Abstract
Mechanical interaction between the cell and its extracellular matrix (ECM) regulates cellular behaviors, including proliferation, differentiation, adhesion, and migration. Cells require the three dimensional (3D) architectural support of the ECM to perform physiologically realistic functions. However, current understanding of cell-ECM and cell-cell mechanical interactions is largely derived from 2D cell traction force microscopy, in which cells are cultured on a flat substrate. 3D cell traction microscopy is emerging for mapping traction fields of single animal cells embedded in either synthetic or natively derived fibrous gels. We discuss here the development of 3D cell traction microscopy, its current limitations, and perspectives on the future of this technology. Emphasis is placed on strategies for applying 3D cell traction microscopy to individual tumor cells migration within collagen gels.
Keywords: Traction force microscopy, cell mechanics, extracellular matrix, collagen, cell migration, 3D imaging
INTRODUCTION
Mechanical forces, whether cell generated or externally applied, critically regulate many cellular functions, including differentiation, growth, adhesion and migration. In their native state, all animal cells live within the context of a three dimensional microenvironment [1, 2]. These cells are supported architecturally by the extracellular matrix (ECM) and exert forces onto the ECM through cell-ECM contacts. The force balance arising from cell-ECM interactions plays an essential role in a number of physiological and pathological processes [3-8]. One well-known pathological example is the association between stiff tissue environment and the poor clinical prognosis of a breast tumor. A recent work from the Weaver lab [5] has demonstrated that breast tumorigenesis is linked to the disruption of force balance through ECM stiffening and increased focal adhesions. More subtly, a number of works have shown that mechanical forces shape morphogenesis during early animal development [9-12].
Quantitative measurements of single cell traction force started about three decades ago through the invention of 2D traction force microscopy (2D TFM) [13-16]. In 2D TFM, animal cells are cultured on the surface of a 2D substrate with tunable stiffness such as polyacrylamide[17, 18] or polydimethylsiloxane (PDMS)[19-21]. The cells are then incubated to allow traction to develop. A detergent or drug disabling cytoskeletal function is then used to release cell traction and the displacements of fluorescent beads embedded on the surface are recorded using fluorescence microscopy. The cellular traction force is calculated from the bead displacements using either a Green’s function[14] or Fourier based approach[15]. 2D TFM has evolved into a mature technology [17, 22-25]. It has played instrumental roles in understanding cell-substrate and cell-cell interaction in cell adhesion [26-30], cell migration [14, 31, 32], tissue formation [33], and tissue migration[34, 35]. For detailed accounts of the 2D TFM, please refer to an excellent review in [ref. 25].
3D cell culture, in which cells are embedded within an ECM, is increasingly accepted by the research community, as many cell types require the biophysical and biochemical cues within a 3D ECM to perform truly physiologically realistic functions [1, 2]. Cells are found to behave very differently on a 2D substrate than they do within 3D biomatrices [2, 36, 37]. In 2D, cells adhere to the substrate only on their basal sides, while in 3D, cells bind to the ECM on all sides and are supported by the 3D ECM architecture. Recent works have shown that dimensionality guides cell migration [37, 38]. Furthermore, molecular mechanisms governing cell adhesion and migration in 2D and 3D do not have apparent correlations [39-44].
As 3D in vitro cell cultures become mainstream [1, 45], 3D traction force microscopy (TFM) technology is rapidly advancing to meet the need of quantifying mechanical forces of single animal cells in 3D. The basic idea behind 3D TFM is similar to that of 2D TFM. It consists of two parts: first, the measurement of fluorescent bead displacements caused by the release of cellular traction force; second, translation of the bead displacements into a cellular traction field. Despite simplicity in its basic design, 3D TFM is still in its infant stage, and it is not widely used. Greater adoption is hindered by the difficulty and cost in imaging sub-micrometer scale features in 3D, knowledge of the mechanical properties of in vitro ECMs, in particular natively derived fibrous ECMs, and the necessity for complex computation algorithms that are not readily accessible to the biology community. In this perspective, we first discuss recent developments in 3D TFM noting that they are all fundamentally limited by modeling the ECM as a linear isotropic elastic continuum. We then discuss the nonlinear and fibrous nature of collagen gels in the context of cell generated forces. Finally, three promising directions are proposed for implementing 3D cell traction microscopy within collagen matrices: 1.) A far-field method that measures the single cell traction generated dipole force; 2.) A near field method that aims at mapping the traction field around a single cell using collagen fiber network modeling; 3.) Methods for mapping the cell traction field using engineered micro or nanoscale stress sensors.
CURRENT STATE OF THE ART IN 3D TRACTION FORCE MICROSCOPY
What is 3D traction force microscopy (3D TFM)?
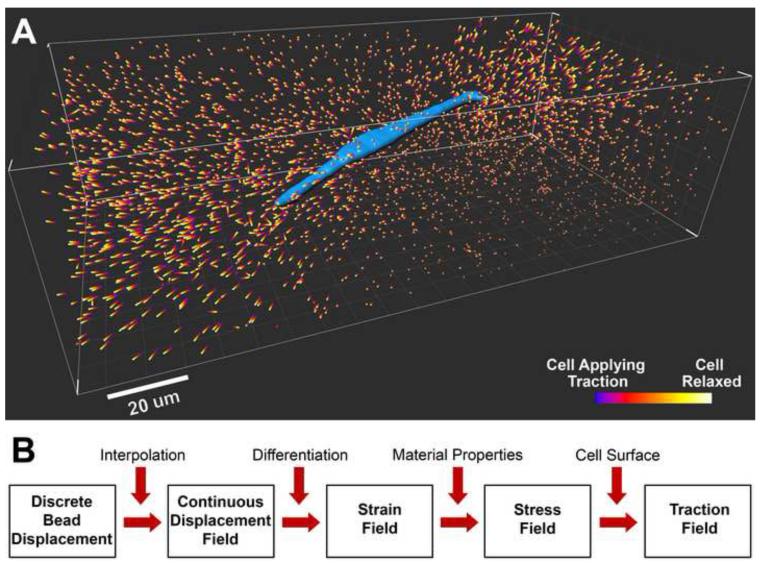
3D TFM is a technology designed to quantitatively measure the traction field of a single animal cell embedded within a 3D ECM. Three major steps are involved in all 3D traction microscopy technologies. First, animal cells are cultured within a 3D ECM embedded with micrometer size fluorescent beads exerting traction. Second, a detergent, enzyme, or drug is used to release the cell traction and the resulting 3D displacements of the fluorescent beads are imaged. Third, a computation algorithm is used to translate the bead displacements into a cellular traction field. One example of the discrete fluorescent bead displacements around a cell is shown in Figure 1A. Here, a breast tumor cell (MDA-MB-231) is embedded in 2.0 mg/ml type I collagen for 16 hours prior to the experiment, and bead positions are imaged before and after the administration of cytochalasin D (see Supplementary Material: Methods for cell relaxation). Cytochalasin D is known to disrupt actin filaments and therefore release cell generated traction force. The resulting displacements of fluorescent beads are computed and rendered in the volume around the 3D cell surface (Figure 1A). Figure 1B is a flow chart for computing 3D cell traction from the measured bead displacements. First, the spatially discrete displacement field is converted into a continuous field by an interpolation method. Second, the strain field is calculated by numerically evaluating the spatial gradient of the displacement field. Third, the stress field is calculated from strain using the known mechanical properties of the biomatrix together with a constitutive model (e. g. linear elastic model, or nonlinear elastic model). Finally, the traction field is evaluated using the 3D stress field in the matrix at the cell surface.
Figure 1. Discrete 3D bead displacements around a cell and forward computation algorithm for mapping cell traction.
A. A breast tumor cell (blue, MDA-MB-231 cell line) is embedded in a type I collagen matrix with concentration of 2 mg/ml. The colored bars are bead displacements caused by the relaxation of the cell after treatment with cytochalasin D. B. Flow chart of a forward computation algorithm to translate bead displacements into a 3D traction field on the cell surface.
Current methods in 3D traction force microscopy
A number of methods for 3D TFM have been developed (see a summary in Table 1) within the past 5 years. While the goal of all single cell 3D TFMs is the same – to measure the 3D traction field of a single animal cell, a variety of different methods have been implemented. Additional relevant methodologies have been developed for investigating 3D tractions in a multi-cellular context including epithelial tissues embedded within a collagen matrix[46], slug migration[47], and confluent endothelial cells[48]. In this section, we discuss the various ECM gel settings, imaging techniques, and computation algorithms employed by each of the methods, as well as their relative limitations and advantages.
Table 1.
