Abstract
OspC is produced by all species of the Borrelia burgdorferi sensu lato complex and is required for infectivity in mammals. To test the hypothesis that the conserved C-terminal motif (C10) of OspC is required for function in vivo, a mutant Borrelia burgdorferi strain (B31∷ospCΔC10) was created in which ospC was replaced with an ospC gene lacking the C10 motif. The ability of the mutant to infect mice was investigated using tick transmission and needle inoculation. Infectivity was assessed by cultivation, qRT-PCR, and measurement of IgG antibody responses. B31∷ospCΔC10 retained the ability to infect mice by both needle and tick challenge and was competent to survive in ticks after exposure to the blood meal. To determine if recombinant OspC protein lacking the C-terminal 10 amino acid residues (rOspCΔC10) can bind plasminogen, the only known mammalian derived ligand for OspC, binding analyses were performed. Deletion of the C10 motif resulted in a statistically significant decrease in plasminogen binding. Although deletion of the C10 motif influenced plasminogen binding, it can be concluded that the C10 motif is not required for OspC to carry out its critical in vivo functions in tick to mouse transmission.
Introduction
Lyme disease is a tick transmitted infection caused by several Borrelia species including Borrelia burgdorferi, B. garinii, and B. afzelii (Benach et al., 1983; Burgdorfer et al., 1982). In nature, numerous species of birds, mammals and lizards serve as reservoirs (Clark et al., 2005; Gern et al., 1998). Newly released case numbers calculated by the Center for Disease Control and Prevention suggest that there are a minimum of 300,000 cases each year in the United States. The disease is also highly prevalent in Europe but the true incidence is not known due to the lack of a uniform reporting system. Humans are incidental hosts and are not significant in the enzootic cycle of the Lyme disease spirochetes. With the exception of the pathognomonic erythema migrans rash, early symptoms of Lyme disease are non-specific (e.g., malaise, low grade fever) (Steere, 2001; Wormser et al., 2006). If Lyme disease goes untreated cardiac, arthritic, dermatological, or neurological sequelae can occur (Steere, 2001; Steere, 2006). Preventive strategies for Lyme disease (acaricides and tick avoidance) are ineffective and a vaccine for use in humans is not available. Outer surface proteins (Osp) OspA, OspC, and recombinant-chimeric OspC derivatives represent the primary focus in efforts to develop new Lyme disease vaccines (reviewed in Earnhart and Marconi, 2008; Marconi and Earnhart, 2010).
The Lyme disease spirochetes must adapt to radically different environmental conditions as they cycle between ticks and mammals. Differential production of outer membrane lipoproteins plays a key role in adaptive responses (Crandall et al., 2006; Narasimhan et al., 2002; Revel et al., 2002; Rogers et al., 2009; Schwan, 2003; Tokarz et al., 2004). OspC, a 22 kDa lipoprotein (Fuchs et al., 1992), is upregulated during tick to mammal transmission and remains expressed during early infection in mammals (Brooks et al., 2003; Gilmore et al., 2001; Schwan et al., 1995; Schwan and Piesman, 2000). Although OspC is an essential virulence factor, its precise and full range of functions are not known (Grimm et al., 2004; Stewart et al., 2006; Tilly et al., 1997; Tilly et al., 2006; Tilly et al., 2007). Structural and bioinformatic analyses of OspC have identified several putative ligand binding domains (Eicken et al., 2001; Kumaran et al., 2001). Several studies suggest that OspC binds mammalian derived plasminogen (Lagal et al., 2006; Onder et al., 2012). Plasminogen binding and activation may facilitate invasion of host tissues and endothelial barriers. The potential binding site for plasminogen on OspC remains undefined. OspC has also been reported to bind to a tick-derived protein, Salp15 (Anguita et al., 2002; Lagal et al., 2006). However, the significance of Salp15 binding is unclear since its interaction is not required for needle inoculated spirochetes to infect and disseminate in mammals (a route independent of Salp15) (Earnhart et al., 2010; Earnhart et al., 2011; Grimm et al., 2004). A putative small ligand binding domain (designated as LBD1) formed along the surface of the dimeric interface of OspC has been demonstrated to be critical for in vivo function (Eicken et al., 2001; Kumaran et al., 2001). A single amino acid substitution within LBD1 (residue E61) eliminated infectivity of B. burgdorferi in mice while other mutations influenced dissemination (Earnhart et al., 2010). While the ligand for LBD1 remains unknown, the E61Q substitution did not affect plasminogen binding indicating that OspC may bind other ligands and have multiple functions critical for transmission and infectivity of the Lyme disease spirochetes(Earnhart et al., 2010).
Approximately 30 distinct OspC phyletic types having been defined (Brisson and Dykhuizen, 2004; Earnhart et al., 2005; Wang et al., 1999). OspC phyletic type identity is defined largely by the sequence of its variable surface-exposed regions. In spite of significant inter-type diversity some OspC regions are highly conserved suggesting a direct (ligand binding) or indirect (maintenance of critical structural determinants) role in its function. One of the most highly conserved regions of OspC is its C-terminal 10 amino acids, henceforth referred to as the C10 motif. To test the hypothesis that the C10 motif is required for OspC function in vivo, the wild type ospC gene of B. burgdorferi B31 was replaced with an ospC gene encoding a truncated protein lacking the C10 motif to yield B31∷ospCΔC10). B31∷ospCΔC10 efficiently infected mice by both needle and tick challenge. Recombinant OspCΔC10 demonstrated attenuated plasminogen binding. The results presented within revealed that the C10 is not required for OspC to function in ticks or mammals raising interesting question about the selective pressures that have maintained its sequence conservation. From a technical perspective, this study is only the second to employ targeted mutagenesis of OspC to assess its functions in vivo.
Materials and Methods
Bacterial strains, cultivation conditions, tick and animal studies
Borrelia were cultivated in BSK-H medium supplemented with 6% bovine serum albumin at 25, 33 or 37 °C in sealed bottles under 3% CO2 (with antibiotics where appropriate). The parental strain employed for genetic manipulation, B. burgdorferi B31-clone 5A4 (henceforth referred to as B31), produces a type A OspC. All strains employed in this study are described in Table 1. E. coli strains NovaBlue and BL21-DE3 were used for plasmid propagation and recombinant protein production, respectively, with growth conditions detailed below. C3H-HeJ mice (Jackson Laboratories) were employed to assess the infectivity of the Borrelia strains. All animal studies were conducted under protocols reviewed and approved by the Virginia Commonwealth University and University of Maryland IACUCs. Ixodes scapularis nymphal stage ticks were obtained from a pathogen free tick colony maintained under 95% humidity at University of Maryland.
