Abstract
Background
The combination of gemcitabine and docetaxel is standard first-line therapy for recurrent or metastatic uterine leiomyosarcoma. There is no standard second-line therapy. Ixabepilone is a semi-synthetic analog of epothilone B that binds to the same site on beta tubulin as paclitaxel and may be a more potent polymerizer of tubulin. We sought to determine activity of ixabepilone as a single agent as second-line treatment for patients with metastatic uterine leiomyosarcoma who had received taxane based therapy.
Methods
Eligible women with unresectable uterine leiomyosarcoma progressing after prior cytotoxic therapy containing a taxane were treated with ixabepilone 40 mg/m2 on day one of a 21 day cycle. Patients with prior pelvic radiation were treated without dose reduction. Response Evaluation Criteria in Solid Tumors (RECIST) response was assessed by computed tomography (CT).
Results
Twenty-three of 26 women were evaluable (two wrong histology, one never treated) with two of 23 receiving one cycle of therapy. There were no complete or partial responses. Stable disease (SD) was seen in four patients (17.4%, median 3.4 months). Seventeen patients (73.9%) had increasing disease (PD) and two patients were inevaluable per RECIST. One patient had SD over six cycles of treatment. Median PFS for all 23 patients was 1.4 months and overall survival was 7.0 months. The predominant grade 3 or 4 toxicity was uncomplicated myelosuppression: neutropenia grade 3 (13%), grade 4 (17%), anemia grade 3 (22%).
Conclusion
Ixabepilone as a single agent is not an active second-line therapy for uterine leiomyosarcoma previously treated with a taxane.
Keywords: Ixabepilone, uterine leiomyosarcoma
Introduction
Uterine sarcomas are rare malignancies, accounting for up to 7% of all uterine cancers and only 1% of all female genital tract malignancies [1]. Of these, leiomyosarcoma is the most common subtype [1-3]. Even when confined to the uterus, the prognosis for uterine leiomyosarcoma may be poor, with overall (all stages) recurrence rates ranging from 53-71% and overall survival (OS) rates ranging from 15-25% with a median survival of approximately 10 months [1-6].
As reflected above, most patients with uterine leiomyosarcoma will experience distant metastases regardless of stage [7]. Although oligometastatic disease may be treated surgically, systemic therapy is utilized in cases where multi-site disease is present, or when there has been a relapse after an attempt at loco regional control with surgical resection. Numerous single agents have been tested in patients with leiomyosarcoma with no agent exceeding the response rate of 25% seen with doxorubicin among a chemotherapy-naïve population [8-23].
Combination therapy has been demonstrated to have higher response rates at the cost of higher toxicity [23-25]. Historically, the highest response rate of 30% was obtained using ifosfamide and doxorubicin in patients with no history of prior treatment [26]. More recently, a Phase II single institution study and the subsequent group study Gynecologic Oncology Group (GOG) 87L have established the best response rate for first line treatment with the combination of gemcitabine plus docetaxel, with an overall response rate (ORR) of 35.8% in the GOG study [27,28]. On the basis of these trials, most women will be treated with this combination, either as adjuvant treatment or in the setting of a first relapse.
Despite the possibility of initial success with this combination, most patients will experience disease recurrence. In the setting of recurrent disease following first line therapy, there is clearly a need for development of new agents for the treatment of recurrent leiomyosarcoma of the uterus, particularly in the setting of progression following docetaxel. GOG 131G demonstrated an ORR of 27.1% for gemcitabine and docetaxel as second line treatment. However, the patients in GOG 131G were not allowed prior docetaxel or gemcitabine for first line therapy [28,29]. At this time, there are no approved agents for recurrent leiomyosarcoma, emphasizing this as an unmet need. While doxorubicin is often used in the second line after gemcitabine and docetaxel in clinical practice, there is no prospective data to suggest its efficacy.
The epothilones are a novel class of non-taxane microtubule stabilizers with unique properties [30,31]. The cytotoxic activity of epothilones, like those of the taxanes, has been linked to suppression of cell growth by promoting accelerated assembly of stable microtubules, which consequently leads to cell cycle arrest at the G2-M transition, and eventual cell death [30,32]. Ixabepilone (BMS-247550) is a semi-synthetic analog of epothilone B that binds to the same site on beta tubulin as paclitaxel and, in preclinical models, appears to be a more potent polymerizer of tubulin than paclitaxel [30,31,33,34]. The unique properties of this agent may allow it to evade both acquired and intrinsic mechanisms of resistance to taxanes. Ixabepilone is a poor substrate for P-glycoprotein and MRP1, therefore potentially avoiding these drug efflux mechanisms. In addition, specific mutations in the ß-tubulin binding sites associated with resistance to paclitaxel were not associated with resistance to ixabepilone in preclinical models, suggesting that this epothilone might be associated with enhanced clinical activity compared to paclitaxel [32].