Summary of current methods in 3D cell traction microscopy.
| Dimensionality | 2.5D | 2.5D | 2.5D | 3D | 3D | 3D |
|---|---|---|---|---|---|---|
| Result | 3D traction mapped over 2D cell surface |
3D traction mapped over 2D cell surface |
3D traction mapped over 2D cell surface |
3D traction mapped over 3D cell surface |
3D traction mapped over 3D multi-cellular tissue surface |
3D strain energy mapped in 3D gel around cell |
| Gel | Polyacrylamide surface functionalized with fibronectin |
Polyacrylamide surface functionalized with fibronectin |
Polyacrylamide surface functionalized with collagen I |
Modified polyethylene glycol diacrylate |
Collagen I (4 mg/ml) |
Collage I (2.4 mg/ml) |
| Imaging Method | Confocal Laser Scanning Microscopy |
Spinning Disk Confocal Microscopy |
Confocal Laser Scanning Microscopy |
Spinning Disk Confocal Microscopy |
Spinning Disk Confocal Microscopy |
Widefield Epifluorescence Microscopy |
|
Average Bead
Spacing [um] |
2.1-5.3 | 1.4-1.7 | 2.2-2.8 | 1.5 (using 2 colors) |
17 | 24 |
|
Displacement
Tracking |
Digital Volume Correlation |
Single Particle Tracking, Digital Volume Correlation |
Single Particle Tracking |
Single Particle Tracking |
Single Particle Tracking |
Single Particle Tracking |
| Solution Type | Forward | Semi-numericala | Inverse | Inverse | Forward | Forward |
| Cell Lines | Swiss 3T3 fibroblast |
Bovine aortic endothelial cells |
AX-2 wild-type Dictyostelium discoideum, NIH 3T3 fibroblast, |
NIH 3T3 fibroblast, human mesenchymal stem cells, bovine pulmonary artery smooth muscle |
EpH4 mouse mammary epithelial cells (multicellular tissue) |
MDA-MB-231, A-125, MCF-7, A-549, A-431 |
|
Cell Relaxation
Method |
Blebbistatin (myosin II disruption) |
Trypsin (proteolysis, cell detachment) |
Shaking buffer droplet (cell detachment), Cell migration |
SDS (cell lysis) |
Triton X-100 (cell lysis) |
Cytochalasin D (actin disruption) |
| Reference | Franck et al.[49, 50] and Maskarinec et al.[51] |
Hur et al.[48, 52] | Delanoë-Ayari and Rieu et al.[47, 53] |
Legant et al.[54] | Gjorevski et al.[46] |
Koch et al.[55] |
Semi-numerical: Hur et al. formulate a 3D boundary value problem and solve with finite element analysis.
Cell-matrix setting and dimensionality
There are two different ways to culture cells in relation to the ECM in the current implementations of 3D TFM. The first method is to culture cells on the surface of a functionalized polyacrylamide gel and embed fluorescent beads within the bulk [47-54]. This method (known as 2.5D [53]) takes advantage of the well characterized, linear and elastic mechanical properties of polyacrylamide gel [55, 56], and is able to map the 3D traction field within the gel directly underneath the cell. The limitation of the method is that cells are in contact with the flat substrate on only one surface rather than in a 3D context. The second method is to embed cells fully within an ECM, a true 3D model. In one case [57], a synthetic gel (a polyethylene glycol (PEG) hydrogel) was developed with appropriate adhesion sites and proteolytically degradable capability specifically for the application of 3D TFM; in another case [58], type I collagen gel was used . The advantage of this fully 3D method is that cells are surrounded and supported by the ECM in all directions. Use of synthetic gels is limited by the difficulty of creating a synthetic gel that both is compatible with cell culture and retains physiologically realistic features of the ECM in vivo. In the case of collagen gel, the limitation is the difficult with which one can manipulate the natively derived gel and its relatively complicated mechanical properties (See section: Bulk mechanical characterization of collagen gels).
Imaging methods for locating fluorescent bead positions and the cell surface
Different imaging methods have been used to record the three dimensional positions of the fluorescent beads and the cell surface embedded within the 3D ECM, balancing spatial and temporal resolution, phototoxicity, and cost. Confocal microscopy has been the main imaging method for 3D TFM as the optical sectioning afforded by the confocal aperture results in high spatial resolution in all three dimensions. This is particularly attractive for imaging the cell surface and fluorescent beads at high concentration. However, each voxel within a 3D volume must be individually illuminated by an excitation laser. In the case of Confocal Laser Scanning Microscopy, the emitted light is captured by a typically low quantum efficiency (~10%) photomultiplier tube compromising temporal resolution and cell viability through phototoxicity. Illuminating and recording multiple pixels in parallel reduces these limitations in Spinning Disk Confocal Microscopy at the cost of reduced spatial resolution. Widefield Fluorescence Microscopy (FM) has recently been used for tracking bead displacements within soft gels by either capturing many closely spaced 2D images to form a 3D image[58] or by using a defocused imaging method [59, 60]. The advantage of FM is that it can both be fast, and limit phototoxicity, however this comes at the cost of poor optical sectioning limiting spatial resolution along the optical axis (z-axis), especially for large homogenous structures like a fluorescently labeled cell. As a result, a true cell traction field has not yet been produced using FM. Looking forward, better microscope objective lenses (e. g. water immersion or silicone immersion), thinner samples, and de-convolution methods [59, 61, 62] could be used to improve the spatial resolution along the optical axis for FM. It should be noted that FM is widely available in biology labs, therefore, a 3D TFM that requires only FM can be more widely adopted and is more cost effective in comparison to methods using confocal microscopy. While the imaging methods above are sufficient to capture a detailed snapshot of cell traction at a given time, achieving this level of detail dynamically will require a method that better combines high spatial and temporal resolution with low phototoxicity. One promising imaging method for this purpose is the recent combination of Structured Illumination Microscopy with Light Sheet Fluorescence Microscopy[63, 64].
Computation algorithms for translating bead positions into traction field
A critical step in 3D TFM is to translate the fluorescent bead displacements into a cell traction field. In this section, we discuss and compare the two main types of computation methods that translate the bead displacements into the traction field: the inverse and the forward computation methods.
The inverse computation method was first introduced in the seminal work of Dembo and Wang [14] in 2D TFM. They model the gel substrate as an infinite half-space of a homogeneous, isotropic, linear, and elastic solid, and used a Green’s function approach to derive the relation between cellular traction and bead displacements. Because of the half space geometry, the solution to the Green’s function (or the displacement field caused by a point force) can be obtained analytically (known as the classical Boussinesq solution) from linear elastic theory [65]. For an isotropic material, only two material parameters are needed: Young’s modulus and the Poisson ratio. Recently this inverse computation method was extended to 3D TFM by Legant et al.[57]. One complication of this extension comes from the irregular boundary between the cell and the surrounding gel, as an analytical solution for the 3D Green’s function is not readily available. This problem was resolved by employing extensive finite element simulations to numerically obtain the 3D Green’s function. The numerically computed 3D Green’s function is used to form a set of linear equations that relate the measured bead displacements to the unknown cellular traction. Solving these equations using an inverse computation algorithm yields the traction. This however is not trivial since this set of equations is usually ill-posed [57], even in the case of 2D TFM as pointed out by Schwarz et al. [16, 66]. This means the cellular traction result may not be unique and can be sensitive to measurement errors in the bead displacement data. To ensure robustness of the traction measurement, additional regularization procedures [57, 66] have been developed. Recently a new approach utilizing adjoint equation was proposed for the inverse problem [67, 68]. In this method, the inverse problem is transformed into a set of coupled equations of the unknown cellular traction and its adjoint state. As a result, it does not require the evaluation of Green’s function and can lead to improved computational efficiency for 3D TFM.
The forward computation method offers a different route from bead displacements to cellular traction. It begins by determining a continuous displacement field from the bead images and then directly evaluates the strain and then the stress field. A schematic of the forward computation method is shown in Fig. 1B. Depending on how the displacement field is determined, the forward method can be further divided into two categories: single particle tracking method and digital volume correlation (DVC).
The single particle tracking method tracks the displacement of individual fluorescent beads, converts discrete displacements into a continuous displacement field using an interpolation algorithm, and then differentiates to find the strain field[59]. First, individual beads are tracked in order to calculate bead displacements by either a nearest neighbor[58, 59], feature volume based[51], feature vector based [57], or auto-regression (Imaris. Bitplane Inc. Zurich, Switzerland) [46] algorithm. Choice of a bead tracking algorithm becomes important for experiments where bead displacement is large relative to bead spacing. Second, a challenging task is to interpolate the displacements of a set of randomly distributed beads into a continuous displacement field. Koch et al.[58] connected the beads into a 3D mesh of tetrahedral elements and used linear interpolation within each element to calculate the strain energy. Questions remain to be addressed before a traction field can be accurately evaluated using this interpolation method: 1) there can be multiple 3D meshes to connect the same set of beads, and it is not clear how this would impact the interpolation; 2) because the displacement is linearly interpolated in each element, its gradient, or the strain field, is discontinuous across neighboring elements, which may limit the resolution of the strain/stress field, especially when the average bead spacing is large. Recently, our labs proposed to use a Moving Least Squares (MLS) method [69] to translate the discrete bead displacements into a continuous displacement field[59]. The main advantages of MLS over other interpolation method (e.g. linear interpolation) are that it is a mesh-free interpolation method; and it can produce an infinitely differentiable displacement field, thus ensuring a smooth strain field. Briefly, we first represent the displacement field by a 3D polynomial function. Unlike the conventional method in which the coefficients in the polynomial function are set to be constants, in MLS method these coefficients are assumed to vary from point to point, which gives the “polynomial” function enough flexibility to represent a very complicated field. At each interpolation point, the polynomial coefficients are determined by a weighted least square method in which beads closer to the interpolation point count more than those further away. The strain field can be easily calculated by differentiating the interpolated displacement field because of its smoothness. This method has been used successfully to map the traction field underneath an indenter [59] with promise for future application to mapping the 3D traction field of single animal cells embedded within collagen gel.
