output buffer so the target picture can be named. The speed with which the output buffer is cleared is affected by the general conceptual properties indexed at the buffer level; the time to clear the production-ready representations of semantically related competitor words is longer than production-ready representations of unrelated competitor words, since the former are potential responses to the target picture.
The PFE is typically localised at the phonological encoding stage within two-stage word production models: When the picture's name and competitor word share the same initial segments, a PFE will occur because of an overlap in activation between the competitor and picture–word's segmental and syllable representations. The increased access to the syllable representation of the picture name results in a facilitation in the encoding of the picture name relative to an unrelated competitor word (Meyer & Schriefers, 1991; Roelofs, 1997). Alternatively, the PFE could be the result of interactive patterns of activation as proposed in interactive activation (IA) models. This model, developed in detail by Dell and his colleagues (Dell, 1986; Dell & O'Seaghdha, 1992; Dell, Schwartz, Martin, Saffran, & Gagnon, 1997; Foygel & Dell, 2000), describes an initially serial but eventual non-discrete process of lexical access in which lexical selection occurs as a result of interactive patterns of activation throughout the network via feedforward and feedback cycles. The presentation of a competitor word eventually activates the target picture's lexical representations via feedback cycles of activation from shared phonological representations. Facilitation effects occur because the competitor word's representations do not receive additional top-down activation as the picture name representation does; therefore, no competitive processes arise (Damian & Martin, 1999).
SUMMARY OF RELATED FINDINGS IN APHASIA
To date, very few studies have been published using the PWIP in individuals with aphasia. Wilshire and colleagues (Wilshire, Keall, Stuart, & O'Donnell, 2007) examined the language performance and naming reaction times on an auditory PWIP in one individual with aphasia, NP. Language performance on a variety of language tasks revealed moderate naming difficulties in which errors were semantic in nature. However, word comprehension abilities were relatively preserved, which suggested a specific deficit in lexical selection for word production. On the auditory PWIP, NP demonstrated a significant semantic facilitation effect at SOA=0 ms while the control group demonstrated a significant SIE at SOA=−200 ms. NP also demonstrated a significant PFE at SOA=0 ms while the control group failed to show any significant PFEs. The presence of semantic facilitation effects implicated the semantic processing stage, according to the differential locus hypothesis (Bloem et al., 2004), which suggests that semantic facilitation effects are localised at a semantic processing stage while SIEs are localised at a lexical level. The semantic facilitation effect at SOA of 0 ms was explained in terms of slowed activation of semantic processes: activation of picture name representations was still incomplete so that the presence of the competitor word facilitated, rather than hampered, naming the picture through activation of shared semantic feature representations. The significant PFEs at SOA=0 ms was thought to originate at the lexical stage rather than phonological encoding stage, because of the relatively early influence of phonological competitor words on lexical access. This finding was felt to be in keeping with IA models since these models allow patterns of activation to feedback from the phonological processing level to the lexical processing levels. Wilshire and colleagues argued that results from the picture–word interference task offered further insight into the nature of NP's naming impairment. Traditional language testing had revealed a specific deficit in lexical selection for word production. However, results from the picture–word interference task provided further evidence that NP's impairment extended to the earlier semantic processing stage, despite adequate performance on semantic comprehension tasks.
The findings from this study provide a tantalising glimpse into the potential uses of the PWIP task in further understanding the nature of lexical access and retrieval processes in individuals with aphasia. However, the broader implications of the task are difficult to predict since there are so few published word interference studies using individuals with aphasia. Other on-line paradigms, such as semantic priming paradigms, offer some insights as to how lexical access occurs in real time in individuals with aphasia.
Semantic priming studies typically report facilitation in lexical decision times for a target word (e.g., carrot) when a semantically related word (e.g., tomato) precedes it (see Neely, 1991, for a review). Shorter SOAs between prime word and target are considered to tap automatic processes, while longer SOAs tap more controlled, strategic processes (de Groot, 1984; Neely, 1991). Semantic priming results have varied when used with Broca's aphasic individuals (see del Toro, 2000, for a review of these studies). Prather and colleagues (Prather, Zurif, Love, & Brownell, 1997; Prather, Zurif, Stern, & Rosen, 1992) found that their Broca's aphasic participants demonstrated the expected semantic priming effects but only at longer SOAs. Thus, Prather and colleagues have argued that individuals with Broca's aphasia demonstrate intact but slowed automatic activation processes. Others have found that Broca's aphasic individuals, contrary to the normal controls, demonstrate inconsistent and abnormal patterns of facilitatory and inhibitory effects (Blumstein et al., 2000; Milberg, Blumstein, & Dworetzky, 1988; Milberg, Blumstein, Katz, Gershberg, & Brown, 1995), longer-lasting reduction of priming effects, or complete loss of priming effects (Utman, Blumstein, & Sullivan, 2001). On the basis of these results, these researchers postulate that individuals with Broca's aphasia have reduced activation of automatic lexical processes.
THE CURRENT STUDY
A PWIP was used to track the activation of semantic and phonological processing during the course of naming in older normal individuals, and individuals with aphasia. Visually presented competitor words, which bore a semantic, phonological, or unrelated relationship to the picture, were presented at SOAs=−300 ms, 0 ms, and +300 ms. These SOAs were chosen in an effort to capture (anticipated) delayed effects exhibited by the aphasic group. The SOA of 0 ms was chosen to replicate previous findings of the literature, which have consistently reported SIEs and PFEs at this SOA. Naming reaction times and naming errors were analysed in both groups.
The rationale for using aphasic individuals was threefold: First, lexical access and retrieval processes in individuals without brain damage is such an automatic, rapid, and typically flawless process, it is difficult to localise the effects that emerge. However, populations with known naming deficits, such as the aphasic population, demonstrate patterns of deficits that indicate breakdowns at certain processing levels. By examining the pattern of semantic and phonological activation during the course of naming in individuals whose deficits are localised to a particular processing stage, it is possible to infer, through the effects that emerge, which stages of naming are disrupted in this population. These findings, when examined in the context of the various hypotheses put forth to explain the PWIP effects, could help clarify what operations govern particular stages of naming. Second, findings from off-line tasks reflect more strategic, rather than automatic, processes that can make the obtained findings difficult to interpret. Recent findings from on-line measures, such as aphasic priming studies, have indicated either slowed or reduced activation of automatic lexical processes. Findings from one aphasic PWIP study (Wilshire et al., 2007) indicated slowed activation processes at the semantic processing stage, a finding that had not been discernible during standard language testing. Thus, latency-based analyses could corroborate and/or augment qualitative, off-line analyses. Finally, very little is known about the temporal course of naming in this population. Although the SOAs employed in the study do not span the entire range when naming processes become activated, the data may reveal patterns more in keeping with discrete two-stage (DTS) models, which propose strictly discrete activation of semantic and phonological processes (Levelt, 1992; Levelt et al., 1991, 1999), or with cascade models, which propose initial serial but eventual cascaded patterns of activation (Caramazza, 1997; Cutting & Ferreira, 1999; Jescheniak & Schriefers, 1998; Rapp & Goldrick, 2000), or with IA models, which propose an initially serial but eventual non-discrete process of word production in which selection of lexical nodes occur as a result of interactive patterns of activation throughout the network via feedforward and feedback cycles (Dell, 1986; Dell & O'Seaghdha, 1992; Dell et al., 1997; Foygel & Dell, 2000).
Predictions for the older normal control group are based on findings from the literature. Semantic competitor words should elicit SIEs while phonological competitor words should elicit PFEs. This group should also demonstrate the same pattern of effects over time as young normal individuals (Taylor & Burke, 2002). The patterns of activation in this group will neither confirm nor refute current models of word production since all models can account for the presence of a mid- PFE or mid-SIE, and it is unlikely that SIE and PFE will be large enough to be measured at the early or late SOAs.
Predictions for the aphasic group are harder to make because of the potential variability of the group. Nevertheless, three general predictions can be made. First, the emergence of greater-than-expected SIEs indicates heightened sensitivity to the presence of semantically related competitor words. Therefore, heightened SIEs in the aphasic group, relative to a matched control group, could indicate some type of impairment at the semantic level of processing (Bloem et al., 2004; Damian & Bowers, 2003; Damian & Martin, 1999; La Heij, 1988; Levelt et al., 1999; Roelofs, 1992; Schriefers et al., 1990; Starreveld & La Heij, 1996) or at the post-lexical phonological processing level (Finkbeiner & Caramazza, 2006; Janssen et al., 2008; Mahon et al., 2007). The presence of greater PFEs in the aphasic group, relative to a matched control group, could indicate heightened sensitivity to the word form of the competitor word. Therefore, the emergence of heightened PFEs could indicate a breakdown at the phonological encoding stage naming (Meyer & Schriefers, 1991; Roelofs, 1997). Second, the nature of the activation pattern may provide support for either the slowed or reduced activation hypotheses. If automatic activation of lexical processes is slowed in the aphasic group, effects may emerge but not unless there is sufficient time for activation levels to reach threshold (Prather et al., 1992, 1997). Therefore, a shift in the pattern of effects should be seen—SIEs would emerge at SOA of −300 ms while PFEs emerge at the late SOA of +300 ms. For the SOA of 0 ms, no SIE may be found if there is insufficient time for activation levels to reach threshold. To the extent that phonological competitor words boost activation levels, PFEs may be found at the SOA of 0 ms despite slowed activation processes. Alternately, other effects may emerge (e.g., semantic facilitation effects at SOA of 0 ms as reported for NP in Wilshire et al.'s 2007 study), depending on the nature of the impairment. If lexical access is characterised by reduced levels of activation, longer time intervals may be needed before activation levels build sufficiently to allow activation to spread (Blumstein et al., 2000; Janse, 2006; Milberg et al., 1995; Utman et al., 2001). Therefore, the presence of semantic competitor words may make it difficult for the system to differentiate target picture representations from the competitor word representations, while the presence of phonological competitors may prevent weakly activated representations to reach threshold for priming effects to emerge. Consequently, the targeted candidate may not be able to compete successfully for lexical selection, resulting in a lack of detectable SIEs across all SOAs (Utman et al., 2001). One exception may be that phonological competitors may boost activation levels sufficiently so that PFEs emerge at the mid-SOA of 0 ms. Finally, with regard to word production models, the DTS model's emphasis on the sequential nature of naming will be confirmed if activation of semantic processes is followed by activation of phonological processes. An overlap of effects at the mid-SOA would provide support for all word production models since all models can account for these findings. An overlap of effects at the late SOA would provide support for both cascade and IA models. Finally, support for IA models would be provided if either an overlap of effects at the early SOA occurs or early phonological effects and/or late semantic effects are obtained, all of which would indicate presence of feedback cycles.
When the matched control and aphasic groups are compared, the aphasic group is expected to produce slower naming RTs compared to the matched control group. The pattern of effects should also differ between the groups. The matched control group's pattern of effects should follow the pattern of effects exhibited by young normal individuals, while the aphasic group will demonstrate qualitatively different patterns of effects, depending on the nature of lexical access/retrieval impairments.