Ixabepilone has demonstrated antitumor activity in both taxane sensitive and taxane refractory cancer cell lines and tumors, including those overexpressing multidrug resistance (MDR) and those with mutations in the beta tubulin gene [35]. In both Phase I and II clinical trials, objective responses with ixabepilone have been shown in a broad range of tumor types, both those considered to be sensitive, as well as those considered relatively insensitive to taxanes [36]. Ixabepilone was chosen in GOG-131H because of the data suggesting responses in taxane resistant disease. The activity of ixabepilone in breast cancer and other solid tumors, even in patients refractory to taxanes, prompted a phase II clinical study with this agent in patients with recurrent or persistent endometrial carcinoma, who had failed one prior chemotherapy regimen [37]. In GOG-129P, ixabepilone was administered to 50 eligible patients with recurrent or persistent endometrial carcinoma. All patients had been treated previously with platinum, and the vast majority had also previously received a taxane. Ixabepilone produced objective responses in six patients (12%), which included one (2%) complete response that has lasted 4.9+ months and 5 (10%) partial responses lasting 4.2 to 19.8 months. Moreover, an additional 30 patients (60%) had stable disease lasting ≥8 weeks.
In light of this promising activity and the current trend to use taxanes as first line therapy for women with uterine leiomyosarcoma, the GOG initiated a study of ixabepilone in women with recurrent leiomyosarcoma previously treated with a taxane.
Materials and Methods
Patients
Women with persistent or recurrent uterine leiomyosarcoma refractory to curative therapy or established treatments were eligible for the study, provided they had received one prior cytotoxic regimen (single or multi-agent) that included a taxane. Patients were allowed to receive up to two prior regimens only if the first regimen did not include a taxane. Prior treatment with a non-cytotoxic agent (biologic/targeted or cytostatic) was permitted. Patients were required to have measurable disease as defined by RECIST 1.1, and at least one target lesion to be used to assess response. Response was assessed by RECIST [38].
Histologic confirmation was performed by central review of the GOG Pathology Committee. Patients were permitted to have had prior pelvic radiotherapy. Patients were required to have GOG performance status of 0-2, and adequate bone marrow function (absolute neutrophil count (ANC) ≥1500/μl, and platelets ≥100,000/μl); renal function (creatinine ≤1.5 × institutional upper limit of normal); hepatic function (bilirubin ≤1.5 × institutional upper limit of normal, and SGOT and alkaline phosphatase ≤2.5 × institutional upper limit of normal); and neurologic function (baseline neuropathy (sensory and motor) ≤ Common Toxicity Criteria grade 1).
All patients signed written, informed consent. The protocol and consent were reviewed and approved annually by participating institutions' Institutional Review Boards.
Treatment plan
All participants had baseline imaging (CT scan of chest, abdomen and pelvis) within four weeks of starting therapy, which was repeated following every other cycle of treatment to assess response. History and physical examination, and assessment of toxicities were performed each cycle. Complete blood counts were monitored weekly and comprehensive metabolic panels on day one of each cycle.
Participants received ixabepilone administered at 40 mg/m2 IV infusion over three hours on day one of a 21 day cycle. Treatment was continued with radiologic disease assessment every other cycle, until evidence of disease progression or unacceptable toxicity despite dose modification. The protocol prohibited the use of prophylactic growth factors such as filgastrim or sargramostin, unless there were recurrent neutropenic complications. Patients were allowed to receive erythropoietin, iron supplements and/or transfusions as clinically indicated for anemia. Recommended pre-medication for ixabepilone infusions were an H1 antagonist and an H2 antagonist one hour prior to infusion to reduce the risk of hypersensitivity reactions. Treatment was continued until time of objective progression of disease, or unacceptable toxicity.
Patients received day one treatment of each cycle provided the ANC was ≥1500/μl and platelet count ≥100,000/μl. Therapy could be delayed for a maximum of two weeks until those values were achieved. Patients with grade 4 thrombocytopenia, febrile neutropenia, or grade 4 neutropenia persisting ≥7 days were reduced one dose level. (One level reduction 32 mg/m2 and two level reduction 25 mg/m2). If cytopenia persisted despite two dose reductions, the patient was removed from study. Treatment was delayed for a maximum of three weeks for grade 2 or greater peripheral neuropathy, grade 2 or greater renal toxicity, grade 3 or greater elevations in liver enzymes, alkaline phosphatase or bilirubin, until these values recovered to grade 1, with treatment resuming at one dose level reduction. Patients with nausea, emesis, diarrhea or constipation that was persistent (greater than 24 hours) grade 3 or greater, in spite of optimal medical management required one dose level reduction and delay in subsequent therapy for a maximum of two weeks until recovered to grade 1. All other non-hematologic toxicities with an impact on organ function of grade 2 or greater required a delay in subsequent therapy for a maximum of three weeks until recovered to grade 1 and a dose reduction with further therapy. Toxicities were graded according to National Cancer Institute Common Toxicity Criteria version 4.0 (CTC 4.0).