The digital volume correlation (DVC) method [70] is a 3D extension of digital image correlation method (DIC) widely used in experimental solid mechanics [71]. The working principle of DVC method is to first divide the imaging domain into volume elements, and then correlate the volume elements before and after deformation by comparing image patterns. The correlation process is realized by minimization of an objective function quantifying the match between the volume elements before and after deformation. Each volume element is assumed to only undergo a rigid translation [70]. Strain fields can be determined by first locally smoothing the displacement field and then numerically evaluating its gradient. Recently the DVC method has been applied to soft gels to image a high density of randomly distributed fluorescent beads which provide the 3D image pattern[49, 72]. Since gels can undergo large deformation, stretch and rotation of individual volume elements need to be taken into account when correlating the volume elements. This was partially considered in Franck et al.[49] by including stretch of volume elements in the correlation algorithm while neglecting rotation.
We emphasize that in the forward computation method, either by single particle tracking or DVC, the strain field is directly determined from the experimental data of bead positions without any assumptions on the mechanical properties of the gel. Once the strain field is determined, the stress field can be calculated by inputting the strain into an appropriate constitutive model that accurately describes the mechanical behavior of the gel. From the stress field σ, the cellular traction can be obtained using where is the unit outward normal vector to the surface of interest.
Inverse versus forward computation method
The irregular boundary between the cell and the 3D ECM can pose challenges to both the inverse and forward computation methods. The current inverse method based on Green’s function, when applied in 3D TFM, requires extensive finite element simulations to numerically constructing the 3D Green’s function, as shown in Legant et al. [57]. Such finite element simulations are performed specifically for a given geometry of the cell-ECM boundary. If the cell actively changes its shape, the boundary changes and a new set of simulations is required. For some occasions, e.g., for migrating cells, this can lead to high computational cost and thus reduced efficiency in the traction field calculation. This difficulty can be circumvented in the adjoint equation based inverse method [67, 68] and the forward computation method. In the forward method, the strain and stress components on the cell-ECM boundary are primarily interpolated from the displacements of beads in the vicinity of the cell surface. Therefore, quality of the cellular traction measurement hinges on the density of beads embedded near the cell and the ability to accurately measure their displacements. This is not the case for the inverse methods which can utilize the displacements of all beads to solve for the cellular traction. The inverse methods developed so far, either based on Green’s function [57] or adjoint equation [67, 68], rely on the assumption of linear isotropic elasticity. This is critical because most fibrous biological gels are nonlinear [73], tend to develop anisotropy as they strain [74, 75], and have other complicated material properties as summarized in next section on characterization of collagen matrix. The most important feature of the forward method is that it does not rely on a particular material model such as linear elasticity to obtain the strain field, and thus can be easily applied when large deformation and/or complex material behavior are encountered. Also, the inverse method requires complex computation algorithms that are not readily accessible to the biology community. These advantages have yet to be capitalized on as all current 3D cell traction microscopy technologies to date have utilized a linear isotropic elastic material model.
CHARACTERIZATION OF COLLAGEN MATRICES FOR 3D TFM
The main challenge in developing 3D TFM within collagen matrices is accounting for the mechanical properties of the fibrous collagen gel. While all current 3D TFM use a linear elastic material model, it has become clear that collagen gel is nonlinear, and exhibits strain stiffening behavior within the strain range that is relevant to cell traction [73]. Furthermore, some cell types (e. g. fibroblasts and malignant breast tumor cells) are capable of plastically deforming collagen by enzymatically degrading or secreting collagen near the cell surface [2, 7]. To develop a more realistic material model for 3D TFM, efforts must be made to better characterize the mechanical properties of the collagen matrices around cells. Here, we discuss current knowledge of the material properties of low concentration collagen gel (up to 5mg/mL) in the context of 3D traction microscopy and cell migration. We emphasize the importance of characterizing collagen microstructure in our quest to understand this complex material.
Cell migration within 3D collagen matrices
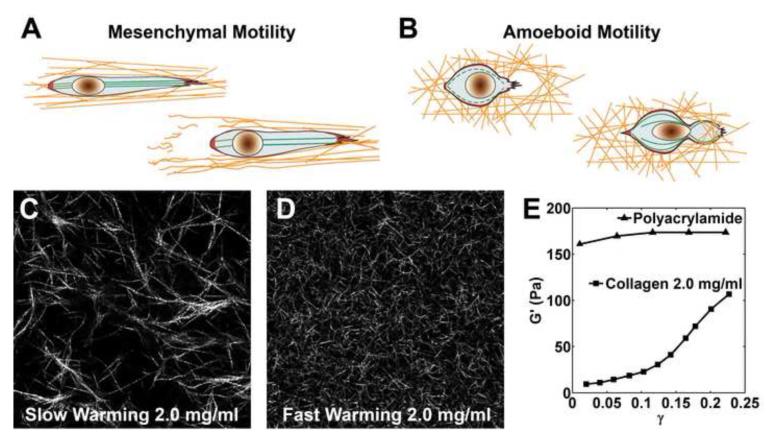
Animal cell migration is critically regulated by the architecture and the mechanical properties of the 3D ECM.. Animal cell migration within a 3D ECM can be broadly categorized into either amoeboid or mesenchymal motility [7, 76]. In amoeboid motility, cells appear ellipsoid in shape, form protrusions, and dynamically change their shape to squeeze through pores within the collagen fiber network (Figure 2B). Traction is distributed over the cell surface through many short lived adhesive contacts with the ECM. In mesenchymal motility, cells appear elongated in shape and climb along the collagen fibers (Figure 2A). Traction is exerted through long-lived, highly condensed integrin-based adhesions. It is important to note that mesenchymal cells are able to remodel the collagen matrix either by aligning fibers through the exertion of the traction force, or by the degradation of the collagen matrix via proteolysis (See Figure 2A). Specific protease inhibitors may be a useful tool for controlling matrix degradation in cell traction experiments [77, 78]. In addition to degrading the matrix, cells can also actively synthesize and deposit new collagen[2, 79]. The dynamic process of matrix remodeling must be taken into consideration in order to accurately measure 3D cell traction in degradable matrices like collagen I.
Figure 2. Cellular Motility Modes and Fibrous Nonlinear Collagen Gel.
A. Mesenchymal Motility. Strong traction at the front and back of the cell aligns surrounding fibers as the cell migrates in a protease-dependent manner leaving degraded collagen fibers in its wake. B. Amoeboid Motility. The cell exerts short lived tractions distributed over its surface and squeezes through a pore in a protease-independent manner. (Both A and B are reproduced from Pathak et al.[7] with permission of the Royal Society of Chemistry.) C –D. Confocal reflectance microscopy images of C slow warming and D fast warming type I collagen gel at 2 mg/mL. Image size is 100 um ×100 um. Increasing warming rate during polymerization dramatically decreases pore size and fiber diameter altering the local and bulk mechanical properties of the gel. E. A collagen gel (2 mg/mL) exhibits strain hardening as its shear storage modulus G’ increases with shear strain γ. This is in contrast to the linearity exhibited by a polyacrylamide gel which has constant G’ at varying γ. (The shear storage moduli G’ for both gels was measured at 10 rad/s with a strain-controlled rheometer (RFS-II Rheometrics). The shear storage modulus G’ approaches the shear modulus G at low frequency. Data is reproduced from Storm et al.[73] with permission of Nature Publishing Group.)
Microstructure of collagen gel
Microstructural features of collagen gels such as pore size and fiber diameter play critical roles in cell migration, and thus traction generation. Features of in vitro collagen gels depend sensitively on polymerization condition including collagen concentration, temperature, ionic strength, pH, collagen source, and pepsinization [80-83]. In addition, in vitro collagen gels can be cross-linked by a variety of enzymatic [84, 85] and non-enzymatic [86-91] processes and aligned by microfluidic flows [92], tensile strain [74], electric current [93], magnetic field [94], or flow of embedded magnetic beads [95]. When varying collagen microstructural features to investigate cellular behavior, each microstructure provides a unique mechanical environment for the cell. The relationship between microstructure and mechanical behavior must therefore be characterized for 3D TFM experiments within collagen gel.
