Abstract
Extracellular ATP is known to permeabilize certain cell types to polyatomic cations like YO-PRO1. Here, we report that extracellularly applied ATP stimulated rapid uptake and accumulation of an otherwise weakly membrane permeable fluorescent DNA-binding cytotoxin, Hoechst 33258, into cervical cancer cells. While ATP stimulated Hoechst 332uptake in 20–70% of cells from seven cervical cancer cell lines, it consistently stimulated uptake in less than 8% of cervical epithelial cells obtained from the normal transformation zone and ectocervix tissue of 10 patients. ATP-evoked Hoechst 33258 uptake was independent of ionotropic P2X receptors, but dependent on activation of P2Y receptors. Thus, we show here that cervical cancer cells can be selectively induced to take up and accumulate an ionic cytotoxin by exposure to extracellular ATP.
Introduction
An overriding objective of cancer research is to develop selective agents for the targeted killing of cancer cells while minimizing collateral damage to surrounding healthy tissue. To this end, the majority of current and in-development anti-cancer drugs are targeted to interfere with critical intracellular components, particularly those involved in cell survival, and proliferation. Examples include drugs that interact directly with DNA (cisplatin derivatives, anthracyclins, and DNA-alkylating agents); drugs that interact with receptors that affect gene regulation (Tamoxifen, Erlotinib); and drugs that interfere with cellular metabolism (5-fluorauracil, methotrexate) (1–3). The caveat of course is that these drugs must first overcome the barrier imposed by the plasma membrane in order to reach these intracellular targets. Several commonly used drugs, including the cisplatin derivatives and the anthracycline, doxorubicin, exhibit relatively poor passive membrane permeability. As a result, a considerable effort has been devoted towards investigating strategies to enhance cell penetration, including the use of nanomaterials to encapsulate drugs and facilitate their entry via passive diffusion or pinocytosis (4), and the use of electrical membrane disruption (5,6).
An alternative, and potentially less invasive, approach is to utilize a cell’s natural transmembrane transport mechanisms to move anticancer drugs to the cell interior. It has long been known that exogenous drugs can serve as substrates for a plethora of facilitative and active transport pumps arrayed on cell membranes. Many of these pumps, such as those of the large multidrug resistance protein family (7), are primarily responsible for the extrusion of drugs from cells, and are a major factor in resistance to anticancer drugs. However, in the case of cisplatin and its derivatives a family of copper transporters are as important for the uptake and accumulation of the drugs in cells as well as their efflux (8). The problem with exploiting these active transport pathways to enhance anticancer drug penetration, though, is that they often have a wide tissue distribution and do not have activity easily regulated pharmacologically.
The experiments described in this paper were conducted based on the long known ability for extracellular adenosine 5′-triphosphate (ATP) to permeabilize certain cell types, such as mast cells and macrophages, to relatively large polyatomic ions including 2-Amino-2-hydroxymethyl-propane-1,3-diol (TRIS), N-methyl-D-glucamine (NMDG+) (9), ethidium (10,11), the Ca2+-sensor Fura-2 and Lucifer Yellow (12,13). A previous study had some success with using ATP-evoked permeabilization in order to load macrophages with doxorubicin and use them as a release vehicle for the drug in tumors (14). The mechanism of ATP-evoked permeabilization is now thought to typically involve ATP binding and activation of the ion channel, P2X7, which subsequently conducts entry of the large cations through its gated transmembrane pore (15,16). However, in some cases, ATP has been reported to permeabilize cells independently of P2X7 activation (17–20).
We hypothesized that cervical cancer cells might be induced to take up and accumulate cytotoxins through a similar ATP-dependent mechanism, thereby lending credence to the idea that some cancer cells might be induced to take up cytotoxins. To test this hypothesis, we treated these cells with one of two DNA-binding cytotoxins, Hoechst 33258 and doxorubicin hydrochloride. Both of these cytotoxins fluoresce upon binding DNA and subsequently stain the cell’s nucleus, allowing their uptake and accumulation to be monitored using basic fluorescence imaging. Hoechst 33258, also known as pibenzimol, is a cationic, weakly permeable DNA-binding dye known to be cytotoxic (21–24), but that was shown to perform poorly against advanced stage pancreatic cancer in Phase I and II clinical trials (25,26). Doxorubicin is a larger anthracycline topoisomerase inhibitor, the electrostatically neutral but water-soluble hydrochloride salt of which is commonly used in the treatment of cancer. Poor and subsequently slow transport of anticancer agents across biological membranes is problematic because it allows for cancer cells to overcome toxin influx via active efflux pathways that utilize multidrug resistance transporters such as P-glycoprotein (27). As we show in this paper, ATP stimulated the rapid uptake and accumulation of one of these toxins, Hoechst 33258, via a mechanism involving activation of purinergic P2Y receptors. This mechanism seems to be preferentially active in cancerous cells, being much less prevalent in cells obtained from normal cervical tissue. Thus, not only do we demonstrate the feasibility of pharmacologically enhancing plasma membrane permeability to a cationic cytotoxin, we also show evidence that this strategy might be utilized to preferentially deliver the drug into cancer cells rather than normal cells.