Statistical design
This study employed an optimal but flexible two-stage design with an early stopping rule intended to limit patient accrual to inactive treatments [39]. During the first stage, the targeted accrual was 23 eligible patients, but was permitted to range from 19 to 26 patients. If more than two out of 19-25, or more than three out of 26 patients responded and medical judgment indicated, accrual to the second stage was to be initiated. Otherwise the study would be stopped and treatment regimen classified as uninteresting. If opened to the second stage, the overall study accrual would be to 48 eligible and evaluable patients, but was permitted to range from 44 to 51. If more than six out of 44-45, or more than seven out of 46 to 51 patients responded, then the regimen would be considered worthy of additional investigation. If the true probability of responding is only 10%, the study design provides a 90% chance of correctly classifying the treatment as inactive. Conversely, if the true response rate is 25%, then the average probability of correctly classifying the treatment as active is 90%. The figures for Progression Free survival (PFS) and overall survival (OS) were constructed using the technique of Kaplan-Meier [40].
Results
Patient characteristics
Twenty-six patients were enrolled from 15 GOG institutions. The first stage of accrual was achieved over 23 months following study activation. Twenty-three women were considered evaluable (one patient ineligible due to wrong primary, one due to wrong cell type, one patient never treated). Patient characteristics are presented in Table 1. All patients had a GOG performance status of 0-1 and most patients (12, 46%) were between 50-59 years of age. Only one patient received two prior regimens; the rest had one prior regimen. The median age was 56.5 years (41-68). Seventeen patients were white and six were African-American. Four of 23 (17%) had received prior pelvic radiation. All patients had received one prior cytotoxic regimen containing a taxane. Seventeen patients (74%) received two cycles of treatment followed by progression of disease.
Table 1. Patient characteristics.
| Characteristics | Number of Cases |
|---|---|
| Age | |
| <50 | 5 |
| 50-59 | 12 |
| 60-69 | 6 |
| Performance Status | |
| 0 | 10 |
| 1 | 13 |
| Race | |
| White | 17 |
| Black | 6 |
| Prior Chemotherapy | 23 |
| Prior Radiotherapy | 6 |
| Courses | |
| 1 | 2 |
| 2 | 17 |
| 4 | 3 |
| 6 | 1 |
Response to treatment and survival
Treatment response is outlined in Table 2. Twenty-one patients were evaluable for response. There were no patients seen with CR or PR. SD was seen in four patients (17.4%) and 17 patients (73.9%) had increasing disease (PD). The median length of stable disease is 3.4 months (2.8, 2.9, 3.8, and 18.9 months).
Table 2. RECIST-defined responses to treatment (n=23).
| Response Category | No. of Cases | % of Cases |
|---|---|---|
| Stable Disease | 4 | 17.4 |
| Increasing Disease | 17 | 73.9 |
| Inevaluable | 2 | 8.7 |
| Total | 23 | 100.0 |
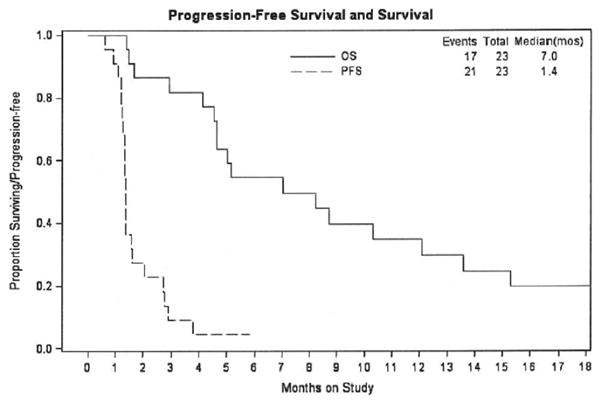
Twenty-one patients received at least two cycles of treatment. The median number of cycles per patient was two (range 1-6). One patient had SD over the course of six cycles of treatment, but ultimately had disease progression. Median progression-free survival (PFS) for all 23 patients was 1.4 months and OS was 7.0 months. PFS and OS curves are demonstrated in Figure 1.
Figure 1. Overall survival (OS) Progression free survival (PFS).