Table 1. Strains used in this study.
| Strain | Description | Missing plasmids | Reference |
|---|---|---|---|
| B31 (5A4 clone) | Parental strain | None | (Kostick et al., 2011) |
| B31∷ospCwt | Transgenic control expressing wt OspC | lp21 | (Earnhart et al., 2010) |
| B31∷ospCΔC10 | Transgenic expressing truncated OspC | lp21 | (Earnhart et al., 2010) |
| B31ΔospC | Transgenic OspC knockout strain | None | This study |
SDS-PAGE and immunoblot analyses
Recombinant proteins or cell lysates were solubilized and fractionated by SDS-PAGE using precast 12% Criterion gels (Biorad). Proteins were transferred to PVDF membranes by electroblotting using standard methods. Non-specific binding to the membranes was blocked with PBS-T (5% non-fat dry milk in PBS; 0.02% Tween-20), and the membranes were screened with primary and secondary antibodies as indicated below. Mouse anti-His mAb (Pierce), mouse anti-OspC (Earnhart et al., 2011), and mouse anti-FlaA antiserum (Pierce), were used at dilutions of 1:10,000, 1:10,000, and 1:40,000, respectively. Antiserum from mice challenged with spirochetes by needle inoculation or by tick feeding were used at a dilution of 1:2000. Antibody binding was detected using goat anti-mouse IgG peroxidase conjugate (1:40,000; Pierce) and chemiluminescent substrate (SuperSignal West Pico; Pierce).
Proteinase K digestion
Cultures were grown at 33 °C and then shifted to 37 °C for 2 days to maximize OspC production (Stevenson et al., 1995). Proteinase K digestion assays were performed as previously detailed (Earnhart et al., 2010). Cells were harvested by centrifugation, washed, suspended in PBS (0.3 OD600 units mL-1) and proteinase K or PBS (negative control) were added. The samples were incubated at 22°C (1 hr) and then the proteinase K was inactivated by the addition of PMSF. The cells were solubilized, separated by SDS-PAGE, and transferred to membranes for subsequent immunoblot analyses.
Immunofluorescence assays
Strains were cultivated at 33 EC, temperature shifted to 37 EC (as detailed above), harvested by centrifugation, and immobilized on slides (Superfrost Plus, Fisher) as previously described (Buckles et al., 2006). Non-specific binding was blocked with PBS-T containing 3% BSA. The slides were screened with mouse anti-OspC antiserum (1:2,000) with secondary detection by Alexa-488 conjugated goat anti-mouse IgG (1:200). Slides were mounted with Prolong Gold (Invitrogen) and the cells were visualized by fluorescence microscopy (Olympus BX51).
Production of recombinant OspC proteins
Full length rOspC (rOspCwt) and a C-terminal (10 aa) truncation variant (rOspCΔC10) were generated by PCR amplification using the OspC20(+)/OspC210(-) and OspC20(+)/OspC200(-) primer sets (Table 2). The final constructs were designed without signal sequences. The amplicons were annealed to the pET46 Ek/LIC vector (Novagen). The plasmids were propagated in NovaBlue cells and plasmids isolated using Qiagen kits. The purified plasmids were transformed into BL21(DE3) cells, protein expressed by IPTG induction, and then the r-proteins purified using nickel affinity chromatography (Earnhart and Marconi, 2007b). Sequences were verified for all constructs described in this study on a fee for service basis (MWG Biotech).
Table 2. Summary of oligonucleotides used in this study.
| Primer | Sequence (5’ to 3’) |
|---|---|
| OspC 20 (+) LIC | GACGACGACAAGATTAATAATTCAGGGAAAGATGGG |
| OspC 210 (-) LIC | GAGGAGAAGCCCGGTTTAAGGTTTTTTTGGACTTTCTGC |
| OspC 200 (-) LIC | GAGGAGAAGCCCGGTTTAGCTTGTAAGCTCTTTAACTGAATT |
| Insert typeA20(+)BspEI | TCCGGAAAAGATGGGAATACATCTGCA |
| Insert type A200(-)BspEI | TCCGGATTAGCTTGTAAGCTCTTTAACTGAATTAG |
Allelic exchange replacement of wild-type ospC
A pCAEV1-based suicide allelic exchange vector was created that encodes an OspC truncation variant lacking the 10 terminal amino acids. This vector is designed to integrate into circular plasmid 26 (cp26). The sequence encoding amino acids 20 to 200 of B31 ospC was amplified using the Insert-typeA20(+)BspEI and Insert-typeA200(-) BspEI primers, ligated into the BspEI sites of pCAEV1, and the plasmid propagated in E. coli NovaBlue (Earnhart et al., 2010). The purified, linearized plasmid was introduced into B. burgdorferi B31-5A4 by electroporation (Samuels et al., 1994). Clonal populations of the transformants were obtained by subsurface plating with streptomycin selection (Earnhart et al., 2010). Colonies were cored from the plates and transferred to BSK-H medium for expansion. The plasmid content of each clone was determined by PCR using plasmid-specific primer sets (Rogers et al., 2009). An aliquot of each culture was boiled in 100 :l water, and 1 :l was used as template in each reaction. Integration of the ospC cassette into the circular plasmid cp26 of B31 was verified by PCR amplification and DNA sequencing (Earnhart et al., 2010). The growth rates of all strains were determined by daily triplicate cell counts of cultures grown at 37°C in BSK-H medium (no antibiotics) using dark-field microscopy.
Assessment of plasminogen binding by wild-type and site-directed mutant proteins
Plasminogen binding to rOspC and rOspC)C10 was assessed by ELISA (Lagal et al., 2006). ELISA plates (Costar 3590) were coated with each protein (1:g well-1) in 100 :l carbonate buffer (pH 9.6; overnight). Immobilized BSA and blank wells served as negative controls and rOspA (an established plasminogen binding protein (Fuchs et al., 1994)) served as a positive control. Non-specific binding was blocked with TBS-T (5% BSA). Plasminogen was added (0.1 :g well-1 in blocking buffer) and the plates incubated for 2 hr at room temperature. After washing with TBS-T, goat anti-human plasminogen was added (1:1000; 1 hr; room temperature; Pierce). After washing, antibody binding was detected with peroxidase-conjugated rabbit anti-goat IgG (1;20,000 dilution; 1 hr; room temperature; Calbiochem) and ABTS chromogen as described by the supplier (Pierce). Absorbance was measured at 405 nm (A405).
