Abstract
Objective
CD5+ B cells have been conceptualized as a possible surrogate for Breg cells. The aim of the present study was to determine the utility of CD5+ B cells as biomarkers in antineutrophil cytoplasmic antibody–associated vasculitis (AAV).
Methods
The absolute and relative numbers (percentages) of CD5+ B cells (explanatory variables) were measured longitudinally during 18 months in 197 patients randomized to receive either rituximab (RTX) or cyclophosphamide (CYC) followed by azathioprine (AZA) for the treatment of AAV (Rituximab in ANCA-Associated Vasculitis [RAVE] trial). Outcome variables included disease activity (status of active disease versus complete remission), responsiveness to induction therapy, disease relapse, disease severity, and, in RTX-treated patients, relapse-free survival according to the percentage of CD5+ B cells detected upon B cell repopulation.
Results
CD5+ B cell numbers were comparable between the treatment groups at baseline. After an initial decline, absolute CD5+ B cell numbers progressively increased in patients in the RTX treatment arm, but remained low in CYC/AZA-treated patients. In both groups, the percentage of CD5+ B cells increased during remission induction and slowly declined thereafter. During relapse, the percentage of CD5+ B cells correlated inversely with disease activity in RTX-treated patients, but not in patients who received CYC/AZA. No significant association was observed between the numbers of CD5+ B cells and induction treatment failure or disease severity. The dynamics of the CD5+ B cell compartment did not anticipate disease relapse. Following B cell repopulation, the percentage of CD5+ B cells was not predictive of time to flare in RTX-treated patients.
Conclusion
The percentage of peripheral CD5+ B cells might reflect disease activity in RTX-treated patients. However, sole staining for CD5 as a putative surrogate marker for Breg cells did not identify a subpopulation of B cells with clear potential for meaningful clinical use. Adequate phenotyping of Breg cells is required to further explore the value of these cells as biomarkers in AAV.
The emergence of B cell depletion strategies for the treatment of immune-mediated disorders has renewed the interest in B cell biology. B cells not only represent a potential source of autoantibodies but also modulate effector, memory, and regulatory T cell responses through antibody-independent mechanisms (1–3). Some of these mechanisms involve antigen-specific suppressive B cells (known as Breg cells), which have been identified and characterized in experimental models and in human disease (1,4–10).
The competency to produce and secrete interleukin-10 (IL-10) is a hallmark of Breg cells. However, more than one phenotypically distinct subpopulation of B cells seems to be able to function in a regulatory capacity (4,11). Breg cells have been described within both the B1 and B2 B cell lineages. In healthy individuals, ~10% of the immature transitional B2-phenotype peripheral B cells produce IL-10 upon CD40 engagement. These cells can limit the polarization of naive CD4 lymphocytes toward the T helper cell subtypes Th1 and Th17, and can promote the conversion of effector CD4 cells into FoxP3+ regulatory T cells (10,12). Of note, abnormalities in the number or function of Breg cells have been demonstrated in patients with different autoimmune disorders (10,12–14), and a positive correlation between increased numbers of transitional B cells, increased serum concentrations of IL-10, and the state of tolerance off immunosuppression has been described in kidney transplant recipients (15).
CD5 is expressed on 80% of B cells in newborns and on 10–30% of B cells in adults (10,16). Most CD5+ B cells are naive and represent either transitional B2 B cells or T cell–independent B1 B cells. CD5 negatively regulates B cell receptor signaling (17), induces the production of IL-10 (16), and is reported to be present in many of the phenotypes attributed to Breg cells (10,18). Therefore, it is conceivable that surface CD5 staining on B cells could identify a subpopulation of cells in which Breg cells are enriched. In antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis (AAV), increased numbers of circulatory CD25+CD5+ B cells have been linked to disease quiescence (19). Recently, an inverse correlation between the percentage of CD5+ B cells and disease activity was described in a group of patients with this disease (20). Following peripheral B cell repopulation after rituximab (RTX) administration, a higher percentage of CD5+ B cells (i.e., >30%) was associated with prolonged remission (20).
The aim of this study was to analyze the kinetics of the putatively regulatory CD19+CD5+ B cell compartment in a large, well-characterized cohort of patients with AAV. In addition, we sought to explore the clinical associations of the change in the absolute and relative numbers of this cell subpopulation.
PATIENTS AND METHODS
Patient groups, treatment regimens, and definitions
The Rituximab in ANCA-Associated Vasculitis (RAVE) study was a multicenter, double-blind, placebo-controlled trial that randomized 197 patients in a 1:1 ratio to receive either RTX (375 mg/m2 intravenously each week for 4 weeks; n = 99) or cyclophosphamide (CYC) (2 mg/kg for 3–6 months) followed by azathioprine (AZA) (2 mg/kg, up to 150 mg/day) (n = 98) (21). Both groups of patients were tapered off prednisone treatment over 5.5 months, and were followed up for a total of 18 months.
Disease activity during the clinical trial was measured using the Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis (BVAS/WG) (maximum score 68) (22) at baseline and at 1, 2, 4, 6, 9, 12, 15, and 18 months. Patients with a BVAS/WG score of ≥1 were considered to have active disease. Complete remission was defined as a BVAS/WG score of 0, following successful completion of the prednisone taper. Severe flare was defined as a BVAS/WG score of ≥3 or the occurrence of at least one major BVAS/WG item requiring treatment with either RTX or CYC, following disease remission. Limited flare was defined as the occurrence (or worsening) of at least one minor BVAS/WG item leading to a score of <3 (21). Induction treatment failure was defined as the occurrence of a severe or limited flare that required RTX or CYC treatment within the first 6 months after initial treatment, inability to adhere to the prespecified prednisone taper due to persistent or recurrent disease activity, the occurrence of a limited flare within the first 6 months that could not be controlled by increasing the prednisone dose, or death caused by persistent disease activity.
