Abstract
In this paper we describe the masking of pure tones in humans and birds by man-made noises and show that similar ideas can be applied when considering the potential effects of noise on fishes, as well as other aquatic vertebrates. Results from many studies on humans and birds, both in the field and in the laboratory, show that published critical ratios can be used to predict the masked thresholds for pure tones when maskers consist of complex man-made and natural noises. We argue from these data that a single, simple measure, the species critical ratio, can be used to estimate the effect of man-made environmental noises on the perception of communication and other biologically relevant sounds. We also reason that if this principle holds for species as diverse as humans and birds, it probably also applies for all other vertebrates, including fishes.
Keywords: man-made noise, sound, masking, behavior, communication
INTRODUCTION
Noise is ubiquitous, and man-made (or anthropogenic) noise is increasing in many environments due to increases in transportation and the exploration for and exploitation of energy sources. While humans and animals are adapted to deal with natural levels of ambient noise, increased ambient noise levels from human activites can potentially have a variety of adverse effects. These could include noise-induced hearing loss and auditory system damage, temporary hearing loss (called Temporary Threshold Shift or TTS), and masking of communication and other important biological sounds (e.g., Adler et al. 1992; Buck et al. 1984; Clark 1991; Le Prell et al. 2012; Miller 1974; Ryals et al. 1999). Increased noise can also cause behavioral and/or physiological changes such as increased stress, sleep loss, and hormonal changes (Miller 1974; Brumm 2004). Moreover, in water, high intensity signals can damage non-auditory tissues (e.g., Cudahy & Parvin 2001; Halvorsen et al. 2012a) and result in death, although death has rarely been documented except when animals are exposed to very high intensity impulsive sound (e.g., Caltrans 2001).
While relatively little is known about the effects of man-made sounds on fishes, a good deal may be inferred about the potential effects, and approaches to the study of those effects, from the wealth of data available from humans and laboratory animals (Buck et al. 1984; Clark 1991; Le Prell et al. 2012; Miller 1974; Passchier-Vermeer & Passchier 2000). For example, we surmise that a similar range of adverse effects found in humans and other mammals occurs in birds (e.g., Slabbekoorn et al. 2010; Francis & Barber 2013; Dooling & Blumenrath 2014) and probably other animals, including fishes. However, it has been difficult to reach a clear consensus on the causal relationships between man-made noise levels and these various adverse effects in fishes (see Popper & Hawkins 2012).
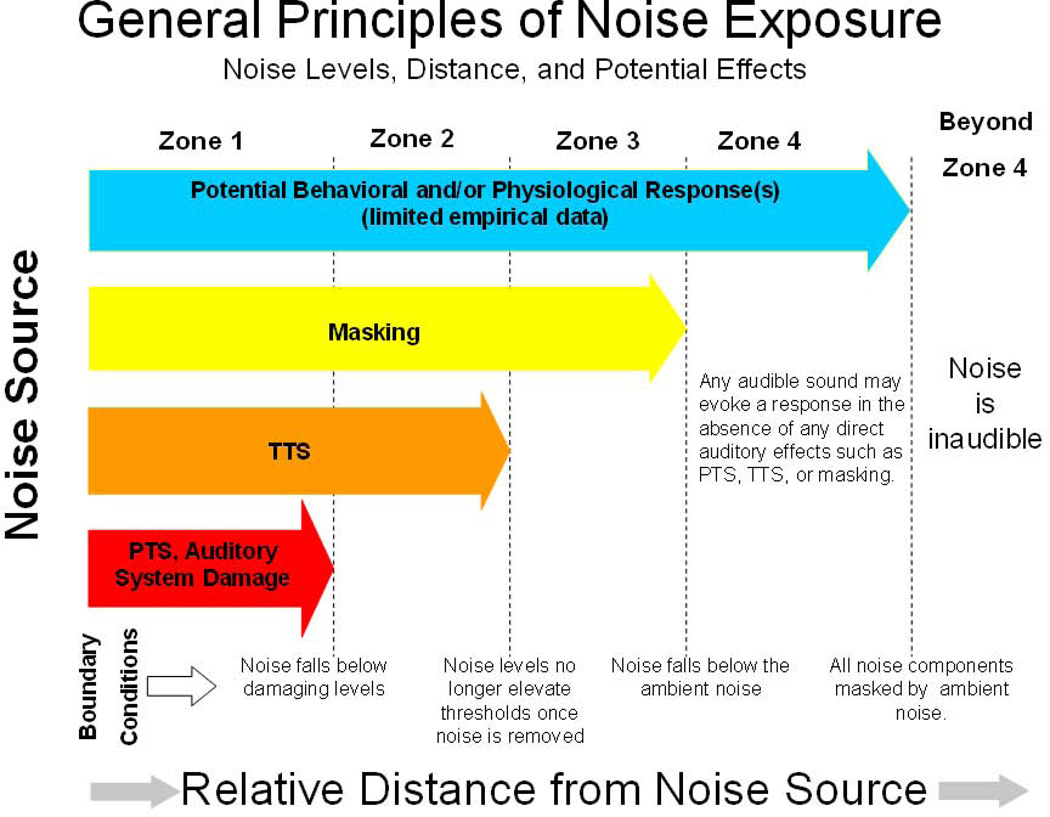
One difficulty in understanding the effects on fishes, or any nonhuman animal, is that while most humans have very similar auditory capabilities and sensitivities, the same is not true for all species or even for closely related species (e.g., Dooling 1982; Dooling et al. 2000; Fay 1988; Ladich & Fay 2013). Thus, sound sources that may have negative consequences for one species may not have similar consequences for another. Another problem is that it is sometimes difficult in animal studies, and in particular for terrestrial species, to definitively identify noise as the principal source of non-auditory effects, such as stress-related behavioral or physiological effects. This is the case since noise is usually accompanied by other visual, tactile, or olfactory stimulation that might also have negative impacts (Forman et al. 2002; Foppen & Reijnen 2004). Thus a causal relationship between stimulus and effect is often not clear. Still another issue is the lack of specificity and the inherently overlapping nature of the different auditory and non-auditory effects of noise. The general effects of noise, and the relationships among these effects, vary with distance from the noise source (Fig. 1).