Experimental Procedures
Cervical cell culture
CXT cervical cancer cell lines and normal human cervical epithelial cells were isolated and cultured from tissue purchased from the Cooperative Human Tissue Network as described elsewhere (28). Normal and CXT cancerous cells were cultured in Keratinocyte Serum-Free Medium (KSFM) supplemented with 50 units/mL penicillin G, and 50 μg/mL streptomycin. Normal cells were used at passages 1–2. HeLa and CaSki cells were cultured in DMEM supplemented with 10% fetal bovine serum, 2mM L-glutamine, 50 units/mL penicillin G, and 50 μg/mL streptomycin. All cells were incubated at 37 °C in a humidified atmosphere of 5% CO2.
Fluorescence Imaging
Cells were seeded onto 13 mm borosilicate glass coverslips in 4-well plates at low density. For experiments, the coverslip was transferred to a perfusion bath on the stage of a Nikon TE200 epifluorescence microscope. The extracellular solution bathing the cells was of the following composition: 140 mM NaCl, 2.5 mM KCl, 10 mM HEPES, 10 mM glucose, 0.1 mM CaCl2 (except when specified otherwise), and pH adjusted to 7.4 with NaOH). Drugs were added in final concentration via peristaltic pump-mediated perfusion. Images were acquired and analyzed using μManager (Professor R. Vale laboratory, University of California, San Francisco, CA).
Dye uptake experiments
Fluorescence was recorded using appropriate excitation and emission filters as follows: Hoechst 33258, 350ex/450em; doxorubicin HCl, 488ex/600em. Images were acquired at 0.1 Hz, 200 ms exposure. Dye fluorescence over time was recorded as the mean from all cells in the field of view.
Ca2+ imaging
Cells were loaded with the Ca2+ indicator Fluo-4/AM for 30 min at ~22°C, washed thoroughly, and then incubated for 15–30 min at 37 °C to allow for deesterification and release of cytosolic Fluo-4. Images were acquired at 0.2 Hz, 200 ms exposure.
Patch clamp electrophysiology
Cell were added to the microscope bath chamber of an Olympus IV-51 inverted microscope. The extracellular solution was of the following composition (in mM): 140 NaCl, 0.1 CaCl2, 10 glucose, 10 HEPES, pH 7.4 (NaOH). Plasma membrane currents were recorded in the broken patch voltage-clamp configuration using an Axopatch 200B amplifier and digitized using a 1440 Digidata (Molecular Devices, CA). The cell membrane potential was held at −60 mV in all cases. Recording pipettes were filled with a standard intracellular solution of the following composition (in mM): 140 CsCl, 10 EGTA, 10 HEPES, pH 7.3 (CsOH). Data were recorded using AxoGraphX (Axograph, CA) software and analyzed offline using IgorPro (Wavemetrics, Inc., OR). Drugs were applied using the Perfusion Fast-Step System SF-77 (Warner Instruments).
Cell viability
Cells were seeded at low density on glass coverslips in 4-well IVF plates. The coverslips were removed, washed, treated with drugs in extracellular solution for 20 min, washed three times with culture media and replaced in the incubator at 37°C. Cell viability was assessed at 24 hrs and 48 hrs after treatment using an Annexin V-FITC/propidium iodide viability assay (Sigma-Aldrich, St. Louis, MO), with all cells showing Annexin V and/or propidium iodide staining deemed non-viable.
Cell Growth
Cell proliferation was assessed by plating cells at low density onto 35 mm culture dishes. Viable adherent cells were then counted using microscopy from 5 fields of view (x4 magnification) at 24 Hr intervals, with the values normalized to the number counted at t=0 for the same plate. Viability was assessed, and clearly apparent, based on adherence and morphology.
Reagents
ATP, UTP, Hoechst 33258, doxorubicin, pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS), U73122, ATPγS, and BAPTA/AM were purchased from Sigma Aldrich (St. Louis, MO). Histamine, A740003, and MRS2179 were purchased from Tocris (Bristol, UK).
Analysis and statistics
Data are presented as the mean ± S.E.M. Significant differences among groups were determined using GraphPad Prism (GraphPad, San Diego, CA) using Student’s t-test or one-way ANOVA with Tukey’s post hoc test as appropriate. A calculated p value ≤0.05 was considered significant.