One important parameter that dramatically impacts collagen microstructure is the temperature under which the collagen is polymerized. Typically, collagen solution derived from rat tails is prepared on ice at 0C, transferred into a device and then left to polymerize at 37C and 100% humidity [80]. The temperature at which collagen is polymerized changes the collagen microstructure, with lower polymerization temperature corresponding to larger pore size and fiber diameter [82, 83, 96-98]. Furthermore, our lab confirms that the ramping time it takes for collagen to reach 37C during polymerization also impacts the resulting microstructure. Figure 2C, D shows the microstructure of two collagen samples, one is fast warming, with the microwell containing the collagen sample directly placed on an iron block preheated to 37C; and the other is slow warming, with the microwell confined inside a plastic Petri dish before being placed inside a 37C incubator (see Supplementary Material: Methods for collagen microstructure characterization). The collagen concentration in both cases is 2 mg/mL.
Bulk mechanical characterization of collagen gels
Characterizing the relationship between the bulk mechanical properties of collagen gels and their fibrous microstructures is critical for computing the 3D traction field in 3D TFM. Recent advances in collagen material characterization have been made possible by the parallel measurements of bulk mechanical material properties at the macro-scale with visualization of the collagen fibers at the micro-scale [73-75, 81, 83, 99, 100]. Low concentration collagen gels with collagen concentration of 5 mg/mL or less exhibit nonlinear strain-stiffening in uniaxial tensile[100] and cyclic rheology[73] experiments that has been correlated with collagen fiber alignment under tension. Figure 2E shows the dependence of dynamic shear storage modulus G’ on the level of shear strain for a polyacrylamide gel and a collagen gel (2 mg/mL concentration) [73]. For linear elastic materials, the shear storage modulus G’ is a constant independent of the shear strain, as is approximately the case for the polyacrylamide gel. In contrast, for the collagen gel, the shear storage modulus G’ increases rapidly relative to shear strain even at low strain, presumably at least in part due to alignment of collagen fibers.
The bulk nonlinear behavior of collagen gels can be categorized into viscoelastic and poroelastic components. Viscoelasticity refers to time dependent viscous and elastic network deformation; while poroelasticity results from the flow of interstitial fluid (mostly water) under the pressure gradient between pores. Paired-incremental uniaxial step and ramp tests[101], dynamical mechanical analysis[102] and shear rheology tests[73, 81, 103, 104] have been designed to characterize the viscoelastic behavior of collagen. Chandran et. al. [99] have also studied the viscoelasticity and poroelasticity of collagen using step and ramp confined compression. It should be noted that the characteristic time of poroelastic flow in general depends on the size of the gel whereas the relaxation time of viscoelastic flow is insensitive to gel dimensions. Carefully designed experiments can therefore separate poroelastic and viscoelastic behavior temporally by controlling the length scale over which poroelastic flow occurs[105].
Modeling low concentration collagen gels as a continuum with constant mechanical properties throughout may have limited utility for 3D TFM near a single cell. This is because continuum models assume that it is possible to assign definite values to physical quantities such as stress and strain associated with a continuum “point”. The requirement is that a continuum “point” is macroscopically small in comparison with typical specimen dimensions. However, the “point” must be sufficiently large to contain a large number of relevant microstructural features (e.g. the number of cross-links between collagen fibers), so that an average quantity independent of this number can be computed. Given that the width of a stretched cell within a collagen matrix of about 10-20 um is comparable to the pore size between collagen fibers in low concentration collagen gels, a continuum model may not be valid at this length scale. It therefore may be necessary to use a fiber network model to fully describe the mechanical properties of the collagen matrix at the cellular length scale (See section: Near-field method: fiber network modeling). If continuum models break down near the cell, it may still be possible to use bulk mechanical properties for 3D TFM by implementing a far-field method (See section: Far-field method: cellular dipole force).
TOWARDS 3D TFM WITHIN COLLAGEN GELS
Current 3D TFM is successful in measuring the 3D traction field of single cells embedded in a linear and elastic material. The next step is to move this technology into fibrous gels, of which the biophysical ( e.g. the strain stiffening properties) and the biochemical (e. g. adhesion sites) parameters are important for many animal cell types to exhibit physiologically realistic behavior.
Here, we present three strategies for applying single cell 3D TFM within collagen gels: 1.) Far-field methods such as the dipole force model 2.)Near-field collagen fiber network modeling, and 3.)Engineering micro-scale and nano-scale stress sensors.
Far-field method: cellular dipole force
One challenge when applying 3D TFM to fibrous gels is that some cell types (e. g. fibroblasts, some malignant tumor cells) modify collagen around the cell surfaces. This makes it difficult to interpret the traction field around the cell, as the collagen material properties there are likely to be inhomogeneous and anisotropic and cannot be obtained a priori.
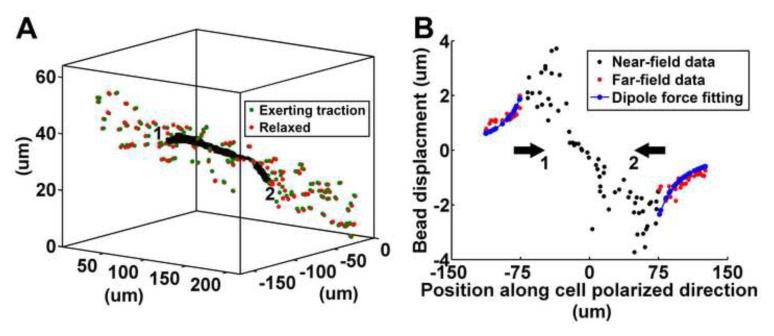
One way to circumvent this problem is by seeking a single numeric quantity that is representative of the total force exerted by the cell on its surroundings. Specifically, one can replace the cell and the region surrounding it where homogeneity of the matrix breaks down by a distribution of body forces. The stress and strain field at distances sufficiently far away from this region can be approximated by a multi-pole expansion caused by these body forces [106]. A special case of importance is when the cell is highly elongated which is the case for the mesenchymal cell phenotype (as shown in Figure 1 and Figure 2A). Since the cell is elongated, the dominant force exerted by the polarized cell on its surrounding can be represented approximately as a dipole force, that is, two equal and opposite forces of magnitude P along the polarized direction of the cell (see Figure 3A). The distance between these forces in the polarized direction are denoted by 2a, which is roughly equal to the length of the cell. In this simplified example, the moments applied by the cell are ignored due to insignificant asymmetry of experimental displacement fields.
Figure 3. Illustration of a cell generated force dipole.
A. Micro-beads in a cylindrical region of radius 10 um along the cell polarized direction are plotted. Red and green beads represent positions before and after deformation. Units of [um] are used in the figure. B. Far field displacements of the micro-beads in the region shown in A (which have been projected onto the cell polarized direction) are compared against fitted displacement using the dipole force calculation. The experiment is performed for a MDA-MB-231 tumor cell for a cytochalasin D experiment in a 2mg/mL collagen gel under fast warming condition. The Young’s modulus and Poisson’s ratio of collagen are assumed to be 50 Pa and 0.2 respectively. The half distance between the force dipole is 48 um.
For an infinite isotropic linear elastic material, the displacement fields along the dipole force direction can be readily determined by,
| (1) |
where P is the magnitude of dipole force, ν is Poisson’s ratio, E is Young’s modulus and x is the position along the dipole force direction. The force P can be determined by fitting the displacements given by (1) to the far-field bead displacement data from a cell traction experiment. Significant remodeling around the cell may shift the maxima in the displacement profile away from the cell tips. To accommodate this remodeling effect, the half distance between the force dipole a can be relaxed as an additional fitting parameter. Figure 3B shows an example of the measured displacements with the fitted displacements. Despite good agreement with the analytic solution in the far field, real displacements close to the cell tips are complicated due to fiber reorientation, plastic deformation, and cellular remodeling. To better account for these processes, this method can easily be extended to nonlinear material models under large deformation, including both the continuum models and medium scale regular cell network models discussed in the next section. When using a nonlinear model, numerical simulation would be necessary to obtain the dipole force from far field displacements. While far-field methods like the dipole force fitting are straightforward to implement, their assumption of a certain 3D stress field means that they are unable to map traction onto the cell surface. Their utility is rather to obtain a single number to quantify the force exerted by a cell. The calculated force can be insensitive to cellular remodeling if only far-field data is considered and account for the nonlinear behavior of collagen by incorporating an appropriate nonlinear material model.