Figure 1.
Noise level, distance, and potential effects on birds from a noise source (traffic noise). The zones indicate distances over which specific effects will be experienced by a bird. In Zone 1 close to the noise source, potential effects include permanent threshold shift (PTS), temporary threshold shift (TTS), and other behavioral or physiological effects. In Zone 4, furthest from the noise source, we would expect only other effects. (Dooling & Popper 2007; Dooling & Blumenrath 2014).
We think it is useful to delineate four overlapping categories of the effects of noise on humans and animals: (1) hearing damage, including permanent threshold shift (PTS), and other non-auditory tissue damage from exposure to very loud sounds; (2) temporary threshold shift (TTS) from acoustic overexposure; (3) masking of communication signals or other important environmental sounds; and (4) changes in behavior or other physiological responses to noises (e.g., changes in hormone levels, stress responses, lack of sleep). At least for the first three of these, direct auditory effects strongly depend on the level and duration of noise exposure which is generally, although not always, correlated with the proximity of the listener to the noise source.
Close to the noise source, where the sound levels are generally highest, non-auditory tissue damage, hearing damage, TTS, and masking, as well as behavioral and physiological effects, may occur simultaneously. At greater distances from the noise source, where noise levels are lower, the more drastic effects are reduced. However, even at these distances noise exposure may be of sufficient duration or intensity to cause TTS in which hearing thresholds are considerably elevated for a sustained period of time. At even greater distances from the noise source, where sound levels would be even lower, damage and TTS may no longer be a problem, but noise above the ambient level could cause masking, interfering with the organism’s ability to hear communication signals or other important sounds.
There is a considerable amount of data on these effects for humans and some terrestrial animals, but far fewer data on marine mammals (e.g., Southall et al. 2007) and fishes (e.g., Popper & Hastings 2009; Normandeau Associates 2012; see also Popper & Hawkins 2012). However, given the well-documented and long history of adverse consequences of elevated noise on humans and other animals, including hearing loss, masking, stress, behavioral and physiological changes, sleep disturbances, and changes in feelings of well-being (Miller 1974), it is likely that there is a similar range of effects in other species, including fishes and other aquatic animals.
Masking effects from increased noise in the environment
Noise may be particularly intrusive when it interferes with the perception of complex sounds and the communication abilities of animals and humans in their natural environments. Here, communication is meant in its broadest sense of gathering critical information for the well-being, health, and preservation of both individuals and populations. This includes the ability (for some species) to convey meaning through the use of sound, to be able to localize and identify important events and entities in the near or far environment (such as predators, prey, and conspecifics), and interpret the status and health of those entities. All of these functions of hearing may be negatively impacted by other irrelevant sounds (i.e., noise) that have acoustic characteristics with some commonalities with the signals of interest (Dooling & Blumenrath 2014).
The effects resulting from masking of complex sounds is particularly challenging to assess in animals. Most of what we know about the effects of masking, especially on complex sounds such as communication signals, comes from laboratory studies on humans using precise psychophysical approaches. Personal experiences provide important additional data - one need only think of the difficulty of conversing in a noisy restaurant to understand the consequences of masking for speech communication. For humans, then, there is a tight correspondence between laboratory measures of masking and problems associated with communicating against a background of noise.
Clearly, it is much more difficult for humans to accurately experience or measure the auditory world of an animal or the effect of noise on that animal’s acoustic communication. In contrast to the situation with most animals, humans, and to a large degree birds, can be tested relatively easily using rigorous, well-established psychophysical procedures. Birds are particularly easy to train by operant conditioning and they make excellent subjects for psychoacoustic studies. Consequently, we now have useful data from a large number of avian species.
Birds, like humans, are also a highly vocal vertebrate group. We have a considerable amount of psychophysical data on masking in birds, including the masking of communication signals. The data show remarkable similarities in masking between humans and birds. Perhaps what has been learned about hearing loss and masking by noise in humans and birds can inform our approaches to understanding the effects of noise on fishes and other animals such as amphibians and reptiles (both in and out of water).