Sample collection, cell preparation, and cell surface staining
Heparinized peripheral blood samples were obtained at baseline, 2 weeks, and 1, 2, 4, 6, 9, 12, 15, and 18 months for analysis of lymphocyte populations and subpopulations by flow cytometry. Whole blood was collected in sodium heparin vacutainers (Becton Dickinson) and shipped ambient overnight to the ITN Flow Cytometry Core (Roswell Park Cancer Institute). Using a stainlyse method, cells from blinded samples were labeled with 5-color monoclonal antibody panels. Marker/fluorochrome combinations have been previously described (21). Following staining, data were acquired on a FACSCanto flow cytometer (BD Biosciences). The data were analyzed using WinList software (Verity Software House).
Lymphocytes that expressed CD19 were categorized as CD19+ B cells (or simply B cells), and the subpopulation of CD19+CD5+ cells was identified by determining the coexpression of CD5 (categorized as CD5+ B cells). B cell depletion was defined as the presence of <10 CD19+ B cells per μl. B cell redetection was defined as the presence of at least 10, but fewer than 69, CD19+ B cells per μl. B cell reconstitution was defined as the presence of ≥69 CD19+ B cells per μl or a return to baseline levels, as described previously (23). The results for CD5+ B cells were expressed as the absolute number of CD19+CD5+ B cells per μl of whole blood and as the relative number (percentage) of CD5+ B cells within the total population of CD19+ B cells.
Statistical analysis
The explanatory variables were the absolute number and percentage of CD5+ B cells at different time points, and the percentage of CD5+ B cells upon peripheral B cell repopulation among RTX-treated patients. Outcome variables included disease activity (status of active disease versus complete remission), any disease relapse, severe relapse, BVAS/WG scores, occurrence of induction treatment failure due to disease activity, ANCA titers, and time to disease flare. Subanalyses according to treatment received (CYC/AZA versus RTX), disease category (new-onset versus relapsing disease at baseline), disease type (granulomatosis with polyangiitis [GPA] versus microscopic polyangiitis [MPA]), ANCA type (myeloperoxidase [MPO] versus proteinase 3 [PR3]), and the presence or absence of renal involvement at baseline were completed. Time to relapse in the RTX cohort was determined according to the percentage of CD5+ B cells detected following peripheral B cell redetection and reconstitution. Relative CD5+ B cell numbers for survival analysis were treated as dichotomous (i.e., >30% CD5+ B cells versus ≤30% CD5+ B cells) (20), categorical (percentage of CD5+ B cells stratified by quartiles), and continuous (logarithmic transformation of the percentage of CD5+ B cells) variables.
Repeated-measures analysis of variance was performed to compare the numbers of CD5+ B cells between the treatment arms at each time point, and adjustment for multiple comparisons was applied using the Tukey method. Statistical associations of the explanatory variables with continuous outcomes were measured using the Mann-Whitney U test for independent observations and Wilcoxon’s signed rank test for paired observations. Statistical associations of the explanatory variables with categorical outcomes were measured using Fisher’s exact test and the chi-square test, as appropriate. Correlation between the CD5+ B cell numbers and ANCA titers at baseline was calculated using Spearman’s rank test. Time-to-event comparisons were done using log-rank tests. Hazard ratios were calculated using the Cox proportional hazards method. SAS version 9.2 (SAS Institute) and the R program version 3.0.0 (http://www.r-project.org) were used for the statistical analyses. All data, as shown in Figures 1–5, are available to the public via the ITN TrialShare system (available at https://www.itntrialshare.org/ar/figures.html).
Figure 1.
Absolute and relative numbers of CD5+ B cells in patients with antineutrophil cytoplasmic antibody–associated vasculitis (AAV) treated with cyclophosphamide/azathioprine (CYC/AZA)–based and rituximab (RTX)–based regimens. Whole blood was obtained at different time points during a period of 18 months from patients with AAV who were treated with RTX (n = 99) or CYC/AZA (n = 98). Cells were stained for CD19 and CD5, and expression was determined by flow cytometry. Results are expressed in A, absolute numbers of CD19+CD5+ B cells/μl and B, percentages of CD5+ B cells within total CD19+ B cells. Groups were compared using repeated-measures analysis of variance with adjustment for multiple testing. Bars show the median and interquartile range. * = P < 0.05.
Figure 5.