Results
ATP-evoked Hoechst 33258 uptake and accumulation in cervical cancer cells
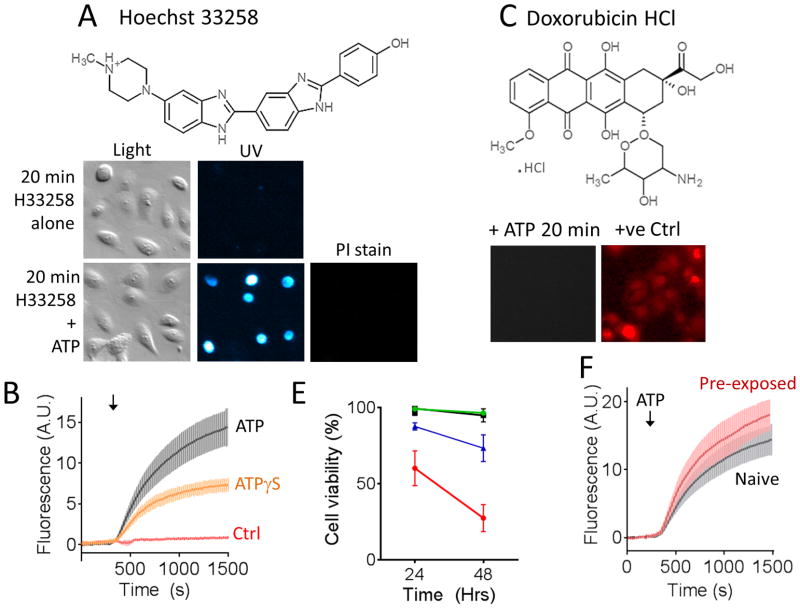
We first examined whether extracellularly applied ATP could increase the plasma membrane penetration of two candidate cytotoxins, Hoechst 33258 and the currently in-use anticancer drug, doxorubicin hydrochloride (HCl). These cytotoxins were chosen due to their fluorescence properties when bound to nuclear DNA, which provided a means by which to assay their time-dependent penetration into the cell. Live cell time-lapse fluorescence imaging was used to monitor cytotoxin uptake and accumulation into adherent cells of a cervical cancer cell line (CXT2) in the absence and presence of extracellular ATP. In control experiments, cells were first exposed to Hoechst 33258 (Figure 1A, and B), or doxorubicin HCl (Figure 1C) alone for 25 min. No cumulative increase in uptake was observed, consistent with these compounds exhibiting poor passive membrane permeability over this time frame. In parallel experiments, cells were exposed to ATP (100 μM) in the continued presence of each of the cytotoxins for 20 min. In cells exposed to Hoechst 33258, application of ATP evoked a rapid uptake and accumulation of the cytotoxin into cells as shown by an increase in nuclear fluorescence (Figure 1A and B). This was not due to ATP-mediated cell death, as cells were still able to exclude the cell viability dye propidium iodide when applied after the experiment (3 μM; 5 min). ATPγS also evoked Hoechst 33258 uptake, showing that the response was not dependent on ATP hydrolysis. In the presence of 30 μM Hoechst 33258, ATP stimulated dye uptake in 58 ± 5 % of CXT2 cells. Over the same 20 min time course, ATP did not evoke uptake and accumulation of up to 100 μM doxorubicin HCl (Figure 1C).
Figure 1. Extracellular ATP enhanced accumulation of DNA-binding fluorescent cytotoxins into cervical cancer cells.
A) Cervical cancer cells exposed to Hoechst 33258 (30 μM) alone for 20 min showed no signs of uptake and nuclear staining. In contrast, cells exposed to extracellular ATP (100 μM) in the continued presence of Hoechst 33258 showed rapid uptake and accumulation of the cytotoxin. Subsequent assay for propidium iodide (PI) exclusion (3 μM; 5 min) showed that cells were still viable. B) Pooled time course data showing Hoechst 33258 uptake in the absence of co-applied purinoceptor agonist (Ctrl) and in presence of ATP or its non-hydrolysable analogue, ATPγS. C) ATP did not enhance uptake of, doxorubicin HCl applied up to 100 μM in concentration over a 20 minute period. Cells staining by exposure to doxorubicin HCl for 2 hrs at 37°C are shown as a positive control. E) Shows the effect of ATP-evoked Hoechst 33258 loading on cell viability over a 48 Hr period. Untreated control cells are shown in black; cells treated for 20 min with ATP (100 μM) alone are shown in green; cells treated with ATP and Hoechst 33258 simultaneously are represented both by the blue (% total cells) and red (% Hoechst-positive cells only) lines. F) ATP (100 μM) still evoked substantial Hoechst 33258 (30 μM) uptake in subcultured cervical cancer cells seeded from the survivors of a previous 20 min treatment with ATP and Hoechst 33258. Error bars represent s.e.m of at least 3 experiments.
To confirm that ATP-stimulated uptake and accumulation of Hoechst 33258 was cytotoxic, we exposed cells to either Vehicle control, ATP alone or ATP in the presence of Hoechst 33258 for 20 min under sterile conditions. The cells were washed thoroughly, replaced in fresh culture medium and incubated for 24 or 48 hrs at 37°C, 5% CO2 in a humidified incubator. Cell viability was then assessed using an Annexin V/propidium iodide assay. ATP-evoked Hoechst 33256 uptake was observed to significant cell death over a period of 48 hrs post-treatment. Total cell viability after treatment with Hoechst 33258 and ATP (i.e. including both cells that took up the toxin and those that did not) was reduced to 73 ± 9 % over 48 hrs compared with 96 ± 0.7% for cells exposed to ATP alone. Among the cells that took up Hoechst 33258 in the presence of ATP, only 27 ± 9 % remained viable (Figure 1E).