Noise exposure in humans has been studied extensively for decades, leading to a considerable number of governmental regulations on acceptable noise levels in industry and in the environment (e.g., Le Prell et al. 2012; National Institute of Occupational Safety and Health 1998). Excessive noise is a public health hazard that often produces significant and permanent loss of hearing function (Rabinowitz 2012). The effects of various sources of hearing impairment are additive and cumulative: exposure to noise concurrently with exposure to industrial solvents or other environmental pathogens results in more hearing loss than either source of damage alone (e.g., Morata & Johnson 2012). Further, the normal aging process in humans typically results in some loss of hearing, which can be exacerbated and accelerated by exposures to excessive noise (e.g., Bielefeld 2012). The functional deficits resulting from significant hearing impairment include psychological and social isolation as communication with family, friends, co-workers, and others is disrupted. Impaired hearing can also be dangerous as the individual may be unaware of alerting sounds of impending danger or other warning signals. We can expect these latter two effects to be disruptive in similar ways for animals that rely extensively on acoustic communication.
Human studies provide important additional insights
Humans know from personal experience that noise in the environment does not need to be so intense as to cause either permanent or temporary loss of auditory function in order to produce significant difficulty in the ability to communicate or hear important environmental sounds. Laboratory studies in humans focused on this issue typically are carried out by asking subjects to respond to a speech signal, such as words or isolated sentences, in the presence of noise with a variety of characteristics for clinical assessment of hearing disorders or for an understanding of how speech and other sounds are processed in the auditory system. The approach is often to either distort the acoustic characteristics of speech or to determine which aspects of a noise (level, frequency content, intermittency, etc.) result in more or less disruption of the target sound perception. For both clinical and basic research applications, the starting measurement usually is the relative levels of target and masking noise that result in accurate perception 50% of the time, a measure that is sometimes referred to as the Speech Reception Threshold (SRT). Note that the SRT is not the relative level that will allow communication to occur, but it is a metric that can be used to compare speech communication across individual listeners and in the presence of different types of speech and noise. In fact, the exact definition of the SRT is somewhat arbitrary and may be defined by an investigator as any value between 0 and 100% correct perception of speech against a background of a particular noise. Regardless of the definition chosen, the relative intensities of speech and noise (i.e., the signal-to-noise ratio, SNR) may be reported at equivalent performance levels (percent correct perceptions) across listeners and conditions. As the SNR increases, communication in the presence of background noise gets progressively easier.
The ability to communicate, or to determine that two sounds are different (e.g., from two different speakers or two different words) or to detect that a sound of some kind is present (the least complex perceptual task) improves with increasing SNR levels. The relationship between SNR level and accuracy of perception for each of these tasks (i.e., communication, discrimination, detection) differs depending on the spectral and temporal characteristics of the background noise.
Greatest masking of communication signals like speech occurs when the frequency spectra of the speech signal and the noise are very similar. Auditory processing in most vertebrates, including some aquatic vertebrates, is based on the concept that the auditory system operates like a series of overlapping band pass filters laid out across the audible spectrum for a given species. According to this concept, a signal can only be heard if its level within a given frequency band is proportional to the level of the masker within that bandwidth (Fletcher 1940). This so-called “power spectrum model” of hearing, also means that masker energy in frequency regions outside a “critical band” of frequencies around the signal frequency do not significantly interfere with perception of the target signal. Though the data from fish are limited, in practical terms this means that the spectrum and the levels of both the man-made noise and the signal the animal is trying to hear are the critical variables for determining how detrimental a certain noise is for an animal.
To facilitate comparisons across species and sound environments, the critical masking bandwidths estimated for different animals have been transformed into a signal-to-noise type metric called the “critical ratio” where proportional constant between the signal level and the noise level within the critical band is equal to 1. In other words, the critical ratio is defined as the level within an internally-based (i.e., within the animal’s auditory system) band of the signal that is equivalent to the level of the masker sound within a similar bandwidth. The critical ratio metric may be considered as that combination of signal and noise that produces an internal SNR of 0 dB. Critical ratios differ among species due to differences in auditory anatomy and physiology. Animals that hear relatively well in noise (i.e., have good frequency resolution) will have smaller critical ratios while poorer listeners in noise will have large critical ratios and experience more masking. Critical ratios specified at a particular frequency region can vary as much as 10 dB or more across species.
For humans, the SNR at which about 50% of speech may be correctly identified in steady state noise is about −5 dB (i.e., the masking noise is about 5 dB greater in intensity than the speech) (Festen & Plomp 1990). To increase identification performance to about 75% correct, the SNR would have to improve such that the noise is only 3.5 dB greater than the speech, and for identification performance of 90% correct, the noise level must be only 2 dB greater than the speech. Another way to think of this is that taking simple detection as the perceptual case with the lowest SNR, an increase in the SNR of about 2 dB would support a discrimination of two different sounds, and a further increase of 2 dB would allow some degree of identification. For speech to be heard at a level that would allow clear communication (or the accurate perception of environmental sounds),a signal-to-noise ratio of about 15 dB would be required (noise 15 dB below the level of the speech: Franklin et al. 2006). In a noisy restaurant, for example, the SNR is likely to be well into the negative range (i.e., noise level much higher than the target speech level), such that conversation is strained for both talkers and listeners. As a consequence, clear and easy communication is not possible.
Parallel laboratory studies on masking in birds
One might reasonably ask whether the relationship between different levels of perception and SNR is a general phenomenon or is only true for humans. Studies have been conducted in birds that are similar to the human studies described (Dooling et al. 2009; Lohr et al. 2003). Masking studies in birds, like those in humans, have shown that the most important acoustic feature for masking a signal is the level of the noise with energy in the frequency region of the signal. Noise falling outside of the frequency band of the signal contributes far less to masking. Most laboratory studies estimating the effects of noise on signal detection use continuous noises with precisely defined bandwidths, intensities, and spectra.