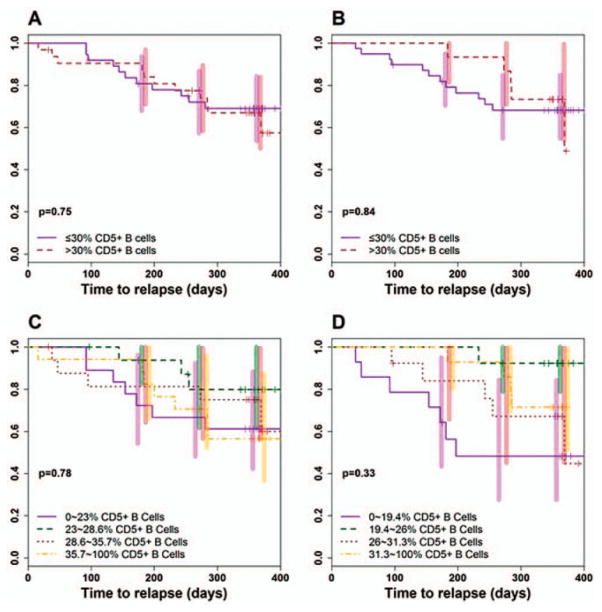
Relapse-free survival in patients with AAV according to the kinetics of CD5+ B cell repopulation after RTX treatment. Kaplan-Meier estimates of the time to relapse from complete remission were determined according to the percentage of CD5+ B cells at the time of B cell redetection (n = 69) and reconstitution (n = 54) after RTX administration. A and B, The percentage of CD5+ B cells was analyzed as a dichotomous predictor of time to relapse (>30% CD5+ B cells [brown lines] versus ≤30% CD5+ B cells [purple lines]) during times of B cell redetection (>30% n = 32 and ≤30% n = 37) (A) and B cell reconstitution (>30% n = 15 and ≤30% n = 39) (B). C and D, The percentage of CD5+ B cells was analyzed as a categorical predictor of time to relapse (stratified in quartiles: first quartile = purple lines, second quartile = green lines, third quartile = brown lines, fourth quartile = yellow lines) during times of B cell redetection (first quartile n = 18, second–fourth quartiles n = 17 each) (C) and B cell reconstitution (first quartile n = 14, second quartile n = 13, third quartile n = 13, fourth quartile n = 14) (D). Bars show the 95% confidence intervals at 183, 274, and 365 days. Results were compared using the log rank test in A and B and the Cox proportional hazards method in C and D. See Figure 1 for other definitions.
RESULTS
Summary of the main clinical outcomes
The clinical outcomes of the RAVE trial, as reported elsewhere (23,24), are summarized here. All subjects had active disease upon study enrollment (mean BVAS/WG score >8). The primary end point, complete remission at 6 months after randomization, was achieved in 64% of patients in the RTX group and 53% of patients in the CYC/AZA group (24). At 12 months and 18 months, 48% and 39% of the patients in the RTX group, respectively, had maintained complete remission, compared with 39% and 33%, respectively, in the CYC/AZA group (23) (Table 1).
Table 1.
Baseline characteristics of the study patients and clinical outcomes by treatment group*
| RTX (n = 99) | CYC/AZA (n = 98) | P | |
|---|---|---|---|
| Age at onset of symptoms, mean ± SD years | 54 ± 16.8 | 55.5 ± 14.1 | 0.26 |
| Sex, % female | 54 | 46 | 0.29 |
| Race or ethnic group, % white | 92 | 95 | 0.64 |
| GPA/MPA, % | 75/24† | 76/24 | 0.61 |
| PR3-ANCA+/MPO-ANCA+, % | 67/33 | 66/34 | >0.99 |
| New-onset disease/relapse, % | 48/52 | 49/51 | 0.62 |
| Renal disease, % | 66 | 66 | 0.92 |
| BVAS/WG score at study entry, mean ± SD | 8.5 ± 3.2 | 8.2 ± 3.2 | 0.38 |
| CD19+ B cells, median (IQR) cells/μl | 234 (124–392) | 193 (113–273) | 0.13 |
| CD5+CD19+ B cells, median (IQR) cells/μl | 16 (7–37) | 18 (7–37) | 0.98 |
| % CD5+ B cells, median (IQR) | 10 (4–15) | 10 (5–15) | 0.56 |
| B cell depletion by 6 months, no./total no. assessed (%) | 98/98 (100)‡ | 69/86 (80)§ | <0.01 |
| Complete remission, % | |||
| At 6 months (primary outcome) | 64 | 53 | 0.13 |
| At 12 months | 48 | 39 | 0.22 |
| At 18 months | 39 | 33 | 0.32 |
| At any time within 18 months | 77 | 71 | 0.42 |
| Patients in complete remission whose disease flared at or before 18 months, no./total no. assessed (%) | 24/76 (32) | 20/70 (29) | 0.16 |
| Severe flares, no. | 9 | 10 | 0.81 |
| Limited flares, no. | 15 | 10 | 0.39 |
Continuous variables were compared between groups using Mann-Whitney U test or Student’s t-test. Categorical variables were compared between groups using Fisher’s exact test. RTX = rituximab; CYC = cyclophosphamide; AZA = azathioprine; GPA = granulomatous with polyangiitis; MPA = microscopic polyangiitis, PR3 = proteinase 3; ANCA = antineutrophil cytoplasmic antibody; MPO = myeloperoxidase; BVAS/WG = Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis (maximum score 68); IQR = interquartile range.
One patient had undetermined ANCA-associated vasculitis.
One-month B cell data were unavailable for 1 RTX-treated patient.
Twelve CYC-treated patients were excluded from this analysis because they had been crossed over or treated according to best medical judgment before 6 months.
CD5+ B cell kinetics in response to RTX- and CYC/AZA-based treatment regimens
We first determined the kinetics of the CD5+ B cell compartment in patients with AAV by treatment group (RTX n = 99 and CYC/AZA n = 98). Baseline characteristics were comparable between the RTX and CYC/AZA cohorts (Table 1). By 6 months after randomization, 100% of patients in the RTX arm and 80% of patients in the CYC/AZA arm achieved peripheral B cell depletion. At baseline, there was no statistically significant difference between groups in terms of the absolute numbers of CD19+ B cells (median 234 B cells/μl in RTX-treated patients and 193 B cells/μl in CYC/AZA-treated patients; P = 0.13), the absolute numbers of B cells bearing the CD5 marker (median 16 CD5+ B cells/μl in RTX-treated patients and 18 CD5+ B cells/μl in CYC/AZA-treated patients; P = 0.98), or the percentage of CD19+ B cells that were also CD5+ (median 10% CD5+ B cells in both groups; P = 0.56) (Table 1).
