Abstract
Objectives
Small cell lung cancers (SCLCs) are characterized by aberrantly-methylated O6-methyl-guanine-DNA methyltransferase (MGMT). Epigenetic silencing of MGMT is associated with loss of MGMT activity and improved sensitivity to alkylating agents in glioblastomas. We have reported the activity of temozolomide, a non-classical alkylating agent, in patients with relapsed sensitive or refractory SCLCs, given at 75 mg/m2/day for 21 of 28 days. However, prolonged myelosuppression was noted. We therefore evaluated a 5-day dosing schedule of temozolomide and examined MGMT as a predictive biomarker for temozolomide treatment in SCLC.
Materials and Methods
Patients with sensitive or refractory SCLCs and progression after one or two prior chemotherapy regimens received temozolomide 200 mg/m2/day for 5 consecutive days in 28-day cycles. The primary endpoint was tolerability. We also assessed MGMT promoter methylation status by PCR and MGMT expression by immunohistochemistry in tumor specimens.
Results
Of 25 patients enrolled, 5 experienced grade 3 or 4 toxicity (anemia, thrombocytopenia, neutropenia, and constipation). The partial response rate was 12% [95% CI: 3–31%], with partial responses in 2 refractory patients. We were able to obtain tumor samples for more than half of patients for MGMT testing.
Conclusion
Temozolomide 200 mg/m2/day for 5 days in 28-day cycles is tolerable and active in patients with relapsed SCLCs. No treatment-limiting prolonged cytopenias were observed, making this our preferred schedule for further studies. Acquisition of archived biospecimens is feasible and necessary in order to continue evaluating the role of MGMT as a predictive biomarker in SCLCs.
Keywords: temozolomide, SCLC, MGMT, biomarker, alkylating agent
Introduction
Only topotecan is FDA approved for the 2nd line treatment of small cell lung cancers (SCLCs).1–3 Since alkylating agents have efficacy in this disease, we studied temozolomide, which has higher central nervous system (CNS) penetration than other drugs in its class. Further, aberrant methylation of the O6-methyl-guanine-DNA methyltransferase (MGMT) has been demonstrated in SCLCs, which leads to loss of MGMT activity and improved sensitivity to alkylating agents in gliomablastomas.4, 5
Three schedules of temozolomide are used to treat recurrent glioblastoma: 200 mg/m2 per day for 5 days6, 75 mg/m2 per day for 21 days every 28 days7, and 150 mg/m2 per day every other week.8 We initially chose the 21 day dosing schedule for our Phase II study.9 While there was a 20% response rate [95% CI, 11–32%], 11% of patients experienced prolonged thrombocytopenia and neutropenia. To avoid prolonged myelosuppression, we evaluated the 5-day dosing schedule in 25 patients with SCLCs. We also examined tissue to determine if MGMT methylation and/or MGMT expression predicted response to temozolomide in patients with SCLCs.9–14
Materials and Methods
This was a single-center study enrolling patients with relapsed sensitive SCLCs (S-SCLCs, progression or relapse ≥60 days after first-line chemotherapy)1 and refractory SCLCs (R-SCLCs, progression during initial therapy or <60 days after first-line treatment). Prior therapies were restricted to ≤2 lines. Other eligibility criteria included Karnofsky Performance Status of ≥60%, measurable disease according to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.015, and adequate liver, kidney, and bone marrow function. Patients with brain metastases were eligible but those with leptomeningeal disease were not. This study was approved by our Institutional Review Board and registered at www.clinicaltrials.gov (NCT00740636) and all patients provided written informed consent.
Patients received temozolomide 200 mg/m2/day orally on days 1–5 of each 28-day cycle and ondansetron 8 mg orally 30 minutes before temozolomide. Therapy was continued until unacceptable toxicity, progression of disease, or withdrawal of consent. Two dose reductions were permitted (150 mg/m2/day and 100 mg/m2/day) in the event of grade 3/4 toxicity. Temozolomide was stopped if a toxicity did not resolve within 21 days, a grade 3 non-hematologic adverse event recurred, or any grade 4 non-hematologic toxicity occurred. Patients with grade ≥3 lymphopenia were given prophylaxis for pneumocystis carinii pneumonia.