To evaluate the effect of masking noise on bird communication, we used an approach that integrates the spectrum and level of the masking noise, the bird’s hearing in quiet and noise, the spectrum and level of a signaling bird’s vocalizations, and the acoustic characteristics of the environment (Dooling et al. 2009; Dooling & Blumenrath 2014). The model assumes that the spectrum and amplitude (level) of the noise and the signaler’s vocalization are both known at the location of the receiving animal. These values can either be measured directly or they can be estimated by applying signal attenuation algorithms to both the noise source and the signals of the sender. The algorithms adjust the spectra and level of the noise and of the signal as if they were transmitted over distance and through different habitats (e.g., meadows, forests) between communicating birds. The challenge for the receiver is to hear the signal in the presence of noise. This is dependent on the species-specific auditory capabilities of the receiver, such as how well it hears in noise (i.e., its critical ratio), and the signal-to-noise ratio at the receiver’s location. Using a human parallel, the model also incorporates the notion that different auditory behaviors (e.g., communicating comfortably versus just being able to detect that something was said) require different SNRs. We know from empirical studies, using highly controlled psychophysical methodologies in the laboratory, that birds require about a 2–3 dB higher SNR to discriminate between two vocalizations than to just detect the presence of a vocalization. And they require a further increase in the SNR of 2–3 dB in order to recognize a vocalization rather than to simply discriminate between two vocalizations. While it cannot be directly measured in animals, birds and other animals are likely to require a significant improvement in SNR to support effortless communication in the presence of background noise, just as humans require a fairly large increment in SNR to allow comfortable and seamless communication (Franklin et al. 2006).
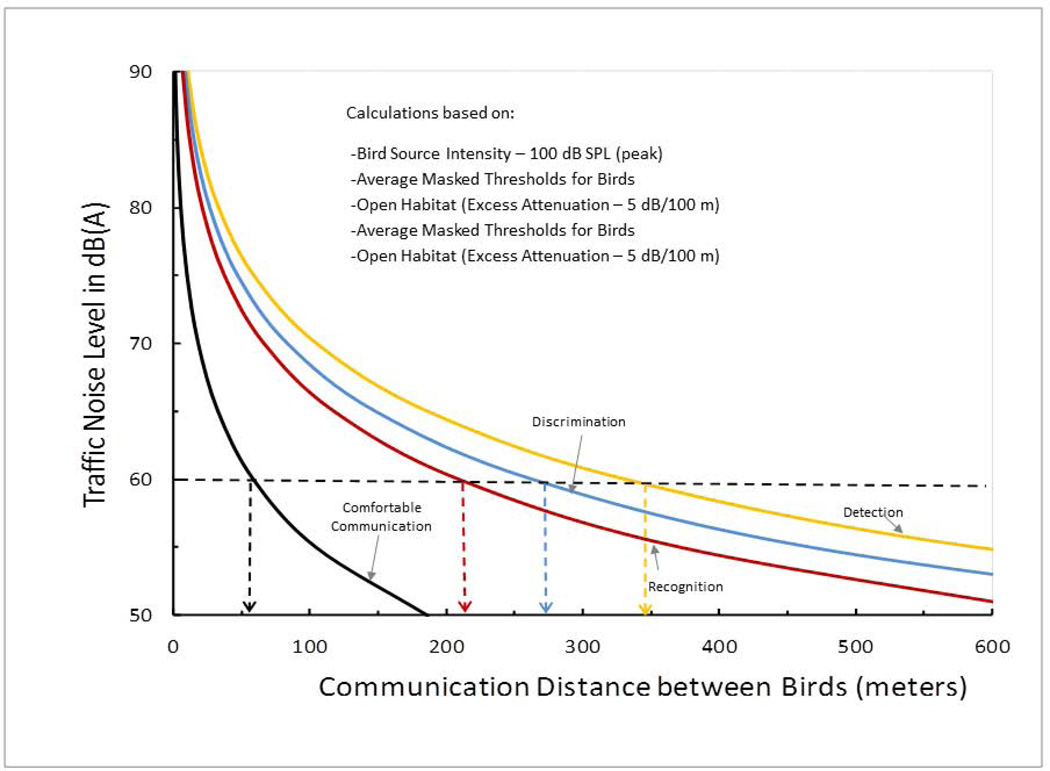
What does this mean for birds in their natural environment exposed to excessive levels of man-made noise? Figure 2 shows the effect of traffic noise on four different auditory behaviors based on the median bird critical ratio function. The specific case illustrated is for a background noise level at the location of the listening bird of 60 dB(A) – a level typical of traffic noise measured roughly 300 meters from a busy six-lane highway (Dooling & Popper2007). This example assumes the calling bird is vocalizing at a peak sound pressure level of 100 dB re 20 µPa through an open area, and the vocalization is affected by excess attenuation (beyond the loss due to spherical spreading) of 5dB/100 meters. In this noise, a comfortable level of communication between two birds requires a distance between them of less than 60 meters. Recognition of a bird vocalization by the receiver can still occur at greater inter-bird distances up to about 220 meters. Discrimination between two vocalizations is possible at inter-bird distances up to 270 meters. And finally, simple detection of another bird’s vocalization can occur at distances up to 345 meters in this noise.
Figure 2.