Near field method: fiber network modeling
As previously discussed, the linear elastic isotropic continuum material model used in all current TFMs is insufficient for describing the material behavior of low concentration collagen gels near the cell. The network of collagen fibers near the cell can be highly inhomogeneous and subjected to remodeling by the cell, which could be a source of non-affine deformation [107]. Therefore, to accurately map cell traction onto the 3D cell surface, it is necessary to include the deformation of individual fibers as part of a deforming network in the mechanical modeling of collagen gels. At the moment, two distinct strategies are available to model the complex nature of collagen gels: 1.) A fiber network model can be used to create a continuum model that reproduces the real fibrous gel behavior such as strain stiffening; or 2.) The detailed geometry and deformation of the fiber network can be imaged and used as an input to a discrete large network model for computing the cellular traction field.
Improved Collagen Continuum Material Model through Network Modeling
A model for the mechanical behavior of individual fibers, together with an artificial network geometry, can be used to create a continuum model that better captures real collagen behavior. The advantage of this approach is that it does not require direct imaging and mapping of the real collagen network around each cell. All that is necessary is to supply the model with parameters that characterize the collagen microstructure (e.g fiber diameter, length, and network connectivity). These parameters can be obtained by imaging a volume of the collagen gel, for instance by using a CLSM in reflectance mode (though note that using CLSM to image fluorescently labeled fibers is preferable[108]). The collagen volume can then be rendered with a commercial software, for example Voxel-View reconstruction software (Vital Images, Inc., Plymouth, MN), and fiber diameter, length, and network connectivity statistics can be manual recorded for input into the network model[100]. Two types of network models that can be used to generate a continuum model with improved accuracy are continuum-scale isolated fiber models [109-112] and medium-scale regular-fiber-cell models [113-115].
For continuum-scale isolated fiber models, each fiber in the network behaves independently and moves affinely as dictated by the macroscopic deformation. A fiber can have nonlinear elastic behavior through application of hyperelastic material properties or an originally undulated configuration. These models require the least detail of network connectivity but require input of structural information such as angular distribution of fiber orientation [109-112] in the calculation of a continuum strain energy density function. Once the energy density function is obtained, the stresses can be computed knowing the deformation. The equilibrium deformations are determined by the minimization of total potential energy of the homogenized network. These simple structural models have been successful in modeling the strain-stiffening behavior of tissues although it is not clear that they can be applied to explain the large Poisson’s ratio and negative Poynting’s effect observed experimentally in collagen [116].
Regular-fiber-cell models, also known as cellular solid models, involve a unit cell usually consisting of 4-20 fibers, in which fiber connectivity and non-affine deformation can be considered. The constitutive behavior of the network is derived by calculating the equilibrium configuration of the unit cell under an applied load. The strain stiffening of collagen gels is explained as resulting from the nonlinearity of individual fibers in combination with non-affine reorientation of the network [114, 115]. These models can predict Poisson’s ratios greater than 1, though predictions based on these models are still substantially less than those observed in experiments. Although regular-fiber-cell models incorporate network connectivity, the network is still artificial and oversimplified when compared to real network geometry. The lack of accurate information about the real orientation and connectivity of the fibers in the real network is a source of error for both isolated fiber and regular-fiber-cell models especially at the cellular scale. Like other continuum models, these models become inaccurate at length scales where a continuum “point” does not incorporate a sufficient number of fibers to be considered homogenous. In the context of 3D TFM, using network modeling to obtain a continuum model is useful for improving the accuracy of far-field techniques, but it may be insufficient to accurately quantify cellular force with near-field data or map cell traction onto the cell surface.
Non-continuum large network modeling with collagen microstructure
Micro-scale large network models can be used to map 3D cell tractions to distinct points of contact between the cell and real collagen fiber network. These large-network models involve thousands of interconnected fibers which have either been randomly simulated, [117, 118] or as is more useful for cell traction microscopy, extracted from CLSM images [119, 120]. A custom automated 3D fiber extraction Matlab algorithm (FIRE) has been developed to reconstruct real network connectivity from CLSM image stacks of fluorescently labeled collagen fibers([120, 121]). Based on such extracted collagen fiber networks, a 3D linear elastic model of the collagen network has been established accounting for the stretching and bending of individual fibers as well as torsion at cross-links [119].
In large-network models, two approaches have been used to compute the macroscopic deformation. One approach determines the non-affine material response by minimizing the total potential energy of the network [119] or equivalently, enforcing local force balance at fiber crosslinks [117]. The other approach uses homogenization or volume averaging technique to obtain continuum strain and stress [118]. So far these models have not been able to predict the large Poisson’s ratio observed in collagen gel experiments. For example, [118] and [119] reported that the Poisson’s ratios in their simulated tension test are close to 1 which are below experimental measurements. Another hybrid network model [117] of collagen-agarose co-gels shows good match with the experimentally measured Poisson’s ratios for varying agarose concentration, however, the initial fiber concentration of the collagen gel is used as an adjustable parameter in their calculation. Finally, we point out that the network model by Stein et al. [119] have been successful in predicting negative Poynting effect in simple shear.
In summary, large network models for collagen are promising for describing the complex mechanical properties of fibrous gels, but further work is necessary to develop a physically-based network model that replicates the real mechanical behavior of collagen networks. . Current models have successfully predicted strain stiffening and a negative Poynting effect with numerical simulation, but the predicted Poisson’s ratio is still underestimated. With the challenges in mind, integrating a network model into 3D TFM has the potential to map cell traction on the cell surface even for cells that have greatly reoriented their surrounding fiber matrix.
Micro-scale and nano-scale stress sensors
Microfabricated devices have been used successfully to measure 2D single cell traction force [122-124], whereby an animal cell is cultured on top of a substrate with a bed of PDMS micropillars. The size of the micropillar is designed such that the cell generated traction will bend the pillar at the micrometer scale. The deformed pillar can be subsequently imaged with bright field or fluorescence microscopy. The micropillar is essentially a force transducer as the amount of bending is proportional to the force applied. This method is straightforward to implement, and has been used extensively to study cell-substrate mechanical interactions [125, 126] and the roles of various cytoskeletal molecules in cellular traction generations [31, 32]. Recently, there has been an attempt to move this method into 3D, where cells are embedded in a 3D collagen matrix, and plated on top of a sparsely populated micro-pillar bed [127]. Using stress sensors at this large micro-meter length scale is unlikely to yield sufficient resolution to map traction to the 3D cell surface. Rather, results would be similar to those for the far-field approach discussed previously. Magnitudes for total forces and moments exerted by the cell could be obtained. Smaller stress sensors are necessary to obtain more detailed information about the spatial distribution of traction exerted by the cell.
A nano-meter scale stress or strain sensor that can be embedded in the ECM would be an ideal tool for probing single cell traction force, in particular for those cells such as fibroblasts that are known to remodel ECMs. With our increasing understanding of force generation at the single molecule level, in particular molecules such as fibronectin that are present along with collagen in the ECM [128, 129], it is conceivable to engineer nano-scale stress sensors for 3D TFM in the near future [130].
Supplementary Material
ACKNOWLEDGEMENTS
This work is mainly supported by the National Center for Research Resources (5R21RR025801-03) and the National Institute of General Medical Sciences (8 R21 GM103388-03) of the National Institutes of Health. MW thanks partial support from the National Cancer Institute (Award No R21CA138366), the Cornell Center on the Microenvironment & Metastasis (Award No U54CA143876 from the National Cancer Institute), and the Cornell Nanoscale Science and Technology and the Cornell Nanobiotechnology Center. All of us would like to thank BJ Kim for his help at every stage of this work.