Adverse events
A summary of all adverse events of all grades is provided in Table 3. The predominant toxicity was myelosuppression: all grade 4 events were myelosuppressive. There were seven patients with grades 3 or 4 leukopenia, seven patients with grades 3 and 4 neutropenia, and five patients with grade 3 anemia. Of note, there were no reports of hypersensitivity in this study. The 4 patients who received prior pelvic radiotherapy contributed 2 of the 4 grade 4 neutropenic events but otherwise did not differ from the entire treatment group with respect to hematologic toxicity; there was one grade 4 anemia in this group and no grade 3 or 4 platelets.
Table 3. Selected adverse events considered at least possibly related to study treatment, all grades, by number of patients experiencing the event.
| Adverse Event | 0 | 1 | 2 | 3 | 4 | Total |
|---|---|---|---|---|---|---|
| Blood/lymphatics | ||||||
| Leukopenia | 5 | 5 | 6 | 5 | 2 | 23 |
| Thrombocytopenia | 17 | 6 | 0 | 0 | 0 | 23 |
| Neutropenia | 8 | 4 | 4 | 3 | 4 | 23 |
| Anemia | 2 | 10 | 6 | 5 | 0 | 23 |
| Platelet count decreased | 17 | 6 | 0 | 0 | 0 | 23 |
| Gastrointestinal | ||||||
| Nausea | 16 | 4 | 3 | 0 | 0 | 23 |
| Vomiting | 19 | 3 | 1 | 0 | 0 | 23 |
| Mucositis | 20 | 2 | 0 | 1 | 0 | 23 |
| Constipation | 15 | 7 | 1 | 0 | 0 | 23 |
| Diarrhea | 18 | 5 | 0 | 0 | 0 | 23 |
| Fatigue | 10 | 10 | 1 | 2 | 0 | 23 |
| Anorexia | 19 | 2 | 2 | 0 | 0 | 23 |
| Musculoskeletal/connective tissue | 19 | 3 | 1 | 0 | 0 | 23 |
| Arthralgia | ||||||
| Myalgia | 20 | 2 | 1 | 0 | 0 | 23 |
| Peripheral sensory neuropathy | 17 | 4 | 2 | 0 | 0 | 23 |
| Dizziness | 20 | 3 | 0 | 0 | 0 | 23 |
| Dyspnea | 19 | 2 | 1 | 1 | 0 | 23 |
| Alopecia | 13 | 4 | 6 | 0 | 0 | 23 |
Discussion
For years, doxorubicin, with or without ifosfamide, was the standard first line chemotherapy treatment for women with metastatic leiomyosarcoma, with no standard options for second line therapy. In the past decade, this first line therapy has almost exclusively changed to the combination of gemcitabine and docetaxel in the first line, based on the data from two recent studies [27,29]. There is, in addition, preliminary data to suggest that women with uterus confined disease at the time of initial diagnosis may benefit from adjuvant treatment with gemcitabine and docetaxel, given with the gemcitabine as a fixed dose rate or as a bolus infusion [41]. Therefore, most women with advanced or recurrent uterine leiomyosarcoma are likely to be treated with a taxane based therapy as first line therapy, and essentially all of these patients will ultimately experience recurrence and be candidates for salvage therapy. Unfortunately, options for second line therapy remain limited, particularly for patients already treated with a taxane. Therefore, second-line treatment remains an unmet medical need.
Ixabepilone was chosen in GOG-131H because of the data suggesting responses in taxane resistant disease. In GOG-129P, ixabepilone was administered to patients with endometrial cancer, and produced an ORR of 12%, with an additional 60% of patients having stable disease [37]. Ixabepilone was very well tolerated in recurrent leiomyosarcoma patients, even in those patients with prior pelvic radiation, with limited to no toxicity other than myelosuppression that was easily managed. Unfortunately, the responses seen in endometrial cancer in GOG-129P and in taxane resistant breast cancer were not seen in the setting of uterine leiomyosarcoma. Similarly, a recent Phase II study in pediatric sarcoma did not show a response rate to ixabepilone [42].