Relationship between traffic noise level and communication distances for detection, discrimination, recognition, and comfortable communication between birds. These curves are based on 100 dB source level of the vocalization, the listener’s masked threshold (here the critical ratio for the average bird), and the habitat’s average excess attenuation (here an open habitat with attenuation of 5 dB/100m) (modified from Dooling et al. 2009; Dooling & Blumenrath 2014).
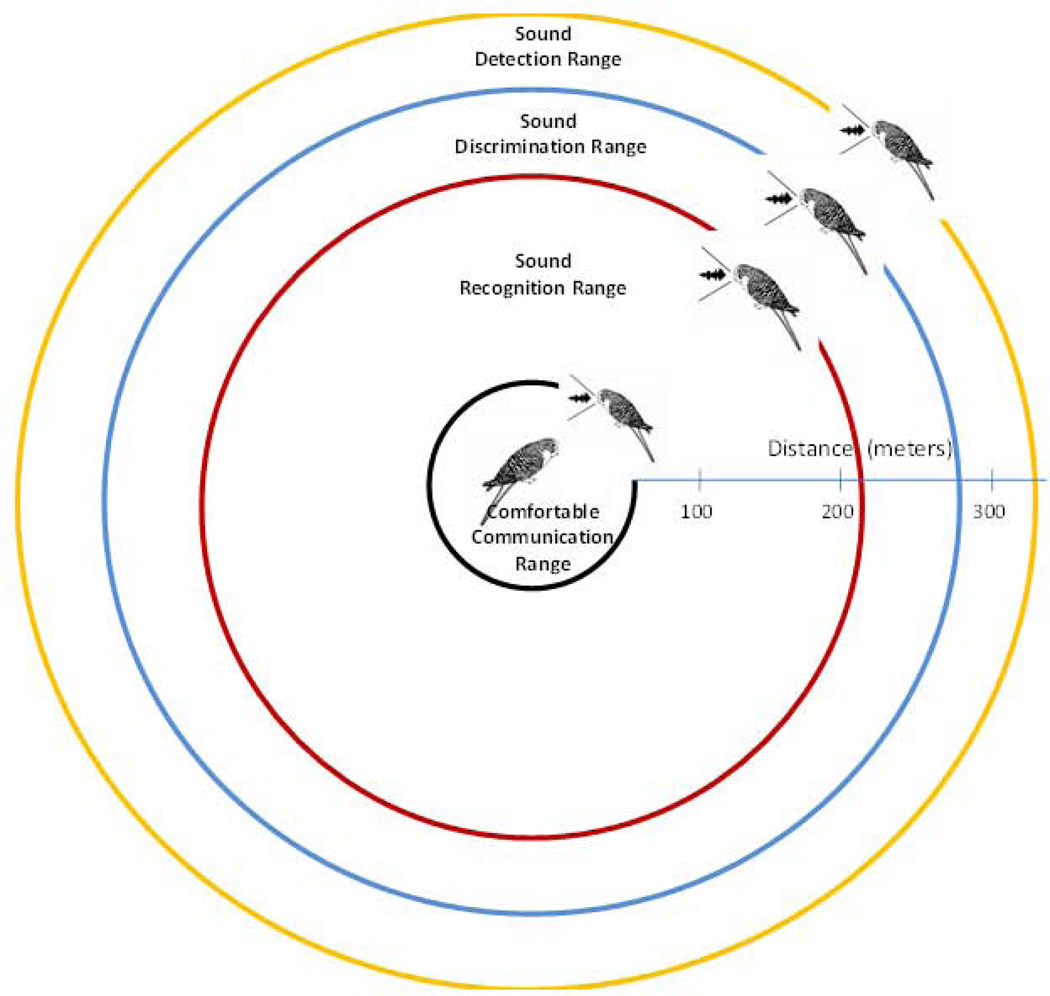
The distance values computed for a 60 dB(A) level of man-made noise (Fig. 2) can be used to construct a receiver-centric map of distances corresponding to the four different auditory communication behaviors (Fig. 3). Communication distance between the sender (calling bird, along the periphery) and the receiver (listening bird, at the center) is represented as the radius for the concentric circles defining the boundaries of each of the four levels of communication. While any increase in the ambient noise level by contributions from man-made sources could affect acoustic communication, which auditory behaviors are affected depends on the noise level. In figure 3, the inner circle represents the case where the sender is closest to the receiver. This represents a signal-to-noise ratio that is sufficiently large (based on humans listening to speech) to allow the sender and receiver to communicate comfortably. As the sender moves away from the receiver, the signal level drops and SNR decreases for the receiver. At this level of separation, the receiver can no longer communicate comfortably but can recognize a sender’s different vocalizations. If the sender moves even further away, the receiver can still discriminate between two vocalizations but cannot reliably recognize them. Finally, at the outer perimeter, the signal level at the receiver results in such a low signal-to-noise ratio that the receiver can detect only that some kind of a sound has occurred. The distance over which masking from man-made noise sources occurs can be quite large. This schematic provides a way of estimating and quantifying the risk to acoustic communication for birds at different distances from a noise source.
Figure 3.
Schematic representation of the distances over which a listening bird can perceive a vocalizing bird in 60 dB traffic noise. Circles represent the active auditory space for four different types of perception: detection, discrimination, recognition, and comfortable communication.