We were curious to see if the fraction (~40 %) of cells that failed to take up Hoechst 33258 during the first exposure in the presence of ATP were permanently resistant to this treatment. CXT2 cells were exposed to ATP (100 μM) and Hoechst (30 μM) for 20 min, washed thoroughly, replaced in media and incubated for 48 hrs. The surviving cells were then reseeded in plates and allowed to grow for 48 hrs before being exposed to a second round of treatment with ATP and Hoechst 33258. These were incubated once again for a further 48 hrs before being reseeded onto coverslips for imaging experiments. Having now been exposed to two consecutive treatments of ATP and Hoechst 33258, we predicted that we would have a subculture with significantly reduced sensitivity to ATP-evoked Hoechst 33258 uptake. Surprisingly, however, we observed ATP-evoked Hoechst 33258 uptake in 62.4 ± 2.5% of these pre-exposed CXT2 cells, which was not significantly different from the uptake fraction in naïve cells (Figure 1F).
In summary, application of extracellular ATP permeabilized cervical cancer cells to the DNA-binding cytotoxin, Hoechst 33258, leading to uptake and accumulation of this cytotoxin. A single short period of ATP-evoked Hoechst 33258 uptake was subsequently sufficient to significantly impair cell viability. Furthermore, cells surviving initial exposures to this treatment subsequently acquired sensitivity, suggesting that enhanced cell viability could be achieved by repeat of sustained exposure to this treatment.
ATP preferentially stimulated Hoechst 33258 uptake in abnormal versus normal cervical epithelial cells
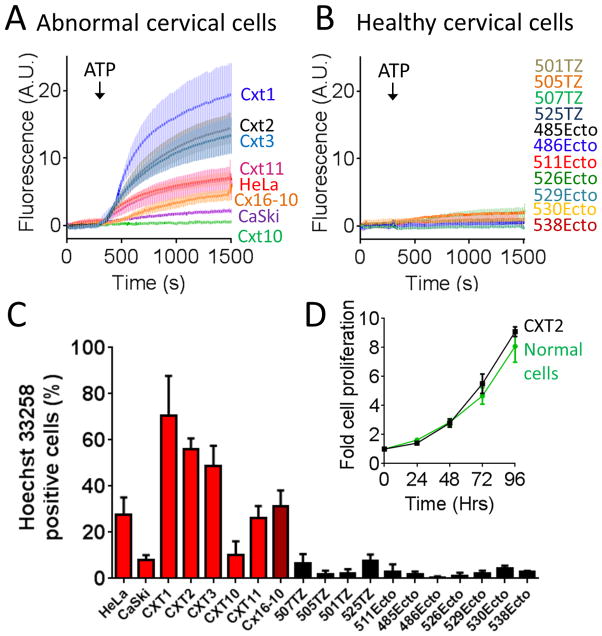
We observed varying degrees of ATP-stimulated Hoechst 33258 uptake in 7 different cervical cancer cell lines (Figure 2A and C). The fraction of cells responding to ATP with Hoechst 33258 uptake was greatest for Cxt1 cells (71 ± 17 %) and least for the commonly used cervical cancer cell line, CaSki (8.0 ± 2.1 %). The fraction of cells exhibiting ATP-evoked Hoechst 33258 uptake in HeLa cells were 23 ± 13 %. We also observed ATP-evoked Hoechst 33258 uptake in a human papilloma virus (HPV)-immortalized cell line, Cx16-10 (26 ± 6%). In contrast, we consistently observed ATP-evoked Hoechst 33258 uptake in less than 8% of transformation zone and ectocervical cells obtained from 11 different healthy patients (Figure 2B and C). Based on these data it may be that cervical cancer cells have an enhanced sensitivity to ATP-stimulated Hoechst 33258 uptake relative to health cervical epithelial cells. The ability for cancer rather than normal cells to appreciably take up Hoechst 33258 in response to ATP was not a function of cell proliferation rate, as CXT2 cells and normal cells exhibited similar growth curves (Figure 2D).
Figure 2. ATP evoked Hoechst 33258 uptake in abnormal versus normal cervical epithelial cells.
A) ATP (100 μM) evoked Hoechst 33258 (30 μM) uptake and accumulation in cells of several cervical cancer cell lines and one HPV-immortalized cell line (CX16-10). B) ATP (100 μM) evoked negligible Hoechst 33258 (30 μM) uptake and accumulation in normal ectocervical and transformation zone epithelial cells from numerous patients (passages 1–2). C) Pooled data showing the fraction of abnormal and normal cervical epithelial cells that accumulated nuclear Hoechst 33258 in the presence of ATP. D) Comparison showing similar 96 Hr growth curves for normal and cancerous cell lines. Error bars represent s.e.m of at least 3 experiments.