The PWIP naming errors produced by the aphasic group would be expected to reflect an interaction between the nature of lexical access/retrieval impairments of the group and the influences of the word interference task. At a minimum, a high rate of no responses and semantic naming errors are expected: The PWIP, unlike a straightforward naming task, is made more difficult with the introduction of stimuli during the course of naming. Therefore, a vulnerable naming system, as is the case in aphasic individuals, is likely to produce no responses (Lambon Ralph, Sage, & Roberts, 2000). A high rate of semantic naming errors might also be expected since naming is a semantically mediated process; semantic errors would be the expected outcome when stress (i.e., naming under time constraints in the presence of competitor words) is introduced to the naming task (Starreveld & LaHeij, 1999; Vitkovitch & Humphreys, 1991). Furthermore, the aim of the word interference task is to induce SIEs, through competition processes between semantically related items, and to produce PFEs, through priming processes between phonologically related items. Since competition processes likely elicit more errors than priming processes, more semantic than phonological naming errors should be produced. Finally, the pattern of naming errors should be consistent with the pattern of naming RT effects. Thus, if analyses of naming RT data reveal heightened SIEs, analyses of naming errors should reveal more errors in semantic conditions relative to the unrelated conditions. Likewise, if analyses of naming RT data reveal heightened PFEs, analyses of naming error patterns should reveal fewer errors in phonological conditions relative to the unrelated conditions.
METHOD
Healthy volunteers
A total of 20 older (M age = 58.85; SD = 12.97) healthy volunteers were recruited either from flyers or the Aging Research Registry maintained by Northwestern University's Buehler Center on Aging. Participants either volunteered or were paid $20.00 for their participation. All met the following inclusionary criteria: (a) completion of high school; (b) normal or corrected-to-normal vision; (c) right-handed dominance as indicated by the Edinburgh Scale (Oldfield, 1971); (d) use of English as primary language; and (e) no history of neurological- or psychiatric-based illnesses or disease, language or learning disabilities, or alcohol or substance abuse. A subset of the older normal participants, closely matched for years of education, age, and gender with each aphasic individual, was selected from the older normal participant group to comprise a direct comparison group (M = 60.5; SD = 10.5). Independent sample t-tests revealed no significant age, t(20) = 0.143, ns, or educational level t(20) = −0.77, ns, differences between the groups.
Aphasic participants
A total of 11 aphasic participants (M = 61.2; SD = 10.3) were recruited from the Northwestern University Aphasia & Neurolinguistics Research Laboratory subject pool, the Northwestern University Speech and Language Clinic, and Chicago area stroke clubs/groups. All met the same selection criteria imposed for the normal controls. Aphasia resulted from a single vascular lesion or haemorrhage in nine participants; two others had undergone surgery for a left aterio-venous malformation and a left frontal meningioma removal (see Table 1).
TABLE 1.
Summary of aphasic participant demographic characteristics
| Participant | Age (years) | Onset | Neuro logical findings |
|---|---|---|---|
| A1 | 62 | 4/10/94 | Head injury with left MCA CVA |
| A2 | 52 | 7/31/90 | Left MCA CVA/left fronto-parietal haemorrhage |
| A3 | 67 | 12/1/93 | Left MCA CVA |
| A4 | 50 | 3/20/80 | Left MCA CVA |
| A5 | 55 | 11/14/90 | Left MCA CVA |
| A6 | 78 | 1/16/95 | Left frontotemporal CVA; including left basal ganglia |
| A7 | 47 | 1/25/00 | Elective left AVM repair |
| A8 | 76 | 1/10/01 | Brainstem/left temporal CVA |
| A9 | 64 | 5/22/98 | Left frontal meningiomas |
| A10 | 68 | 8/19/96 | Left temporal subarachnoid haemorrhage |
| A11 | 55 | 10/97 | Basal ganglia/temporo-parietal haemorrhage |
MCA = middle cerebral artery; CVA = cerebral vascular accident.
Language testing using the Western Aphasia Battery Aphasia Quotient (WAB AQ; Kertesz, 1982) indicated mild to moderate impairments for all aphasic participants, with AQs ranging from 77.1 to 93.5 (see Table 2). Two of the aphasic participants (A8, A9) performed in the normal range of language functioning (WAB AQ cut-off score of 93.8); nevertheless, these same two individuals demonstrated naming deficits on the Boston Naming Test (BNT; Kaplan, Goodglass, & Weintraub, 2001) and were therefore included in the study. All participants demonstrated relatively preserved auditory comprehension skills. Verbal output was judged to be nonfluent to some degree in all participants; analyses of narrative discourse in four of the participants (A2, A3, A5, A11) in previous studies (Faroqi-Shah & Thompson, 2004; Kim & Thompson, 2003) had revealed production patterns consistent with agrammatic aphasia. The aphasic participants' ability to read and name all experimental stimuli was pre-tested. All participants accurately named all items by second presentation. Oral reading pre-test scores ranged from 90% to 100% accuracy.
TABLE 2.
Summary of aphasic group's performance on various language and psycholinguistic tests
| SUBTEST | Norms M; SD | A1 | A2 | A3 | A4 | A5 | A6 | A7 | A8 | A9 | A10 | A11 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WAB | ||||||||||||
| Aphasia Quotient | 77.1 | 85.8 | 75.6 | 89.8 | 78.6 | 89.5 | 82.7 | 93.5 | 93.4 | 89.2 | 75.8 | |
| PPTT | ||||||||||||
| Word Association Match (n = 52) | 47 | 48 | 48 | 48 | 52 | 51 | 47 | 48 | 50 | 48 | DNT | 47 |
| PALPA-Visual Lexical Decision | ||||||||||||
| Words (n = 60) | – | 60 | 59 | 58 | 56 | 60 | 59 | 59 | 59 | 59 | 59 | 58 |
| Nonwords (n = 60) | 59.8; 0.45 | 60 | 54 | 59 | 59 | 56 | 58 | 59 | 59 | 57 | 49 | 58 |
| PALPA Auditory Lexical Decision | ||||||||||||
| Words (n = 80) | – | 80 | 76 | 77 | 80 | 79 | 78 | 77 | 80 | 80 | DNT | 77 |
| Nonwords (n = 80) | 76; 4.27 | 80 | 79 | 73 | 80 | 76 | 68 | 73 | 78 | 80 | DNT | 79 |
| PALPA – Semantic Subtests | ||||||||||||
| Spoken Word Picture Match (n = 40) | 39.2; 1.07 | 40 | 39 | 40 | 40 | 40 | 39 | 39 | 39 | 39 | 39 | 39 |
| Written Word Picture Match (n = 40) | 39.4; 1.01 | 38 | 38 | 40 | 40 | 40 | 38 | 38 | 40 | 40 | DNT | 39 |
| Semantic Associates-HI (n = 15) | 13.4; 1.26 | 12 | 12 | 12 | 13 | 13 | 11 | 10 | 14 | 9 | 13 | 11 |
| Semantic Associates-LI (n = 15) | 12.2; 1.82 | 10 | 12 | 11 | 12 | 15 | 11 | 11 | 14 | 10 | 10 | 11 |
| Auditory Synonym-HI (n = 30) | – | 28 | 30 | 28 | 27 | 30 | 25 | 29 | 29 | 28 | 28 | 29 |
| Auditory Synonym-LI (n = 30) | – | 20 | 29 | 25 | 23 | 30 | 19 | 26 | 30 | 26 | 26 | 21 |
| Written Synonym–HI (n = 30) | 28.9; 0.85a | 30 | 29 | 29 | 29 | 29 | 30 | 26 | 30 | 30 | 28 | 29 |
| Written Synonym–LI (n = 30) | 27.8; 1.69a | 22 | 27 | 28 | 28 | 30 | 29 | 26 | 30 | 27 | 29 | 25 |
| PALPA – Phonological Subtests | ||||||||||||
| Auditory Word Repetition (n = 24) | – | 22 | 24 | 24 | 23 | 24 | 23 | 24 | 24 | 24 | DNT | 23 |
| Auditory Nonword Repetition (n = 30) | – | 21 | 30 | 28 | 26 | 25 | 28 | 20 | 30 | 27 | DNT | 19 |
| Auditory Rhyme Judgment | ||||||||||||
| Spelling Pattern (n =15) | – | 15 | 15 | 14 | 14 | 10 | 15 | 15 | 15 | 15 | DNT | 15 |
| Spelling Pattern Control (n = 15) | – | 8 | 14 | 14 | 14 | 9 | 14 | 14 | 14 | 14 | DNT | 13 |
| Phonological Rhyme (n =15) | – | 11 | 13 | 13 | 11 | 10 | 13 | 11 | 13 | 13 | DNT | 11 |
| Phonological Control (n = 15) | – | 10 | 14 | 14 | 14 | 9 | 14 | 14 | 14 | 14 | DNT | 14 |
| Written Rhyme Judgment | ||||||||||||
| Spelling Pattern (n = 15) | 14.6; 0.49 a | 14 | 14 | 13 | 14 | 12 | 13 | 12 | 15 | 14 | 15 | 14 |
| Spelling Pattern Control (n = 15) | 11.5; 2.9 a | 8 | 12 | 14 | 9 | 7 | 13 | 14 | 14 | 5 | 13 | 14 |
| Phonological Rhyme (n = 15) | 13.8; 1.78 a | 10 | 14 | 12 | 10 | 11 | 14 | 11 | 14 | 12 | 13 | 9 |
| Phonological Control (n = 15) | 13.1; 1.78 a | 8 | 15 | 15 | 15 | 10 | 14 | 14 | 15 | 15 | 14 | 14 |
| Homophone Decision | ||||||||||||
| Real Words (n = 20) | 18.5; 0.93 a | 12 | 18 | 15 | 16 | 20 | 15 | 20 | 18 | 15 | 15 | 17 |
| Exception (n = 20) | 18.5; 1.12 a | 8 | 19 | 17 | 16 | 19 | 11 | 19 | 20 | 16 | 15 | 17 |
| Nonwords (n = 20) | 17.9; 1.68 a | 8 | 16 | 20 | 14 | 11 | 10 | 16 | 18 | 13 | 15 | 14 |
WAB = Western Aphasia Battery (Kertesz, 1982); PPTT = Pyramids & Palm Trees Test (Howard & Patterson, 1992); PALPA = Psycholinguistic Assessments of Language Processing in Aphasia (Kay et al., 1992); DNT = did not test; LI = low imageability; HI = high imageability.
Norms taken from Nickels and Cole-Virtue (2004).
Results of the BNT revealed naming deficits for all aphasic participants as indicated by a score at least one standard deviation below reported test norms (Kaplan et al., 2001; Saxton et al., 2000; Welch, Doineau, Johnson, & King, 1996). BNT error responses were coded as either (a) semantic paraphasias: a word substitution that was semantically related to the picture name (e.g., bowl → cup); (b) phonological paraphasias: a word substitution that was phonologically related to the picture name (e.g., trunk → tank); or (c) no responses (NRs). Number of errors by type was as follows: semantic paraphasias (n = 36), phonological paraphasias (n = 5), and NRs (n = 95). Analysis of naming errors by type, using the Friedman's Ranks Tests showed significant differences between error types χ2 = 17.59, p<.001, with follow-up comparisons indicating significantly more NRs than either semantic or phonological errors, and significantly more semantic than phonological errors. All aphasic participants benefited from phonemic cues.
Further testing of linguistic functions was undertaken using the Pyramids & Palm Trees Tests – Three Picture Version (PPTT; Howard & Patterson, 1992) and the Psycholinguistic Assessment of Language Processing in Aphasia (PALPA; Kay, Lesser, & Coltheart, 1992). Table 2 provides a summary of test results.