In most patients, the absolute number of CD5+ B cells decreased to <5 cells/μl within 8 weeks of randomization regardless of treatment allocation, and remained at that level through 6 months (Figure 1A). As expected, this initial decline was significantly faster and more pronounced in the RTX group. After 6 months, CD5+ B cell numbers progressively increased in the RTX-treated subjects, but remained low in the CYC/ AZA group. At 18 months, the median number of CD5+ B cells/μl was 29 (interquartile range [IQR] 16–51) in patients in the RTX arm, compared with a median of 4 (IQR 2–15) in those in the CYC/AZA arm (P < 0.0001).
In contrast, the percentage of CD5+ B cells within the CD19+ B cell compartment significantly increased during remission induction, reaching a peak at 4 months in the RTX group (median CD5+ B cells 40%, IQR 20–67%) and 6 months in the CYC/AZA group (median CD5+ B cells 27%, IQR 18–36%), and gradually declined in both groups thereafter (Figure 1B). At 18 months, the median percentage of CD5+ B cells was 24% (IQR 21–28%) in the RTX group and 23% (IQR 17–29%) in the CYC/AZA group (P > 0.99).
No other baseline variables examined, including specific diagnosis (GPA versus MPA), ANCA type (PR3 versus MPO), disease presentation (new-onset versus relapse), or the presence of renal involvement upon enrollment, had any significant influence on the dynamics of the CD5+ B cell subpopulation in either group (data available from the corresponding author upon request). In addition, there was no statistically significant correlation between baseline percentage of CD5+ B cells and baseline ANCA titers (rs = 0.01, P = 0.89).
CD5+ B cells as a marker of disease activity
To test the hypothesis that the relative numbers of CD5+ B cells are inversely correlated with disease activity, we analyzed a group of 146 patients who achieved complete remission, and divided them by treatment allocation into the categories of nonrelapsers (RTX n = 52, CYC/AZA n = 50) and relapsers (RTX n = 24, CYC/AZA n = 20). Relapse was defined as any disease exacerbation (severe or limited flare) that occurred after the achievement of complete remission. The percentages of CD5+ B cells were then compared within each subgroup at specific time points, including active disease at baseline, time of first complete remission, 18 months (for nonrelapsers), and active disease during a subsequent disease relapse (for relapsers) (Figure 2). Fifty-one patients among the original cohort of 197 did not achieve complete remission during the trial and therefore were not included in this analysis.
Figure 2.
Percentage of CD5+ B cells in AAV patients treated with CYC/AZA or RTX during times of active disease and complete remission. Percentages of CD5+ B cells were serially obtained from patients treated with CYC/AZA or RTX who achieved and maintained complete remission with their original treatment until month 18 (nonrelapsers; RTX n = 52, CYC/AZA n = 50), and those who achieved complete remission with their original treatment but subsequently experienced a disease flare (relapsers; RTX n = 24, CYC/ AZA n = 20). The percentages of CD5+ B cells were compared within groups using Wilcoxon’s signed rank test. The × symbols represent values obtained from individual patients during active disease at baseline, circles represent values obtained during complete remission (e.g., first complete remission in all subgroups and at 18 months in nonrelapsers), and asterisks represent values obtained during disease flare (in relapsers). The remaining 51 subjects from the original cohort of 197 patients did not achieve complete remission and were therefore not included in this analysis. See Figure 1 for other definitions.
We found that the percentage of CD5+ B cells significantly increased in most patients in both treatment groups as disease activity declined toward remission, regardless of whether the subjects experienced a subsequent disease relapse. Among RTX-treated patients who were nonrelapsers, the median percentages of CD5+ B cells at baseline and at complete remission were 7% (IQR 3–12%) and 32% (IQR 13–57%), respectively (P < 0.0001). Among RTX-treated patients who were relapsers, the median percentages of CD5+ B cells at baseline and at complete remission were 12% (IQR 5–16%) and 28% (IQR 23–41%), respectively (P < 0.001). Among the CYC/AZA-treated patients, the median percentages of CD5+ B cells at baseline and at complete remission were 11% (IQR 8–15%) and 27% (IQR 18–36%), respectively, for nonrelapsers (P < 0.0001), and 6% (IQR 2–13%) and 26% (IQR 19–35%), respectively, for relapsers (P < 0.002). Among these 4 patient subsets—RTX nonrelapsers, RTX relapsers, CYC/AZA nonrelapsers, and CYC/AZA relapsers—there were no significant differences in the median percentage of CD5+ B cells at either baseline or complete remission.
Nonrelapsers, by definition, stayed in complete remission through month 18. This group experienced a non–statistically significant reduction in the percentage of CD5+ B cells by the end of followup (median CD5+ B cells 24%, IQR 20–28% in RTX-treated patients [P = 0.16]; median CD5+ B cells 25%, IQR 18–31% in CYC/AZA-treated patients [P = 0.14]). Within the relapsers, the percentage of CD5+ B cells was not significantly different at the time of disease exacerbation compared with the period of complete remission among CYC/AZA-treated patients (median CD5+ B cells 19%, IQR 12–27% [P = 0.24]). The corresponding analysis in the RTX-treated patients, however, demonstrated a modest but significant decline in the percentage of CD5+ B cells during disease relapse (median 23%, IQR 16–33% [P = 0.04]) (Figure 2).