The results discussed above are generated assuming a constant, flat spectrum noise such as used in the laboratory (e.g., a relatively continuous, constant spectrum and intensity). The principle is based on the notion that noise in frequencies immediately surrounding the signal frequencies is most effective in masking the signal. This is quite easy to calculate from continuous, flat spectrum noise. But the same principle works for birds in the laboratory even when more complex noises are used, such as those produced by a helicopter, snowmobile, or chain-saw (Dooling, unpublished data). Even though the spectrum may be steeply sloping from low to high frequencies, it is still the energy in the frequency region surrounding the signal frequencies that determines the masking threshold level for birds (Dooling et al. 2013). A core element and general predictor across species of the effect of masking is the SNR in the spectral region of the signal, whether involving simple signals and noises, or complex, natural signals and complex natural or man-made noises. This general principle also operates across humans and other land animals with both simple and complex sounds. We suggest that this principle will also apply to fishes and other aquatic animals.
Fishes and environmental noise
Over the past decades it has become apparent that man-made noise also has the potential to impact the lives of aquatic organisms, including sharks, bony fishes, marine turtles, and marine mammals (reviewed in Southall et al. 2007; Normandeau Associates 2012; papers in Popper & Hawkins 2012). While there is strong evidence that very intense, and particularly impulsive, sounds can damage tissues and potentially result in mortal effects (e.g., Bolle et al. 2012; Casper et al. 2012, 2013a, b; Halvorsen et al. 2012a, b; Hastings et al. 2008; McCauley et al. 2003; Popper et al. 2005, 2007), far more fish are likely to be exposed to sounds at some distance from the source where the intensity is lower that nearer the source and any effects are likely to be behavioral rather than physical. Moreover, in addition to behavioral effects resulting from impulsive sounds, it is also highly likely that general and continuous increases in background sounds, such as those produced by shipping and other wind farm operation may have behavioral effects on fishes. Effects may range from only small, and inconsequential, changes in behavior to long-term effect on reproduction or feeding. However, very little is known about potential behavioral effects of sound on fishes (e.g., Slabbekoorn et al. 2010; Normandeau Associates 2012).
Still, it is clear that the basic questions on effects of man-made sound on fishes are the same as for birds and mammals – do man-made sounds impact the fishes and cause PTS, TTS, masking, or other behavioral effects (e.g., Slabbekoorn et al. 2010; Ladich 2013). Indeed, it is reasonable to expect that sounds will impact fishes in much the same way as has been described for birds, and terrestrial and marine mammals. We make this suggestion since while the hearing mechanisms and capabilities of fishes differ somewhat from those of mammals, fishes do exhibit many of the same basic auditory phenomena as found in birds and mammals, including critical ratios (Fay & Megela Simmons 1999). Indeed, the potential effects of man-made noise described above for humans, birds and other animals are likely to be encountered in fishes (e.g., Slabbekoorn et al. 2010). Thus, the critical ratio may be a valuable metric for comparing noise effects on fishes as well. In other words, if the general principles described above hold for species as different as humans and birds (Figs. 1 – 3), they are also very likely to hold for fishes. The relevant metric is the critical ratio, but sufficient data are available for only a few species of fishes (Fay 1988; Ladich 2013).
We therefore argue that future studies in fishes should focus on many of the same questions about detectability and effects on communication as discussed for birds and humans. In fact, it is important that future studies of fishes and aquatic organisms take into consideration the fact that these animals, just like their terrestrial relatives, rely on hearing for a major portion of the sensory information they collect about the world around them (Fay & Popper 2012). As a consequence, masking acoustic communication, which impacts the ability to detect, discriminate, and understand behaviorally important sounds, can significantly affect an aquatic animal’s fitness and survival. In fact, the critical issue in reaching a full understanding of the effects of man-made sounds on fishes (and other animals) lies with observations of the effects on their natural behavior.
ACKNOWLEDGMENTS
This work was supported in part by NIH grants to RJD, and a Senior Research Career Scientist Award to MRL from the Department of Veterans Affairs Rehabilitation Research and Development Service. The contents of this article do not represent the views of the Department of Veterans Affairs or the U.S. Government. We thank Dr. Peggy Edds-Walton for her thorough review of the MS and her excellent suggestions for improvement.
