Abstract
Background
Although Brazil has model HIV care programs, many patients continue to present late to care. We studied the frequency of tuberculosis (TB) diagnosed at HIV diagnosis in Rio de Janeiro, Brazil, in order to quantify missed opportunities for TB prevention.
Methods
People living with HIV (PLHIV) and enrolled in the TB/HIV in Rio (THRio) study between 1 September 2005 and 31 August 2009 were included. Prevalent TB was defined as TB diagnosed within 60 days of HIV diagnosis or HIV diagnosis during TB therapy. Survival was measured from HIV diagnosis. We conducted Kaplan-Meier survival plots and Cox regression analyses.
Results
4,548 newly diagnosed PLHIV were enrolled; 476(10.5%) with prevalent TB. Prevalent TB cases were older, had lower CD4 counts and higher viral loads than those without TB. Median time to receiving highly active antiretroviral therapy (HAART) in prevalent TB cases was 99 days(IQR=58–191) vs. 126 days(IQR=63–301) in those without TB(p=0.021). Among prevalent TB cases, 17% died during follow-up compared to 8% among non-TB cases(p<0.001). After adjustment for sex, age, baseline CD4 and baseline viral load, risk of death remained significantly higher among prevalent TB cases[aHR=1.72(CI 95% 1.19–2.48)].
Conclusions
More than 10% of newly PLHIV in our study presented to care with concurrent active TB disease and thus missed the opportunity for TB preventive therapy. Despite initiating HAART more quickly, these were at significantly greater risk of death. Earlier HIV diagnosis is necessary to provide earlier initiation of HAART and TB preventive therapy to reduce morbidity and mortality in PLHIV.
Introduction
Although Brazil has been recognized as having model HIV/AIDS care programs1, many patients continue to present late to care2, thus, missing opportunities for preventive interventions and prolonging survival3. In the early era of HIV/AIDS, co-trimoxazole prophylaxis became widely adopted as an effective measure to prevent Pneumocysitis jirovecii pneumonia, and subsequently was shown to reduce morbidity and mortality in people living with HIV (PLHIV) in developing countries4,5. Tuberculosis (TB) is now the leading killer of PLHIV6, and highly active antiretroviral therapy (HAART) is the most effective way to reduce the risk of TB among them7. However, mounting scientific evidence8 shows that isoniazid preventive therapy (IPT) reduces the risk of active TB and may reduce the risk of death in PLHIV with latent tuberculosis infection (LTBI)9. In Brazil, studies of IPT in PLHIV demonstrate reduced incidence of active TB10 and improved survival11 that is additive to the impact of HAART. Brazil has a high burden of TB in PLHIV12,13,14, with most recent estimates stating that 9% of HIV deaths are due to TB15. Brazilian guidelines recommending tuberculin skin tests and isoniazid preventive therapy for PLHIV have existed since 199516 and recent recommendations from the World Health Organization restate the need for IPT for PLHIV, though evidence in Brazil suggests that physicians do not adhere strictly to these guidelines17.
Persons diagnosed with both HIV and TB simultaneously are missed opportunities for TB prevention. We report the frequency of TB at the time of HIV diagnosis in new patients entering the TB/HIV in Rio (THRio)18,19 study to quantify missed opportunities for TB prevention and to measure the impact on survival of newly diagnosed PLHIV co-infected with active TB disease in this population.
Methods
The THRio study was a cluster-randomized trial assessing the impact of implementing TB screening and IPT in public HIV clinics. THRio followed over 19,000 PLHIV receiving care in 29 public HIV clinics in Rio de Janeiro18,19. Trained data collectors routinely abstracted clinical and laboratory data from clinic medical records in a standardized manner. TB in Brazil is commonly diagnosed without microbiological confirmation, thus for purposes of this analysis, we included TB diagnosed either through microbiological confirmation or clinical suspicion based on symptoms and chest radiographic findings as recorded in the medical records. Anti-tuberculosis therapy is only available through the public sector in Brazil and must be reported, thus we supplemented our case detection through linkage of THRio patients with the Brazilian Health System for Mandatory Reporting Diseases (SINAN). Deaths were ascertained through linkage to the Brazilian Health Information Systems for Death (SIM), as described elsewhere20.