Overall, the aphasic group's performance on various linguistic tests can be summarised as follows: First, WAB performance profiles revealed relatively preserved comprehension abilities and verbal output that was nonfluent to some degree in all participants and agrammatic in four of the participants. Second, nearly intact performance on the naming and oral reading pre-tests indicated that participants were able to name and read most of the experimental stimuli. Third, participants demonstrated spared lexical input processing of real words on the PALPA Auditory/Visual Lexical Decision subtest; some participants (A2, A5, A9, A10) demonstrated reduced visual input processing of nonwords. Fourth, BNT scores revealed naming deficits in all aphasic participants. Significantly more NRs were produced compared to either semantic or phonological errors, and significantly more semantic than phonological errors were produced. All aphasic participants benefited from phonemic cues. Fifth, performance on the PPTT and PALPA semantic subtests revealed relatively intact semantic abilities with the exception of performance on the PALPA Auditory Synonym subtest, where participants exhibited mild to moderate impairment primarily with low-imageability items. However, a comparison of high- and low-imageability items on the PALPA semantic subtests revealed isolated instances of an imageability effect; thus, this was not a trend found in the group as a whole. Finally, performance on the PALPA Auditory Nonword Repetition, PALPA Visual Rhyme and PALPA Homophone subtests revealed fairly consistent deficits at the phonological processing levels. These deficits were more evident on tasks that required manipulation of phonological information through reading.
Materials
A total of 25 nouns were selected for the experimental study, and corresponding pictures were selected from a commercial clip-art program. Each noun represented a different semantic category, although certain categories were represented more than once if members within the category could be grouped distinctly and separately (e.g., animals – wild/domesticated/sea). The number of living and nonliving things was roughly equal (i.e., 11 living object, 14 nonliving objects). Familiarity ratings were obtained from Snodgrass and Vanderwart (1980) or by the first author who used the same instructions and rating scale described by Snodgrass and Vanderwart (1980). A comparison of the average familiarity ratings for living and nonliving objects revealed no significant differences, t(23)=0.328, ns. The picture stimuli were normed on 10 English speaking volunteers.
The semantic (SEM) condition (n=75) was created by pairing each picture with three different semantically related competitor words. Native English-speaking volunteers (n>20) rated the degree of categorical relatedness using a 1.0 (very related) to 7.0 (very unrelated) point scale. Only pairs that received a score of ≤2.5 were kept. Another set of native English-speaking volunteers (n>20) rated stimulus pairs for associative relatedness using the same 7-point rating scale. Pairs with rating of ≥4.5 were considered to be weakly associated. Pairs that received both a strong semantic-categorical rating and a weak associative rating were selected as stimuli. No phonological or orthographic relationship existed between semantically related competitors and targets. (See Appendix A for semantic–categorical pairs and their average categorical and associative ratings). The phonological (PHO) condition (n=75) was created by pairing each picture with a word that shared the same initial consonant–vowel sound or consonant cluster–vowel sound, the same first two letters, the same stress pattern, and when possible, the same number of syllables as the target picture name. Appendix B lists the phonological pairs. The unrelated (UNR) condition (n=75) was created by using a word that had been selected for the semantic and phonological conditions and pairing it with a picture such that neither a semantic–categorical or phonologic–orthographic relationship existed between the competitor word and picture. Filler pairs (n=90), which were included to reduce the possibility of strategic processing, were created by choosing pictures and competitor words that were unrelated to any of the stimuli used in the experimental conditions.
Using the CELEX Lexical Database, which provides written frequency counts per million (Baayen, Piepenbrock, & Van Rijn, 1993), the semantic and phonological mean frequency ratings comparison was found to be non-significant, t(151)=0.449, ns. Mean syllable length of the semantic competitor words was 1.61 (SD=0.71), and mean syllable length of the phonological competitor words was 1.62 (SD=0.6).
Stimuli sets
Each picture was presented a total of 12 times, once per each SOA×competitor type condition. Altogether, each study participant named a total of 450 pictures. Each picture was paired with a different competitor word at each of the SOAs. The competitor word was one of three that had been previously judged as having a strong semantic–categorical or phonological relationship with the picture. Each picture–word pair was also rotated through the SOAs so that a particular picture–word pair was used with different SOAs. These steps were taken to reduce any strategic processing that might take place. Six different sets were created. Each participant was tested with one of the six sets.
Apparatus
The pictures were edited and stored as picture files using Adobe Photoshop Program. The visual competitor words were presented in bold Times Roman font at size 64 point. The experiment was conducted on SuperLab 1.74. Naming responses were recorded using Olympus Digital Voice Recorder, D-330. These recorded files were then transferred into SoundEdit 16 Version 2.0 for naming RT analyses.
Procedure
All participants were tested either in the Northwestern University Aphasia and Neurolinguistics Research Laboratory or in their home. Practice trials were provided to familiarise the participants with the task and reduce naming errors during the actual experiment. Stimuli pictures were randomly presented with a series of χs, one χ for every letter in the name, then presented with competitor words that were comparable to those used in the experimental condition. Incorrect responses were corrected. Following the practice trials, there were two 30-minute to 1-hour experimental sessions. For both groups, half of an experimental set, 225 pairs or five lists of 45 word–picture pairs, was presented during the first session. At least 1 week later, the remaining five lists were presented during a second session. The first author administered all training and experimental sessions.
For SOAs ±300 ms, each trial consisted of the following series of events: first, a fixation point was displayed in the centre of the screen; second, the first stimulus component, either the word or the target picture, appeared 500 ms later after the investigator pressed a key on the keyboard to initiate the trials; third, the second stimulus component, word or picture, appeared on the screen together with the first stimulus component after the appropriate SOA exposure; fourth, both picture and word remained on the screen for 500 ms; finally, there was an interval of 3000 ms during which time the participant was expected to name the picture. For SOA=0 ms, the series of events was the same as above, except that the picture and word appeared together. For all trials, the pictures were centred on the computer screen, and the competitor words appeared in the middle of the target picture at the appropriate time.
Reliability
Intrajudge agreement was obtained on all of the participants' accurate naming reaction time (RT) responses. All original data were re-analysed by the primary author for reliability purposes. RTs were judged to be in agreement if there was a ≤3-ms difference between the original and subsequent analyses. If more than a 3-ms difference existed between two obtained RTs, that trial was re-analysed. Items were re-analysed three times before all RTs were found to be in agreement.
Interjudge agreement was obtained by two judges who were trained by the primary author. A total of 962 responses were analysed. The judges' RTs were compared to the primary author's RTs. Items were judged to be in agreement if the judges' RTs were within 10 ms of the primary author's RTs for a particular item. For items that were not in agreement, the primary author and the judge examined how the RTs had been obtained for the discrepant items. Any procedural differences were then resolved. Judges were then asked to re-analyse the discrepant item for another (final) comparison. Using these criteria, inter-judge agreement was 79%. Inter-judge agreement was also obtained on naming errors. A judge was given the definitions of error types to use as a guide for scoring. Inter-judge agreement was 86% for word interference naming errors, and 87% for the Boston Naming Test errors.
RESULTS
Errors, invalid responses, and outliers were removed from the data set. An error was an incorrect name (e.g., car→bus), a self-corrected response (e.g., apple, no, banana), or oral reading errors produced by aphasic participants during the reading pre-test. An invalid response was a response made during a computer malfunction or when the participant was talking or laughing during a trial. For the normal participants, responses produced after 2000 ms were considered outliers. For the aphasic participants, outliers were determined by computing the grand mean plus two standard deviations above the mean of each aphasic individual's naming RTs and averaging them across all aphasic individuals. The average value was 2747 ms. Therefore, responses produced after 3000 ms were considered outliers for the aphasic participants. Removal of errors, invalid responses, and outliers resulted in the exclusion of 1.8% (112/6000) and 9.87% (326/3300) of the data from the older normal and aphasic group, respectively. Data for both groups were further trimmed if RTs were longer than the mean plus two standard deviations above the mean for a particular condition. In those cases, RTs were replaced with the mean plus the two standard deviations above the mean value for that particular condition.
Individual group naming RTs were submitted to a 3 (PHO, SEM, UNR) × 3 (−300 ms, 0 ms, +300 ms) repeated-measures ANOVA. Between-group naming RTs were analysed using a mixed model ANOVA, which included two within-participants variables, Competitor Type (SEM, PHO, UNR), and SOAs (−300 ms, 0 ms, and +300 ms), and one between-participants variable, Group. To correct for the skew in the distribution of scores, all values were log transformed to approximate a normal distribution. Results are reported using the log-transformed values. Analyses were performed across both participant data (denoted as F1 and t1 analyses) and across items (denoted as F2 and t2 analyses). All results reported as significant reached at least p ≤ .05.
Reaction time analyses
Older normal group
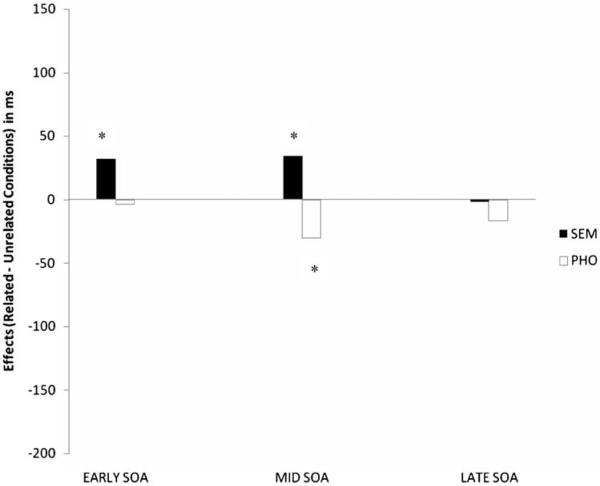
Table 3 lists the untransformed naming RTs by competitor type, relatedness, and SOA for the older normal group. Figure 1 provides the semantic and phonological effects across SOAs. As can be seen, the semantic competitors appeared to produce SIEs at both the early and mid-SOAs, while phonological competitors yielded PFEs at the late SOA. When log transformed RTs were analysed, significant main effects of Competitor Type F1(2, 38) = 37.72, η2 = .66; F2(2,48) = 26.46; η2 = .52, and SOA, F1(2, 38) = 81.48, η2 = .81; F2(2, 48) = 122.79, η2.83, were obtained. The SOA × Competitor Type interaction was also significant, F1(4, 76) = 6.05, η2 = .24; F2 (4, 96) = 4.44, η2 = .15. Follow-up pair-wise comparisons with Bonferroni corrections indicated a significant mid-PFE, t1(19) = −4.21; t2(24) = −3.53, a significant early SIE, t1(19) = 4.48; t2(24) = 3.04, and a significant mid-SIE, t1(19) = 3.77; t2(24) = 3.
TABLE 3.