Role of purinergic receptors in mediating ATP-stimulated Hoechst uptake and accumulation
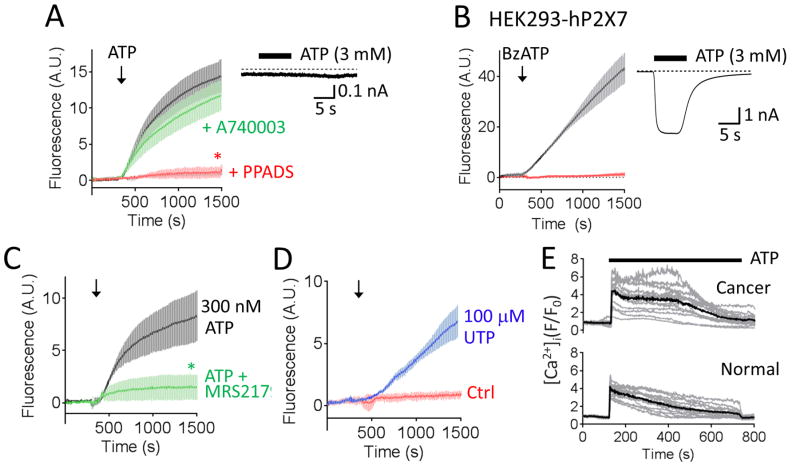
Stimulated Hoechst 33258 uptake into CXT2 cells was dependent on the concentration of ATP applied (Figure 3A) and the concentration of Hoechst 33258 present (Figure 3B). In addition, we observed that the ATP-evoked Hoechst 33258 uptake was also affected by extracellular Ca2+ [[Ca2+]o), and within the physiological range (Figure 3C). Previous studies have shown that the human P2X7 receptor, a non-selective ATP-gated cation channel, can conduct relatively large ionic species, such as YO-PRO1, and that current through these channels is inhibited by extracellular divalents (29). There is biochemical evidence of P2X7 receptor expression in cervical epithelial cells, although it appears that cervical cancer cells upregulate a non-functional splice variant (30,31). Nevertheless, we set out to test the hypothesis that P2X7 receptors might be responsible for the observed ATP-evoked uptake in cervical cancer cells. However, in contrast to the broad spectrum purinergic receptor inhibitor, PPADS, the putative selective P2X7 receptor antagonist, A-740003, had little effect on ATP-evoked Hoechst 33258 uptake into CXT2 cells (Figure 4A). We also observed no ATP-evoked transmembrane currents in CXT2 cells subjected to whole cell broken patch clamp electrophysiology, suggestive of an absence of functional P2X receptors in the plasma membrane of these cells. Interestingly, we observed that activation of the recombinant human P2X7 receptor expressed in HEK293 cells to facilitate a marked uptake of Hoechst 33258 uptake; an effect abolished by A-740003 (Figure 4B).
Figure 3. Effect of varying ATP, Hoechst 33258 and extracellular Ca2+ on ATP-evoked Hoechst 33258 uptake.
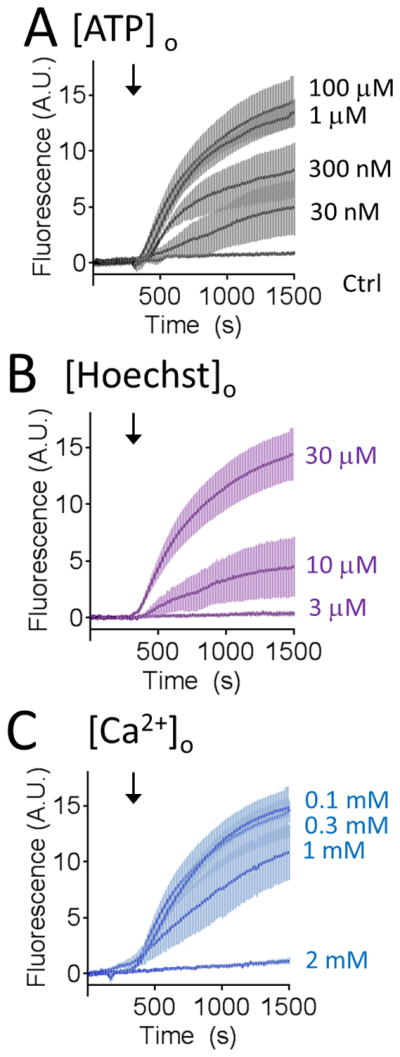
A) Hoechst 33258 (30 μM) uptake into CXT2 cells was dependent on the concentration of ATP applied. B) Amplitude of ATP (100 μM)-evoked cytotoxic dye uptake varied with Hoechst 33258 concentration. C) ATP (100 μM)-evoked Hoechst 33258 (30 μM) uptake was inhibited by increasing extracellular Ca2+ concentration ([Ca2+]o). Error bars represent s.e.m of at least 3 experiments.
Figure 4. ATP-stimulated Hoechst 33258 uptake is mediated by activation of P2Y purinergic receptors.