CD5+ B cells prior to disease relapse
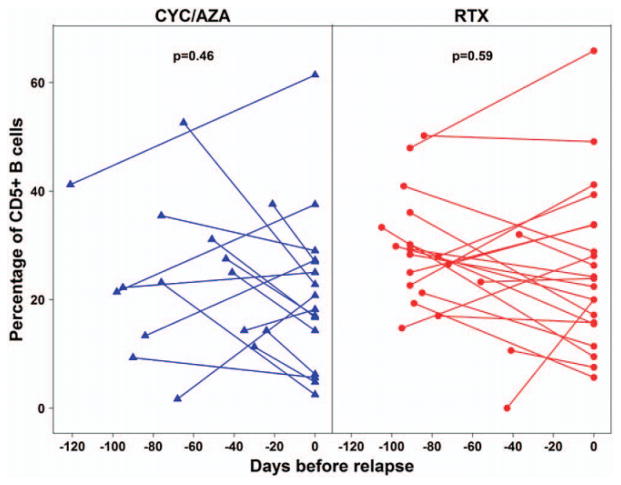
To determine whether reductions in the relative number of CD5+ B cells preceded disease relapse, we compared the percentage of CD5+ B cells by treatment group immediately before and at the time of vasculitis flare in 37 patients (RTX n = 21, CYC/AZA n = 16). Using, for each subject, a single measurement 20–120 days prior to disease exacerbation and a second measurement during the disease flare, the median percentage of CD5+ B cells was found to be 28% (IQR 21–32%) before disease exacerbation and 24% (IQR 16–34%) during the exacerbation in the RTX group (P = 0.59), while the median percentage of CD5+ B cells was 23% (IQR 14–32%) before disease exacerbation and 19% (IQR 12–27%) during the exacerbation in the CYC/AZA group (P = 0.46) (Figure 3). Thus, we did not observe a significant decline in the percentage of CD5+ B cells before disease relapse.
Figure 3.
Percentage of CD5+ B cells prior to and during disease relapse in AAV patients treated with RTX or CYC/AZA. The percentage of CD5+ B cells was compared immediately before and during disease relapse in 37 patients with AAV according to treatment group (CYC/AZA n = 16, RTX n = 21). Symbols joined by lines represent individual patients. Results were compared using Wilcoxon’s signed rank test. See Figure 1 for other definitions.
CD5+ B cells in relation to response to induction of remission and clinical course
To evaluate whether the initial change in the relative numbers of CD5+ B cells was correlated with early response to treatment, we compared the percentage of CD5+ B cells at baseline and during their peak levels between 4 and 6 months among patients who achieved complete remission (n = 115) or failed induction treatment (n = 55) by 6 months. The analysis showed no significant association between these variables (data available from the corresponding author upon request).
When divided by treatment group, longitudinal measurements of the absolute and relative CD5+ B cell numbers failed to discriminate between patients who maintained disease remission and those who experienced disease relapse (Figures 4A and B). Furthermore, when individual patients were analyzed from the time of their documented complete remission forward, there was no significant difference in the trend of the CD5+ B cell subpopulation between patients who subsequently experienced a disease exacerbation and those whose condition stayed in remission until the end of the study (data available from the corresponding author upon request).
Figure 4.
Absolute and relative numbers of CD5+ B cells in RTX-and CYC/AZA-treated patients who developed either a relapsing or nonrelapsing disease course. The absolute numbers (A) and relative numbers (percentage) (B) of CD5+ B cells were measured longitudinally in 146 AAV patients over a period of 18 months according to relapsing phenotype (those without relapse during the study [nonrelapsers] and those with at least one relapse during the study [relapsers]) and treatment group (RTX nonrelapsers n = 52 and RTX relapsers n = 24; CYC/AZA nonrelapsers n = 50 and CYC/AZA relapsers n = 20). Bars show the median and interquartile range. The remaining 51 subjects from the original cohort of 197 patients did not achieve complete remission and were therefore not included in this analysis. See Figure 1 for other definitions.
We also looked into the potential correlation between change in the CD5+ B cell fraction during induction of remission and subsequent disease severity (e.g., glomerulonephritis or alveolar hemorrhage flare). However, patients who developed poor outcomes during the study due to severe vasculitis had comparable baseline and peak percentages of CD5+ B cells (data available from the corresponding author upon request).
Relative numbers of CD5+ B cells upon peripheral B cell repopulation after RTX administration as a predictor of disease relapse
In the RTX group, 77 patients achieved complete remission at any time during the 18 months of the clinical trial (Table 1). B cell redetection (≥10 CD19+ B cells/μl) occurred in 69 patients after a mean period of time of 309 days, and B cell reconstitution (≥69 CD19+ B cells/μl) occurred in 54 patients after a mean period of time of 366 days. To study the hypothesis that the percentage of CD5+ B cells at the time of peripheral B cell repopulation after RTX administration is predictive of relapse-free survival (20), we divided patients according to the CD5+ B cell fraction upon B cell redetection and B cell reconstitution, and performed time-to-event analyses. Glucocorticoid use was not included as a confounding factor in these comparisons because only 3 and 5 patients were still receiving prednisone at the time of B cell redetection and reconstitution, respectively. By the end of followup, B cells had not reached the threshold of redetection and reconstitution in 8 and 23 patients, respectively, and therefore these subjects were excluded from the analysis.
Based on prior reports (20), we first investigated whether the time to disease relapse was significantly longer for individuals with >30% CD5+ B cells at the time of B cell redetection. We found that in this group of patients, the 80th percentile of time to flare from complete remission was 233 days (95% confidence interval [95% CI] 47–369), compared to 197 days (95% CI 135–infinite) in subjects who demonstrated ≤30% CD5+ B cells at the same time point.