Mean reaction times
|
−300 ms SOA
|
0 ms SOA
|
+300 ms SOA |
||||
|---|---|---|---|---|---|---|
| Condition | RT | SD | RT | SD | RT | SD |
| Older normal group | ||||||
| Semantic | 720 | 98 | 811 | 108 | 677 | 98 |
| Phonological | 684 | 97 | 746 | 105 | 663 | 85 |
| Unrelated | 688 | 100 | 776 | 105 | 679 | 94 |
|
| ||||||
| Aphasic group | ||||||
| Semantic | 1216 | 314 | 1281 | 228 | 1065 | 254 |
| Phonological | 1069 | 227 | 1028 | 230 | 1025 | 201 |
| Unrelated | 1115 | 288 | 1178 | 222 | 1084 | 217 |
Mean reaction times (RT) in milliseconds (ms), and standard deviation (SD) by stimulus onset asynchrony (SOA) in semantic, phonological, and unrelated conditions for both groups.
Figure 1.
Effects of competitor types in the older normal group. Competitor effects at each of the SOAs obtained by subtracting mean RTs in the semantic (SEM) and phonological (PHO) conditions from the mean RTs in the unrelated condition.
Aphasic group
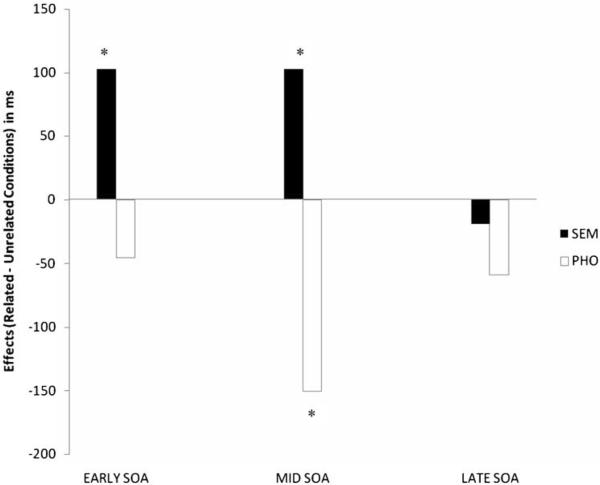
Table 3 lists the untransformed naming RTs by competitor type, relatedness, and SOA for the aphasic group. Figure 2 plots the semantic and phonological effects over SOAs. The effects, with one exception, were in the expected direction; semantic competitors appeared to yield SIEs while phonological competitors yielded PFEs. The exception was at the late semantic SOA where a facilitation effect was seen. Additionally, the largest effects appeared to be at the mid-SOA. Analyses of log-transformed RTs revealed significant main effects of Competitor Type F1(2, 20) = 17.01, η2 = .63; F2 (2, 48) = 13.65, η2 = .36, and SOA F1(2, 20) = 3.97, η2 = .28; F2(2, 48) = 13.01, η2 = .35. A significant Competitor Type × SOA interaction was also obtained, F1(4, 40) = 6.31, η2 = .38; F2 (4, 96) = 5.78, η2 = .19. Follow-up pair-wise comparisons with Bonferroni corrections indicated a significant mid-PFE, t1(10) = −5.13; t2(24) = −4.23, a significant early SIE, t1(10) = 3.96; t2(24) = 2.35, and a significant mid-SIE, t1(10) = 3.21; t2(24) = 2.70.
Figure 2.
Effects of competitor types in the aphasic group. Competitor effects at each of the SOAs obtained by subtracting mean RTs in the semantic (SEM) and phonological (PHO) conditions from the mean RTs in the unrelated condition.
Matched control–aphasia group comparison
A group comparison was performed between a subset of the matched control and aphasic groups in order to examine whether differences existed if factors such as education and age were controlled. Since an analysis of homogeneity of variance revealed significant variances (p < .05) in the two groups, the data were log transformed. The homogeneity variance test on log-transformed data revealed non-significant variances across all condition in the two groups. Therefore, log-transformed data were used for the group comparison.
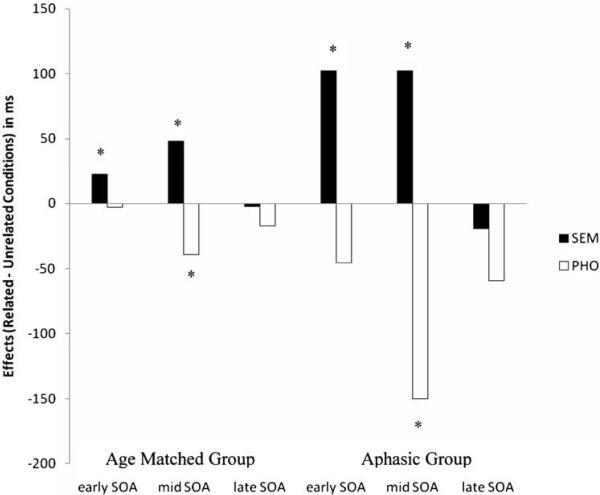
The naming RTs were longer for the aphasic group than for the matched control group F1(1, 20) = 38.65, η2 = .65; F2(1, 48) = 484.75, η2 = .91 (see Figure 3). A significant main effect of Competitor Type, F1(2, 40) = 29.8, η2 = .59; F2(2, 96) = 27.03, η2 = .36, and a significant main effect of SOA, F1(2, 40) = 26.15, η2 = .56; F2(2, 96) = 64.54, η2 = .57, were obtained. There were also significant two-way interaction effects: a SOA × Group effect, F1(2, 40) = 3.27, η2 = .14; F2 (2, 96) = 5.44, η2 = .10, a Competitor Type × SOA effect, F1(2, 40) = 4.68, η2 = .35, F2(4, 192) = 9.06, η2 = .15, and a Competitor Type × Group effect, F1(2, 40) = 4.66, η2 = .18; F2(2, 96) = 3.22, η2 = .06. The overall three-way interaction, Competitor Type × SOA × Group was non-significant, indicating no overall group differences in how the competitor types varied across SOAs.
Figure 3.
Effects of competitor types across matched control and aphasic group. Competitor effects at each of the SOAs obtained by subtracting mean RTs in the semantic (SEM) and phonological (PHO) conditions from the mean RTs in the unrelated condition.
The interaction effect, SOA × Group, indicated group differences in how the timing of competitors affected naming RTs. Overall, the matched control group had significantly shorter naming RTs across SOAs compared to the aphasic group. The matched control group demonstrated significantly longer naming RTs in the mid-SOA relative to the other SOAs, while the aphasic group demonstrated significantly longer naming RTs in the mid-SOA relative to the late SOAs. The Competitor Type × Group effect indicated group differences in how competitor types affected naming RTs. Both types of competitors elicited longer naming RTs in the aphasic group compared to the matched control group. Follow-up comparisons of the different competitor types for the matched control group revealed non-significant differences between semantic and unrelated conditions as well as non-significant differences between phonological and unrelated conditions. In contrast, the same comparisons in the aphasic group revealed significant differences between semantic and unrelated conditions as well as significant differences between phonological and unrelated conditions.
It should be noted that the naming RTs were examined on an individual basis; however, only two individuals performed differently from the others on more than one of the significant SOAs. For the semantic conditions, A4 and A10 demonstrated an early semantic facilitation effect while A4 demonstrated a mid-semantic facilitation effect. Their behavioural performances did not stand out from the others. When individuals' performances over all SOAs were examined, a number of individuals demonstrated late phonological interference effects and late semantic facilitation effects. These effects were either not very large (in the case of phonological competitors) or variable (in the case of semantic competitors) which would explain why these effects weren't significant.
Naming error analyses
Naming errors produced were placed into one of the following categories: (a) semantic paraphasias; (b) phonological paraphasias; (c) unrelated errors; (d) NRs; (e) competitor errors: a substitution in which the word was the competitor word; (f) neologisms: nonwords that were either phonologically related to the picture name (e.g., /kornIn/ → crayon) or unrelated to the picture name (e.g., /sor/ → toaster); and (g) other: responses that were descriptive in nature (e.g., playing piano, that sea animal again). The older normal participants produced 61 naming errors, which comprised 1% of the data. They produced the following errors: NR (n = 19), semantic paraphasias (n = 18), competitor errors (n = 12), unrelated errors (n = 10), phonological paraphasias (n = 1), and other (n = 1). The Friedman Ranks Tests revealed a significant difference in the distribution of error types, χ2 = 23.73, p = .001. A series of follow-up pairwise comparisons revealed significantly more semantic paraphasias, NRs, and competitor errors relative to phonological paraphasias. All other analyses were non-significant.
The aphasic group produced a total of 319 errors, which constituted 9.66% (319/3300) of the trials: NR (n = 162), semantic paraphasias (n = 61), competitor errors – semantic condition (n = 47), competitor errors – phonological conditions (n = 5), unrelated errors (n = 24), phonological paraphasias (n = 3), neologisms (n = 16), and other (n = 1). Analysis of the errors was performed on all error types (collapsing competitor errors) using the Friedman Ranks Tests, which revealed a significant difference in the distribution of error types, χ2 = 40.98, p < .001. In order to determine where the differences existed, follow-up pairwise comparisons were performed. Only the rates of semantic errors, phonological errors, competitor errors, NRs, and unrelated errors were considered for the follow-up comparisons. The analysis revealed significantly more NRs than all other error types with the exception of semantic paraphasias; no significant difference was found between the rates of NRs and semantic paraphasias. There were also significantly more semantic, unrelated, and competitor errors relative to phonological errors. These findings were similar to error types generated by the BNT, which had revealed significantly more NRs than any other type of errors, and significantly more semantic than phonological errors.
The errors by conditions were submitted to the Friedman Ranks Test, which revealed significant differences, χ2 = 9.94, p < .05. Using Wilcoxon Signed Ranks Tests, the follow-up pairwise comparisons revealed significantly more errors in the semantic condition relative to the semantically unrelated condition (p < .05) and significantly more errors in the semantic condition relative to the phonological condition (p < .01). All other analyses were non-significant.
DISCUSSION
The aim of the study was to track the pattern of semantic and phonologic activation over the course of naming in older normal and aphasic individuals. A PWIP was employed in which visually presented competitor words that were semantically related, phonologically related, or unrelated to the target pictures were presented at SOAs of −300 ms, 0 ms, and +300 ms. The influence of these different types of relationships was determined by analysing the groups' naming RTs and naming errors.
The PWIP elicited a significant early SIE, a significant mid-SIE, as well a significant mid-PFE in the older normal control group. The finding of significant effects at the mid-SOA was in accordance with results obtained in previous word interference studies, which have reported a maximum effect close to the mid-SOA of 0 ms, for both young and older participants (Bloem et al., 2004; Damian & Bowers, 2003; Damian & Martin, 1999; Glaser & Dungelhoff, 1984; LaHeij et al., 1990; Schriefers et al., 1990; Starreveld & La Heij, 1995, 1996; Taylor & Burke, 2002). The analyses of naming error types in the older normal group revealed significantly more semantic paraphasias, NRs, and competitor errors relative to phonological paraphasias. High rates of NRs and semantic paraphasias appeared to reflect the fact that the task was not only challenging for this group (Starreveld & LaHeij, 1999) but that the paradigm was perhaps too effective. Since naming is a semantically mediated process, any stress placed on the naming system will result in higher rates of no responses and semantic paraphasias than any other naming error types. The stressor in this particular case was the pressure to name in the face of competitor words under timed conditions. Moreover, the high rates of semantic paraphasias and competitor errors found in semantic conditions were likely due to the nature of the task: Competitor words that elicited competitive processes produced more errors than competitor words that elicited priming processes; therefore, more semantic and semantic-competitor errors were produced than phonological naming errors. Thus, the naming error findings in the older normal group seemed to reflect the fact that these participants found the task more challenging than the younger normal participants reported in the literature (who generally demonstrate negligible rates of errors).