In this analysis, PLHIV entering care between 01 September 2005 and 31 August 2009 were included and followed until death (primary outcome of interest) or administratively censored at 31 December 2009. Prevalent TB was defined as TB diagnosed within 60 days following an HIV diagnosis, or a new HIV diagnosis made during the 180 days of standard TB therapy. All patients diagnosed with HIV should be screened for TB, thus we allowed for 60 days following HIV diagnosis for screening and laboratory results to be conducted and a TB diagnosis to be made. All patients diagnosed with TB should be screened for HIV; however, this screening is often delayed. Thus, we allowed for HIV results to be conducted during TB treatment and assumed that a positive HIV diagnosis during these 6 months meant that the patient had HIV at time of TB diagnosis. Thus, these two definitions were both considered to be prevalent TB in this analysis. Patients diagnosed with TB more than 180 days prior to HIV diagnosis were excluded from this analysis.
Survival was measured from HIV diagnosis date, comparing those with prevalent TB to those who entered the cohort TB-free (not prevalent or not concurrent TB). Only death or administrative censoring ended follow-up; thus other opportunistic infections, including TB, were not censoring variables. Patient demographics and other characteristics related to HIV care were compared between prevalent and TB-free cases using the chi-square test and t-test. We generated Nelson-Aalen cumulative hazard plots and we conducted Cox proportional hazards regression analyses. All analyses were performed using STATA statistical package (version 11; College Station, Texas).
Results
In the study period, 4,548 patients entered care with newly diagnosed HIV, of whom 476 (10.5%) were diagnosed with prevalent TB. There were 168 patients who were diagnosed with HIV during TB therapy and 308 patients diagnosed with TB within the first 60 days following HIV diagnosis. These two groups were similar regarding sex, age, baseline viral load, and days from HIV diagnosis until HAART began, and death, however, the group with TB diagnosed first had higher baseline CD4 (206 vs 133; p=0.041). There were 162 (3.6%) patients who had a TB diagnosis more than 180 days before their HIV diagnosis date and were excluded from the present analysis.
Prevalent TB patients were more likely to be male and slightly older than non-prevalent TB patients (Table 1). Prevalent TB patients had significantly lower CD4 counts (median: 162 cells/mm3 (interquartile range (IQR: 62–297) vs. 315 cells/mm3 (IQR: 148–511), p<0.001) at HIV diagnosis and slightly higher viral loads (median: 4.4 log (IQR: 2.3–5.1) vs. 4.1 log (IQR: 3.0–4.8), p=0.34) than those without TB. The median time to initiation of HAART after HIV diagnosis in prevalent TB cases was 99 days (IQR=58–191) compared to 126 days (IQR=63–301) in those without TB (p=0.02); 75% of the prevalent TB cases ever started HAART compared to 60% among non-prevalent TB patients (p<0.001).
Table 1.
Characteristics of prevalent and TB-free patients among newly HIV-diagnosed patients entering the THRio cohort, 2005–2009 (n=4,548).
| Characteristics | Prevalent TB (n=476) |
No concurrent TB (n=4,072) |
p-value |
|---|---|---|---|
| Sex (male) | 330 (69%) | 2,552 (63%) | 0.004 |