Cox proportional hazard regression analysis showed a hazard ratio for disease flare of 1.14 (95% CI 0.49–2.64; P = 0.75) in patients whose CD5+ B cell percentage was >30% compared with patients in whom the CD5+ B cell percentage was ≤30%. The wide confidence intervals for both the 80th percentile relapse-free times and the hazard ratio for disease flare indicate that there was substantial variability in the association between percentage of CD5+ B cells and propensity to flare. The estimated relative risk was not significantly different from 1.0 and, in fact, the point estimate was consistent with longer flare-free times for patients with lower percentages of CD5+ B cells at the time of B cell redetection (Figure 5A).
Because the estimation of percentages within a very small population of cells carries an inherent risk of being inaccurate, we also conducted analyses in patients divided on the basis of the relative number of CD5+ B cells at the time of B cell reconstitution. The results showed that the 80th percentile of relapse-free survival time from complete remission was 284 days (95% CI 0–369) and 181 days (95% CI 95–infinite) in patients who demonstrated >30% CD5+ B cells and those who demonstrated ≤30% CD5+ B cells at this time point, respectively. In patients whose peripheral B cell pool was reconstituted with >30% CD5+ B cells, the hazard ratio for disease flare was 0.9 (95% CI 0.31–2.55), which was again not significantly different from that in subjects whose peripheral B cell pool was reconstituted with ≤ 30% CD5+ B cells (P = 0.84) (Figure 5B).
We supplemented the initial dichotomous approach with continuous variable– and categorical variable–based analyses. Nonetheless, we found no evidence of a significant or consistent trend. When we used the logarithmic transformation of the percentage of CD5+ B cells at the time of redetection and reconstitution as a continuous predictor, we observed no independent effect of this variable on the time to disease exacerbation (P = 0.73 for redetection, P = 0.12 for reconstitution).
Finally, after dividing patients based on quartiles of CD5+ B cell percentage upon B cell repopulation, we did not find any statistically significant difference in terms of relapse-free survival when the higher quartiles were compared with the lower quartiles. The hazard ratios for disease flare were 1.05 (95% CI 0.72–1.54; P = 0.78) and 0.80 (95% CI 0.52–1.24; P = 0.33) at B cell redetection and reconstitution, respectively, with no trend according to the order of the strata (Figures 5C and D).
DISCUSSION
Biomarkers that predict clinical outcomes accurately and in a timely manner have been elusive in AAV. Our results, which come from the analysis of a large cohort of patients who underwent rigorous clinical monitoring and systematic blood sampling for a period of 18 months while receiving what is now considered standard of care, do not support the notion that CD19+CD5+ B cells are useful as biomarkers in current clinical practice in this disease.
We did find a statistically significant association between disease activity and the relative number of CD5+ B cells in the RTX treatment group. However, such an association was not observed in patients treated with CYC/AZA. In addition, we cannot completely exclude the possibility that the main driver for the changes in the CD5+ B cell subpopulation was the effect of immunosuppression, because the fraction of CD5+ B cells at the time of relapse in RTX-treated patients had declined only modestly compared with the same measurement at the time of complete remission, and most subjects who remained in complete remission also demonstrated a slow decline in their percentage of CD5+ B cells.
In contrast to previous reports (19,20), we found no significant association between the percentage of CD5+ B cells and disease relapse. We did not observe a decline in the percentage of these cells immediately before disease exacerbation, nor did we find any correlation between the kinetics of the CD5+ B cells and the relapsing phenotype. In addition, early measurements of absolute and relative CD5+ B cell numbers (e.g., during the first 6 months) were not significantly different between the patient subsets who achieved or failed to achieve complete remission or between patients who did and those who did not experience severe disease relapses upon further followup.
In an analysis of 19 cases, Bunch et al (20) suggested that the percentage of CD5+ B cells at B cell repopulation after RTX administration may be of prognostic importance in terms of estimating the duration of disease remission. Our results using 2 different cutoffs (B cell redetection, defined as 10–68 CD19+ B cells/μl; B cell reconstitution, defined as ≥69 CD19+ B cells/μl) showed that the relative numbers of CD5+ B cells were not a predictor of relapse-free survival. We observed no significant difference in the time to disease flare following complete remission among patients whose peripheral B cell pool was replenished with >30% CD5+ B cells as opposed to ≤30% CD5+ B cells. Furthermore, the division of patients by quartiles of CD5+ B cell percentage upon B cell repopulation failed to show any significant effect of the higher quartiles on the time to relapse, nor was there any trend according to the order of magnitude of the strata.
We did not perform intracellular staining for IL-10, labeling for other surface antigens (e.g., CD24, CD38, CD1d, CD27), or functional assays for a more accurate characterization of Breg cells. This represents an important limitation of our study. The flow cytometry assays described herein were performed in real-time throughout the RAVE trial. At the time that these studies were conceived, knowledge about Breg cell biology was relatively limited. Since then, the characterization of Breg cells and their role in experimental models and human autoimmune disease has considerably improved (1,4,11). Subsequent research has shown that more than one B cell subpopulation is capable of immunoregulation, and these subpopulations exert their regulatory functions through IL-10–dependent and IL-10–independent mechanisms. Breg cells with an immature/transitional phenotype (e.g., CD19+CD24high CD38high) (10) and those with a mature/memory phenotype (e.g., CD19+CD24highCD27+) (18) have been described.
In healthy humans, up to 30% of peripheral CD19+ B cells express CD5 (10). Within the CD19+ CD5+ B cell compartment, ~10% of the cells are CD1dhigh and roughly 90% are CD1dlow/−. About 70% of CD19+CD5+CD1dhigh B cells—known to be regulatory in animal models (8)—are contained within the CD19+CD24highCD38high subpopulation in humans (10). In contrast, the exact percentage of CD19+CD5+ CD1dlow/− B cells that is included within the CD19+ CD24highCD38high phenotype has not been reported. In addition, although CD19+CD24highCD38high Breg cells are known to be either numerically reduced or functionally deficient in patients with rheumatoid arthritis (RA) (12) and patients with systemic lupus erythematosus (SLE) (10), data regarding the numbers or functionality of CD19+CD5+CD1dlow/− B cells in RA and SLE have not been published.