A) BzATP (100 μM) activation of recombinant human P2X7 receptors expressed in HEK293 cells evoked Hoechst 33258 uptake in a manner blocked completely by the P2X7-selective antagonist A740003. Voltage clamp current trace obtained at a holding potential of −60 mV showing ATP-evoked current in HEK293 cell transfected with human P2X7 receptor (inset). B) ATP-evoked Hoechst 33258 uptake in cancer cells was inhibited by PPADS but unaffected by the selective P2X7 antagonist, A740003. Voltage clamp current trace obtained at a holding potential of −60 mV showing no measurable ATP-evoked current in CXT2 cells (inset). Error bars represent s.e.m. C) The P2Y1 receptor antagonist, MRS2179, significantly suppressed Hoechst 33258 uptake evoked by a submaximal concentration of ATP (300 nM). D) The P2Y2 agonist, UTP (100 μM), evoked a slow rate of Hoechst 33258 uptake. Error bars represent s.e.m of at least 3 experiments. Asterisks indicates values significantly different from Ctrl (p<0.05). E) Representative traces showing ATP (100 μM) stimulated sustained elevations in [Ca2+]i in both Fluo-4-loaded cancerous (upper panel) and normal (lower panel) cervical epithelial cells. Solid black line indicated mean fluorescence from all cells in the visual field; grey lines indicate individual single cell fluorescence values.
ATP can also activate G protein-coupled P2Y receptors, of which the Gq protein/Phospholipase C (PLC) pathway-linked P2Y1 and P2Y2 receptors are reportedly expressed in cervical epithelial cells (32,33). The rate of Hoechst 33258 uptake stimulated by a submaximal concentration of ATP (300 nM) was significantly reduced by a maximally effective concentration of the selective P2Y1 antagonist MRS 2179 (3 μM) (Figure 4C). We also observed a significant but slow rate of Hoechst 33258 uptake upon exposure of cells to UTP, which activates P2Y2 receptors. These data suggest that the ATP-stimulated uptake of Hoechst 33258 is chiefly mediated through P2Y1 receptors with perhaps a small contribution from P2Y2 receptors.
While it was tempting to speculate that the lack of ATP-stimulated Hoechst 33258 uptake in normal cervical epithelial cells might be due to reduced expression of P2Y receptors, we observed no obvious mean difference in the amplitude and profile of ATP-stimulated elevations in [Ca2+]i as assayed using the Ca2+ indicator, Fluo-4 (Figure 4E). Thus, further investigation is needed to identify the mechanism by which P2Y receptor activation stimulates Hoechst 33258 uptake, and to determine why this phenomenon occurs preferentially in abnormal cervical epithelial cells relative to health cells. Nevertheless, these experiments provide compelling evidence that activation of endogenous plasma membrane receptors can stimulate signaling pathways that functionally permeabilize cancer cells to otherwise weakly membrane permeable cytotoxins. Furthermore, in the case of cervical epithelial cells, this mechanism is disproportionately active in cancerous cells versus normal cells, potentially conferring an element of specificity.
Discussion
In this paper we report that extracellular ATP can stimulate the rapid uptake and accumulation of the ionic DNA-binding cytotoxin, Hoechst 33258 (Pibenzimol), into cervical cancer cells. Thus, in the presence of ATP Hoechst 33258 penetration of the plasma membrane was virtually immediate, which is in stark contrast to the several hours required for cells to substantially accumulate the commonly employed anticancer drug, doxorubicin HCl (34)(Figure 1C). Notably, uptake of doxorubicin HCl was not stimulated by ATP over this time course. The reason for this disparity will be subjected to further investigation, but likely lies in the selectivity of the transport mechanism, whether an active transporter of ion channel. Doxorubicin HCl differs from Hoechst 33258 in terms of its size, geometry and charge, which might impact its ability to utilize the transport pathway responsible for ATP-evoked uptake. Interestingly, the inability of doxorubicin to utilize the pathway implies that the mechanism by which ATP permeabilizes cervical cancer cells is different from that responsible for allowing macrophages to be loaded with doxorubicin as reported in an earlier study (14).
Contrary to our initial hypothesis, uptake of Hoechst 33258 was not primarily due to permeation through the P2X7 receptor, an ATP-gated ion channel known to be permeable to large cationic species (29). This is despite evidence presented in this paper showing that the recombinant hP2X7 can nevertheless conduct Hoechst 33258 entry into transfected HEK293 cells, and so could potentially perform the role of drug delivery conduit in other cell types. The reason we do not observe P2X7-mediated uptake in cervical cancer is consistent with previous reports indicating that this channel is significantly down regulated during carcinogenesis in these cells (30), and possibly because the concentration of ATP used is relatively low for activation of human P2X7 receptors (35). Instead, ATP stimulation of Hoechst 33258 uptake appears to be dependent on activation of P2Y receptors.
Importantly, the ATP-evoked Hoechst 33258 uptake phenomenon was not merely a quirk of the CXT2 cancer cell line. We observed ATP-evoked uptake in >20% of cells in 5 of 7 cancer cell lines and 1 HPV-immortalized cell line. Importantly, cells that survived an initial treatment, consisting largely of cells that did not take up Hoechst 33258 at all, nevertheless demonstrated the same degree of sensitivity when challenged again at a later time. A possible explanation for this is that the mechanism responsible for ATP-evoked Hoechst 33258 uptake is expressed by cells in a time-dependent fashion, such that cells not expressing the transport mechanism during an initial 20 min treatment may nevertheless upregulate it later on, perhaps in a cell cycle-dependent manner, rendering them sensitive to a subsequent treatment. This is significant, because the susceptible fraction of cancer cells would be predicted to increase simply as a result of repeated exposure to ATP and Hoechst 33248.