Given the lack of activity of ixabepilone in previously treated sarcoma patients, it is likely that future studies will need to investigate novel agents and targeted agents, which might be tested with or without cytotoxic therapy. The work done in other soft tissue sarcomas may provide a useful starting point for the treatment of uterine leiomyosarcoma. For example, there has been promising data with respect to targeted agents and soft tissue sarcomas. One Phase III randomized double blind placebo controlled study in patients with metastatic and recurrent soft tissue sarcoma demonstrated a survival advantage for pazopanib over placebo, with an increase in PFS of 4.6 months vs 1.6 months (HR: 0.31, 95%CI: 0.24–0.40; p < 0.0001) and an increase in OS of 12.5 months vs 10.7 months with placebo (HR: 0.86, 95%CI: 0.67–1.1; p = 0.25)[43]. The combined data specifically regarding uterine sarcomas from this study as well as the data from a recent EORTC phase II trial [44] were presented at the American Society of Clinical Oncology (ASCO) in 2014.[45] Of the 386 patients with intermediate or high grade soft tissue sarcoma treated in these two studies, 44 patients had uterine sarcoma and 39 of these had uterine leiomyosarcoma. There were 5 PR (11.4%) and 25 SD (56.8%) responses seen in the uterine leiomyosarcoma group; these response rates were almost identical to the response rates seen in the group as a whole (10.7% and 57.2%) suggesting that pazopanib induces similar response rates for patients with uterine sarcoma as compared to other soft tissue sarcomas. Morevoer, the median overall survival (OS) for patients with uterine sarcoma was longer compared to other subtypes in this small group (uterine OS 17.5 months compared to 11.1 months)
A scientific rationale to combine ixabepilone with pazopanib in patients with uterine leiomyosarcoma may exist, as a recent Phase I study in patients with solid tumors reported at American Association for Cancer Research (AACR) in 2013 has defined a maximally tolerated dose of the combination with a high rate of disease stabilization [46]. Not surprisingly, the main toxicity was myelosuppression. Ridaforolimus, a mammalian target of rapamycin (mTOR) inhibitor, has also been studied in patients with advanced bone and soft tissue sarcoma, with a clinical benefit rate (CR+PR+SD) of 33.3% [47]. Future study of recurrent uterine leiomyosarcoma may also benefit from combining these tumors with other soft tissue sarcomas, given the rarity of disease and the subsequent slow accrual to clinical trials. The subset analysis presented at ASCO 2014 as described above suggests that it is reasonable to include uterine leiomyosarcoma with other intermediate or high grade soft tissue sarcomas.
In conclusion, ixabepilone as a single agent is not a clinically active drug in patients with uterine leiomyosarcoma who have been treated with a prior taxane. Future investigation in this population should focus on new targeted agents and individualization of therapy where possible.
Research Highlights.
First line therapy in metastatic uterine leiomyosarcoma is gemcitabine and docetaxel
There is no standard second line therapy for metastatic uterine leiomyosarcoma
Ixabepilone is not active in second line for disease previously treated with taxane
Acknowledgments
This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group (GOG) Administrative Office (CA 27469) and the Gynecologic Oncology Group Statistical Office (CA 37517) and the NRG Oncology Grant (1 U10 CA180822). The following Gynecologic Oncology Group member institutions participated in the primary treatment studies: Abington Memorial Hospital, Walter Reed National Military Medical Center, University of Mississippi Medical Center, University of North Carolina at Chapel Hill, Rush University Medical Center, Cleveland Clinic Foundation, Washington University School of Medicine, Women's Cancer Center of Nevada, University of Oklahoma Health Sciences Center, University of Virginia, University of Chicago, Case Western Reserve University, University of Wisconsin Hospital and Clinics, Women and Infants Hospital, and Carolinas Medical Center/Levine Cancer Institute.
Footnotes
This study was registered at www.clinicaltrials.gov, NCT01220609, on October 12, 2010.
Conflicts of Interest: Dr. Don Dizon is employed at UpToDate as Deputy Editor, Oncology. All other coauthors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Linda R. Duska, Email: lduska@virginia.edu.
John A. Blessing, Email: blessing@gogstats.org.
Jacob Rotmensch, Email: jacob_rotmensch@rush.edu.
Robert S. Mannel, Email: Robert-mannel@ouhsc.edu.
Parviz Hanjani, Email: phanjani@amh.org.
Peter G. Rose, Email: rosep@ccf.org.
Don S. Dizon, Email: ddizon@partners.org.