An observational study found that the fractions of circulating CD19+CD5+B cells, CD19+CD5+CD1d+B cells, and CD19+CD5+CD1d+IL-10+ B cells are diminished in patients with early RA and increase significantly as patients enter into disease remission with immunomodulatory therapy, but no functional evaluation was performed (25). Reduced frequencies of CD19+CD5+CD1d+IL-10+ B cells and/or CD19+ CD24highCD38high B cells have also been described in patients with Crohn’s disease and myasthenia gravis (13,14). Unfortunately, no data pertaining to the number or function of CD19+CD5+ (CD1dlow/−) B cells in these patients are available.
Breg cells in AAV have been recently studied by 3 independent groups (26–28). However, the different phenotypic characterizations of this cell subpopulation used in those analyses, none of which included the CD5 marker, preclude comparisons with our results. Moreover, it is important to recognize that the patients in those studies were sampled on only a single occasion, while receiving a variety of immunosuppressive agents. In a study of 41 subjects, the percentage of CD19+IL-10+ B cells was significantly lower in patients compared with healthy controls, but no significant difference was detected between patients with active disease and those with inactive disease (26). Another study of 53 subjects found a reduction in the fraction of CD19+ CD24highCD38high B cells in PR3- and MPO-ANCA–positive patients with quiescent AAV and PR3-ANCA–positive patients with active AAV, when compared with healthy controls (27).
Finally, a recent study evaluated 2 putative Breg cell subsets (i.e., CD19+CD24highCD38high B cells and CD19+CD24highCD27+ B cells) in 48 PR3-ANCA–positive patients with AAV (28). The numbers of CD19+CD24highCD38high B cells were significantly reduced in patients in remission compared with patients whose disease was active or healthy controls. On the other hand, CD19+CD24highCD27+ B cell numbers were significantly decreased both in patients with active disease and in patients with inactive disease compared with healthy controls. The capacity of CD19+ B cells to produce IL-10 and suppress the activation of monocytes, however, was not altered in these patients.
In conclusion, the sole staining for CD5 as a surrogate marker for Breg cells did not identify a subpopupation of CD19+ B cells with clinically meaningful value. Longitudinal studies using an expanded repertoire of surface CD markers (e.g., CD1d, CD24, CD38), intracellular staining for IL-10, and functional inhibitory assays are required to explore more thoroughly the question of Breg cells as biomarkers in AAV before any firm conclusions regarding their clinical utility are warranted.
Acknowledgments
The data in this study are from the Rituximab in ANCA-Associated Vasculitis (RAVE) trial, which was performed as a project of the Immune Tolerance Network (NIH contract N01-AI-15416), an international clinical research consortium headquartered at the Benaroya Research Institute and supported by the National Institute of Allergy and Infectious Diseases, NIH.
Dr. Miloslavsky has received honoraria for Advisory Board service from Genentech (less than $10,000). Dr. Langford has participated in clinical trials at the Cleveland Clinic that were funded by Genentech. Dr. Spiera has served as an investigator in clinical trials funded by Roche and Genentech. Dr. Kallenberg has received consulting fees from Novo Nordisk, Eli Lilly, MedImmune, and Chemo-Centyx (less than $10,000 each). Dr. Stone has received consulting fees, speaking fees, and/or honoraria from Genentech and Roche (less than $10,000 each).
Footnotes
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Stone had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Unizony, Phippard, Tchao, Iklé, Merkel, Seo, St.Clair, Langford, Spiera, Hoffman, Specks, Stone.
Acquisition of data. Unizony, Phippard, Tchao, Iklé, Asare, Merkel, Seo, St.Clair, Langford, Spiera, Hoffman, Kallenberg, Specks, Stone.
Analysis and interpretation of data. Unizony, Lim, Phippard, Carey, Miloslavsky, Tchao, Iklé, Asare, Merkel, Monach, St.Clair, Langford, Kallenberg, Specks, Stone.