| Age (median; IQR) | 37 (30–45) | 35 (29–44) | 0.002 |
| Baseline CD4 (median; IQR, cells/mm3) | 162 (62–297) | 315 (148–511) | <0.001 |
| CD4 assay performed (baseline) | 85% | 90% | <0.001 |
| Baseline viral load log (median; IQR) | 4.4 (2.3–5.1) | 4.1 (3.0–4.8) | 0.338 |
| HAART (ever) | 75% | 60% | <0.001 |
| Days to HAART (median; IQR) | 99 (58–191) | 126 (63–301) | 0.021 |
| Death | 81 (17%) | 317 (8%) | <0.001 |
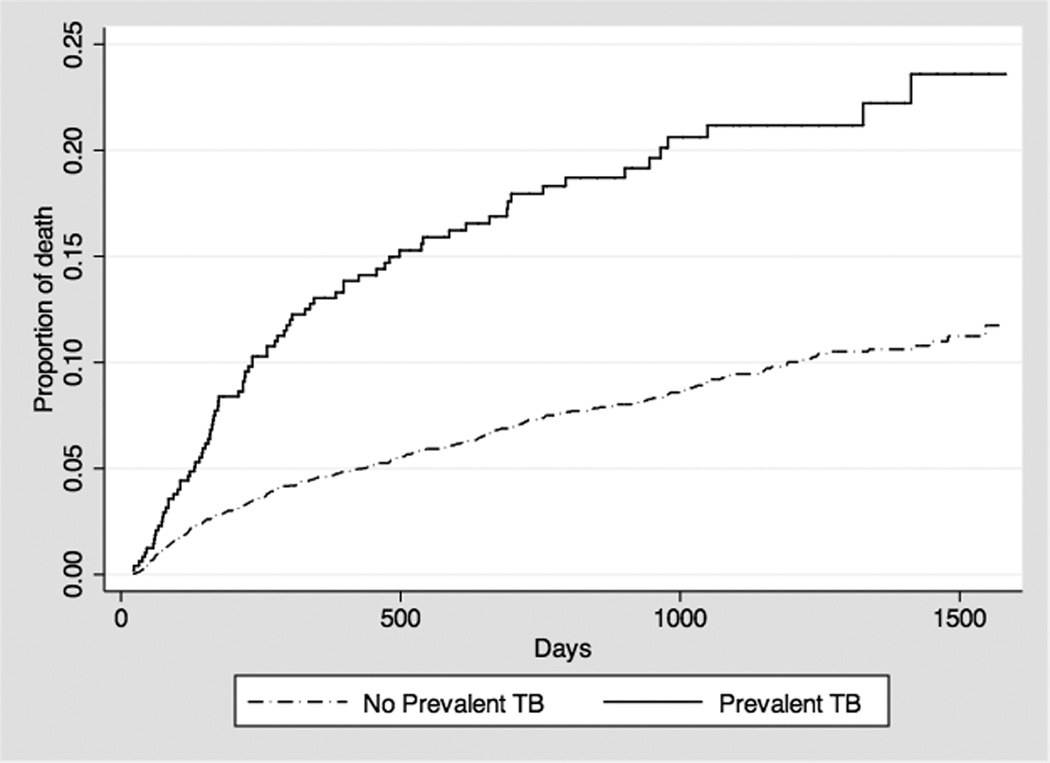
Among prevalent TB patients, 81 (17%) died during follow-up versus 317 (8%) among TB-free patients (p<0.0001) and the hazard of death among prevalent TB cases was significantly greater (log-rank test, p<0.001; Figure 1). Among prevalent TB patients, those who died were less likely to initiate HAART than those who survived (65% vs. 77%, p=0.02) and received HAART for a shorter median period of time (181 (IQR: 29 – 376) vs. 828 days (IQR: 451 – 1,157), p=0.02). Prevalent TB patients who died were less likely to have a CD4 count (52% vs. 92%; p<0.001) or a viral load (46% vs. 87%; p<0.001) assay performed at baseline than those who survived (Table 2).
Figure 1.
Cumulative hazard of death in newly HIV-diagnosed patients in the THRio cohort, prevalent TB versus no concurrent TB.
Table 2.
Characteristics of deceased and alive patients at the end of follow-up among those diagnosed with prevalent TB (n=476).
| Characteristics | Dead (n=81) |
Not dead N=395) |
p-value |
|---|---|---|---|
| Known CD4 at baseline | 52% | 92% | <0.001 |
| Known viral load at baseline | 46% | 87% | <0.001 |
| HAART ever | 65% | 77% | 0.022 |
| Median time on HAART (days) | 181 | 828 | 0.021 |
| Median survival time (days) | 218 | 975 | 0.002 |
Cox proportional hazards regression analysis showed that prevalent TB patients had a greater risk of death versus patients without prevalent TB [HR=2.33 (95% CI 1.82–2.97) p< 0.001]. After adjustment for sex, age, baseline CD4 cell count and baseline viral load, the hazard of death remained significantly greater for prevalent TB cases [aHR=1.72 (CI 95% 1.19–2.48)] (Table 3). Risk of death was inversely associated with baseline CD4 cell count.
Table 3.
Characteristics associated with death, multivariable Cox proportional hazards analysis.
| Characteristics | Adjusted Hazard Ratio |
95% CI | p-value |
|---|---|---|---|
| Prevalent TB | 1.72 | 1.19 – 2.48 | 0.004 |
| Sex (male) | 0.93 | 0.69 – 1.26 | 0.642 |
| Age (10 years) | 1.10 | 0.97 – 1.26 | 0.148 |
| CD4 < 200* | 1.00 | - | - |
| CD4 200 – 349* | 0.46 | 0.31 – 0.68 | <0.001 |
| CD4 350 – 499* | 0.43 | 0.28 – 0.68 | <0.001 |
| CD4 >=500* | 0.24 | 0.14 – 0.42 | <0.001 |
| Viral load (log) | 1.33 | 1.20 – 1.48 | <0.001 |
cells/mm3
Discussion
In this cohort of newly diagnosed PLHIV, a concurrent TB diagnosis was detected in 10.5% of patients and these patients had a significantly greater risk of death despite receiving HAART earlier than newly diagnosed PLHIV without TB. PLHIV are at great risk for TB and though effective preventive therapy is available10, patients newly diagnosed with TB at the time of HIV diagnosis represent missed opportunities for prevention. These patients, in addition to the 3.6% of the population with a prior TB diagnosis at time of HIV diagnosis, suggest that HIV diagnoses are not being made early enough in Brazil.