In the case of the aphasic group, the PWIP also elicited a significant early SIE, a significant mid-SIE, as well a significant mid-PFE. Thus, the aphasic group's pattern of activation during naming was the same as that found in the normal control group. The matched control–aphasic group comparison revealed differences in latency of naming response: The aphasic group produced significantly slower RTs compared to the matched control group. When the timing of competitors was examined, the aphasic group had significantly longer naming RTs across SOAs compared to the matched control group. The aphasic group demonstrated significantly longer naming RTs in the mid-SOA relative to the late SOAs while the matched control group demonstrated significantly longer naming RTs in the mid-SOA relative to the other SOAs. Thus, both groups demonstrated significantly longer naming RTs when picture and word were simultaneously presented. When the effects of competitor types on naming RTs were examined, both types of competitors produced longer naming RTs in the aphasic group compared to the matched control group. Naming RTs were in the expected direction for both groups: semantically related competitors increased naming RTs when compared to unrelated conditions, while phonologically related competitors decreased naming RTs when compared to unrelated competitors. However, while the matched control group demonstrated non-significant differences between both semantic and phonological conditions compared to the unrelated condition, the aphasic group demonstrated significant differences between both semantic and phonological conditions relative to the unrelated condition. This difference indicated a heightened sensitivity in the aphasic group to competitors over the course of naming. However, no overall significant effect was found in the group comparison, indicating no group differences in the effects of competitor types across SOAs. Thus, the findings indicated that the temporal activation of semantic and phonological processes during naming did not differ across these two groups when individuals were matched on demographic variables of age and educational level. These results were unexpected given the abnormal word interference findings exhibited by NP, the aphasic participant in the PWIP study (Wilshire et al., 2007) as well as the evidence from the aphasic priming literature, where results have indicated either slowed (Prather et al., 1992, 1997) or reduced automatic activation (Blumstein et al., 2000; Milberg et al., 1995; Utman et al., 2001) of lexical access. The implications of these findings are discussed more fully in the next section.
Analyses of the aphasic group's error types on the word interference task revealed significantly more NRs than any other error types, and significantly more semantic paraphasias, unrelated, and competitor errors relative to phonological paraphasias. The high rates of NRs indicated that the task was difficult for this group (Lambon Ralph et al., 2000; Starreveld & LaHeij, 1999). The nature of the task also appeared to play a role in the types of errors that emerged: The high rates of semantic paraphasias not only reflected the fact that the aphasic group's (semantically mediated) naming system was being stressed by the task, just as had been the case with the older normal group, but that these errors as well as semantic competitor errors occurred because of competition processes between semantically related items. Since competition processes are more likely to elicit errors than priming processes (as was the case when phonological competitors appeared with the picture), more semantic than phonological naming errors were produced. When naming errors were examined by condition, the aphasic group produced significantly more errors in the semantically related condition relative to the unrelated conditions. However, no differences were found between phonologically related and unrelated conditions. Thus, only partial support was found for the prediction that the naming error patterns would support naming RTs patterns. It should be noted that Starreveld and LaHeij (1999), when examining naming error rates in a speeded naming variant of their original word interference study (Starreveld & LaHeij, 1996), also found only partial replication of the word interference study: Although SIEs were obtained, (i.e., significantly more errors were produced in the semantic condition compared to the unrelated condition), no significant PFE was obtained. It may be that the word interference task is better suited to tracking naming RT data than naming error performance, and may therefore be a less sensitive measure of naming error performance (Starreveld & LaHeij, 1999).
The aphasic group was also tested on a variety of language measures. The BNT, which was used to measure naming abilities, revealed significantly more NRs than any other error types, followed by semantic errors, and finally, phonological errors. The high rates of NRs and semantic paraphasias typically indicate an under-specification or degradation of semantic representations. However, the group's relatively intact semantic/comprehension abilities make it unlikely that the BNT naming errors stemmed from a breakdown of the semantic system. Additional support comes from the fact that all participants benefited from phonemic cues. Typically, phonemic cues produce positive effects for individuals who have impaired access to the phonological system. Presumably, the cue boosts activation of the targeted lexical item over and beyond other semantically related competitors so that accurate naming occurs (Hillis & Caramazza, 1995; Howard & Gatehouse, 2006; Wilshire & Saffran, 2005). The aphasic individuals in this study bear a strong resemblance to other aphasic individuals reported in the literature who have benefited from phonemic cueing; case studies report that these individuals demonstrate, among other things, relatively preserved semantic/comprehension abilities and a predominant production of semantic errors or circumlocutions. Thus, it seems unlikely that the locus of breakdown occurred at the semantic processing level. Instead, it is more likely that the errors were a result of an inability to access subsequent stages of naming.
Other measures of language functioning were obtained using the PPTT and PALPA subtests. While performance on PALPA semantic subtests revealed relatively preserved semantic abilities, performance on the PALPA phonological subtests revealed deficits at the phonological processing level(s). More specifically, a range of mild to moderate deficits were demonstrated when the auditory nonword repetition, auditory/visual rhyme, and homophone decision abilities were examined; furthermore, there was a predominant pattern in which the aphasic participants demonstrated better auditory rhyme judgements and auditory nonword repetition skills than visual rhyme judgements and homophone decisions. Within the context of Monsell's (1987) auditory-verbal short-term memory model, performance on these different phonological tasks are a function of different input and output buffers. The input phonological buffer, which is a pre-lexical phonological store, codes item order. Therefore, processes such as auditory rhyme judgements, which involve the segmentation of the phonological string, are thought to be performed at the input buffer. The output buffer, or post-lexical phonological store, retains information in phonological form. Visual input information also initially accesses the output buffer before accessing the input buffer for further processing. Therefore, homophone decisions, which require a comparison of whole phonological strings, are thought to be performed at the output buffer, and visual information, such as written rhyme judgements, access the output store before being processed further at the phonological input store. The input-to-output link is involved in nonword repetition, while the output-to-input link is needed in order to transfer visual information to the input buffer for further processing.
When the patterns of performance across the three tasks of auditory rhyme judgement, visual rhyme judgement, and homophone decision were examined in the aphasic group, most of the aphasic participants demonstrated a profile consistent with a breakdown at the phonological output buffer (Howard & Franklin, 1987, 1990, 1993; Howard & Nickels, 2005; Nickels et al., 1997). However, it should be noted that the aphasic group's overall performance profiles was not completely consistent with a breakdown of the phonological output buffer. Other neuropsychological evidence that supports the existence of such a buffer comes from individuals who demonstrate relatively preserved comprehension abilities as well as fluent verbal output characterised by phonological paraphasias and neologisms. These individuals also produce phonologically related errors on tasks of repeating, reading aloud, and writing nonwords to dictation, and the relative distribution of substitution, addition, deletion, and transposition errors is remarkably similar across each task (Bub et al., 1987; Caramazza et al., 1986; Shallice et al., 2000). Although the aphasic participants of this study demonstrated relatively preserved comprehension abilities, verbal output was judged to be nonfluent to some degree, and semantic naming errors and NRs predominated when naming pictured objects. Thus, the suggestion of a breakdown of the phonological output buffer in this group of aphasic individuals is not completely supported by the group's behavioural performance profiles.2
Nevertheless, the group's performance provides sufficient evidence to argue that, at a minimum, there was a breakdown surrounding the phonological system: When taking into account the types of naming errors produced on the BNT and performance on PALPA phonological subtests, it appeared that weakened connections or reduced activation levels resulted in an inability to access the phonological system. Although the evidence was not as compelling, the aphasic group's difficulties with visual rhyme judgements and homophone decisions also implicated the phonological output buffer, which is thought to play a role in holding phonological representations in store while the representations are being manipulated by different conversion processes.
Aphasic group's behavioural performance and PWIP performance
It was argued earlier that on-line measures obtained from the word interference task could further augment and/or corroborate findings obtained from behavioural testing. One measure, the strength of the types of word interference effects that emerged during the course of naming, might further indicate which stages of naming are disrupted in a group of aphasic individuals whose deficits, through behavioural testing, have been localised to a particular processing level. When competitor types were examined between groups, the aphasic group demonstrated heightened SIEs and PFEs. It had been predicted that greater SIEs in the aphasic group, compared to a matched control group, could indicate some type of impairment either at the semantic processing level or at the post-lexical phonological processing level, while the presence of greater PFEs in the aphasic group, relative to a matched control group, could indicate a breakdown at the phonological encoding stage of naming. The behavioural performance of the aphasic group, which seemed to suggest either difficulty in accessing phonological processes or a disruption in phonological processes, converges nicely with on-line findings if one considers heightened SIEs to be indicative of a post-lexical phonological processing level (Finkbeiner & Caramazza, 2006; Janssen et al., 2008; Mahon et al., 2007), while heightened PFEs would indicate a breakdown at the phonological encoding level.
Another reason for using the PWIP in individuals with aphasia was to examine the nature of temporal activation during naming just as priming paradigms have been used successfully to discern the nature of activation processes during lexical access. Predictions were made based on the priming literature, which suggests that individuals with Broca's or nonfluent aphasia demonstrate patterns indicative of slowed activation (Prather et al., 1992, 1997) or reduced activation of lexical access processes (Blumstein et al., 2000; Janse, 2006; Milberg et al., 1995; Utman et al., 2001). Under the slowed activation hypothesis, it had been expected that SIEs and PFEs might emerge if there was a sufficient time lag between the word and picture presentation. At a minimum, a distinctive pattern of early SIEs and late PFEs was expected. Effects at the mid-SOA may have also emerged depending on the strength of activation. Under the reduced activation hypothesis, it had been predicted that weakened activation levels would result in a lack of SIEs across all SOAs, and a lack of PFEs at the early and late SOAs. However, the presence of phonological competitors at the mid-SOA may have boosted activation levels sufficiently so that PFEs may have emerged at the mid-SOA of 0 ms. Surprisingly, neither of the hypotheses received support from the present study. Although the aphasic group did demonstrate early SIEs as predicted by the slowed activation hypothesis, this pattern of effects was also demonstrated by the normal control group. These findings indicated that the temporal patterns of activation during naming did not differ as a function of mild language deficits (at least of the kind described for the participants in this study). This is not to say that the aphasic group's naming processes was not abnormal; the fact that the aphasic group demonstrated significantly longer naming latencies in all RT analyses highlights the fact that there were indeed problems of access and/or activation during naming. However, it was the temporal pattern of activation that did not differ in the aphasic group when compared to a matched control group. It should be noted that one of the main reasons for the lack of support for either of the semantic priming hypotheses may be due to the fact that priming tasks differ crucially from word interference tasks: priming tasks tap input, lexical access processes while word interference paradigms tap both input (word recognition) and output (word production) processes.