Toxicity was assessed using the National Cancer Institute Common Toxicity Criteria version 3.0. Response was evaluated using RECIST 1.0 with CT scans of the chest and other relevant sites at the end of cycles 1 and 2, and every 2 cycles thereafter. Wherever available, archival tissue samples from patients were evaluated for MGMT promoter methylation by PCR and MGMT expression by immunohistochemistry.9
The primary endpoint was the toxicity of the regimen. The acceptable rate of severe toxicity, defined as ≥ grade 3 toxicity (excluding lymphopenia), was 10% and the unacceptable severe toxicity rate was 40%. If ≥3 patients experienced severe toxicity among the first 10 enrolled, ≥5 among the first 20 patients, or ≥6 among the 25 patients, the trial would close. This design ensured a <10% probability of stopping the trial if the true toxicity rate was 10% or less and >95% probability of stopping the trial if the true toxicity rate was 40% or higher.
Secondary endpoints included overall response rate [ORR: complete response (CR) plus partial response (PR)], overall survival (OS), and time to progression (TTP); response rates in patient groups stratified by second- or third-line treatment, and presence or absence of brain metastases; and presence of MGMT promoter hypermethylation by PCR and MGMT expression by immunohistochemistry in available tumor samples.
Results
Over 12 months, we enrolled 25 patients (16 relapsed sensitive and 9 refractory). Baseline characteristics are listed in Table 1. Fifteen patients (60%) received one prior line of therapy and 10 (40%) received two prior lines of therapy. Ten patients had new or progressive brain metastases, including two patients previously treated with whole brain radiation therapy (WBRT). The median number of cycles administered was one (range 0.2 to 9 cycles). Reasons for discontinuation of temozolomide were disease progression (N=19), intercurrent illness/symptomatic deterioration (N=5), and toxicity (N=1).
Table 1.
Baseline Characteristics
| Characteristic | Total (N=25) |
|---|---|
| Median age (range) | 62 (35–84) |
| Men | 9 (36%) |
| Women | 16 (64%) |
| Karnofsky performance status | |
| ≥90% | 5 (20%) |
| 80% | 9 (36%) |
| 70% | 7 (28%) |
| 60% | 4 (16%) |
| Stage at diagnosis (%) | |
| Limited | 7 (28%) |
| Extensive | 18 (72%) |
| Sensitive | 16 (64%) |
| Refractory | 9 (36%) |
| Previous lines of therapy | |
| Onea | 15 (60%) |
| Twob | 10 (40%) |
| Brain metastases | 10 (40%) |
First-line treatment was a platinum/etoposide doublet except for 3 patients: 1 received platinum/etoposide with irinotecan, 1 received platinum and irinotecan, and 1 received platinum/etoposide and IMC-A12.
Second-line treatments included rechallenge with platinum/etoposide (N=3), cyclophosphamide/doxorubicin/vincristine (N=3), topotecan (N=2), taxanes (N=1), and platinum with irinotecan (N=1).
Toxicity
Table 2 lists the most common treatment-related toxicities. Five patients experienced grade 3 or 4 treatment-related toxicity (excluding lymphopenia but including anemia, thrombocytopenia, neutropenia, and constipation). Grade 3 and 4 thrombocytopenia occurred in 4 patients with a median duration of 12 days (range 7–14 days). Lymphopenia due to temozolomide was quite common: grade 3 in 17 patients (68%) and grade 4 in 2 patients (8%). However, further therapy was not limited by cytopenias and no bleeding events occurred. The only grade 3 or 4 non-hematologic toxicity was grade 3 constipation in one patient and may have been due to the ondansetron taken before temozolomide doses. One patient came off study for altered mental status due to radiation necrosis from whole brain radiation therapy received prior to enrollment. There were no treatment related deaths.
Table 2.