Late diagnosis of HIV in Brazil has been well documented21,22, with over 40% of patients considered late entries into care22. In our study, patients with prevalent TB at HIV diagnosis entered care later than expected, as evidenced by lower median CD4 cell counts (162 cells/mm3) than the TB-free population (315 cells/mm3) at time of HIV diagnosis. In Brazil, access to HIV care and universal provision of antiretroviral therapy should prompt earlier HIV diagnosis, thus providing PLHIV more opportunities for prevention of opportunistic infections21. Access to antiretroviral therapy has been shown to improve survival since the early days of combined antiretroviral therapy22, and in a scenario of universal access to HAART like Brazil, a reduction of 43% in mortality has been shown2, comparable to outcomes in the developed world23. Research in Brazil suggests that late entry into HIV care is likely greatest in regions of lower economic development where there are barriers to accessing health care22. Thus, our data adds to the evidence that despite free access to HIV diagnosis and testing in Brazil, uptake is poor.
In people with both TB and HIV diseases, HAART significantly improves survival when given within the first two months of TB therapy24–27, but survival is still poorer after a TB episode27–29. Late presentation to care among people living with HIV can reduce the odds of survival, and in Brazil late entry increased AIDS mortality probability in the first year in 36% of patients2, and has impaired the outcome of people living with HIV in the US as well3. Prevalent TB at baseline or soon after HIV diagnosis were strongly associated with elevated mortality risk in a recent South Africa study30. In our study, death among patients presenting with prevalent TB was higher for those who never received HAART; the median time to HAART among the prevalent TB cases was 99 days, strongly suggesting that “unmasking TB” following HAART initiation was not a concern, as has been suggested by others31.
Early provision of HAART is the primary benefit of early HIV diagnosis, though, given the high prevalence of TB at or immediately after HIV diagnosis in high TB incidence settings, the window of opportunity to provide IPT and prevent TB was closed for many as TB was present at time of HIV diagnosis. Scaling up of programs to implement IPT for PLHIV are expanding globally, but uptake remains low, particularly for patients presenting with advanced HIV infection32. The THRio study aimed to implement IPT according to Brazilian guidelines and was successful in reducing both the time to tuberculin skin testing (TST) and to IPT after a positive TST, and reduced TB incidence and death in this population19.
Active TB case finding among newly diagnosed PLHIV is a World Health Organization recommendation as part of the 3 I’s Initiative33, which also strongly recommends IPT preventive therapy. In our study population, TB diagnosis led to an HIV diagnosis in 168 patients, and TB was diagnosed in the first 60 days after the HIV diagnosis in 308 patients. Delays in diagnosing PLHIV are life-threatening, and the prognosis for HIV-infected patients with prevalent TB is poor compared to those who are TB free30. Efforts to increase HIV testing are necessary, as is further integration of HIV and TB services if progress in the battle against this dual epidemic is to be made34.
Conclusion
More than 10% of newly diagnosed PLHIV in our study presented to care with concurrent active TB disease and thus missed the opportunity for TB preventive therapy. Despite initiating HAART more quickly, these patients were at significantly greater risk of death. Only achieving consistent and widely accessible early HIV diagnosis will lead to early initiation of HAART and IPT and corresponding reductions in morbidity and mortality in Brazil.
Acknowledgments
Source of Funding: Support for this work was provided by the Bill & Melinda Gates Foundation grant for the Consortium to Respond Effectively to the AIDS/TB Epidemic (CREATE) and National Institutes of Health grants AI066994, AI001637, and the International Clinical Operational and Health Services Research and Training Award (ICOHRTA), 5U2RTW006885.
Footnotes
Conflicts of Interest:
The authors have no conflicts of interest to declare. No manuscript resembling results reported in this manuscript has been or will be published elsewhere.
Author Contributions
VS, JEG and REC drafted the manuscript; VS, BD, JEG, SC, LHM, and APC conducted or contributed to the analyses; All authors contributed to data interpretation and drafting the manuscript.