REFERENCES
- Adler HJ, Kenealy JF, Dedio RM, Saunders JC. Threshold shift, hair cell loss, and hair bundle stiffness following exposure to 120 and 125 dB pure tones in the neonatal chick. Acta Otolaryngologica. 1992;112:444–454. doi: 10.3109/00016489209137425. [DOI] [PubMed] [Google Scholar]
- Bielefeld EC. Effects of early noise exposure on subsequent age-related changes in hearing. In: LePrell CG, Henderson D, Fay RR, Popper AN, editors. Noise-Induced Hearing Loss: Scientific Advances. New York: Springer Science+Business Medica LCC; 2012. pp. 205–222. [Google Scholar]
- Bolle LJ, de Jong CAF, Bierman SM, et al. Common sole larvae survive high levels of pile-driving sound in controlled exposure experiments. PLoS One. 2012;7(3):e33052. doi: 10.1371/journal.pone.0033052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumm H. The impact of environmental noise on song amplitude in a territorial bird. Journal of Animal Ecology. 2004;73:434–440. [Google Scholar]
- Buck K, Dancer A, Franke R. Effect of the temporal pattern of a given noise dose on TTS in guinea pigs. Journal of the Acoustical Society of America. 1984;76:1090–1097. doi: 10.1121/1.391401. [DOI] [PubMed] [Google Scholar]
- Caltrans. PIDP EA 012081, Caltrans Contract 04A0148. San Francisco: Oakland Bay Bridge East Span Seismic Safety Project; 2001. Pile Installation Demonstration Project, Fisheries Impact Assessment. [Google Scholar]
- Casper BM, Popper AN, Matthews F, Carlson TJ, Halvorsen MB. Recovery of barotrauma injuries in Chinook salmon, Oncorhynchus tshawytscha from exposure to pile driving sound. PLoS ONE. 2012;7(6):e39593. doi: 10.1371/journal.pone.0039593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casper BM, Smith ME, Halvorsen MB, Sun H, Carlson TJ, Popper AN. Effects of exposure to pile driving sounds on fish inner ear tissues. Comparative Biochemistry and Physiology, A. 2013a;166:352–360. doi: 10.1016/j.cbpa.2013.07.008. [DOI] [PubMed] [Google Scholar]
- Casper BM, Halvorsen MB, Matthews F, Carlson TJ, Popper AN. Recovery of barotrauma injuries resulting from exposure to pile driving sounds in two sizes of hybrid striped bass. PLoS ONE. 2013b;8(9):e73844. doi: 10.1371/journal.pone.0073844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudahy E, Parvin S. The effects of underwater blasts on divers. Naval Submarine Medical Research Laboratory; 2001. NSMRL Report 1218, Available at: http://www.dtic.mil/cgi-bin/GetTRDoc?AD=ADA404719. [Google Scholar]
- Clark WW. Recent studies of temporary threshold shift (TTS) and permanent threshold shift (PTS) in animals. Journal of the Acoustical Society of America. 1991;90:155–163. doi: 10.1121/1.401309. [DOI] [PubMed] [Google Scholar]
- Dooling RJ, Popper AN. The Effects of Highway Noise on Birds. Report for The California Department of Transportation, Division of Environmental Analysis, 1120 N Street Sacramento, CA 94274 2007. 2007 Available from URL: http://www.dot.ca.gov/hq/env/bio/files/caltrans_birds_10-7-2007b.pdf.
- Dooling RJ. Auditory perception in birds. In: Kroodsma D, Miller E, editors. Acoustic Communication in Birds. Vol. 1. New York: Academic Press; 1982. pp. 95–130. [Google Scholar]
- Dooling RJ, Leek MR, West EW. Predicting the effects of masking noise on communication distance in birds. Journal of the Acoustical Society of America. 2009;125:2517. [Google Scholar]
- Dooling RJ, Blumenrath SH. Avian sound perception in noise. In: Brumm H, editor. Animal Communication and Noise. Heidelberg: Springer; 2014. pp. 229–250. [Google Scholar]
- Fay RR. Hearing in Vertebrates: A Psychophysics Databook. Winnetka, IL: Hill-Fay Associates; 1988. [Google Scholar]
- Fay RR, Megela Simmons A. The sense of hearing in fishes and amphibians. In: Fay RR, Popper AN, editors. Comparative hearing: Fish and Amphibians. New York: Springer-Verlag; 1999. pp. 269–318. [Google Scholar]
- Fay RR, Popper AN. Fish hearing: New perspectives from two “senior” bioacousticians. Brain, Behaviour and Evolution. 2012;792:215–217. doi: 10.1159/000338719. [DOI] [PubMed] [Google Scholar]
- Festen JM, Plomp R. Effects of fluctuating noise and interfering speech on the speech-reception threshold for impaired and normal hearing. Journal of the Acoustical Society of America. 1990;88:1725–1736. doi: 10.1121/1.400247. [DOI] [PubMed] [Google Scholar]
- Fletcher H. Auditory patterns. Reviews of Modern Physics. 1940;12:47–65. [Google Scholar]
- Francis CD, Barber JR. A framework for understanding noise impacts on wildlife: an urgent conservation priority. Frontiers in Ecology and the Environment. 2013;11:305–313. [Google Scholar]
- Franklin CA, Jr, Thelin JW, Nabelek AK, Burchfield SB. The effect of speech presentation level on acceptance of background noise in listeners with normal hearing. Journal of the American Academy of Audiology. 2006;17:141–146. doi: 10.3766/jaaa.17.2.6. [DOI] [PubMed] [Google Scholar]
- Foppen R, Reijnen R. The effects of car traffic on breeding bird populations in woodland. II. Breeding dispersal of male willow warblers (Phylloscopus trochilus) in relation to the proximity of a highway. Journal of Applied Ecology. 2004;31:95–101. [Google Scholar]
- Forman RTT, Reineking B, Hersperger AM. Road traffic and nearby grassland bird patterns in a suburbanizing landscape. Environmental Management. 2002;29:782–800. doi: 10.1007/s00267-001-0065-4. [DOI] [PubMed] [Google Scholar]