References
- 1.D'Angelo E, Prat J. Uterine sarcomas: a review. Gynecol Oncol. 2010;116:131–139. doi: 10.1016/j.ygyno.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 2.Nordal RR, Thoresen SO. Uterine sarcomas in Norway 1956-1992: incidence, survival and mortality. Eur J Cancer. 1997;33:907–911. doi: 10.1016/s0959-8049(97)00040-3. [DOI] [PubMed] [Google Scholar]
- 3.Oláh KS, Dunn JA, Gee H. Leiomyosarcomas have a poorer prognosis than mixed mesodermal tumours when adjusting for known prognostic factors: the result of a retrospective study of 423 cases of uterine sarcoma. Br J Obstet Gynaecol. 1992;99:590–594. doi: 10.1111/j.1471-0528.1992.tb13827.x. [DOI] [PubMed] [Google Scholar]
- 4.Major FJ, Blessing JA, Silverberg SG, Morrow CP, Creasman WT, Currie JL, et al. Prognostic factors in early-stage uterine sarcoma. A Gynecologic Oncology Group study. Cancer. 1993;71(4 Suppl):1702–1709. doi: 10.1002/cncr.2820710440. [DOI] [PubMed] [Google Scholar]
- 5.Abeler VM, Royne O, Thoresen S, Danielsen HE, Nesland JM, Kristensen GB Uterine sarcomas in Norway. A histopathological and prognostic survey of a total population from 1970 to 2000 including 419 patients. Histopathology. 2009;54:355–364. doi: 10.1111/j.1365-2559.2009.03231.x. [DOI] [PubMed] [Google Scholar]
- 6.D'Angelo E, Spagnoli LG, Prat J. Comparative clinicopathologic and immunohistochemical analysis of uterine sarcomas diagnosed using the World Health Organization classification system. Hum Pathol. 2009;40:1571–1585. doi: 10.1016/j.humpath.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 7.Gadducci A, Landoni F, Sartori E, Zola P, Maggino T, Lissoni A, et al. Uterine leiomyosarcoma: analysis of treatment failures and survival. Gynecol Oncol. 1996;62:25–32. doi: 10.1006/gyno.1996.0185. [DOI] [PubMed] [Google Scholar]
- 8.Thigpen JT, Blessing JA, Beecham J, Homesley H, Yordan E. Phase II trial of cisplatin as first-line chemotherapy in patients with advanced or recurrent uterine sarcomas: a Gynecologic Oncology Group study. J Clin Oncol. 1991;9:1962–1966. doi: 10.1200/JCO.1991.9.11.1962. [DOI] [PubMed] [Google Scholar]
- 9.Thigpen JT, Blessing JA, Wilbanks GD Cisplatin as second-line chemotherapy in the treatment of advanced or recurrent leiomyosarcoma of the uterus. A phase II trial of the Gynecologic Oncology Group. Am J Clin Oncol. 1986;9:18–20. doi: 10.1097/00000421-198602000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Muss HB, Bundy BN, Adcock L, Beecham J Mitoxantrone in the treatment of advanced uterine sarcoma. A phase II trial of the Gynecologic Oncology Group. Am J Clin Oncol. 1990;13:32–34. doi: 10.1097/00000421-199002000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Asbury R, Blessing JA, Buller R, Malfetano JH, Walker J, Sevin BU. Amonafide in patients with leiomyosarcoma of the uterus: a phase II Gynecologic Oncology Group study. Am J Clin Oncol. 1998;21:145–146. doi: 10.1097/00000421-199804000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Rose PG, Blessing JA, Soper JT, Barter JF. Prolonged oral etoposide in recurrent or advanced leiomyosarcoma of the uterus: a gynecologic oncology group study. Gynecol Oncol. 1998;70:267–271. doi: 10.1006/gyno.1998.5080. [DOI] [PubMed] [Google Scholar]
- 13.Slayton RE, Blessing JA, Angel C, Berman M. Phase II trial of etoposide in the management of advanced and recurrent leiomyosarcoma of the uterus: a Gynecologic Oncology Group Study. Cancer Treat Rep. 1987;71:1303–1304. [PubMed] [Google Scholar]
- 14.Slayton RE, Blessing JA, Look K, Anderson B A phase II clinical trial of diaziquone (AZQ) in the treatment of patients with recurrent leiomyosarcoma of the uterus. A Gynecologic Oncology Group study. Invest New Drugs. 1991;9:207–208. doi: 10.1007/BF00175091. [DOI] [PubMed] [Google Scholar]
- 15.Miller DS, Blessing JA, Kilgore LC, Mannel R, Van Le L. Phase II trial of topotecan in patients with advanced, persistent, or recurrent uterine leiomyosarcomas: a Gynecologic Oncology Group Study. Am J Clin Oncol. 2000;23:355–357. doi: 10.1097/00000421-200008000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Gallup DG, Blessing JA, Andersen W, Morgan MA. Evaluation of paclitaxel in previously treated leiomyosarcoma of the uterus: a gynecologic oncology group study. Gynecol Oncol. 2003;89:48–51. doi: 10.1016/s0090-8258(02)00136-1. [DOI] [PubMed] [Google Scholar]