Infectivity studies
Strains B31 (wild type strain not subjected to genetic manipulation), B31∷ospCwt (B31 which has a ospC wild type- type A-strep cassette inserted as a control), B31∷ospCΔC10, or B31∷ospC were needle inoculated (subcutaneous; 104 spirochetes) into C3H-HeJ mice (n = 5 per group). After four weeks the mice were euthanized and ear punch biopsies (2 mm), urinary bladders, and blood were collected. To determine if the spirochetes established an infection, skin biopsies and bladders were placed in BSK-H complete medium supplemented with rifampicin, fosfomycin, and amphotericin B (Sigma); the cultures were maintained at 33°C and their growth monitored by dark field microscopy. To assess antibody responses, serum was harvested and IgG titers were determined by ELISA as previously described (Earnhart and Marconi, 2007c). In brief, B31 cells (0.01 OD600 well-1), rOspC, and rOspCΔC10 were immobilized in ELISA plate wells overnight in carbonate buffer (pH9.6) to saturation. Non-specific antibody binding was blocked with 1% BSA in PBS-T. The immobilized antigens were screened with serial dilutions of mouse sera (1:50 - 1:109,150) infected with the appropriate strain and antibody binding detected using peroxidase conjugated goat anti-mouse IgG (1:20,000) and ABTS chromogen.
Transmission analyses
To assess the ability of each strain to transit from ticks into mice, naïve, unfed I. scapularis nymphs (15 ticks per group) were infected by microinjection as previously described (Zhang et al., 2009). After a two day recovery period, the ticks were placed on naïve mice (5 ticks per mouse; 3 mice per group), fed to repletion, and collected. Fourteen days after the ticks dropped off, the mice were euthanized, and heart, blood, and skin biopsies were collected. The presence of actively growing spirochetes in mouse tissues and in the fed ticks was assessed using qRT-PCR as previously described (Zhang et al., 2009). To assess the antibody response to infection, immunoblots of cell lysates were screened with serum from each mouse (1:2000), and anti-B. burgdorferi IgG titers determined as described above.
Results
Generation and analysis of a B. burgdorferi strain expressing a C-terminally truncated OspC protein
PCR analyses confirmed that the wild-type cp26 (circular plasmid 26 kb) carried ospC gene of strain B31 (clone 5A4) was successfully replaced with a cassette encoding antibiotic resistance and an ospC gene lacking the C-terminal ten amino acids (C10 motif) (data not shown). Clonal populations of the transformed strain, designated as B31∷ospCΔC10, were obtained by subsurface plating (Sung et al., 2001) and screened for plasmid content using plasmid-specific PCR primers (Labandeira-Rey and Skare, 2001; McDowell et al., 2001). Several clones harboring all plasmids present in the parental strain, with the exception of linear plasmid lp21, were identified (data not shown). The absence of lp21 is not relevant to this study as it is not required to infect mice or ticks and its absence has no effect on dissemination in mammals (Earnhart et al., 2010). The growth rate of B31∷ospCΔC10 was found to be similar to that of the control strains (Figure 1, panel A). Immunoblot analyses verified production of full length OspC by B31 and B31∷ospCwt, production of truncated OspC by B31∷ospC)C10, and no OspC production by B31∷ospC (Figure 1, panel B). Proteinase K digestion assays (Figure 1, panel B) and IFA analyses (Figure 1, panel C) demonstrated that OspC is exported and presented on the cell surface by each recombinant strain in a manner similar to wild type. It can be concluded that deletion of C10 and expression of antibiotic resistance does not alter growth rate, or the expression, production and export of OspC to the outer membrane.
Figure 1.
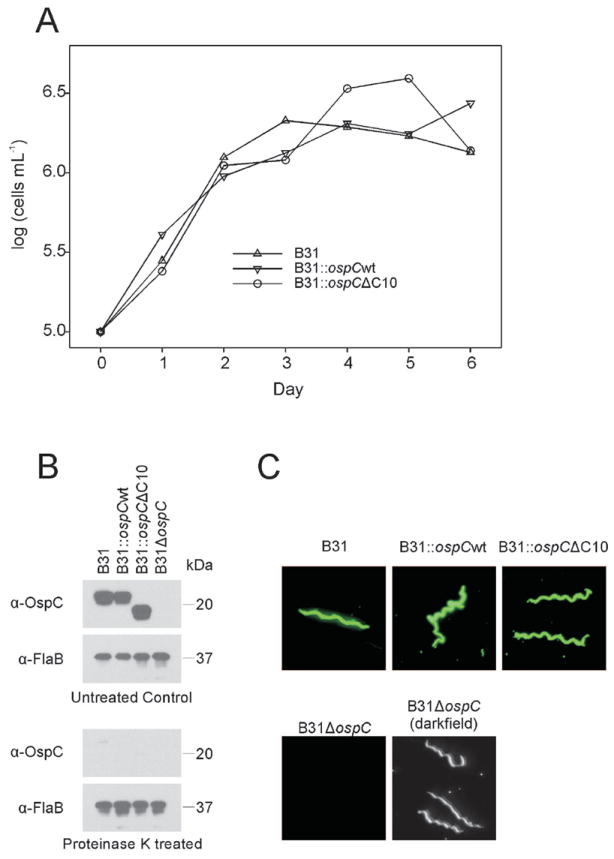
Characterization of wild type and mutant B. burgdorferi strains. In panel A growth rates were determined by daily triplicate cell counts using dark field microscopy of cultures grown at 37°C in BSK-H medium (no antibiotics). In panel B the production and surface presentation of OspC by each strain was assessed by immunoblotting and proteinase K treatment, respectively. Proteinase K treated or untreated cells were fractionated by SDS-PAGE, immunoblotted and screened with polyclonal anti-OspC (phyletic type A) or anti-FlaB antiserum. Surface presentation was further demonstrated through indirect immunofluorescence assays using polyclonal anti-OspC antiserum (Panel D). All methods are detailed in text.