- Halvorsen MB, Casper BM, Woodley CM, Carlson TJ, Popper AN. Threshold for onset of injury in Chinook salmon from exposure to impulsive pile driving sounds. PLoS ONE. 2012a;7(6):e38968. doi: 10.1371/journal.pone.0038968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halvorsen MB, Casper BM, Matthews F, Carlson TJ, Popper AN. Effects of exposure to pile driving sounds on the lake sturgeon, Nile tilapia, and hogchoker. Proceedings of the Royal Society of London, Series B, Biological Sciences. 2012b;279:4705–4714. doi: 10.1098/rspb.2012.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings MC, Reid CA, Grebe CC, et al. The effects of seismic airgun noise on the hearing sensitivity of tropical reef fishes at Scott Reef, Western Australia. Underwater noise measurement, impact and mitigation. Proceedings of the Institute of Acoustics. 2008;30(5):102–110. [Google Scholar]
- Ladich F. Effects of noise on sound detection and acoustic communication in fishes. In: Brumm H, editor. Animal Communication and Noise. Vol. 2. Heidelberg: Springer; 2013. pp. 65–90. [Google Scholar]
- Ladich F, Fay RR. Auditory evoked potential audiometry in fish. Reviews in Fish Biology and Fisheries. 2013 doi: 10.1007/s11160-012-9297-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Prell CG, Henderson D, Fay RR, Popper AN, editors. Noise-Induced Hearing Loss: Scientific Advances. New York: Springer Science+Business Medica LLC; 2012. [Google Scholar]
- Lohr B, Wright TF, Dooling RJ. Detection and discrimination of natural calls in masking noise by birds: estimating the active space signal. Animal Behaviour. 2003;65:763–777. [Google Scholar]
- McCauley RD, Fewtrell J, Popper AN. High intensity man-made sound damages fish ears. Journal of the Acoustical Society of America. 2003;113:638–642. doi: 10.1121/1.1527962. [DOI] [PubMed] [Google Scholar]
- Miller JD. Effects of noise on people. Journal of the Acoustical Society of America. 1974;56:729–763. doi: 10.1121/1.1903322. [DOI] [PubMed] [Google Scholar]
- Morata TC, Johnson A-C. Effects of exposure to chemicals on noise-induced hearing loss. In: Le Prell CG, Henderson D, Fay RR, Popper AN, editors. Noise-Induced Hearing Loss: Scientific Advances. New York: Springer Science+Business Medica LLC; 2012. pp. 223–256. [Google Scholar]
- National Institute of Occupational Safety and Health (NIOSH) Criteria for a recommended standard: Occupational noise exposure revised criteria 1998, Cincinnati DHHS. 1998 Available from URL: http://www.cdc.gov/niosh/docs/98-126/pdfs/98-126.pdf.
- Normandeau Associates, Inc. A Literature Synthesis for the U.S. Dept. of the Interior. Bureau of Ocean Energy Management; 2012. Effects of Noise on Fish, Fisheries, and Invertebrates in the U.S. Atlantic and Arctic from Energy Industry Sound-Generating Activities; p. 135. Contract # M11PC00031. [Google Scholar]
- Passchier-Vermeer W, Passchier WF. Noise exposure and public health. Environmental Health Perspectives. 2000;108(supplement 1):123–131. doi: 10.1289/ehp.00108s1123. Available from URL: ://www.ncbi.nlm.nih.gov/pmc/articles/PMC1637786/pdf/envhper00310-0128.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popper AN, Halvorsen MB, Casper BM, Carlson TJ. U.S. Dept. of the Interior, Bureau of Ocean Energy Management, Headquarters, Herndon, VA. Effects of Pile Sounds on Non-Auditory Tissues of Fish. 2013 OCS Study BOEM 2012-105. [Google Scholar]
- Popper AN, Halvorsen MB, Kane E, et al. The effects of high-intensity, low-frequency active sonar on rainbow trout. Journal of the Acoustical Society of America. 2007;122:623–635. doi: 10.1121/1.2735115. [DOI] [PubMed] [Google Scholar]
- Popper AN, Hastings MC. Effects of man-made sources of sound on fishes. Journal of Fish Biology. 2009;75:455–498. doi: 10.1111/j.1095-8649.2009.02319.x. [DOI] [PubMed] [Google Scholar]
- Popper AN, Smith ME, Cott PA, et al. Effects of exposure to seismic airgun use on hearing of three fish species. Journal of the Acoustical Society of America. 2005;117:3958–3971. doi: 10.1121/1.1904386. [DOI] [PubMed] [Google Scholar]
- Popper AN, Hawkins A, editors. The Effects of Noise on Aquatic Life. New York: Springer Science+Business Media LLC; 2012. [Google Scholar]
- Rabinowitz PM. The public health significance of noise-induced hearing loss. In: LePrell CG, Henderson D, Fay RR, Popper AN, editors. Noise-Induced Hearing Loss: Scientific Advances. New York: Springer Science+Business Medica LLC: 2012. pp. 13–26. [Google Scholar]
- Ryals BM, Dooling RJ, Westbrook E, Dent ML, MacKenzie A, Larsen ON. Avian species differences in susceptibility to noise exposure. Hearing Research. 1999;131:71–88. doi: 10.1016/s0378-5955(99)00022-2. [DOI] [PubMed] [Google Scholar]
- Slabbekoorn H, Bouton N, van Opzeeland I, Coers A, ten Cate C, Popper AN. A noisy spring: the impact of globally rising underwater sound levels on fish. Trends in Ecology & Evolution. 2010;25:419–427. doi: 10.1016/j.tree.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Southall BL, Bowles AE, Ellison WT, et al. Marine mammal noise exposure guidelines: initial scientific recommendations. Aquatic Mammals. 2007;33:411–521. [Google Scholar]