Abbreviations
- 2D
(Two Dimensional)
- 3D
(Three Dimensional)
- AFM
(Atomic Force Microscopy)
- DMEM
(Dulbecco’s Modified Eagle Medium)
- ECM
(Extracellular Matrix)
- FM
(Widefield Fluorescence Microscopy)
- PDMS
(Polydimethylsiloxane)
- PEG
(Polyethylene glycol)
- TFM
(Traction Force Microscopy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol. 2006;7(3):211–24. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- 2.Cukierman E, et al. Taking cell-matrix adhesions to the third dimension. Science. 2001;294(5547):1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 3.DuFort CC, Paszek MJ, Weaver VM. Balancing forces: architectural control of mechanotransduction. Nature Reviews Molecular Cell Biology. 2011;12(5):308–319. doi: 10.1038/nrm3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paszek MJ, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8(3):241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Levental KR, et al. Matrix Crosslinking Forces Tumor Progression by Enhancing Integrin Signaling. Cell. 2009;139(5):891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kostic A, Lynch CD, Sheetz MP. Differential Matrix Rigidity Response in Breast Cancer Cell Lines Correlates with the Tissue Tropism. PLoS One. 2009;4(7) doi: 10.1371/journal.pone.0006361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pathak A, Kumar S. Biophysical regulation of tumor cell invasion: moving beyond matrix stiffness. Integrative Biology. 2011;3(4):267–278. doi: 10.1039/c0ib00095g. [DOI] [PubMed] [Google Scholar]
- 8.Jaalouk DE, Lammerding J. Mechanotransduction gone awry. Nature Reviews Molecular Cell Biology. 2009;10(1):63–73. doi: 10.1038/nrm2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Culver JC, Dickinson ME. The effects of hemodynamic force on embryonic development. Microcirculation. 2010;17(3):164–78. doi: 10.1111/j.1549-8719.2010.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hove JR, et al. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature. 2003;421(6919):172–177. doi: 10.1038/nature01282. [DOI] [PubMed] [Google Scholar]
- 11.Vermot J, et al. Reversing blood flows act through klf2a to ensure normal valvulogenesis in the developing heart. PLoS biology. 2009;7(11):e1000246. doi: 10.1371/journal.pbio.1000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.le Noble F, et al. Flow regulates arterial-venous differentiation in the chick embryo yolk sac. Development. 2004;131(2):361–375. doi: 10.1242/dev.00929. [DOI] [PubMed] [Google Scholar]
- 13.Harris AK, Wild P, Stopak D. SILICONE-RUBBER SUBSTRATA - NEW WRINKLE IN THE STUDY OF CELL LOCOMOTION. Science. 1980;208(4440):177–179. doi: 10.1126/science.6987736. [DOI] [PubMed] [Google Scholar]
- 14.Dembo M, Wang YL. Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophysical Journal. 1999;76(4):2307–2316. doi: 10.1016/S0006-3495(99)77386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butler JP, et al. Traction fields, moments, and strain energy that cells exert on their surroundings. American Journal of Physiology-Cell Physiology. 2002;282(3):C595–C605. doi: 10.1152/ajpcell.00270.2001. [DOI] [PubMed] [Google Scholar]
- 16.Sabass B, et al. High resolution traction force microscopy based on experimental and computational advances. Biophysical Journal. 2008;94(1):207–220. doi: 10.1529/biophysj.107.113670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pelham RJ, Jr., Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(25):13661–5. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang YL, Pelham RJ. Preparation of a flexible, porous polyacrylamide substrate for mechanical studies of cultured cells. Molecular Motors and the Cytoskeleton, Pt B. 1998;298:489–496. doi: 10.1016/s0076-6879(98)98041-7. [DOI] [PubMed] [Google Scholar]
- 19.Lee J, et al. TRACTION FORCES GENERATED BY LOCOMOTING KERATOCYTES. Journal of Cell Biology. 1994;127(6):1957–1964. doi: 10.1083/jcb.127.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oliver T, Jacobson K, Dembo M. Design and use of substrata to measure traction forces exerted by cultured cells. Molecular Motors and the Cytoskeleton, Pt B. 1998;298:497–521. doi: 10.1016/s0076-6879(98)98042-9. [DOI] [PubMed] [Google Scholar]
- 21.Merkel R, et al. Cell force Microscopy on elastic layers of finite thickness. Biophysical Journal. 2007;93(9):3314–3323. doi: 10.1529/biophysj.107.111328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310(5751):1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 23.Reinhart-King CA, Dembo M, Hammer DA. Cell-Cell Mechanical Communication through Compliant Substrates. Biophysical Journal. 2008;95(12):6044–6051. doi: 10.1529/biophysj.107.127662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maruthamuthu V, et al. Cell-ECM traction force modulates endogenous tension at cell-cell contacts. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(12):4708–4713. doi: 10.1073/pnas.1011123108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang JHC, Lin JS. Cell traction force and measurement methods. Biomechanics and Modeling in Mechanobiology. 2007;6(6):361–371. doi: 10.1007/s10237-006-0068-4. [DOI] [PubMed] [Google Scholar]
- 26.Gardel ML, et al. Traction stress in focal adhesions correlates biphasically with actin retrograde flow speed. Journal of Cell Biology. 2008;183(6):999–1005. doi: 10.1083/jcb.200810060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stricker J, et al. Optimization of traction force microscopy for micron-sized focal adhesions. Journal of Physics-Condensed Matter. 2010;22(19) doi: 10.1088/0953-8984/22/19/194104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith LA, et al. Neutrophil traction stresses are concentrated in the uropod during migration. Biophysical Journal. 2007;92(7):L58–L60. doi: 10.1529/biophysj.106.102822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCain ML, et al. Cooperative coupling of cell-matrix and cell-cell adhesions in cardiac muscle. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(25):9881–9886. doi: 10.1073/pnas.1203007109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mertz AF, et al. Cadherin-based intercellular adhesions organize epithelial cell-matrix traction forces. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(3):842–847. doi: 10.1073/pnas.1217279110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jannat RA, et al. Neutrophil adhesion and chemotaxis depend on substrate mechanics. Journal of Physics-Condensed Matter. 2010;22(19) doi: 10.1088/0953-8984/22/19/194117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jannat RA, Dembo M, Hammer DA. Traction forces of neutrophils migrating on compliant substrates. Biophysical Journal. 2011;101(3):575–84. doi: 10.1016/j.bpj.2011.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mertz AF, et al. Scaling of Traction Forces with the Size of Cohesive Cell Colonies. Physical Review Letters. 2012;108(19) doi: 10.1103/PhysRevLett.108.198101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trepat X, et al. Physical forces during collective cell migration. Nature Physics. 2009;5(6):426–430. [Google Scholar]
- 35.Tambe DT, et al. Collective cell guidance by cooperative intercellular forces. Nature Materials. 2011;10(6):469–475. doi: 10.1038/nmat3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cukierman E, Bassi DE. Physico-mechanical aspects of extracellular matrix influences on tumorigenic behaviors. Seminars in Cancer Biology. 2010;20(3):139–145. doi: 10.1016/j.semcancer.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang SS, et al. Guidance of cell migration by substrate dimension. Biophysical Journal. 2013;104:313–321. doi: 10.1016/j.bpj.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Konstantopoulos K, Wu PS, Wirtz D. Dimensional Control of Cancer Cell Migration. Biophysical Journal. 2013;104(2):279–280. doi: 10.1016/j.bpj.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fraley SI, et al. A distinctive role for focal adhesion proteins in three-dimensional cell motility. Nature Cell Biology. 2010;12(6):598–U169. doi: 10.1038/ncb2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyer AS, et al. 2D protrusion but not motility predicts growth factor-induced cancer cell migration in 3D collagen. Journal of Cell Biology. 2012;197(6):721–729. doi: 10.1083/jcb.201201003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harunaga JS, Yamada KM. Cell-matrix adhesions in 3D. Matrix Biology. 2011;30(7-8):363–368. doi: 10.1016/j.matbio.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hakkinen KM, et al. Direct Comparisons of the Morphology, Migration, Cell Adhesions, and Actin Cytoskeleton of Fibroblasts in Four Different Three-Dimensional Extracellular Matrices. Tissue Engineering Part A. 2011;17(5-6):713–724. doi: 10.1089/ten.tea.2010.0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kubow KE, Horwitz AR. Reducing background fluorescence reveals adhesions in 3D matrices. Nature Cell Biology. 2011;13(1):3–5. doi: 10.1038/ncb0111-3. author reply 5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jayo A, Parsons M. Imaging of cell adhesion events in 3D matrix environments. European journal of cell biology. 2012;91(11-12):824–833. doi: 10.1016/j.ejcb.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 45.Kim BJ, Wu M. Microfluidics for Mammalian Cell Chemotaxis Annals of Biomedical Engineering. 2012;40(6):1316–1327. doi: 10.1007/s10439-011-0489-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gjorevski N, Nelson CM. Mapping of Mechanical Strains and Stresses around Quiescent Engineered Three-Dimensional Epithelial Tissues. Biophysical Journal. 2012;103(1):152–162. doi: 10.1016/j.bpj.2012.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rieu JP, Delanoe-Ayari H. Shell tension forces propel Dictyostelium slugs forward. Physical Biology. 2012;9(6) doi: 10.1088/1478-3975/9/6/066001. [DOI] [PubMed] [Google Scholar]