References
- 1.Galvão J. Brazil and access to HIV/AIDS drugs: a question of human rights and public health. Am J Public Health. 2005;95:1110–1116. doi: 10.2105/AJPH.2004.044313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grangeiro A, Escuder MM, Menezes PR, et al. Late entry into HIV care: estimated impact on AIDS mortality rates in Brazil, 2003–2006. PLoS ONE. 2011;6:e14585. doi: 10.1371/journal.pone.0014585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Althoff KN, Gange SJ, Klein MB, et al. Late presentation for human immunodeficiency virus care in the United States and Canada. Clin Infect Dis. 2010;50:1512–1520. doi: 10.1086/652650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guidelines for prophylaxis against Pneumocystis carinii pneumonia for persons infected with human immunodeficiency virus. MMWR Morb Mortal Wkly Rep. 1989;38(Suppl 5):1–9. [PubMed] [Google Scholar]
- 5.Date AA, Vitoria M, Granich R, et al. Implementation of co-trimoxazole prophylaxis and isoniazid preventive therapy for people living with HIV. Bull World Health Organ. 2010;88:253–259. doi: 10.2471/BLT.09.066522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO; 2011. Oct 4, Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings. Available at: http://www.who.int/hiv/pub/tb/9789241500708/en/index.html. [Google Scholar]
- 7.Suthar AB, Lawn SD, del Amo J, et al. Antiretroviral Therapy for Prevention of Tuberculosis in Adults with HIV: A Systematic Review and Meta-Analysis. PLoS Med. 2012;9:e1001270. doi: 10.1371/journal.pmed.1001270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Comstock GW, Baum C, Snider DE., Jr Isoniazid prophylaxis among Alaskan Eskimos: a final report of the bethel isoniazid studies. Am Rev Respir Dis. 1979;119:827–830. doi: 10.1164/arrd.1979.119.5.827. [DOI] [PubMed] [Google Scholar]
- 9.Akolo C, Adetifa I, Shepperd S, et al. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev. 2010:CD000171. doi: 10.1002/14651858.CD000171.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Golub JE, Saraceni V, Cavalcante SC, et al. The impact of antiretroviral therapy and isoniazid preventive therapy on tuberculosis incidence in HIV-infected patients in Rio de Janeiro, Brazil. AIDS. 2007;21:1441–1448. doi: 10.1097/QAD.0b013e328216f441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Pinho AM, Santoro-Lopes G, Harrison LH, et al. Chemoprophylaxis for tuberculosis and survival of HIV-infected patients in Brazil. AIDS. 2001;15:2129–2135. doi: 10.1097/00002030-200111090-00008. [DOI] [PubMed] [Google Scholar]
- 12.Jamal LF, Moherdaui F. Tuberculosis and HIV infection in Brazil: magnitude of the problem and strategies for control. Rev Saude Publica. 2007;41(Suppl 1):104–110. doi: 10.1590/s0034-89102007000800014. [DOI] [PubMed] [Google Scholar]
- 13.Boletim Epidemiológico. Ministério da Saúde. Secretaria de Vigilância em Saúde. 2014 Apr 18; Available at: http://portalsaude.saude.gov.br/images/pdf/2014/abril/10/Boletim-Tuberculose-2014.pdf.