The overall results of this study differed from those obtained by Wilshire and colleagues (2007). NP, their aphasic participant, demonstrated a behavioural performance (i.e., relatively preserved single word comprehension skills, reduced naming with prominent semantic errors), which was felt to indicate an impairment at the lexical selection stage. His PWIP performance, however, indicated an abnormally prolonged semantic processing stage, as indicated by the significant semantic facilitation effects found at SOA of 0 ms, which contrasted with the normal control group's significant SIE at SOA = −200 ms. The other significant finding, PFE at SOA = 0 ms, was found for NP but not for the control group. Thus, there were not only different temporal patterns of activation but different effects that emerged in NP relative to the control group. Although the aphasic group in this current study demonstrated similar behavioural performance profiles as NP, the group nevertheless demonstrated similar temporal patterns and similar effects during naming as the matched control group. These differences may stem from the fact that Wilshire and colleagues (2007) used an auditory PWIP, which could account for temporal differences in the emergence of effects (Damian & Martin, 1999). More importantly, individual differences were not examined in this study; an investigation of individual performances may have yielded similar results as NP, particularly since most of the group in this study exhibited similar behavioural performance as NP.
Early SIE effects
One of the unexpected findings of the study was the appearance of the SIE, which was found in all RT analyses performed. Word interference studies that examine SIEs across a range of SOAs do not report an interference effect beyond an SOA of −100 ms when visual competitors are used (Alario, Segui, & Ferrand., 2000; Damian & Martin, 1999; Glaser & Düngelhoff, 1984; La Heij et al., 1990; Starreveld & La Heij, 1996). In competitive lexical selection accounts, the lack of interference effects at large negative SOAs is presumably due to the fact that competitor word processing has been completed by the time the picture is presented. One of the factors that might explain the appearance of a SIE is the type of semantic relationship that exists between competitor word and picture. Studies that have examined the effects of different types of semantic relationships on word interference results have found that a categorical relationship must exist between competitor word and picture in order for a SIE to appear (Cutting & Ferreira, 1999; La Heij et al., 1990; Lupker, 1979). On the basis of these results, the stimuli pairs that were chosen for the study were ones that had a strong semantic–categorical, rather than a semantic–associative, relationship. All stimuli pairs were judged twice by separate judges, once to determine the degree of categorical relatedness, and again to determine the degree of associative relatedness. Only pairs that demonstrated a strong categorical – weak associative relationship were used in the study in order to ensure that a SIE would be elicited. To date, there is only one known study (La Heij et al., 1990) that has examined the effects of semantic–categorical and semantic–associative relationships across SOAs. The use of an SOA of −400 ms in their study did not reveal any semantic–categorical interference effects. However, it may have been the case that an SIE may have been present at an SOA of −300 ms (if effects had been measured at that SOA), but had become too small to be measured at an SOA of −400 ms. An alternate explanation is that the competitor words may have had a distant semantic, rather than a close semantic, relationship with the picture. Mahon and colleagues (2007) found consistently smaller SIEs when pairs enjoyed a close within-category semantic relationship (e.g., zebra – HORSE) as opposed to a distant within-category relationship (e.g., whale – HORSE). Accordingly, it may be the case that the pairs used in this study, although not associative in nature, may have been pairs that were semantically distant. In any case, the early SIE findings remain open to interpretation, and the question remains whether the effects obtained in this study can be attributed to the study design, stimuli, or instead reflects an actual representation of activation patterns that occur during naming.
Implications for current word production models
Although the SOA range used in the current study did not span the entire time when naming processes become activated, the results could at least confirm the validity of existing word production models by examining the degree to which these models accommodate the results found with aphasic individuals. In fact, some have argued that theoretical models cannot be considered a comprehensive account of speech production unless they can accommodate the patterns of performance seen in individuals with impaired language systems (Caramazza, 1997; Nickels, 2000; Semenza, Luzzatti, & Mondini, 1999; Wilshire & McCarthy, 1996). According to DTS models (Levelt, 1992; Levelt et al., 1999), naming is primarily a serial process in which feedforward patterns of activation result in the flow of information in one direction only. Cascade models (Rapp & Goldrick, 2000) describe an initially serial but eventual cascaded process of word production in which the selection of the targeted word occurs as a result of feedforward but cascaded cycles. Finally, IA models (Dell, 1986; Dell et al., 1997) describe an initially serial but eventual non-discrete process of word production in which lexical selection occurs as a result of interactive patterns of activation. Although the findings did not provide differential support for one model over another, the findings derived from both groups did confirm the sequential nature of naming. Thus, the primary activation pattern found in the three word production models received further validation from the performance of a group of older, neurologically intact individuals as well as a group of aphasic individuals.
CONCLUSIONS AND FUTURE DIRECTIONS
Of particular interest in the current study was to examine the temporal sequence of semantic and phonological activation during the course of naming in aphasic individuals. The fact that a group of aphasic individuals could complete the task, and that these individuals were sensitive to the manipulations inherent in the paradigm bodes well for its future use in this population. This is an important factor to consider since analyses of naming latencies offers a means of uncovering processes and/or pinpointing the locus of impairments that would otherwise be unattainable when using standard, off-line measures. When behavioural performance profiles were compared to PWIP findings, the data converged to indicate disruptions around the phonological system. Thus, in the case of the aphasic group in this study, the PWIP findings corroborated findings obtained from standard testing. When naming RTs were analysed, the aphasic group performed similarly as the matched control group in the types of effects that were elicited as well as the pattern of effects that were found over the course of naming. Specifically, the temporal patterns of semantic and phonological activation in these individuals when naming pictures were similar in both groups. This finding was unexpected given the results from the priming literature, which has increasingly supported the idea that aphasic individuals demonstrate slowed or reduced patterns of activation (Blumstein et al., 2000; Janse, 2006; Milberg et al., 1995; Prather et al., 1992, 1997; Utman et al., 2001); however, given the fact that the paradigms differ crucially in what processes are being tapped, the discrepant findings may not, upon closer examination, be so surprising. The naming error data provided another means to examine lexical access and retrieval processes in the aphasic participants. The types of naming errors produced on the word interference task included high rates of NRs, semantic paraphasias, and semantic-competitor errors. These errors, which were similar to the types produced by the older normal group, were thought to be a direct result of naming under stressful conditions as well as the use of competitor words that elicited competitive processes during naming. When the naming error patterns were analysed by condition, SIEs but no PFEs were found, thus providing only partial replication of naming RT patterns.
Since a primary aim of the study was to determine if the PWIP could be used meaningfully in aphasic individuals, an examination of group patterns was necessary. Consequently, individual patterns were masked. This is not a trivial matter where individual variability commonly exists in aphasic individuals. Thus, the PWIP might prove most fruitful in individual case studies where efforts to localise naming impairments can be accomplished by using both standard, behavioural measures and PWIP measures (e.g., Wilshire et al., 2007). Another avenue to pursue in future studies would be to increase the range of SOAs used in the PWIP paradigm. Subsequent studies should consider additional SOAs to obtain a fuller picture of the when processes become activated during naming in aphasic individuals. Finally, the fact that interpretable PWIP results could be obtained with aphasic individuals is promising in terms of its potential clinical applications. A series of treatment studies based on PWIP findings may provide further treatment options for this population.
Acknowledgments
The research reported in this study comprised the first author's doctoral dissertation at Northwestern University. The authors wish to thank Drs James Booth, Mark Jung-Beeman, and Michael Dickey for their valuable input as committee members. We wish to thank all participants for generous participation in the study. This research was funded in part by NIH Grant R01-DC01948 awarded to Cynthia K. Thompson, and the Northwestern University Dissertation Year Fellowship awarded to Naomi Hashimoto.
APPENDIX A.
Experimental stimuli list: Categorical-semantic condition
| Picture | Comp 1 | Categ | Assoc | Comp 2 | Categ | Assoc | Comp 3 | Categ | Assoc |
|---|---|---|---|---|---|---|---|---|---|
| banana | peach | 1.77 | 5.52 | cherry | 1.69 | 5.23 | grapes | 1.83 | 5.25 |
| lion | monkey | 1.82 | 5.31 | zebra | .87 | 5.85 | giraffe | 1.38 | 5.65 |
| corn | corn | 1.57 | 5.52 | corn | 1.55 | 5.9 | peas | 1.82 | 5.1 |
| piano | flute | 1.56 | 5.31 | trumpet | 1.58 | 5.1 | violin | 1.53 | 5.55 |
| bus | truck | 1.56 | 5.04 | car | 1.69 | 4.6 | van | 1.52 | 5.1 |
| butterfly | ladybug | 1.49 | 5.33 | grasshopper | .92 | 6.15 | beetle | .93 | 6.1 |
| hammer | wrench | 1.41 | 5.0 | pliers | 1.77 | 4.84 | drill | 1.87 | 4.78 |
| crayons | chalk | 1.67 | 4.72 | pencils | 1.94 | 4.75 | pens | 1.29 | 4.75 |
| rose | daisy | 1.41 | 5.08 | tulip | 1.62 | 5.12 | lily | 1.74 | 5.25 |
| Pig | cow | 1.89 | 5.15 | sheep | 1.67 | 5.2 | horse | 1.13 | 5.78 |
| pretzel | cookie | 1.81 | 5.36 | popcorn | 1.95 | 4.4 | crackers | 1.58 | 4.75 |
| candle | flashlight | 1.3 | 5.22 | lamp | 1.47 | 4.94 | lantern | 1.71 | 4.3 |
| seal | dolphin | 1.83 | 5.25 | otter | 1.74 | 5.0 | whale | 1.68 | 5.75 |
| toaster | mixer | 1.10 | 6.2 | griddle | .84 | 6.47 | blender | 1.66 | 5.45 |
| dominos | cards | 1.46 | 5.4 | dice | 1.88 | 5.1 | chess | 1.23 | 5.5 |
| hand | nose | 1.53 | 5.4 | toe | 1.46 | 5.55 | ear | 1.41 | 5.9 |
| tank | submarine | 1.83 | 5.15 | ship | 1.24 | 5.89 | helicopter | 1.53 | 6.05 |
| belt | shoes | 2.03 | 5.05 | hat | 1.98 | 4.38 | socks | .93 | 6.26 |
| duck | flamingo | 2.4 | 6.45 | owl | 1.8 | 6.5 | swan | 1.05 | 4.93 |
| sword | knife | 2.25 | 6.4 | arrow | 2.0 | 6.8 | axe | 2 22 | 7.0 |
| cop | lawyer | 2.26 | 5.3 | judge | 2.0 | 6.8 | fireman | 2.0 | 6.4 |
| chair | couch | 1.8 | 6.2 | stool | 1.26 | 4.73 | bed | 2.3 | 6.6 |
| ring | watch | 2.44 | 6.7 | bracelet | 1.6 | 6.7 | necklace | 1.7 | 6.7 |
| bowl | cup | 2.4 | 6.6 | mug | 1.9 | 6.7 | glass | 2.2 | 6.9 |
| mushroom | tomato | 1.94 | 5.7 | onion | 1.55 | 4.8 | carrot | 1.3 | 4.5 |
Comp = competitor word; Categ = categorical ratings; Assoc = associative ratings.
APPENDIX B.