- 48.Hur SS, et al. Roles of cell confluency and fluid shear in 3-dimensional intracellular forces in endothelial cells. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(28):11110–11115. doi: 10.1073/pnas.1207326109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Franck C, et al. Three-dimensional Full-field Measurements of Large Deformations in Soft Materials Using Confocal Microscopy and Digital Volume Correlation. Experimental Mechanics. 2007;47(3):427–438. [Google Scholar]
- 50.Maskarinec SA, et al. Quantifying cellular traction forces in three dimensions. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(52):22108–22113. doi: 10.1073/pnas.0904565106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hur SS, et al. Live Cells Exert 3-Dimensional Traction Forces on Their Substrata. Cellular and Molecular Bioengineering. 2009;2(3):425–436. doi: 10.1007/s12195-009-0082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Franck C, et al. Three-Dimensional Traction Force Microscopy: A New Tool for Quantifying Cell-Matrix Interactions. Plos One. 2011;6(3) doi: 10.1371/journal.pone.0017833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Legant WR, et al. Multidimensional traction force microscopy reveals out-of-plane rotational moments about focal adhesions. Proceedings of the National Academy of Sciences. 2013;110(3):881–886. doi: 10.1073/pnas.1207997110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Delanoe-Ayari H, Rieu JP, Sano M. 4D Traction Force Microscopy Reveals Asymmetric Cortical Forces in Migrating Dictyostelium Cells. Physical Review Letters. 2010;105(24) doi: 10.1103/PhysRevLett.105.248103. [DOI] [PubMed] [Google Scholar]
- 55.Yeung T, et al. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motility and the Cytoskeleton. 2005;60(1):24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- 56.Reinhart-King CA, Dembo M, Hammer DA. Endothelial cell traction forces on RGD-derivatized polyacrylamide substrata. Langmuir. 2003;19(5):1573–1579. [Google Scholar]
- 57.Legant WR, et al. Measurement of mechanical tractions exerted by cells in three-dimensional matrices. Nature Methods. 2010;7(12):969–U113. doi: 10.1038/nmeth.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koch TM, et al. 3D Traction Forces in Cancer Cell Invasion. Plos One. 2012;7(3) doi: 10.1371/journal.pone.0033476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hall MS, et al. Mapping Three-Dimensional Stress and Strain Fields within a Soft Hydrogel Using a Fluorescence Microscope. Biophysical Journal. 2012;102(10):2241–2250. doi: 10.1016/j.bpj.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bloom RJ, et al. Mapping local matrix remodeling induced by a migrating tumor cell using three-dimensional multiple-particle tracking. Biophysical Journal. 2008;95(8):4077–4088. doi: 10.1529/biophysj.108.132738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shaw P. Comparison of Widefield/Deconvolution and Confocal Microscopy for Three-Dimensional Imaging. In: Pawley JB, editor. Handbook Of Biological Confocal Microscopy. Springer US; 2006. pp. 453–467. [Google Scholar]
- 62.Fischer RS, et al. Microscopy in 3D: a biologist’s toolbox. Trends in Cell Biology. 2011;21(12):682–691. doi: 10.1016/j.tcb.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Planchon TA, et al. Rapid three-dimensional isotropic imaging of living cells using Bessel beam plane illumination. Nature methods. 2011;8(5):417–U68. doi: 10.1038/nmeth.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gao L, et al. Noninvasive Imaging beyond the Diffraction Limit of 3D Dynamics in Thickly Fluorescent Specimens. Cell. 2012;151(6):1370–1385. doi: 10.1016/j.cell.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Landau LD, Lifshitz EM. Theory of Elasticity. 3rd ed Butterworth-Heinemann; 1986. [Google Scholar]
- 66.Schwarz US, et al. Calculation of forces at focal adhesions from elastic substrate data: The effect of localized force and the need for regularization. Biophysical Journal. 2002;83(3):1380–1394. doi: 10.1016/S0006-3495(02)73909-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vitale G, Preziosi L, Ambrosi D. A numerical method for the inverse problem of cell traction in 3D. Inverse Problems. 2012;28(9) [Google Scholar]
- 68.Vitale G, Preziosi L, Ambrosi D. Force traction microscopy: An inverse problem with pointwise observations. Journal of Mathematical Analysis and Applications. 2012;395(2):788–801. [Google Scholar]
- 69.Belytschko T, Lu YY, Gu L. Element-Free Galerkin Methods. International Journal for Numerical Methods in Engineering. 1994;37(2):229–256. [Google Scholar]
- 70.Bay BK. Methods and applications of digital volume correlation. Journal of Strain Analysis for Engineering Design. 2008;43(8):745–760. [Google Scholar]
- 71.Pan B, et al. Two-dimensional digital image correlation for in-plane displacement and strain measurement: a review. Measurement Science & Technology. 2009;20(6) [Google Scholar]
- 72.Huang JY, et al. A DIGITAL VOLUME CORRELATION TECHNIQUE FOR 3-D DEFORMATION MEASUREMENTS OF SOFT GELS. International Journal of Applied Mechanics. 2011;3(2):335–354. [Google Scholar]
- 73.Storm C, et al. Nonlinear elasticity in biological gels. Nature. 2005;435(7039):191–194. doi: 10.1038/nature03521. [DOI] [PubMed] [Google Scholar]
- 74.Vader D, et al. Strain-Induced Alignment in Collagen Gels. Plos One. 2009;4(6) doi: 10.1371/journal.pone.0005902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Voytik-Harbin SL, et al. Simultaneous mechanical loading and confocal reflection microscopy for three-dimensional microbiomechanical analysis of biomaterials and tissue constructs. Microscopy and Microanalysis. 2003;9(1):74–85. doi: 10.1017/S1431927603030046. [DOI] [PubMed] [Google Scholar]
- 76.Wirtz D, Konstantopoulos K, Searson PC. The physics of cancer: the role of physical interactions and mechanical forces in metastasis. Nature Reviews Cancer. 2011;11(7):512–522. doi: 10.1038/nrc3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fisher JF, Mobashery S. Recent advances in MMP inhibitor design. Cancer and Metastasis Reviews. 2006;25(1):115–136. doi: 10.1007/s10555-006-7894-9. [DOI] [PubMed] [Google Scholar]
- 78.Murphy G, Nagase H. Progress in matrix metalloproteinase research. Molecular Aspects of Medicine. 2008;29(5):290–308. doi: 10.1016/j.mam.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gelse K, Poschl E, Aigner T. Collagens - structure, function, and biosynthesis. Advanced Drug Delivery Reviews. 2003;55(12):1531–1546. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 80.Cross VL, et al. Dense type I collagen matrices that support cellular remodeling and microfabrication for studies of tumor angiogenesis and vasculogenesis in vitro. Biomaterials. 2010;31(33):8596–8607. doi: 10.1016/j.biomaterials.2010.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang Y.-l., Kaufman LJ. Rheology and Confocal Reflectance Microscopy as Probes of Mechanical Properties and Structure during Collagen and Collagen/Hyaluronan Self-Assembly. Biophysical Journal. 2009;96(4):1566–1585. doi: 10.1016/j.bpj.2008.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang Y.-l., Motte S, Kaufman LJ. Pore size variable type I collagen gels and their interaction with glioma cells. Biomaterials. 2010;31(21):5678–5688. doi: 10.1016/j.biomaterials.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 83.Raub CB, et al. Noninvasive assessment of collagen gel microstructure and mechanics using multiphoton Microscopy. Biophysical Journal. 2007;92(6):2212–2222. doi: 10.1529/biophysj.106.097998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Orban JM, et al. Crosslinking of collagen gels by transglutaminase. Journal of Biomedical Materials Research Part A. 2004;68A(4):756–762. doi: 10.1002/jbm.a.20110. [DOI] [PubMed] [Google Scholar]
- 85.Siegel RC. COLLAGEN CROSS-LINKING - SYNTHESIS OF COLLAGEN CROSS-LINKS INVITRO WITH HIGHLY PURIFIED LYSYL OXIDASE. Journal of Biological Chemistry. 1976;251(18):5786–5792. [PubMed] [Google Scholar]
- 86.Inoue N, et al. A novel collagen hydrogel cross-linked by gamma-ray irradiation in acidic pH conditions. Journal of Biomaterials Science-Polymer Edition. 2006;17(8):837–858. doi: 10.1163/156856206777996835. [DOI] [PubMed] [Google Scholar]
- 87.Damink L, et al. GLUTARALDEHYDE AS A CROSS-LINKING AGENT FOR COLLAGEN-BASED BIOMATERIALS. Journal of Materials Science-Materials in Medicine. 1995;6(8):460–472. [Google Scholar]