Treatment Related Toxicities
| Grade 1 N (%) |
Grade 2 N (%) |
Grade 3 N (%) |
Grade 4 N (%) |
|
|---|---|---|---|---|
| Thrombocytopenia | 7 (28) | 1 (4) | 1 (4) | 3 (12) |
| Constipation | 5 (20) | 3 (12) | 1 (4) | 0 (0) |
| Anemia | 6 (24) | 1 (4) | 1 (4) | 0 (0) |
| Nausea | 6 (24) | 2 (8) | 0 (0) | 0 (0) |
| Transaminitis | 6 (24) | 1 (4) | 0 (0) | 0 (0) |
| Vomiting | 3 (12) | 2 (8) | 0 (0) | 0 (0) |
| Fatigue | 5 (20) | 0 (0) | 0 (0) | 0 (0) |
| Anorexia | 3 (12) | 0 (0) | 0 (0) | 0 (0) |
| Neutropenia | 0 (0) | 0 (0) | 1 (4) | 1 (4) |
| Pruritus | 0 (0) | 1 (4) | 0 (0) | 0 (0) |
| Sensory neuropathy | 0 (0) | 1 (4) | 0 (0) | 0 (0) |
| Rash | 1 (4) | 0 (0) | 0 (0) | 0 (0) |
| Mucositis | 1 (4) | 0 (0) | 0 (0) | 0 (0) |
Some patients experienced multiple toxicities. Highest grade of a toxicity experienced by a patient during the study is reported. All grade 3 and 4 toxicities were experienced by 5 patients.
Efficacy
Two patients were inevaluable due to the lack of repeat disease assessment imaging. One patient died of disease prior to repeat imaging after receiving two doses of temozolomide. Another patient died of acute respiratory failure secondary to pneumonia and pulmonary embolism that were deemed unrelated to temozolomide treatment.
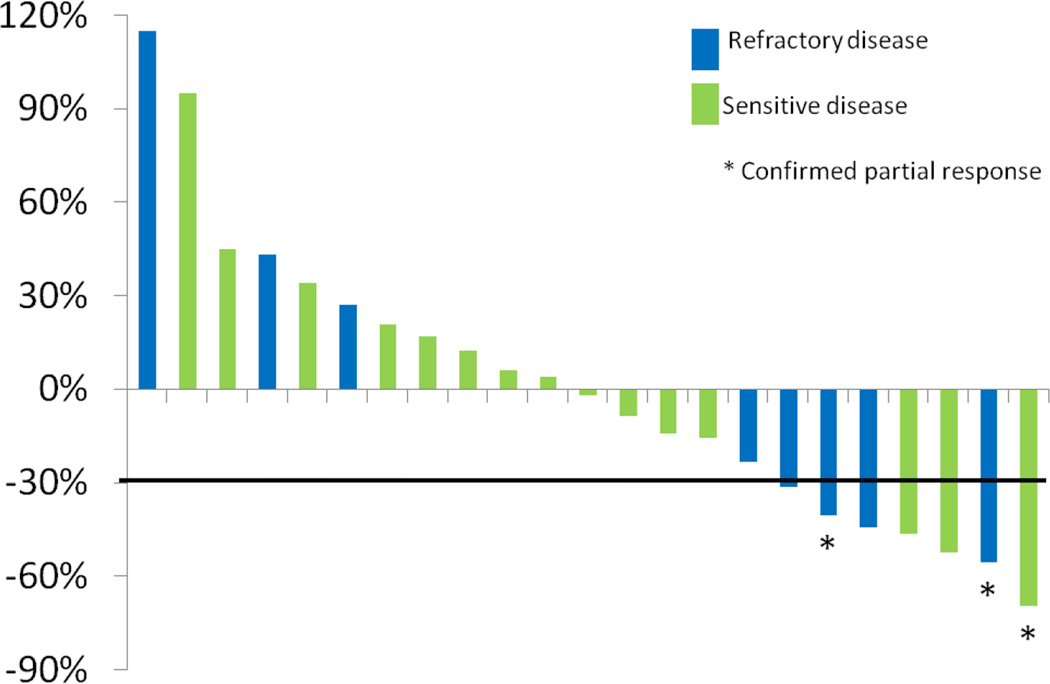
The overall partial response rate was 12% [3/25; 95% CI: 3–31%], two in patients with refractory SCLCs and one in a sensitive relapse patient. An additional eight patients had stable disease for a median of 2 cycles (range 1–5). Figure 1 shows the best response of indicator lesions for the 23 evaluable patients.
Figure 1.
Of the eight patients with target lesions in the brain, four had stable brain lesions [50%, 95% CI: 16–84%], and three had progression in the brain [38%, 95% CI: 9–76%]. The median time to progression and overall survival for all treated patients were 1.8 months [95% CI: 0.9–3.5 months] and 5.8 months [95% CI: 3.3–9.8 months], respectively. One-year overall survival was 26% [95% CI: 11–45%].