- 17.Sutton G, Blessing JA, Ball H. Phase II trial of paclitaxel in leiomyosarcoma of the uterus: a gynecologic oncology group study. Gynecol Oncol. 1999;74:346–349. doi: 10.1006/gyno.1999.5463. [DOI] [PubMed] [Google Scholar]
- 18.McMeekin DS, Sill MW, Darcy KM, Stearns-Kurosawa DJ, Webster K, Waggoner S, et al. A phase II trial of thalidomide in patients with refractory leiomyosarcoma of the uterus and correlation with biomarkers of angiogenesis: a gynecologic oncology group study. Gynecol Oncol. 2007;106:596–603. doi: 10.1016/j.ygyno.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 19.Smith HO, Blessing JA, Vaccarello L. Trimetrexate in the treatment of recurrent or advanced leiomyosarcoma of the uterus: a phase II study of the Gynecologic Oncology Group. Gynecol Oncol. 2002;84:140–144. doi: 10.1006/gyno.2001.6482. [DOI] [PubMed] [Google Scholar]
- 20.Sutton GP, Blessing JA, Barrett RJ, McGehee R. Phase II trial of ifosfamide and mesna in leiomyosarcoma of the uterus: a Gynecologic Oncology Group study. Am J Obstet Gynecol. 1992 Feb;166(2):556–559. doi: 10.1016/0002-9378(92)91671-v. [DOI] [PubMed] [Google Scholar]
- 21.Sutton GP, Blessing JA, Photopulos G, Berman ML, Homesley HD. Early phase II Gynecologic Oncology Group experience with ifosfamide/mesna in gynecologic malignancies. Cancer Chemother Pharmacol. 1990;(26 Suppl):S55–58. doi: 10.1007/BF00685421. [DOI] [PubMed] [Google Scholar]
- 22.Look KY, Sandler A, Blessing JA, Lucci JA, 3rd, Rose PG. Phase II trial of gemcitabine as second-line chemotherapy of uterine leiomyosarcoma: a Gynecologic Oncology Group (GOG) Study. Gynecol Oncol. 2004;92:644–647. doi: 10.1016/j.ygyno.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 23.Omura GA, Major FJ, Blessing JA, Sedlacek TV, Thigpen JT, Creasman WT, et al. A randomized study of adriamycin with and without dimethyl triazenoimidazole carboxamide in advanced uterine sarcomas. Cancer. 1983;52:626–632. doi: 10.1002/1097-0142(19830815)52:4<626::aid-cncr2820520409>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 24.Muss HB, Bundy B, DiSaia PJ, Homesley HD, Fowler WC, Jr, Creasman W, et al. Treatment of recurrent or advanced uterine sarcoma. A randomized trial of doxorubicin versus doxorubicin and cyclophosphamide (a phase III trial of the Gynecologic Oncology Group) Cancer. 1985;55:1648–1653. doi: 10.1002/1097-0142(19850415)55:8<1648::aid-cncr2820550806>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 25.Currie J, Blessing JA, Muss HB, Fowler J, Berman M, Burke TW. Combination chemotherapy with hydroxyurea, dacarbazine (DTIC), and etoposide in the treatment of uterine leiomyosarcoma: a Gynecologic Oncology Group study. Gynecol Oncol. 1996;61:27–30. doi: 10.1006/gyno.1996.0091. [DOI] [PubMed] [Google Scholar]
- 26.Sutton G, Blessing JA, Malfetano JH. Ifosfamide and doxorubicin in the treatment of advanced leiomyosarcomas of the uterus: a Gynecologic Oncology Group study. Gynecol Oncol. 1996;62:226–229. doi: 10.1006/gyno.1996.0220. [DOI] [PubMed] [Google Scholar]
- 27.Hensley ML, Maki R, Venkatraman E, Geller G, Lovegren M, Aghajanian C, et al. Gemcitabine and docetaxel in patients with unresectable leiomyosarcoma: results of a phase II trial. J Clin Oncol. 2002;20:2824–2831. doi: 10.1200/JCO.2002.11.050. [DOI] [PubMed] [Google Scholar]
- 28.Hensley ML, Blessing JA, Mannel R, Rose PG. Fixed-dose rate gemcitabine plus docetaxel as first-line therapy for metastatic uterine leiomyosarcoma: a Gynecologic Oncology Group phase II trial. Gynecol Oncol. 2008;109:329–334. doi: 10.1016/j.ygyno.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hensley ML, Blessing JA, Degeest K, Abulafia O, Rose PG, Homesley HD. Fixed-dose rate gemcitabine plus docetaxel as second-line therapy for metastatic uterine leiomyosarcoma: a Gynecologic Oncology Group phase II study. Gynecol Oncol. 2008;109:323–328. doi: 10.1016/j.ygyno.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bollag DM, McQueney PA, Zhu J, Hensens O, Koupal L, Liesch J, et al. Epothilones, a new class of microtubule-stabilizing agents with a taxol-like mechanism of action. Cancer Res. 1995;55:2325–2333. [PubMed] [Google Scholar]