In contrast to our observations in cancer cells, only 0.5–8% of normal cervical epithelial cells exhibited ATP-evoked Hoechst 33258. The possibility exists, then, that ATP might preferentially permeabilize cancerous and HPV- immortalized cells relative to normal cells, imparting some specificity to the susceptibility of cells to the toxic effects of Hoechst 33258. Determining the mechanism by which ATP evoked the uptake of Hoechst 33258, and establishing why this mechanism is preferentially expressed in cancer cells versus normal cells is a goal of future work.
This apparent ability to pharmacologically activate plasma membrane uptake and accumulation of a drug is reminiscent of a recent series of studies involving delivery of an anesthetic into pain- and itch processing sensory neurons. Here, the investigators discovered that they could selectively deliver an otherwise membrane impermeable lidocaine derivative into pain and itch processing cells through the activated TRPV1 and TRPA1 channels respectively (36–39). It remains to be determined whether an ion channel activated downstream of P2Y receptors is responsible for the final transport of Hoechst 33258 that we observe in cervical cancer cells, and exploring this possibility will be the focus of future experiments.
To conclude, we have demonstrated the feasibility of pharmacologically permeabilizing cervical cancer cells to an otherwise weakly membrane permeable cationic cytotoxin. In cervical cells, the observed P2Y receptor-dependent drug delivery mechanism appears to be preferentially active in cancerous cells, possibly due to the upregulation of some or several required components of the mechanism. This provides a potential selectivity bonus by which ionic cytotoxins that would not be easily transported into healthy cells could have their uptake into cancer cells facilitated.
Highlights.
Application of extracellular adenosine 5′-triphosphate (ATP) stimulated uptake and accumulation of the ionic DNA-binding cytotoxin, Hoechst 33258, into cervical cancer cells
Cervical cancer cells exhibited a higher sensitivity to ATP-stimulated Hoechst 33258 uptake than cervical epithelial cells obtained from normal transformation zone and ectocervical tissue
Acknowledgments
We would like to thank Stanko Stojilkovic (National Institute of Health, Bethesda, MD) for the gift of the human P2X7 cDNA-containing plasmid. This work was partially supported by an award from NCI 1R15CA173703-01. The authors thank the Cooperative Human Tissue network for providing samples of normal and malignant human cervical tissue.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zhao Y, Butler EB, Tan M. Cell death & disease. 2013;4:e532. doi: 10.1038/cddis.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gurova K. Future oncology. 2009;5:1685–1704. doi: 10.2217/fon.09.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porporato PE, Dhup S, Dadhich RK, Copetti T, Sonveaux P. Frontiers in pharmacology. 2011;2:49. doi: 10.3389/fphar.2011.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li H, Nelson CE, Evans BC, Duvall CL. Current pharmaceutical design. 2011;17:293–319. doi: 10.2174/138161211795049642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hampton T. JAMA : the journal of the American Medical Association. 2011;305:549–551. [Google Scholar]
- 6.Janigro D, Perju C, Fazio V, Hallene K, Dini G, Agarwal MK, Cucullo L. BMC cancer. 2006;6:72. doi: 10.1186/1471-2407-6-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keppler D. Handbook of experimental pharmacology. 2011:299–323. doi: 10.1007/978-3-642-14541-4_8. [DOI] [PubMed] [Google Scholar]
- 8.Kuo MT, Chen HH, Song IS, Savaraj N, Ishikawa T. Cancer metastasis reviews. 2007;26:71–83. doi: 10.1007/s10555-007-9045-3. [DOI] [PubMed] [Google Scholar]
- 9.Nuttle LC, Dubyak GR. The Journal of biological chemistry. 1994;269:13988–13996. [PubMed] [Google Scholar]
- 10.Wiley JS, Chen R, Jamieson GP. Archives of biochemistry and biophysics. 1993;305:54–60. doi: 10.1006/abbi.1993.1392. [DOI] [PubMed] [Google Scholar]
- 11.Tatham PE, Cusack NJ, Gomperts BD. European journal of pharmacology. 1988;147:13–21. doi: 10.1016/0014-2999(88)90628-0. [DOI] [PubMed] [Google Scholar]
- 12.Steinberg TH, Newman AS, Swanson JA, Silverstein SC. The Journal of biological chemistry. 1987;262:8884–8888. [PubMed] [Google Scholar]