B31∷ospCΔC10 retains the ability to infect mice when administered by needle inoculation
To determine if the C10 motif mediates a function that is required for B. burgdorferi to infect mice, B31∷ospCΔC10 and control strains (B31, B31ΔospC, B31∷ospCwt) were needle inoculated into mice. Cultivation of tissue biopsies and urinary bladder collected four weeks post infection revealed that all mice inoculated with B31∷ospCΔC10 became infected whereas mice inoculated with the ospC deletion mutant (B31ΔospC; 10-4 spirochetes) did not. Twelve of the 15 mice inoculated with B31∷ospCΔC10 or the positive control strains (B31, B31∷ospCwt) were culture positive from the bladder and skin with the remaining three mice being culture positive from the bladder only (Table 3). It can be concluded that the C-terminus of OspC is not required for spirochetes to infect mice, persist, and disseminate.
Table 3.
Tissue culture results of mice inoculated with B31 and ospC mutant strains*
| Ear | Bladder | |
|---|---|---|
| B31 (untransformed) | 4/5 | 5/5 |
| B31∷ospCwt | 5/5 | 5/5 |
| B31∷ospCΔC10 | 3/5 | 5/5 |
| B31ΔospC | 0/5 | 0/5 |
ratios indicate the number of mice that were culture positive from a total of 5
Tick transmission analyses
To determine if deletion of C10 prevents OspC from participating in functions required for transmission from ticks to mammals, ticks infected by microinjection were fed to repletion on naïve mice and collected. qRT-PCR was used to verify the presence of viable spirochetes in a sampling of the micro-injected tick. B. burgdorferi flaB mRNA (a constitutively expressed gene) was detected in all microinjected ticks (data not shown). The mice were euthanized 2 weeks post tick drop off and blood, tissue biopsies, and hearts were harvested. A portion of the heart was placed in BSK-H medium (with antibiotics) to assess the ability of each strain to establish infection via the tick transmission route. Positive cultures were obtained from all mice except those that were fed on by ticks that were infected with the B31ΔospC (Table 4). To screen for the presence of viable spirochetes in tissue collected from around the tick bite site, flaB qRT-PCR was performed. Transcript was detected in mice infected with B31, B31∷ospCΔC10 and B31∷ospCwt whereas flaB mRNA was not detected in mice infected with B31ΔospC (Figure 2; ANOVA, p<.0001). These results demonstrate that deletion of C10 does not block transmission via natural tick feeding.
Table 4.
Tissue culture results of mice infected by ticks microinjected with B31 and ospC mutant strains*
| Heart | |
|---|---|
| B31 (untransformed) | 3/3 |
| B31∷ospCwt | 3/3 |
| B31∷ospCΔC10 | 3/3 |
| B31ΔospC | 0/3 |
ratios indicate the number of mice that were culture positive from a total of 3
Figure 2.
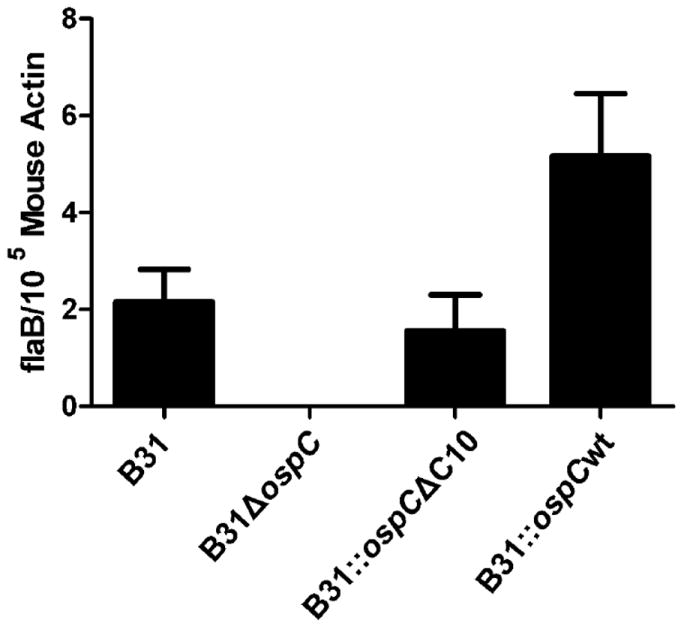
Quantitative RT-PCR of spirochetes in mouse skin. The relative number of spirochetes in tissue biopsies was determined by qRT-PCR with primers specific for the flaB gene of B. burgdorferi. Normalization was achieved using primers that target the mouse actin gene. All methods are detailed in text.
Immune responses to B. burgdorferi infection via needle and tick delivery approaches
The IgG response of mice infected by tick bite or needle inoculation was assessed through determination of anti-B. burgdorferi IgG antibody titers. ELISA analyses using B. burgdorferi B31-5A4 as the immobilized antigen revealed that all infected mice, regardless of infection route, mounted a significant IgG response (Figure 3, panels A and B). As expected, and consistent with data published in previous studies, a significant IgG response was not detected in mice infected with B31ΔospC (data not shown) (Earnhart et al., 2010; Earnhart et al., 2011). To determine if deletion of C10 influences the anti-OspC IgG response, the antibody titer in each mouse infected by needle inoculation was determined by ELISA using rOspC (full length) and rOspCΔC10 as immobilized antigens. Mice infected with B31, B31∷ospCwt, and B31∷ospCΔC10 had comparable anti-OspC antibody titers for the rOspC and rOspCΔC10 proteins (data not shown). Serum harvested from mice infected by needle inoculation and by tick bite were used to screen cell lysates of B. burgdorferi B31. Representative results are presented in Figure 4 (panels A and B). Both B31 and B31∷ospCΔ10 elicited IgG responses to numerous antigens. As expected, an IgG response was not observed with B31ΔospC. Hence, C10 does not appear to be an immunodominant epitope and its deletion does not significantly influence IgG responses directed at OspC.
Figure 3.