Experimental stimuli list: Phonological condition
| Target | Comp 1 | Comp 2 | Comp 3 |
|---|---|---|---|
| banana | balloon | barrette | baton |
| lion | lightning | library | lighthouse |
| corn | cork | corpse | cord |
| piano | pitcher | pillow | pizza |
| bus | bud | button | bun |
| butterfly | buckle | bulb | bubble |
| hammer | hanger | handcuff | hamster |
| crayons | cradle | crane | crate |
| rose | robe | robot | rope |
| Pig | pill | pistol | pin |
| pretzel | present | predator | president |
| candle | camera | calendar | cactus |
| seal | seed | seat | seesaw |
| toaster | toga | tollbooth | tobacco |
| dominos | donkey | dolly | dollar |
| hand | ham | hatchet | hag |
| tank | table | tape | tail |
| belt | beggar | bench | bell |
| duck | swarm | swamp | swing |
| sword | cot | cotton | cod |
| cop | chain | channel | chapel |
| chair | rink | rim | river |
| ring | dust | dummy | dusk |
| bowl | boat | bow | bone |
| mushroom | muffler | muscle | mustard |
Comp = competitor word.
Footnotes
Words that indicate competitors are presented in italicised lower case font, words that indicate the picture are in capital font.
It should be noted that in order to say with any great certainty that a particular process was disrupted, a more comprehensive evaluation should have been carried out. For example, a test of the integrity of the phonological output buffer should have included tasks that involved single word reading, repetition, and writing as well as nonword reading and nonword repetition. While the present study did incorporate tests that tapped into some of these output processes (with the exception of writing), studies that have investigated the phonological output buffer use an extensive battery of tests in order to examine the data for length effects, similarity in types of output errors, and proportion of errors across tasks (Bub et al., 1987; Caramazza et al., 1986; Shallice et al., 2000).
REFERENCES
- Alario FX, Segui J, Ferrand L. Semantic and associative priming in picture naming. Quarterly Journal of Experimental Psychology. 2000;53A:741–764. doi: 10.1080/713755907. [DOI] [PubMed] [Google Scholar]
- Baayen RH, Piepenbrock R, van Rijn H. Linguistic Data Consortium. University of Pennsylvania; USA: 1993. The CELEX Lexical Database. [Google Scholar]
- Best W. When racquets are baskets but baskets are biscuits, where do words come from? A single case study of formal paraphasic errors in aphasia. Cognitive Neuropsychology. 1996;13:443–480. [Google Scholar]
- Bloem I, van den Boogaard S, LaHeij W. Semantic facilitation and semantic interference in language production; Further evidence for the conceptual selection model of lexical access. Journal of Memory & Language. 2004;51:307–323. [Google Scholar]
- Blumstein SE, Milberg W, Brown T, Hutchinson A, Kurowski K, Burton MW. The mapping from sound structure to the lexicon in aphasia: Evidence from rhyme and repetition priming. Brain & Language. 2000;72:75–99. doi: 10.1006/brln.1999.2276. [DOI] [PubMed] [Google Scholar]
- Bub D, Black S, Howell J. Speech output processes and reading. In: Coltheart M, Job R, Sartori G, editors. The cognitive neuropsychology of language. Lawrence Erlbaum Associates Ltd; Hove, UK: 1987. pp. 79–110. [Google Scholar]
- Butterworth B, Howard D, McLoughlin P. The semantic deficit in aphasia: The relationship between semantic errors in auditory comprehension and picture naming. Neuropsychologia. 1984;22:409–426. doi: 10.1016/0028-3932(84)90036-8. [DOI] [PubMed] [Google Scholar]
- Caramazza A. How many levels of processing are there in lexical access? Cognitive Neuropsychology. 1997;14:177–208. [Google Scholar]
- Caramazza A, Berndt RS, Basili AG. The selective impairment of phonological processing: A case study. Brain & Language. 1983;18:128–174. doi: 10.1016/0093-934x(83)90011-1. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Hillis AE. Where do semantic errors come from? Cortex. 1990;26:95–122. doi: 10.1016/s0010-9452(13)80077-9. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Hillis AE. Lexical organization of nouns and verbs in the brain. Nature. 1991;349:788–790. doi: 10.1038/349788a0. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Miceli G, Villa G. The role of the (output) phonological buffer in reading, writing and repetition. Cognitive Neuropsychology. 1986;3:37–76. [Google Scholar]
- Cutting JC, Ferreira VS. Semantic and phonological information flow in the production lexicon. Journal of Experimental Psychology: Learning, Memory, & Cognition. 1999;25:318–344. doi: 10.1037//0278-7393.25.2.318. [DOI] [PubMed] [Google Scholar]
- Damian MF, Bowers JS. Locus of semantic interference in picture-word interference tasks. Psychonomic Bulletin & Review. 2003;10:111–117. doi: 10.3758/bf03196474. [DOI] [PubMed] [Google Scholar]
- Damian MF, Martin RC. Semantic and phonological codes interact in single word production. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1999;25:345–361. doi: 10.1037//0278-7393.25.2.345. [DOI] [PubMed] [Google Scholar]
- deGroot AMB. Primed lexical decision: Combined effects of the proportion of related prime–target pairs and the stimulus-onset asynchrony of prime and target. Quarterly Journal of Experimental Psychology. 1984;36A:253–280. [Google Scholar]
- del Toro JF. An examination of automatic versus strategic semantic priming effects in Broca's aphasia. Aphasiology. 2000;14:925–947. [Google Scholar]
- Dell GS. A spreading activation theory of retrieval in sentence production. Psychological Review. 1986;92:283–321. [PubMed] [Google Scholar]
- Dell GS, O'Seaghdha PG. Stages of lexical access in language production. Cognition. 1992;42:287–314. doi: 10.1016/0010-0277(92)90046-k. [DOI] [PubMed] [Google Scholar]
- Dell GS, Schwartz MF, Martin N, Saffran EM, Gagnon DA. Lexical access in normal and aphasic speech. Psychological Review. 1997;104:801–838. doi: 10.1037/0033-295x.104.4.801. [DOI] [PubMed] [Google Scholar]
- Ellis AW, Miller D, Sin G. Wernicke's aphasia and normal language processing: A case study in cognitive neuropsychology. Cognition. 1983;15:111–144. doi: 10.1016/0010-0277(83)90036-7. [DOI] [PubMed] [Google Scholar]
- Faroqi-Shah Y, Thompson CK. Semantic, lexical, and phonological influences on the production of verb inflections in agrammatic aphasia. Brain & Language. 2004;89:454–498. doi: 10.1016/j.bandl.2003.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkbeiner M, Caramazza A. Now you see it, now you don't: On turning semantic interference into facilitation in a Stroop-like task. Cortex. 2006;42:790–796. doi: 10.1016/s0010-9452(08)70419-2. [DOI] [PubMed] [Google Scholar]
- Foygel D, Dell GS. Models of impaired lexical access in speech production. Journal of Memory and Language. 2000;43:182–216. [Google Scholar]
- Franklin S, Howard D, Peterson K. Abstract word anomia. Cognitive Neuropsychology. 1995;12:549–566. [Google Scholar]
- Gainotti G, Miceli G, Caltagirone C, Silveri MC, Masullo C. The relationship between type of naming error and semantic-lexical discrimination in aphasic patients. Cortex. 1981;17:401–410. doi: 10.1016/s0010-9452(81)80028-7. [DOI] [PubMed] [Google Scholar]
- Glaser WR, Düngelhoff FJ. The time course of picture–word interference. Journal of Experimental Psychology: Human Perception and Performance. 1984;10:640–654. doi: 10.1037//0096-1523.10.5.640. [DOI] [PubMed] [Google Scholar]
- Glaser WR, Glaser MO. Context effects in Stroop-like word and picture processing. Journal of Experimental Psychology: General. 1989;118:13–42. doi: 10.1037//0096-3445.118.1.13. [DOI] [PubMed] [Google Scholar]
- Goldrick M, Rapp B. Lexical and post-lexical phonological representations in spoken production. Cognition. 2007;102:219–260. doi: 10.1016/j.cognition.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Wingfield A. Word-finding deficits in aphasia: Clinical symptomatology and brain–behavior relationships. In: Goodglass H, Wingfield A, editors. Anomia. Academic Press; San Diego, CA: 1997. pp. 3–27. [Google Scholar]
- Hillis AE, Caramazza A. The compositionality of lexical semantic representations: Clues from semantic errors in object naming. Memory. 1995;3:333–358. doi: 10.1080/09658219508253156. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Rapp B, Romani C, Caramazza A. Selective impairment of semantics in lexical processing. Cognitive Neuropsychology. 1990;7:191–243. [Google Scholar]
- Howard D, Franklin S. Three ways for understanding written words, and their use in two contrasting cases of surface dyslexia. In: Allport DA, MacKay D, Prinz W, Scheerer E, editors. Language perception and production: Common processes in listening, speaking, reading, and writing. Academic Press; London: 1987. pp. 340–366. [Google Scholar]
- Howard D, Franklin S. Memory without rehearsal. In: Shallice T, Vallar G, editors. Neuropsychological impairments of short-term memory. Cambridge University Press; Cambridge, UK: 1990. [Google Scholar]
- Howard D, Franklin S. Dissociations between component mechanisms in short-term memory: Evidence from brain-damaged patients. In: Meyer DE, Kornblum S, editors. Attention and performance XIV: Synergies in experimental psychology, artificial intelligence, and cognitive neuroscience. The MIT Press; Cambridge, MA: 1993. [Google Scholar]
- Howard D, Gatehouse C. Distinguishing semantic and lexical word retrieval deficits in people with aphasia. Aphasiology. 2006;20:921–950. [Google Scholar]
- Howard D, Nickels L. Separating input and output phonology: Semantic, phonological, and orthographic effects in short term memory impairment. Cognitive Neuropsychology. 2005;22:42–77. doi: 10.1080/02643290342000582. [DOI] [PubMed] [Google Scholar]
- Howard D, Orchard-Lisle V. On the origin of semantic errors in naming: Evidence from the case of a global aphasic. Cognitive Neuropsychology. 1984;1:163–190. [Google Scholar]
- Howard D, Patterson K. The Pyramids and Palm Trees Test. Thames Valley Test Company; Bury St. Edmunds, UK: 1992. [Google Scholar]
- Janse E. Lexical competition effects in aphasia: Deactivation of lexical candidates in spoken word processing. Brain & Language. 2006;97:1–11. doi: 10.1016/j.bandl.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Janssen N, Schrim W, Mahon BZ, Caramazza A. Semantic interference in a delayed naming task: Evidence for the response exclusion hypothesis. Journal of Experimental Psychology: Learning, Memory, & Cognition. 2008;34:249–256. doi: 10.1037/0278-7393.34.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jescheniak JD, Schriefers H. Discrete serial versus cascaded processing in lexical access in speech production: Further evidence from the coactivation of near-synonyms. Journal of Experimental Psychology. 1998;24:1256–1274. [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Second edition Lippincott Williams & Wilkins Publishers; Philadelphia, PA: 2001. [Google Scholar]
- Kay J, Ellis A. A cognitive neuropsychological case study of anomia. Brain. 1987;110:613–629. doi: 10.1093/brain/110.3.613. [DOI] [PubMed] [Google Scholar]