- 13.Yan Z, Li S, Liang Z, Tomic M, Stojilkovic SS. The Journal of general physiology. 2008;132:563–573. doi: 10.1085/jgp.200810059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munerati M, Cortesi R, Ferrari D, Di Virgilio F, Nastruzzi C. Biochimica et biophysica acta. 1994;1224:269–276. doi: 10.1016/0167-4889(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 15.Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. Science (New York, NY) 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- 16.Browne LE, Compan V, Bragg L, North RA. J Neurosci. 2013;33:3557–3566. doi: 10.1523/JNEUROSCI.2235-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donnelly-Roberts DL, Namovic MT, Faltynek CR, Jarvis MF. The Journal of pharmacology and experimental therapeutics. 2004;308:1053–1061. doi: 10.1124/jpet.103.059600. [DOI] [PubMed] [Google Scholar]
- 18.Faria RX, Reis RA, Casabulho CM, Alberto AV, de Farias FP, Henriques-Pons A, Alves LA. American journal of physiology. 2009;297:C28–42. doi: 10.1152/ajpcell.00476.2008. [DOI] [PubMed] [Google Scholar]
- 19.Cankurtaran-Sayar S, Sayar K, Ugur M. Molecular pharmacology. 2009;76:1323–1332. doi: 10.1124/mol.109.059923. [DOI] [PubMed] [Google Scholar]
- 20.Schachter J, Motta AP, de Souza Zamorano A, da Silva-Souza HA, Guimaraes MZ, Persechini PM. Journal of cell science. 2008;121:3261–3270. doi: 10.1242/jcs.029991. [DOI] [PubMed] [Google Scholar]
- 21.Bailly C. Current medicinal chemistry. 2000;7:39–58. doi: 10.2174/0929867003375489. [DOI] [PubMed] [Google Scholar]
- 22.Chen AY, Yu C, Gatto B, Liu LF. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:8131–8135. doi: 10.1073/pnas.90.17.8131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woynarowski JM, McHugh M, Sigmund RD, Beerman TA. Molecular pharmacology. 1989;35:177–182. [PubMed] [Google Scholar]
- 24.Chen AY, Yu C, Bodley A, Peng LF, Liu LF. Cancer research. 1993;53:1332–1337. [PubMed] [Google Scholar]
- 25.Kraut EH, Fleming T, Segal M, Neidhart JA, Behrens BC, MacDonald J. Investigational new drugs. 1991;9:95–96. doi: 10.1007/BF00194556. [DOI] [PubMed] [Google Scholar]
- 26.Patel SR, Kvols LK, Rubin J, O’Connell MJ, Edmonson JH, Ames MM, Kovach JS. Investigational new drugs. 1991;9:53–57. doi: 10.1007/BF00194545. [DOI] [PubMed] [Google Scholar]
- 27.Bellamy WT. Annual review of pharmacology and toxicology. 1996;36:161–183. doi: 10.1146/annurev.pa.36.040196.001113. [DOI] [PubMed] [Google Scholar]
- 28.Woodworth CD, Diefendorf LP, Jette DF, Mohammed A, Moses MA, Searleman SA, Stevens DA, Wilton KM, Mondal S. Virology. 2011;421:19–27. doi: 10.1016/j.virol.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rassendren F, Buell GN, Virginio C, Collo G, North RA, Surprenant A. The Journal of biological chemistry. 1997;272:5482–5486. doi: 10.1074/jbc.272.9.5482. [DOI] [PubMed] [Google Scholar]
- 30.Li X, Qi X, Zhou L, Catera D, Rote NS, Potashkin J, Abdul-Karim FW, Gorodeski GI. Gynecologic oncology. 2007;106:233–243. doi: 10.1016/j.ygyno.2007.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X, Zhou L, Feng YH, Abdul-Karim FW, Gorodeski GI. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2006;15:1906–1913. doi: 10.1158/1055-9965.EPI-06-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okuda A, Furuya K, Kiyohara T. Cell biochemistry and function. 2003;21:61–68. doi: 10.1002/cbf.992. [DOI] [PubMed] [Google Scholar]
- 33.Gorodeski GI, Burfeind P, Gan SU, Pal D, Abdul-Karim FW. The American journal of physiology. 1998;275:C758–765. doi: 10.1152/ajpcell.1998.275.3.C758. [DOI] [PubMed] [Google Scholar]
- 34.van Hell AJ, Melo MN, van Blitterswijk WJ, Gueth DM, Braumuller TM, Pedrosa LR, Song JY, Marrink SJ, Koning GA, Jonkers J, Verheij M. Scientific reports. 2013;3:1949. doi: 10.1038/srep01949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Donnelly-Roberts DL, Namovic MT, Han P, Jarvis MF. British journal of pharmacology. 2009;157:1203–1214. doi: 10.1111/j.1476-5381.2009.00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Binshtok AM, Bean BP, Woolf CJ. Nature. 2007;449:607–610. doi: 10.1038/nature06191. [DOI] [PubMed] [Google Scholar]
- 37.Puopolo M, Binshtok AM, Yao GL, Oh SB, Woolf CJ, Bean BP. Journal of neurophysiology. 2013 doi: 10.1152/jn.00012.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roberson DP, Binshtok AM, Blasl F, Bean BP, Woolf CJ. British journal of pharmacology. 2011;164:48–58. doi: 10.1111/j.1476-5381.2011.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberson DP, Gudes S, Sprague JM, Patoski HA, Robson VK, Blasl F, Duan B, Oh SB, Bean BP, Ma Q, Binshtok AM, Woolf CJ. Nature neuroscience. 2013;16:910–918. doi: 10.1038/nn.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]