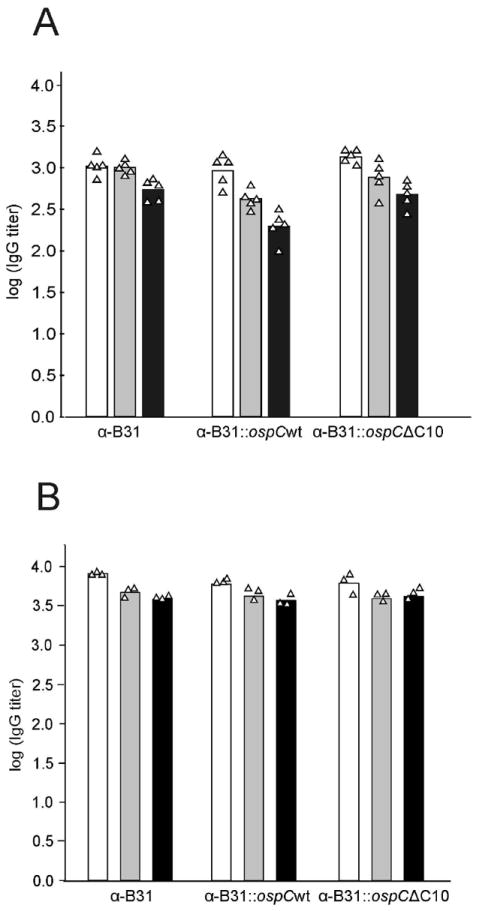
Determination of IgG titer to B. burgdorferi and recombinant OspC proteins in mice infected with B. burgdorferi B31 and ospC mutant strains. Serum was harvested from mice infected by needle inoculation (panel A) or tick bite (panel B), serially diluted, and screened against immobilized whole B. burgdorferi B31 cells (white bar), rOspC (gray bar) and rOspCΔC10 (black bar). Individual mouse titers (indicated by triangles) were calculated as the log of the inverse of the dilution corresponding to 1/3 of the ODmax plateau on the sigmoidal curve. Bars denote the geometric mean titer. All methods are detailed in text.
Figure 4.
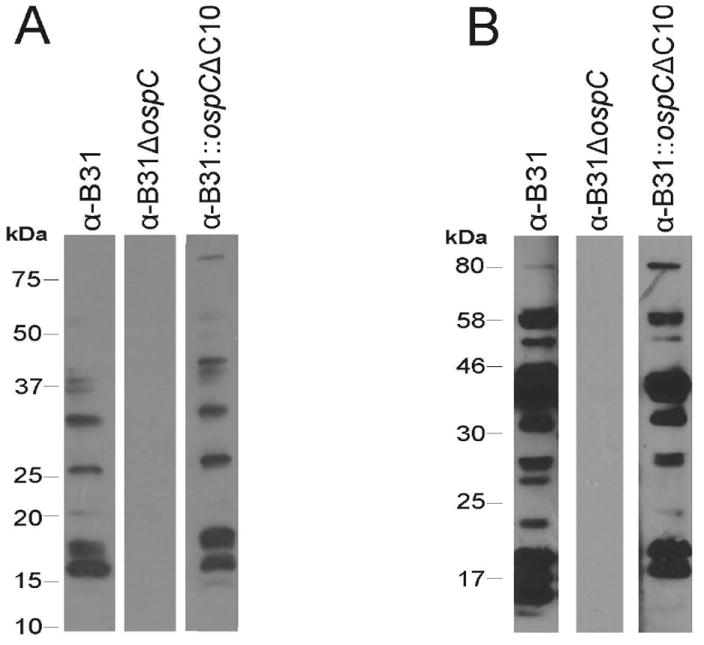
The immunoreactive profiles of mice infected with control and mutant strains by tick bite and needle inoculation. In panel A, ticks were microinjected with each strain and then fed on naïve mice to repletion. Serum was collected after 3 weeks, diluted 1:2000, and used to screen immunoblot of B. burgdorferi B31 cell lysate. In panel B, antiserum from mice challenged with 104 spirochetes of each strain was diluted 1:2000 and used to screen immunoblot of B. burgdorferi B31 cell lysate. Representative data are shown. All methods are detailed in text.
Plasminogen binding to recombinant proteins
It was previously demonstrated that OspC binds plasminogen (Earnhart et al., 2010; Lagal et al., 2006; Onder et al., 2012). However, the binding determinants within OspC that are required for plasminogen binding have not been identified. To determine if the C10 motif is directly or indirectly required for this interaction, recombinant proteins were tested for plasminogen binding using an ELISA format. rOspA, a known plasminogen binding protein (Fuchs et al., 1994), served as a positive control. Deletion of the C10 motif resulted in a small but statistically significant decrease in binding (Figure 5). It remains to be determined if this decrease is a direct or indirect effect associated with the C1- motif.
Figure 5.
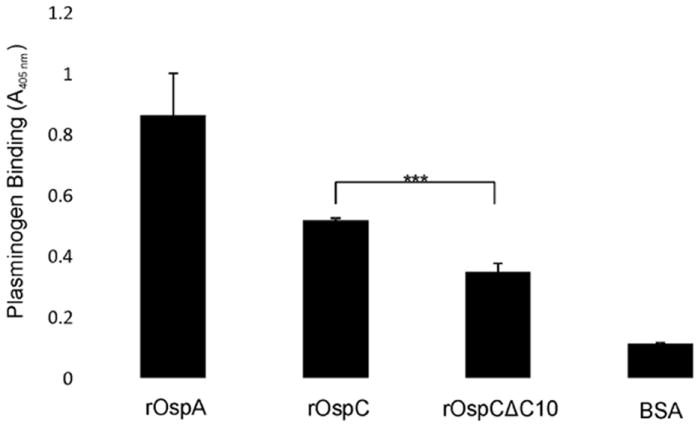
Plasminogen binding to rOspC and rOspC )C10. rOspA (positive control), rOspC, rOspCΔC10, and BSA (negative control) were immobilized in ELISA plate wells and screened with 1μg mL-1 of human plasminogen. Binding was detected by goat anti-human plasminogen antibody and peroxidase-conjugated rabbit anti-goat IgG. Representative data is shown. All methods are detailed in text.
Discussion
The OspC determinants that participate in its critical, yet largely undefined, in vivo functions are not fully known. To date, only two published studies have investigated the potential contribution of specific amino acid residues of OspC in its in vivo function (Earnhart et al., 2010; Earnhart et al., 2011). A solvent accessible, surface exposed region of OspC, designated as ligand binding domain 1 (LBD1), likely is a key determinant in OspC function as single or double amino acid substitutions introduced within LBD1 render B. burgdorferi non-infectious or alter its dissemination patterns (Earnhart et al., 2010). The results obtained through site directed mutagenesis are important as they refute earlier suggestions that OspC serves a non-specific function membrane stabilizing function during infection that can be complemented by other divergent outer surface proteins (Xu et al., 2008). In a second study, allelic exchange mutagenesis was performed (Earnhart et al., 2011) to test the hypothesis that OspC’s in vivo function is dependent on the formation of higher order protein arrays in the spirochetal membrane (Lawson et al., 2006; Zuckert et al., 2001). Residue C130 is the sole cysteine of OspC and its spatial location on the surface of the OspC dimer raises the possibility that it could form inter-dimeric disulfide bonds. Substitution of C130 with Alanine and subsequent allelic exchange replacement of wild type ospC (to yield strain B31∷C130A) had no effect on infectivity. These data indicate that higher order oligomerization (i.e., tetramers, etc) is not required for OspC function in vivo.