- Kay J, Lesser R, Coltheart M. Psycholinguistic Assessment of Language Processes in Aphasia (PALPA) Lawrence Erlbaum Associates Ltd; Hove, UK: 1992. [Google Scholar]
- Kertesz A. Western Aphasia Battery. University of Western Ontario Press; London, Ontario: 1982. [Google Scholar]
- Kim M, Thompson CK. Semantic anomaly judgement in individuals with probable Alzheimer's disease. Aphasiology. 2003;17:1103–1113. doi: 10.1080/02687030344000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn SE, Goodglass H. Picture naming in aphasia. Brain & Language. 1985;24:266–283. doi: 10.1016/0093-934x(85)90135-x. [DOI] [PubMed] [Google Scholar]
- Kohn SE, Smith KL. Distinctions between two phonological output deficits. Applied Psycholinguistics. 1994;15:75–95. [Google Scholar]
- Lambon Ralph MA, Sage K, Roberts J. Classical anomia; A neuropsychological perspective on speech production. Neuropsychologia. 2000;38:186–202. doi: 10.1016/s0028-3932(99)00056-1. [DOI] [PubMed] [Google Scholar]
- La Heij W. Components of Stroop-like interference in picture naming. Memory & Cognition. 1988;16:400–410. doi: 10.3758/bf03214220. [DOI] [PubMed] [Google Scholar]
- La Heij W, Dirkx J, Kramer P. Categorical interference and associative priming in picture naming. British Journal of Psychology. 1990;81:511–525. [Google Scholar]
- Levelt WJM. Accessing words in speech production: Stages, processes, and representations. Cognition. 1992;42:1–22. doi: 10.1016/0010-0277(92)90038-j. [DOI] [PubMed] [Google Scholar]
- Levelt WJM, Roelofs A, Meyer AS. A theory of lexical access in speech production. Behavioral & Brain Sciences. 1999;22:1–75. doi: 10.1017/s0140525x99001776. [DOI] [PubMed] [Google Scholar]
- Levelt WJM, Schriefers H, Vorberg D, Meyer AS, Pechmann T, Havinga J. The time course of lexical access in speech production: A study of picture naming. Psychological Review. 1991;98:122–142. [Google Scholar]
- Lupker SJ. The semantic nature of response competition in the picture–word interference task. Memory & Cognition. 1979;7:485–495. [Google Scholar]
- Lupker SJ. The role of phonetic and orthographic similarity in picture–word interference. Canadian Journal of Psychology. 1982;36:349–367. doi: 10.1037/h0080652. [DOI] [PubMed] [Google Scholar]
- Mahon BZ, Costa A, Peterson R, Vargas KA, Caramazza A. Lexical selection is not by competition: A reinterpretation of semantic interference and facilitation effects in the picture–word interference paradigm. Journal of Experimental Psychology: Learning, Memory, & Cognition. 2007;33:503–535. doi: 10.1037/0278-7393.33.3.503. [DOI] [PubMed] [Google Scholar]
- Martín P, Serrano JM, Iglesias J. Phonological/semantic errors in two Spanish-speaking patients with anomic aphasia. Aphasiology. 1999;13:225–236. [Google Scholar]
- Meyers AS, Schriefers H. Phonological facilitation in picture–word interference experiments: Effect of stimulus onset asynchrony and type of interfering stimuli. Journal of Experimental Psychology: Learning, Memory, & Cognition. 1991;17:1146–1160. [Google Scholar]
- Miceli G, Benvegnu B, Capasso R, Caramazza A. Patterns of dissociation in comprehension and production of nouns and verbs. Aphasiology. 1997;2:351–358. [Google Scholar]
- Milberg W, Blumstein SE, Dworetzky B. Phonological processing and lexical access in aphasia. Brain & Language. 1988;34:279–293. doi: 10.1016/0093-934x(88)90139-3. [DOI] [PubMed] [Google Scholar]
- Milberg W, Blumstein SE, Katz D, Gershberg F, Brown T. Semantic facilitation effects of time and expectancy. Journal of Cognitive Neuroscience. 1995;7:33–50. doi: 10.1162/jocn.1995.7.1.33. [DOI] [PubMed] [Google Scholar]
- Miller D, Ellis AW. Speech and writing errors in “Neologistic jargonaphasia”: A lexical activation hypothesis. In: Coltheart M, Job R, Sartori G, editors. The cognitive neuropsychology of language. Lawrence Erlbaum Associates Ltd.; Hove, UK: 1987. pp. 253–271. [Google Scholar]
- Monsell S. On the relation between lexical input and output pathways for speech. In: Allport DA, MacKay D, Prinz W, Scheerer E, editors. Language perception and production: Common processes in listening, speaking, reading and writing. Academic Press; London: 1987. pp. 273–311. [Google Scholar]
- Neely JH. Semantic priming effects in visual word recognition: A selective review of current findings and theories. In: Besner D, Humphreys GW, editors. Basic processes in reading: Visual word recognition. Lawrence Erlbaum Associates Inc.; Hillsdale, NJ: 1991. pp. 264–336. [Google Scholar]
- Nickels L. Getting it right? Using aphasic naming errors to evaluate theoretical models of spoken word production. Language and Cognitive Processes. 1995;10:13–45. [Google Scholar]
- Nickels L. When the words won't come: Relating impairments and models of spoken word production. In: Wheeldon LR, editor. Aspects of language production. Psychology Press; Philadelphia, PA: 2000. [Google Scholar]
- Nickels L, Cole-Virtue J. Reading tasks from PALPA: How do controls perform on visual lexical decision, homophony, rhyme, and synonym judgements? Aphasiology. 2004;18:103–126. [Google Scholar]
- Nickels L, Howard D. A frequent occurrence? Factors affecting the production of semantic errors in aphasic naming. Cognitive Neuropsychology. 1994;11:289–320. [Google Scholar]
- Nickels L, Howard D, Best W. Fractionating the articulatory loop: Dissociations and associations in phonological recoding in aphasia. Brain and Language. 1997;56:161–182. doi: 10.1006/brln.1997.1732. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Peterson RR, Savoy P. Lexical selection and phonological encoding during language production: Evidence for cascaded processing. Journal of Experimental Psychology: Learning, Memory, & Cognition. 1998;24:539–557. [Google Scholar]
- Prather P, Zurif E, Stern C, Rosen TJ. Slowed lexical access in nonfluent aphasia: A case study. Brain and Language. 1992;43:336–348. doi: 10.1016/0093-934x(92)90134-z. [DOI] [PubMed] [Google Scholar]
- Prather PA, Zurif E, Love T, Brownell H. Speed of lexical activation in nonfluent Broca's aphasia and fluent Wernicke's aphasia. Brain and Language. 1997;59:391–411. doi: 10.1006/brln.1997.1751. [DOI] [PubMed] [Google Scholar]
- Rapp B, Goldrick M. Discreteness and interactivity in spoken word production. Psychological Review. 2000;107:460–499. doi: 10.1037/0033-295x.107.3.460. [DOI] [PubMed] [Google Scholar]
- Rayner E, Posnansky C. Stages of processing in word identification. Journal of Experimental Psychology: General. 1978;107:64–80. doi: 10.1037//0096-3445.107.1.64. [DOI] [PubMed] [Google Scholar]
- Rayner E, Springer CJ. Graphemic and semantic similarity effects in the picture–word interference task. British Journal of Psychology. 1986;77:207–222. doi: 10.1111/j.2044-8295.1986.tb01995.x. [DOI] [PubMed] [Google Scholar]
- Roelofs A. A spreading activation theory of lemma retrieval in speaking. Cognition. 1992;42:107–142. doi: 10.1016/0010-0277(92)90041-f. [DOI] [PubMed] [Google Scholar]
- Roelofs A. The WEAVER++ model of word-form encoding in speech production. Cognition. 1997;64:249–284. doi: 10.1016/s0010-0277(97)00027-9. [DOI] [PubMed] [Google Scholar]
- Rosinski RR. Picture–word interference is semantically based. Child Development. 1977;48:643–647. [Google Scholar]
- Rosinski RR, Golinkoff RM, Kukish KS. Automatic semantic processing in a picture–word interference task. Child Development. 1975;46:247–253. [Google Scholar]
- Saxton J, Ratcliff G, Munro CA, Coffey E, Becker JT, Fried L, et al. Normative data on the Boston Naming Test and two equivalent 30-item short for ms. The Clinical Neuropsychologist. 2000;14:526–534. doi: 10.1076/clin.14.4.526.7204. [DOI] [PubMed] [Google Scholar]
- Schriefers H, Meyer AS, Levelt WJM. Exploring the time course of lexical access in language production: Picture–word interference studies. Journal of Memory and Language. 1990;29:86–102. [Google Scholar]
- Semenza C, Luzzatti C, Mondini S. Lemma theory and aphasiology. Behavioral & Brain Sciences. 1999;22:56. [Google Scholar]
- Shallice T, Rumiati RI, Zadini A. The selective impairment of the phonological output buffer. Cognitive Neuropsychology. 2000;17:517–546. doi: 10.1080/02643290050110638. [DOI] [PubMed] [Google Scholar]
- Smith MC, Magee LE. Tracing the time course of picture–word processing. Journal of Experimental Psychology: General. 1980;109:373–392. doi: 10.1037//0096-3445.109.4.373. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: Norms for name agreement, image agreement, familiarity, and visual complexity. Journal of Experimental Psychology: Human Learning and Memory. 1980;6:174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Starreveld PA, La Heij W. Semantic interference, orthographic facilitation, and their interaction in naming tasks. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1995;21:686–698. [Google Scholar]
- Starreveld PA, La Heij W. Time-course analysis of semantic and orthographic context effects in picture naming. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1996;22:896–918. [Google Scholar]
- Starreveld PA, La Heij W. Word substitution errors in a speeded picture–word task. American Journal of Psychology. 1999;112:521–553. [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. [Google Scholar]
- Taylor JK, Burke DM. Asymmetric aging effects on semantic and phonological processes: Naming in the picture–word interference task. Psychology and Aging. 2002;17:662–676. doi: 10.1037//0882-7974.17.4.662. [DOI] [PubMed] [Google Scholar]
- Underwood G. Semantic interference from unattended printed words. British Journal of Psychology. 1976;67:327–338. [Google Scholar]
- Utman JA, Blumstein SE, Sullivan K. Mapping from sound to meaning: Reduced lexical activation in Broca's aphasics. Brain and Language. 2001;79:444–472. doi: 10.1006/brln.2001.2500. [DOI] [PubMed] [Google Scholar]
- Vitkovitch M, Humphreys GW. Perseverant responding in speeded naming of pictures: It's in the links. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1991;17:664–680. [Google Scholar]
- Welch LW, Doineau D, Johnson S, King D. Educational and gender normative data for the Boston Naming Test in a group of older adults. Brain and Language. 1996;53:260–266. doi: 10.1006/brln.1996.0047. [DOI] [PubMed] [Google Scholar]
- Wilshire CE. Where do aphasic phonological errors come from? Evidence from phoneme movement errors in picture naming. Aphasiology. 2002;16:169–197. [Google Scholar]
- Wilshire CE, Keall LM, Stuart EJ, O'Donnell DJ. Exploring the dynamics of aphasic word production using the picture-word interference task: A case study. Neuropsychologia. 2007;45:939–953. doi: 10.1016/j.neuropsychologia.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Wilshire CE, McCarthy RA. Experimental investigations of an impairment in phonological encoding. Cognitive Neuropsychology. 1996;13:1059–1098. [Google Scholar]
- Wilshire CE, Saffran EM. Contrasting effects of phonological priming in aphasic word production. Cognition. 2005;95:31–71. doi: 10.1016/j.cognition.2004.02.004. [DOI] [PubMed] [Google Scholar]