References
- 1.Lund FE, Randall TD. Effector and regulatory B cells: modulators of CD4+ T cell immunity. Nat Rev Immunol. 2010;10:236–47. doi: 10.1038/nri2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dorner T, Radbruch A, Burmester GR. B-cell-directed therapies for autoimmune disease. Nat Rev Rheumatol. 2009;5:433–41. doi: 10.1038/nrrheum.2009.141. [DOI] [PubMed] [Google Scholar]
- 3.Eming R, Nagel A, Wolff-Franke S, Podstawa E, Debus D, Hertl M. Rituximab exerts a dual effect in pemphigus vulgaris. J Invest Dermatol. 2008;128:2850–8. doi: 10.1038/jid.2008.172. [DOI] [PubMed] [Google Scholar]
- 4.Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol. 2012;30:221–41. doi: 10.1146/annurev-immunol-020711-074934. [DOI] [PubMed] [Google Scholar]
- 5.Carter NA, Rosser EC, Mauri C. Interleukin-10 produced by B cells is crucial for the suppression of Th17/Th1 responses, induction of T regulatory type 1 cells and reduction of collagen-induced arthritis. Arthritis Res Ther. 2012;14:R32. doi: 10.1186/ar3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–50. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 7.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16:219–30. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 8.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–50. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 9.Dalwadi H, Wei B, Schrage M, Spicher K, Su TT, Birnbaumer L, et al. B cell developmental requirement for the Gαi2 gene [published erratum appears in J Immunol 2004;173:695] J Immunol. 2003;170:1707–15. doi: 10.4049/jimmunol.170.4.1707. [DOI] [PubMed] [Google Scholar]
- 10.Blair PA, Norena LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, et al. CD19+CD24hiCD38hi B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity. 2010;32:129–40. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Kalampokis I, Yoshizaki A, Tedder TF. IL-10-producing regulatory B cells (B10 cells) in autoimmune disease. Arthritis Res Ther. 2013;15 (Suppl 1):S1. doi: 10.1186/ar3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flores-Borja F, Bosma A, Ng D, Reddy V, Ehrenstein MR, Isenberg DA, et al. CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Sci Transl Med. 2013;5:173ra23. doi: 10.1126/scitranslmed.3005407. [DOI] [PubMed] [Google Scholar]
- 13.Oka A, Ishihara S, Mishima Y, Tada Y, Kusunoki R, Fukuba N, et al. Role of regulatory B cells in chronic intestinal inflammation: association with pathogenesis of Crohn’s disease. Inflamm Bowel Dis. 2014;20:315–28. doi: 10.1097/01.MIB.0000437983.14544.d5. [DOI] [PubMed] [Google Scholar]
- 14.Sun F, Ladha SS, Yang L, Liu Q, Shi SX, Su N, et al. Interleukin-10 producing-B cells and their association with responsiveness to rituximab in myasthenia gravis. Muscle Nerve. 2014;49:487–94. doi: 10.1002/mus.23951. [DOI] [PubMed] [Google Scholar]
- 15.Newell KA, Asare A, Kirk AD, Gisler TD, Bourcier K, Suthanthiran M, et al. Identification of a B cell signature associated with renal transplant tolerance in humans. J Clin Invest. 2010;120:1836–47. doi: 10.1172/JCI39933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dalloul A. CD5: a safeguard against autoimmunity and a shield for cancer cells. Autoimmun Rev. 2009;8:349–53. doi: 10.1016/j.autrev.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Mageed RA, Garaud S, Taher TE, Parikh K, Pers JO, Jamin C, et al. CD5 expression promotes multiple intracellular signaling pathways in B lymphocyte. Autoimmun Rev. 2012;11:795–8. doi: 10.1016/j.autrev.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Iwata Y, Matsushita T, Horikawa M, Dilillo DJ, Yanaba K, Venturi GM, et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117:530–41. doi: 10.1182/blood-2010-07-294249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eriksson P, Sandell C, Backteman K, Ernerudh J. B cell abnormalities in Wegener’s granulomatosis and microscopic polyangiitis: role of CD25+-expressing B cells. J Rheumatol. 2010;37:2086–95. doi: 10.3899/jrheum.100074. [DOI] [PubMed] [Google Scholar]
- 20.Bunch DO, McGregor JG, Khandoobhai NB, Aybar LT, Burkart ME, Hu Y, et al. Decreased CD5+ B cells in active ANCA vasculitis and relapse after rituximab. Clin J Am Soc Nephrol. 2013;8:382–91. doi: 10.2215/CJN.03950412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Specks U, Merkel PA, Hoffman GS, Langford CA, Spiera R, Seo P, et al. Design of the Rituximab in ANCA-Associated Vasculitis (RAVE) trial. Open Arthritis J. 2011;4:1–18. [Google Scholar]
- 22.Stone JH, Hoffman GS, Merkel PA, Min YI, Uhlfelder ML, Hellmann DB, et al. for the International Network for the Study of the Systemic Vasculitides (INSSYS) A disease-specific activity index for Wegener’s granulomatosis: modification of the Birmingham Vasculitis Activity Score. Arthritis Rheum. 2001;44:912–20. doi: 10.1002/1529-0131(200104)44:4<912::AID-ANR148>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 23.Specks U, Merkel PA, Seo P, Spiera R, Langford CA, Hoffman GS, et al. for the RAVE-ITN Research Group. Efficacy of remission-induction regimens for ANCA-associated vasculitis. N Engl J Med. 2013;369:417–27. doi: 10.1056/NEJMoa1213277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, et al. for the RAVE-ITN Research Group. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363:221–32. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma L, Liu B, Jiang Z, Jiang Y. Reduced numbers of regulatory B cells are negatively correlated with disease activity in patients with new-onset rheumatoid arthritis. Clin Rheumatol. 2014;33:187–95. doi: 10.1007/s10067-013-2359-3. [DOI] [PubMed] [Google Scholar]
- 26.Wilde B, Thewissen M, Damoiseaux J, Knippenberg S, Hilhorst M, van Paassen P, et al. Regulatory B cells in ANCA-associated vasculitis. Ann Rheum Dis. 2013;72:1416–9. doi: 10.1136/annrheumdis-2012-202986. [DOI] [PubMed] [Google Scholar]
- 27.Todd SK, Pepper RJ, Draibe J, Tanna A, Pusey CD, Mauri C, et al. Regulatory B cells are numerically but not functionally deficient in anti-neutrophil cytoplasm antibody-associated vasculitis. Rheumatology (Oxford) 2014;53:1693–703. doi: 10.1093/rheumatology/keu136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lepse N, Abdulahad WH, Rutgers A, Kallenberg CG, Stegeman CA, Heeringa P. Altered B cell balance, but unaffected B cell capacity to limit monocyte activation in anti-neutrophil cytoplasmic antibody-associated vasculitis in remission. Rheumatology (Oxford) 2014;53:1683–92. doi: 10.1093/rheumatology/keu149. [DOI] [PubMed] [Google Scholar]