The goal of this study was to test the hypothesis that the highly conserved C10 motif of OspC (PVVAESPKKP) directly or indirectly plays a role in OspC’s in vivo functions. It had previously been suggested that the C10 motif may assume a polyproline II helical conformation (Mathiesen et al., 1998), a secondary structure motif that may participate in protein-protein interactions (Brady et al., 2010). While the C10 motif has been demonstrated to be solvent accessible, NMR analyses suggest it to be relatively unstructured (Huang et al., 1999). To assess the potential role of C10 in OspC in vivo activity, a B. burgdorferi mutant strain (B31∷ospCΔC10) was generated that produces an OspC protein lacking the C10 motif. The B31∷ospCΔC10 strain, generated through allelic exchange, was found to have similar growth kinetics and OspC production patterns as the parental wild type strain. Deletion of the C10 motif did not disrupt OspC trafficking and presentation on the cell surface.
The potential of B31∷ospCΔC10 to infect mice by needle inoculation was assessed. Positive cultures of B31∷ospCΔC10 were obtained from 5/5 bladder samples and 3/5 tissue biopsies. B31∷ospCΔC10 also elicited anti-B. burgdorferi IgG titers that were equivalent to that observed for mice infected with B31 and B31∷ospCwt. The ability of B31∷ospCΔC10 to transit from ticks to mammals was also assessed using I. scapularis ticks infected by micro-injection. The infected ticks were fed on naïve mice and the ticks were collected after feeding to repletion. Spirochetes were readily detected in tissue biopsies harvested near the tick bite site for all mice except those exposed to the B31ΔospC strain. In addition, all mice that were culture positive also seroconverted as assessed by ELISA and immunoblot.
Antibody responses to several OspC epitopes including the loop 5, alpha helix 5, and C10 motifs have been demonstrated to develop during early infection (Buckles et al., 2006; Gilmore and Mbow, 1999). The loop 5 and alpha helix 5 epitopes are linear epitopes that elicit OspC type specific antibody responses that are protective (Buckles et al., 2006; Earnhart et al., 2007; Earnhart and Marconi, 2007a; Earnhart and Marconi, 2007b; Earnhart and Marconi, 2007c; Gilmore and Mbow, 1999). The C10 motif is also a linear epitope, and it has been reported to be immunodominant (Mathiesen et al., 1998). The C10 motif may also contribute to the formation of a conformational epitope that includes the N-terminus of OspC (Gilmore and Mbow, 1999). To determine if deletion of C10 influences antigenicity, anti-OspC IgG responses were measured using rOspC and rOspCΔC10 as immobilized antigens. The proteins were screened with serum from mice infected by needle challenge. Statistically insignificant differences in titer to rOspC and rOspCΔC10 were observed suggesting that the C10 motif is not an immunodominant epitope.
As mentioned above, plasminogen is the only mammalian derived ligand for OspC identified to date. Recent studies suggest that OspC is the primary mediator of the plasminogen-B. burgdorferi interaction (Onder et al., 2012). The contact points for plasminogen on OspC have yet to be identified. To determine if deletion of the C10 motif impacts plasminogen binding, recombinant full length and the C-terminally truncated OspC proteins were tested for plasminogen binding. A reproducible, albeit minor, decrease in plasminogen binding was observed with the truncated protein. However, this decrease proved not to influence infectivity as the B31∷ospCΔC10 mutant remained infectious. This does not imply that OspC is not important in plasminogen binding. It is possible that decreased plasminogen binding by OspC is compensated for by one of several other proteins produced by B. burgdorferi that have plasminogen binding activity.
The genetic stability of the C10 motif suggest that there has been significant positivie selective pressure to maintain this OspC C-terminal sequence. Sequence conservation of a given motif often indicates an important structural or functional role. Based on this general paradigm we speculated that deletion of C10 would adversely affect the ability of OspC to carry out its critical, yet unknown, in vivo function(s). However as detailed above, deletion the C10 motif slightly attenuated plasminogen binging but had no discernable effect on the ability of B. burgdorferi to survive in ticks, infect mice and disseminate. While the data suggest that C10 is dispensable, it is possible that C10 may directly or indirectly mediate functions that are necessary for survival in other hosts or in other environmental conditions not assessed in this study. Additional mutational analyses of OspC will serve to further define its functional determinants. The identification of critical functional residues or domains of OspC may ultimately allow for the definitive determination of its potential ligands and precise biological functions.
Acknowledgments
This work was supported by NIH NIAID grant AI67830 to RTM, a Virginia Innovative Partnership award to RTM and JAC and NIH awards (AI080615 and AI076684) to UP.
Footnotes
The authors of this study do not have a real or perceived conflict of interest that would influence the interpretation of the results presented within.