- 88.Roy R, Boskey A, Bonassar LJ. Processing of type I collagen gels using nonenzymatic glycation. Journal of Biomedical Materials Research Part A. 2010;93A(3):843–851. doi: 10.1002/jbm.a.32231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Paik DC, et al. The nitrite/collagen reaction: Non-enzymatic nitration as a model system for age-related damage. Connective Tissue Research. 2001;42(2):111–122. doi: 10.3109/03008200109014253. [DOI] [PubMed] [Google Scholar]
- 90.Sundararaghavan HG, et al. Genipin-induced changes in collagen gels: Correlation of mechanical properties to fluorescence. Journal of Biomedical Materials Research Part A. 2008;87A(2):308–320. doi: 10.1002/jbm.a.31715. [DOI] [PubMed] [Google Scholar]
- 91.Duan X, Sheardown H. Crosslinking of collagen with dendrimers. Journal of Biomedical Materials Research Part A. 2005;75A(3):510–518. doi: 10.1002/jbm.a.30475. [DOI] [PubMed] [Google Scholar]
- 92.Lee P, et al. Microfluidic alignment of collagen fibers for in vitro cell culture. Biomedical Microdevices. 2006;8(1):35–41. doi: 10.1007/s10544-006-6380-z. [DOI] [PubMed] [Google Scholar]
- 93.Cheng XG, et al. An electrochemical fabrication process for the assembly of anisotropically oriented collagen bundles. Biomaterials. 2008;29(22):3278–3288. doi: 10.1016/j.biomaterials.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 94.Torbet J, Ronziere MC. MAGNETIC ALIGNMENT OF COLLAGEN DURING SELF-ASSEMBLY. Biochemical Journal. 1984;219(3):1057–1059. doi: 10.1042/bj2191057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Guo C, Kaufman LJ. Flow and magnetic field induced collagen alignment. Biomaterials. 2007;28(6):1105–1114. doi: 10.1016/j.biomaterials.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 96.Wood GC, Keech MK. FORMATION OF FIBRILS FROM COLLAGEN SOLUTIONS .1. EFFECT OF EXPERIMENTAL CONDITIONS - KINETIC AND ELECTRON-MICROSCOPE STUDIES. Biochemical Journal. 1960;75:588. doi: 10.1042/bj0750588. &. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McPherson JM, et al. COLLAGEN FIBRILLOGENESIS INVITRO - A CHARACTERIZATION OF FIBRIL QUALITY AS A FUNCTION OF ASSEMBLY CONDITIONS. Collagen and Related Research. 1985;5(2):119–135. doi: 10.1016/s0174-173x(85)80034-0. [DOI] [PubMed] [Google Scholar]
- 98.Carey SP, et al. Biophysical control of invasive tumor cell behavior by extracellular matrix microarchitecture. Biomaterials. 2012;33(16):4157–4165. doi: 10.1016/j.biomaterials.2012.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chandran PL, Barocas VH. Microstructural mechanics of collagen gels in confined compression: Poroelasticity, viscoelasticity, and collapse. Journal of Biomechanical Engineering-Transactions of the Asme. 2004;126(2):152–166. doi: 10.1115/1.1688774. [DOI] [PubMed] [Google Scholar]
- 100.Roeder BA, et al. Tensile mechanical properties of three-dimensional type I collagen extracellular matrices with varied microstructure. Journal of Biomechanical Engineering-Transactions of the Asme. 2002;124(2):214–222. doi: 10.1115/1.1449904. [DOI] [PubMed] [Google Scholar]
- 101.Pryse KM, et al. Incremental mechanics of collagen gels: New experiments and a new viscoelastic model. Annals of Biomedical Engineering. 2003;31(10):1287–1296. doi: 10.1114/1.1615571. [DOI] [PubMed] [Google Scholar]
- 102.Krishnan L, et al. Design and application of a test system for viscoelastic characterization of collagen gels. Tissue Engineering. 2004;10(1-2):241–252. doi: 10.1089/107632704322791880. [DOI] [PubMed] [Google Scholar]
- 103.Arevalo RC, Urbach JS, Blair DL. Size-Dependent Rheology of Type-I Collagen Networks. Biophysical Journal. 2010;99(8):L65–L67. doi: 10.1016/j.bpj.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Arevalo RC, Urbach JS, Blair DL. Four-dimensional structural dynamics of sheared collagen networks. Chaos. 2011;21(4) doi: 10.1063/1.3666225. [DOI] [PubMed] [Google Scholar]
- 105.Kalcioglu ZI, et al. From macro- to microscale poroelastic characterization of polymeric hydrogels via indentation. Soft Matter. 2012;8(12):3393–3398. [Google Scholar]
- 106.Chen CS, et al. Geometric control of cell life and death. Science. 1997;276(5317):1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 107.Wen Q, et al. Non-affine deformations in polymer hydrogels Soft Matter. 2012:8039–8049. doi: 10.1039/c2sm25364j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jawerth LM, et al. A Blind Spot in Confocal Reflection Microscopy: The Dependence of Fiber Brightness on Fiber Orientation in Imaging Biopolymer Networks. Biophysical Journal. 2010;98(3):L1–L3. doi: 10.1016/j.bpj.2009.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lanir Y. A structural theory for the homogeneous biaxial stress-strain relationships in flat collagenous tissues. Journal of biomechanics. 1979;12(6):423–36. doi: 10.1016/0021-9290(79)90027-7. [DOI] [PubMed] [Google Scholar]
- 110.Lanir Y. CONSTITUTIVE EQUATIONS FOR FIBROUS CONNECTIVE TISSUES. 1982 doi: 10.1016/0021-9290(83)90041-6. [DOI] [PubMed] [Google Scholar]
- 111.Nagel T, Kelly DJ. Remodelling of collagen fibre transition stretch and angular distribution in soft biological tissues and cell-seeded hydrogels. Biomechanics and modeling in mechanobiology. 2012;11(3-4):325–39. doi: 10.1007/s10237-011-0313-3. [DOI] [PubMed] [Google Scholar]
- 112.Raghupathy R, Barocas VH. A closed-form structural model of planar fibrous tissue mechanics. Journal of biomechanics. 2009;42(10):1424–8. doi: 10.1016/j.jbiomech.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kabla a., Mahadevan L. Nonlinear mechanics of soft fibrous networks. Journal of the Royal Society, Interface / the Royal Society. 2007;4(12):99–106. doi: 10.1098/rsif.2006.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kuhl E, et al. Remodeling of biological tissue: Mechanically induced reorientation of a transversely isotropic chain network. Journal of the Mechanics and Physics of Solids. 2005;53(7):1552–1573. [Google Scholar]
- 115.Susilo ME, et al. Development of a three-dimensional unit cell to model the micromechanical response of a collagen-based extracellular matrix. Acta Biomaterialia. 2010;6(4):1471–86. doi: 10.1016/j.actbio.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 116.Mihai LA, Goriely A. Positive or negative Poynting effect ? The role of adscititious inequalities in hyperelastic materials Subject collections Positive or negative Poynting effect ? The role of adscititious inequalities in hyperelastic materials. Aug, 2011.
- 117.Lake SP, et al. Mechanics of a fiber network within a non-fibrillar matrix: model and comparison with collagen-agarose co-gels. Annals of Biomedical Engineering. 2012;40(10):2111–21. doi: 10.1007/s10439-012-0584-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stylianopoulos T, Barocas VH. Volume-averaging theory for the study of the mechanics of collagen networks. Computer Methods in Applied Mechanics and Engineering. 2007;196(31-32):2981–2990. [Google Scholar]
- 119.Stein AM, et al. The Micromechanics of Three-Dimensional Collagen-I Gels. 2010:1–7. 00(00) [Google Scholar]
- 120.Stein AM, et al. An algorithm for extracting the network geometry of three-dimensional collagen gels. Journal of Microscopy. 2008;232(3):463–475. doi: 10.1111/j.1365-2818.2008.02141.x. [DOI] [PubMed] [Google Scholar]
- 121.Wu J, et al. Analysis of orientations of collagen fibers by novel fiber-tracking software. Microscopy and Microanalysis. 2003;9(6):574–580. doi: 10.1017/S1431927603030277. [DOI] [PubMed] [Google Scholar]
- 122.Tan JL, et al. Cells lying on a bed of microneedles: An approach to isolate mechanical force. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(4):1484–1489. doi: 10.1073/pnas.0235407100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.le Digabel J, et al. Microfabricated substrates as a tool to study cell mechanotransduction. Medical & Biological Engineering & Computing. 2010;48(10):965–976. doi: 10.1007/s11517-010-0619-9. [DOI] [PubMed] [Google Scholar]
- 124.Balaban NQ, et al. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nature Cell Biology. 2001;3(5):466–472. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- 125.Ghibaudo M, et al. Traction forces and rigidity sensing regulate cell functions. Soft Matter. 2008;4(9):1836–1843. [Google Scholar]
- 126.Tee S-Y, et al. Cell Shape and Substrate Rigidity Both Regulate Cell Stiffness. Biophysical Journal. 2011;100(5):L25–L27. doi: 10.1016/j.bpj.2010.12.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ghibaudo M, et al. Mechanics of cell spreading within 3D-micropatterned environments. Lab on a Chip. 2011;11(5):805–812. doi: 10.1039/c0lc00221f. [DOI] [PubMed] [Google Scholar]
- 128.Smith ML, et al. Force-induced unfolding of fibronectin in the extracellular matrix of living cells. PLoS biology. 2007;5(10):e268–e268. doi: 10.1371/journal.pbio.0050268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Klotzsch E, et al. Fibronectin forms the most extensible biological fibers displaying switchable force-exposed cryptic binding sites. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(43):18267–18272. doi: 10.1073/pnas.0907518106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kong HJ, et al. FRET measurements of cell-traction forces and nano-scale clustering of adhesion ligands varied by substrate stiffness. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(12):4300–4305. doi: 10.1073/pnas.0405873102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.