- 31.Cortes J, Baselga J. Targeting the microtubules in breast cancer beyond taxanes: the epothilones. Oncologist. 2007;12:271–280. doi: 10.1634/theoncologist.12-3-271. [DOI] [PubMed] [Google Scholar]
- 32.Kavallaris M, Verrills NM, Hill BT. Anticancer therapy with novel tubulin-interacting drugs. Drug Resist Updat. 2001;4:392–401. doi: 10.1054/drup.2002.0230. [DOI] [PubMed] [Google Scholar]
- 33.Lee FY, Borzilleri R, Fairchild CR, Kim SH, Long BH, Reventos-Suarez C, et al. BMS-247550: a novel epothilone analog with a mode of action similar to paclitaxel but possessing superior antitumor efficacy. Clin Cancer Res. 2001;7:1429–1437. [PubMed] [Google Scholar]
- 34.Mani S, McDaid H, Hamilton A, Hochster H, Cohen MB, Khabelle D, et al. Phase I clinical and pharmacokinetic study of BMS-247550, a novel derivative of epothilone B, in solid tumors. Clin Cancer Res. 2004;10:1289–1298. doi: 10.1158/1078-0432.ccr-0919-03. [DOI] [PubMed] [Google Scholar]
- 35.Lee FY, Smykla R, Johnston K, Menard K, McGlinchey K, Peterson RW, et al. Preclinical efficacy spectrum and pharmacokinetics of ixabepilone. Cancer Chemother Pharmacol. 2009;63:201–212. doi: 10.1007/s00280-008-0727-5. [DOI] [PubMed] [Google Scholar]
- 36.Pivot X, Dufresne A, Villanueva C. Efficacy and safety of ixabepilone, a novel epothilone analogue. Clin Breast Cancer. 2007;7:543–549. doi: 10.3816/CBC.2007.n.009. [DOI] [PubMed] [Google Scholar]
- 37.Dizon DS, Blessing JA, McMeekin DS, Sharma SK, Disilvestro P, Alvarez RD. Phase II trial of ixabepilone as second-line treatment in advanced endometrial cancer: gynecologic oncology group trial 129-P. J Clin Oncol. 2009;27:3104–3108. doi: 10.1200/JCO.2008.20.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 39.Chen TT, NG T. Optimal flexible designs in phase II clinical trials. Statistics in Medicine. 1998;17:2301–2312. doi: 10.1002/(sici)1097-0258(19981030)17:20<2301::aid-sim927>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 40.Kaplan EL, Meier P. Non parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 41.Hensley ML, Wathen JK, Maki RG, Araujo DM, Sutton G, Priebat DA, et al. Adjuvant therapy for high-grade, uterus-limited leiomyosarcoma: results of a phase 2 trial (SARC 005) Cancer. 2013;119:1555–1561. doi: 10.1002/cncr.27942. [DOI] [PubMed] [Google Scholar]
- 42.Jacobs S, Fox E, Krailo M, Hartley G, Navid F, Wexler L, et al. Phase II trial of ixabepilone administered daily for five days in children and young adults with refractory solid tumors: a report from the children's oncology group. Clin Cancer Res. 2010;16:750–754. doi: 10.1158/1078-0432.CCR-09-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Graaf WT, Blay JY, Chawla SP, Kim DW, Bui-Nguyen B, Casali PG, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379:1879–1886. doi: 10.1016/S0140-6736(12)60651-5. [DOI] [PubMed] [Google Scholar]
- 44.Sleijfer S, Ray-Coquard I, Papai Z, Le Cesne A, Scurr M, Schöffski P, et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: a phase II study from the European organisation for research and treatment of cancer-soft tissue and bone sarcoma group (EORTC study 62043) J Clin Oncol. 2009;27:3126–3132. doi: 10.1200/JCO.2008.21.3223. [DOI] [PubMed] [Google Scholar]
- 45.Ray-Coquard I, Sleijfer S, Litière S, Blay J, Le Cesne A, Papai Z, et al. Pazopanib in uterine sarcoma: Review of two European Organisation for Research and Treatment of Cancer (EORTC) and GSK clinical trials 62043 and 62072 on pazopanib for soft tissue sarcoma (STS) J Clin Oncol. 2014;32(suppl):5s. abstr 10579. [Google Scholar]
- 46.Ganesan C, Obulareddy S, Fischer JH, Antonysamy MA, Jha G, Bliss RL, et al. Phase I study of pazopanib and ixabepilone in patients with solid tumors. Am J Clin Oncol. 2014 Feb 26; doi: 10.1097/COC.0000000000000053. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 47.Chawla SP, Staddon AP, Baker LH, Schuetze Sm, Tolcher AW, D'Amato GZ, et al. Phase II study of the mammalian target of rapamycin inhibitor ridaforolimus in patients with advanced bone and soft tissue sarcomas. J Clin Oncol. 2012;30:78–84. doi: 10.1200/JCO.2011.35.6329. [DOI] [PubMed] [Google Scholar]