Literature cited
- Anguita J, Ramamoorthi N, Hovius JW, Das S, Thomas V, Persinski R, Conze D, Askenase PW, Rincon M, Kantor FS, Fikrig E. Salp15, an ixodes scapularis salivary protein, inhibits CD4(+) T cell activation. Immunity. 2002;16(6):849–59. doi: 10.1016/s1074-7613(02)00325-4. [DOI] [PubMed] [Google Scholar]
- Benach JL, Bosler EM, Hanrahan JP, Coleman JL, Habicht GS, Bast TF, Cameron DJ, Ziegler JL, Barbour AG, Burgdorfer W, Edelman R, Kaslow RA. Spirochetes isolated from the blood of two patients with Lyme disease. N Engl J Med. 1983;308(13):740–2. doi: 10.1056/NEJM198303313081302. [DOI] [PubMed] [Google Scholar]
- Brady LJ, Maddocks SE, Larson MR, Forsgren N, Persson K, Deivanayagam CC, Jenkinson HF. The changing faces of Streptococcus antigen I/II polypeptide family adhesins. Mol Microbiol. 2010;77(2):276–86. doi: 10.1111/j.1365-2958.2010.07212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson D, Dykhuizen DE. ospC diversity in Borrelia burgdorferi: Different hosts are different niches. Genetics. 2004;168:713–722. doi: 10.1534/genetics.104.028738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks CS, Hefty PS, Joliff SE, Akins DR. Global analysis of Borrelia burgdorferi genes regulated by mammalian host-specific signals. Infection and Immunity. 2003;71:3371–3383. doi: 10.1128/IAI.71.6.3371-3383.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckles EL, Earnhart CG, Marconi RT. Analysis of antibody response in humans to the type A OspC loop 5 domain and assessment of the potential utility of the loop 5 epitope in Lyme disease vaccine development. Clin Vaccine Immunol. 2006;13(10):1162–5. doi: 10.1128/CVI.00099-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP. Lyme disease--a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- Clark K, Hendricks A, Burge D. Molecular identification and analysis of Borrelia burgdorferi sensu lato in lizards in the southeastern United States. Appl Environ Microbiol. 2005;71(5):2616–25. doi: 10.1128/AEM.71.5.2616-2625.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall H, Dunn DM, Ma Y, Wooten RM, Zachary JF, Weis JH, Weiss RB, Weis JJ. Gene expression profiling reveals unique pathways associated with differential severity of lyme arthritis. J Immunol. 2006;177(11):7930–42. doi: 10.4049/jimmunol.177.11.7930. [DOI] [PubMed] [Google Scholar]
- Earnhart C, Marconi RT. Lyme disease. In: Barrett AD, Stanberry LR, editors. Vaccines for Biodefense and Emerging and Neglected Diseases. Elsevier; 2008. [Google Scholar]
- Earnhart CG, Buckles EL, Dumler JS, Marconi RT. Demonstration of OspC type diversity in invasive human Lyme disease isolates and identification of previously uncharacterized epitopes that define the specificity of the OspC murine antibody response. Infect Immun. 2005;73(12):7869–77. doi: 10.1128/IAI.73.12.7869-7877.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnhart CG, Buckles EL, Marconi RT. Development of an OspC-based tetravalent, recombinant, chimeric vaccinogen that elicits bactericidal antibody against diverse Lyme disease spirochete strains. Vaccine. 2007;25(3):466–80. doi: 10.1016/j.vaccine.2006.07.052. [DOI] [PubMed] [Google Scholar]
- Earnhart CG, Marconi RT. OspC phylogenetic analyses support the feasibility of a broadly protective polyvalent chimeric Lyme disease vaccine. Clin Vaccine Immunol. 2007a;14(5):628–34. doi: 10.1128/CVI.00409-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnhart CG, Marconi RT. Construction and analysis of variants of a polyvalent Lyme disease vaccine: approaches for improving the immune response to chimeric vaccinogens. Vaccine. 2007b;25(17):3419–27. doi: 10.1016/j.vaccine.2006.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnhart CG, Marconi RT. An octavalent lyme disease vaccine induces antibodies that recognize all incorporated OspC type-specific sequences. Hum Vaccin. 2007c;3(6):281–9. doi: 10.4161/hv.4661. [DOI] [PubMed] [Google Scholar]
- Earnhart CG, Leblanc DV, Alix KE, Desrosiers DC, Radolf JD, Marconi RT. Identification of residues within ligand-binding domain 1 (LBD1) of the Borrelia burgdorferi OspC protein required for function in the mammalian environment. Mol Microbiol. 2010;76(2):393–408. doi: 10.1111/j.1365-2958.2010.07103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnhart CG, Rhodes DV, Marconi RT. Disulfide-Mediated Oligomer Formation in Borrelia burgdorferi Outer Surface Protein C, a Critical Virulence Factor and Potential Lyme Disease Vaccine Candidate. Clin Vaccine Immunol. 2011;18(6):901–6. doi: 10.1128/CVI.05004-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eicken C, Sharma V, Klabunde T, Owens RT, Pikas DS, Hook M, Sacchettini JC. Crystal structure of Lyme disease antigen outer surface protein C from Borrelia burgdorferi. J Biol Chem. 2001;276(13):10010–5. doi: 10.1074/jbc.M010062200. [DOI] [PubMed] [Google Scholar]
- Fuchs H, Wallich R, Simon MM, Kramer MD. The outer surface protein A of the spirochete Borrelia burgdorferi is a plasmin(ogen) receptor. Proc Natl Acad Sci U S A. 1994;91(26):12594–8. doi: 10.1073/pnas.91.26.12594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs R, Jauris S, Lottspeich F, Preac-Mursic V, Wilske B, Soutschek E. Molecular analysis and expression of a Borrelia burgdorferi gene encoding a 22 kDa protein (pC) in Escherichia coli. Molecular Microbiology. 1992;6:503–509. doi: 10.1111/j.1365-2958.1992.tb01495.x. [DOI] [PubMed] [Google Scholar]
- Gern L, Estrada-Pena A, Frandsen F, Gray JS, Jaenson TG, Jongejan F, Kahl O, Korenberg E, Mehl R, Nuttall PA. European reservoir hosts of Borrelia burgdorferi sensu lato. Zentralbl Bakteriol. 1998;287(3):196–204. doi: 10.1016/s0934-8840(98)80121-7. [DOI] [PubMed] [Google Scholar]
- Gilmore RD, Mbow ML. Conformational nature of the Borrelia burgdorferi B31 outer surface protein C protective epitope. Infection and Immunity. 1999;67:5463–5469. doi: 10.1128/iai.67.10.5463-5469.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore RD, Jr, Mbow ML, Stevenson B. Analysis of Borrelia burgdorferi gene expression during life cycle phases of the tick vector Ixodes scapularis. Microbes Infect. 2001;3(10):799–808. doi: 10.1016/s1286-4579(01)01435-6. [DOI] [PubMed] [Google Scholar]
- Grimm D, Tilly K, Byram R, Stewart PE, Krum JG, Bueschel DM, Schwan TG, Policastro PF, Elias AF, Rosa PA. Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc Natl Acad Sci U S A. 2004;101(9):3142–7. doi: 10.1073/pnas.0306845101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Link K, Koide A, Dunn JJ, Luft BJ, Koide S. 1H, 13C, and 15N NMR backbone assignments of 37 kDa surface antigen OspC from Borrelia burgdorferi. J Biomol NMR. 1999;14(3):283–4. doi: 10.1023/a:1008398527355. [DOI] [PubMed] [Google Scholar]