MGMT Analyses
Archival tissue was available to assess MGMT expression by immunohistochemistry in 52% (13 of 25) of patients. One (8%) was MGMT-negative by immunohistochemistry and experienced a partial response. Eight specimens were also tested for the presence of MGMT promoter methylation by PCR. Seven had evidence of promoter methylation and, of these, 1 [14%, 95% CI: 0–40%] had a partial response.
Discussion
This study assessed the toxicity of a 5-day dosing regimen of temozolomide in patients with relapsed SCLCs. The primary end-point of this trial was met with only 5 patients experiencing grade 3 or 4 treatment-related toxicity (excluding lymphopenia as per prespecified trial endpoint). Overall, temozolomide was well-tolerated and toxicities were mild. Responses were noted in 12% of patients [95% CI: 3–31%]. Two patients with refractory SCLCs and one patient with relapsed sensitive SCLC had partial responses. However, despite the known efficacy of temozolomide in brain tumors, no responses were noted in the brain in this patient cohort, which differs from our previous experience.
Only promoter methylation of MGMT has been validated as a biomarker for temozolomide therapy in gliomablastomas. In order to explore the possible validity of MGMT promoter methylation and/or MGMT expression as a predictive biomarker for temozolomide therapy in SCLCs, we obtained archival tissue in more than half of patients. Unfortunately, the small sample size limits our assessment. Furthermore, we do not have any information on changes in MGMT expression and/or MGMT promoter methylation between initial therapy and recurrent disease. While we treated patients with recurrent disease, we only had archival tissue from initial diagnosis available for testing and changes in MGMT over time, during therapy, or with recurrence may occur. MGMT remains an intriguing possible biomarker worthy of furtherstudy.
A comparison of the toxicities observed with the 5-day and 21-day dosing regimens is noted in Table 3.9 The 5-day regimen resulted in less non-hematologic toxicity, without any grade 3 or 4 fatigue or rash as observed in the 21-day schedule of temozolomide. Anemia and neutropenia were similar with the two dosing regimens. Although the observed incidence of thrombocytopenia was higher in the 5-day regimen, the duration of this adverse effect was shorter and did not prohibit further therapy like it did with the 21-day dosing schedule, where 11% of patients could receive no further therapy due to persistent cytopenias.9 The overall response rate for the two regimens was similar (5-day dosing 12% [95% CI: 3–31%] versus 21-day dosing 20% [95% CI: 11–32%]) and comparable to other studies in patients with relapsed SCLCs. Notably, the proportion of patients with sensitive SCLCs in the 21-day dosing trial was higher than in the 5-day dosing study (75% versus 64%) which may have contributed to the observed difference in response rate. Based on this, we recommend the 5-day schedule in ongoing studies.
Table 3.
Comparison of Grade 3 and 4 Toxicity with Different Dosing Regimens of Temozolomide
| 5-day regimen (N=25) N (%) |
21-day regimen (N=64) N (%) |
|
|---|---|---|
| Thrombocytopenia | 4 (16) | 6 (10) |
| Neutropenia | 2 (8) | 3 (5) |
| Anemia | 1 (4) | 2 (3) |
| Fatigue | 0 (0) | 2 (3) |
| Rash | 0 (0) | 2 (3) |
| Constipation | 1 (4) | 0 (0) |
The addition of a second agent targeting DNA repair may potentiate the activity of temozolomide and possibly overcome resistance. Preclinical data suggest that poly-ADP ribose polymerase-1 (PARP-1) may interfere with temozolomide’s activity by repairing alkylated bases. In combination with the PARP-1 inhibitor valiparib, temozolomide causes anti-tumor responses in lung cancer models.16 We are exploring this combination in a phase II multicenter, double-blind, randomized trial of temozolomide with or without valiparib (www.clinicaltrials.gov, NCT01638546). This trial will further explore MGMT promoter methylation and MGMT expression as possible predictive biomarkers in this larger cohort.
Supplementary Material
Acknowledgements
Supported in part by Merck & Co., Inc.