- 14.Soares ECC, Saraceni V, Lauria LM, et al. Tuberculosis as a disease defining acquired immunodeficiency syndrome: ten years of surveillance in Rio de Janeiro, Brazil. J Bras Pneumol. 2006;32:444–448. [PubMed] [Google Scholar]
- 15.Saraceni V, King BS, Cavalcante SC, et al. Tuberculosis as primary cause of death among AIDS cases in Rio de Janeiro, Brazil. Int J Tuberc Lung Dis. 2008;12:769–772. [PMC free article] [PubMed] [Google Scholar]
- 16.Conde MB, Melo FAF, Marques AMC, et al. III Diretrizes para Tuberculose da Sociedade Brasileira de Pneumologia e Tisiologia. J Bras Pneumol. 2009 Aug 17;35(10) doi: 10.1590/s1806-37132009001000011. (online) 2011 Available at: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1806-37132009001000011&lng=pt&nrm=iso&tlng=pt. [DOI] [PubMed] [Google Scholar]
- 17.Saraceni V, Pacheco AG, Golub JE, et al. Physician adherence to guidelines for tuberculosis and HIV care in Rio de Janeiro, Brazil. Braz J Infect Dis. 2011;15:249–252. doi: 10.1016/s1413-8670(11)70184-2. [DOI] [PubMed] [Google Scholar]
- 18.Moulton LH, Golub JE, Durovni B, et al. Statistical design of THRio: a phased implementation clinic-randomized study of a tuberculosis preventive therapy intervention. Clin Trials. 2007;4:190–199. doi: 10.1177/1740774507076937. [DOI] [PubMed] [Google Scholar]
- 19.Durovni B, Saraceni V, Moulton LH, et al. Effect of improved tuberculosis screening and isoniazid preventive therapy on incidence of tuberculosis and death in patients with HIV in clinics in Rio de Janeiro, Brazil: a stepped wedge, cluster-randomised trial. Lancet Infect Dis. 2013;13:852–858. doi: 10.1016/S1473-3099(13)70187-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pacheco AG, Saraceni V, Tuboi SH, et al. Validation of a hierarchical deterministic record-linkage algorithm using data from 2 different cohorts of human immunodeficiency virus-infected persons and mortality databases in Brazil. Am J Epidemiol. 2008;168:1326–1332. doi: 10.1093/aje/kwn249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schechter M, Pacheco AG. Late diagnosis of HIV infection in Brazil despite over 15 years of free and universal access to treatment. AIDS Res Hum Retroviruses. 2012;28:1541–1542. doi: 10.1089/AID.2012.0077. [DOI] [PubMed] [Google Scholar]
- 22.Grangeiro A, Escuder MM, Pereira JC. Late entry into HIV care: lessons from Brazil, 2003 to 2006. BMC Infect Dis. 2012;12:99. doi: 10.1186/1471-2334-12-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 24.Grinsztejn B, Veloso VG, Pilotto JH, et al. Comparison of clinical response to initial highly active antiretroviral therapy in the patients in clinical care in the United States and Brazil. J Acquir Immune Defic Syndr. 2007;45:515–520. doi: 10.1097/QAI.0b013e3180decb6a. [DOI] [PubMed] [Google Scholar]
- 25.Santoro-Lopes G, de Pinho AMF, Harrison LH, et al. Reduced risk of tuberculosis among Brazilian patients with advanced human immunodeficiency virus infection treated with highly active antiretroviral therapy. Clin Infect Dis. 2002;34:543–546. doi: 10.1086/338641. [DOI] [PubMed] [Google Scholar]
- 26.Havlir DV, Kendall MA, Ive P, et al. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med. 2011;365:1482–1491. doi: 10.1056/NEJMoa1013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdool Karim SS, Naidoo K, Grobler A, et al. Integration of antiretroviral therapy with tuberculosis treatment. N Engl J Med. 2011;365:1492–1501. doi: 10.1056/NEJMoa1014181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dheda K, Lampe FC, Johnson MA, et al. Outcome of HIV-associated tuberculosis in the era of highly active antiretroviral therapy. J Infect Dis. 2004;190:1670–1676. doi: 10.1086/424676. [DOI] [PubMed] [Google Scholar]
- 29.López-Gatell H, Cole SR, Margolick JB, et al. Effect of tuberculosis on the survival of HIV-infected men in a country with low tuberculosis incidence. AIDS. 2008;22:1869–1873. doi: 10.1097/QAD.0b013e32830e010c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta A, Wood R, Kaplan R, et al. Prevalent and Incident Tuberculosis Are Independent Risk Factors for Mortality among Patients Accessing Antiretroviral Therapy in South Africa. PLoS One. 2013;8:e55824. doi: 10.1371/journal.pone.0055824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lawn SD, Wilkinson RJ, Lipman MC, et al. Immune reconstitution and "unmasking" of tuberculosis during antiretroviral therapy. Am J Respir Crit Care Med. 2008;177:680–685. doi: 10.1164/rccm.200709-1311PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Granich R, Getahun H, Hirnschall G, et al. Hurry up and wait? Accelerating access to the Three I's for HIV-TB. Int J Tuberc Lung Dis. 2012;16:853–854. doi: 10.5588/ijtld.12.0406. [DOI] [PubMed] [Google Scholar]
- 33.WHO; 2011. Oct 17, WHO_3Is_meeting_report.pdf (online) Available at: http://www.who.int/hiv/pub/meetingreports/WHO_3Is_meeting_report.pdf. [Google Scholar]
- 34.Nemes M, Melchior R, Basso C, et al. The variability and predictors of quality of AIDS care services in Brazil. BMC Health Services Research. 2009;9:51. doi: 10.1186/1472-6963-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]