Footnotes
No other financial disclosures
References
- 1.von Pawel J, Schiller JH, Shepherd FA, et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol. 1999;17:658–667. doi: 10.1200/JCO.1999.17.2.658. [DOI] [PubMed] [Google Scholar]
- 2.Eckardt JR, von Pawel J, Pujol JL, et al. Phase III study of oral compared with intravenous topotecan as second-line therapy in small-cell lung cancer. J Clin Oncol. 2007;25:2086–2092. doi: 10.1200/JCO.2006.08.3998. [DOI] [PubMed] [Google Scholar]
- 3.O'Brien ME, Ciuleanu TE, Tsekov H, et al. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol. 2006;24:5441–5447. doi: 10.1200/JCO.2006.06.5821. [DOI] [PubMed] [Google Scholar]
- 4.Esteller M, Herman JG. Generating mutations but providing chemosensitivity: the role of O6-methylguanine DNA methyltransferase in human cancer. Oncogene. 2004;23:1–8. doi: 10.1038/sj.onc.1207316. [DOI] [PubMed] [Google Scholar]
- 5.Toyooka S, Toyooka KO, Maruyama R, et al. DNA methylation profiles of lung tumors. Mol Cancer Ther. 2001;1:61–67. [PubMed] [Google Scholar]
- 6.Hammond LA, Eckardt JR, Baker SD, et al. Phase I and pharmacokinetic study of temozolomide on a daily-for-5-days schedule in patients with advanced solid malignancies. J Clin Oncol. 1999;17:2604–2613. doi: 10.1200/JCO.1999.17.8.2604. [DOI] [PubMed] [Google Scholar]
- 7.Brandes AA, Tosoni A, Cavallo G, et al. Temozolomide 3 weeks on and 1 week off as first-line therapy for recurrent glioblastoma: phase II study from gruppo italiano cooperativo di neuro-oncologia (GICNO) Br J Cancer. 2006;95:1155–1160. doi: 10.1038/sj.bjc.6603376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wick A, Felsberg J, Steinbach JP, et al. Efficacy and tolerability of temozolomide in an alternating weekly regimen in patients with recurrent glioma. J Clin Oncol. 2007;25:3357–3361. doi: 10.1200/JCO.2007.10.7722. [DOI] [PubMed] [Google Scholar]
- 9.Pietanza MC, Kadota K, Huberman K, et al. Phase II trial of temozolomide in patients with relapsed sensitive or refractory small cell lung cancer, with assessment of methylguanine-DNA methyltransferase as a potential biomarker. Clin Cancer Res. 2012;18:1138–1145. doi: 10.1158/1078-0432.CCR-11-2059. [DOI] [PubMed] [Google Scholar]
- 10.Gilbert M, Wang M, Aldape K, Stupp R, Hegi M, Jaeckle K. A randomized phase III trial comparing standard adjuvant temozolomide with a dose-dense schedule in newly diagnosed glioblastoma. J Clin Oncol. 2011;29 doi: 10.1200/JCO.2013.49.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paz MF, Yaya-Tur R, Rojas-Marcos I, et al. CpG island hypermethylation of the DNA repair enzyme methyltransferase predicts response to temozolomide in primary gliomas. Clin Cancer Res. 2004;10:4933–4938. doi: 10.1158/1078-0432.CCR-04-0392. [DOI] [PubMed] [Google Scholar]
- 12.Esteller M, Garcia-Foncillas J, Andion E, et al. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med. 2000;343:1350–1354. doi: 10.1056/NEJM200011093431901. [DOI] [PubMed] [Google Scholar]
- 13.Sciuscio D, Diserens AC, van Dommelen K, et al. Extent and patterns of MGMT promoter methylation in glioblastoma- and respective glioblastoma-derived spheres. Clin Cancer Res. 2011;17:255–266. doi: 10.1158/1078-0432.CCR-10-1931. [DOI] [PubMed] [Google Scholar]
- 14.Hegi ME, Liu L, Herman JG, et al. Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J Clin Oncol. 2008;26:4189–4199. doi: 10.1200/JCO.2007.11.5964. [DOI] [PubMed] [Google Scholar]
- 15.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 16.Palma JP, Wang YC, Rodriguez LE, et al. ABT-888 confers broad in vivo activity in combination with temozolomide in diverse tumors. Clin Cancer Res. 2009;15:7277–7290. doi: 10.1158/1078-0432.CCR-09-1245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.