- Kostick JL, Szkotnicki LT, Rogers EA, Bocci P, Raffaelli N, Marconi RT. The diguanylate cyclase, Rrp1, regulates critical steps in the enzootic cycle of the Lyme disease spirochetes. Mol Microbiol. 2011 doi: 10.1111/j.1365-2958.2011.07687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D, Eswaramoorthy S, Luft BJ, Koide S, Dunn JJ, Lawson CL, Swaminathan S. Crystal structure of outer surface protein C (OspC) from the Lyme disease spirochete Borrelia burgdorferi. EMBO J. 2001;20:971–978. doi: 10.1093/emboj/20.5.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labandeira-Rey M, Skare JT. Decreased infectivity in Borrelia burgorferi strain B31 is associated with the loss of either linear plasmid 25 or 28-1. Infection and Immunity. 2001;69:446–455. doi: 10.1128/IAI.69.1.446-455.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagal V, Portnoi D, Faure G, Postic D, Baranton G. Borrelia burgdorferi sensu stricto invasiveness is correlated with OspC-plasminogen affinity. Microbes Infect. 2006;8(3):645–52. doi: 10.1016/j.micinf.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Lawson CL, Yung BH, Barbour AG, Zuckert WR. Crystal structure of neurotropism-associated variable surface protein 1 (Vsp1) of Borrelia turicatae. J Bacteriol. 2006;188(12):4522–30. doi: 10.1128/JB.00028-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconi RT, Earnhart C. Lyme disease vaccines. In: Samuels DS, Radolf J, editors. Borrelia: Molecular biology, host interaction and pathogenesis. Norfolk: Caister Academic Press; 2010. pp. 467–486. [Google Scholar]
- Mathiesen MJ, Holm A, Christiansen M, Blom J, Hansen K, Ostergaard S, Theisen M. The dominant epitope of Borrelia garinii outer surface protein C recognized by sera from patients with neuroborreliosis has a surface-exposed conserved structural motif. Infect Immun. 1998;66(9):4073–9. doi: 10.1128/iai.66.9.4073-4079.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell JV, Sung SY, Labandeira-Rey M, Skare JT, Marconi RT. Analysis of mechanisms associated with loss of infectivity of clonal populations of Borrelia burgdorferi B31MI. Infect Immun. 2001;69(6):3670–7. doi: 10.1128/IAI.69.6.3670-3677.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan S, Santiago F, Koski RA, Brei B, Anderson JF, Fish D, Fikrig E. Examination of the Borrelia burgdorferi transcriptome in Ixodes scapularis during feeding. J Bacteriol. 2002;184(11):3122–5. doi: 10.1128/JB.184.11.3122-3125.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onder O, Humphrey PT, McOmber B, Korobova F, Francella N, Greenbaum DC, Brisson D. OspC Is Potent Plasminogen Receptor on Surface of Borrelia burgdorferi. J Biol Chem. 2012;287(20):16860–8. doi: 10.1074/jbc.M111.290775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel AT, Talaat AM, Norgard MV. DNA microarray analysis of differential gene expression in Borrelia burgdorferi, the Lyme disease spirochete. Proc Natl Acad Sci. 2002;99:1562–1567. doi: 10.1073/pnas.032667699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers EA, Terekhova D, Zhang HM, Hovis KM, Schwartz I, Marconi RT. Rrp1, a cyclic-di-GMP-producing response regulator, is an important regulator of Borrelia burgdorferi core cellular functions. Mol Microbiol. 2009;71(6):1551–1573. doi: 10.1111/j.1365-2958.2009.06621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels DS, Mach K, Garon CF. Genetic transformation of the Lyme disease agent Borrelia burgdorferi with coumarin-resistant gyrB. Journal of Bacteriology. 1994;176:6045–6049. doi: 10.1128/jb.176.19.6045-6049.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan TG, Piesman J, Golde WT, Dolan MC, Rosa PA. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci USA. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan TG, Piesman J. Temporal changes in outer surface proteins A and C of the lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J Clin Microbiol. 2000;38(1):382–8. doi: 10.1128/jcm.38.1.382-388.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan TG. Temporal regulation of outer surface proteins of the Lyme-disease spirochaete Borrelia burgdorferi. Biochem Soc Trans. 2003;31(Pt 1):108–12. doi: 10.1042/bst0310108. [DOI] [PubMed] [Google Scholar]
- Steere AC. Lyme disease. New England Journal of Medicine. 2001;345:115–125. doi: 10.1056/NEJM200107123450207. [DOI] [PubMed] [Google Scholar]
- Steere AC. Lyme borreliosis in 2005, 30 years after initial observations in Lyme Connecticut. Wien Klin Wochenschr. 2006;118(21-22):625–33. doi: 10.1007/s00508-006-0687-x. [DOI] [PubMed] [Google Scholar]
- Stevenson B, Schwan TG, Rosa PA. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1995;63(11):4535–9. doi: 10.1128/iai.63.11.4535-4539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PE, Wang X, Bueschel DM, Clifton DR, Grimm D, Tilly K, Carroll JA, Weis JJ, Rosa PA. Delineating the requirement for the Borrelia burgdorferi virulence factor OspC in the mammalian host. Infect Immun. 2006;74(6):3547–53. doi: 10.1128/IAI.00158-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung SY, McDowell JV, Marconi RT. Evidence for the contribution of point mutations to vlsE variation and for apparent constraints on the net accumulation of sequence changes in vlsE during infection with Lyme disease spirochetes. J Bacteriol. 2001;183(20):5855–61. doi: 10.1128/JB.183.20.5855-5861.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly K, Casjens S, Stevenson B, Bono JL, Samuels DS, Hogan D, Rosa P. The Borrelia burgdorferi circular plasmid cp26: conservation of plasmid structure and targeted inactivation of the ospC gene. Molecular Microbiology. 1997;25:361–373. doi: 10.1046/j.1365-2958.1997.4711838.x. [DOI] [PubMed] [Google Scholar]
- Tilly K, Krum JG, Bestor A, Jewett MW, Grimm D, Bueschel D, Byram R, Dorward D, Vanraden MJ, Stewart P, Rosa P. Borrelia burgdorferi OspC protein required exclusively in a crucial early stage of mammalian infection. Infect Immun. 2006;74(6):3554–64. doi: 10.1128/IAI.01950-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly K, Bestor A, Jewett MW, Rosa P. Rapid Clearance of Lyme Disease Spirochetes Lacking OspC from Skin. Infect Immun. 2007;75(3):1517–1519. doi: 10.1128/IAI.01725-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokarz R, Anderton JM, Katona LI, Benach JL. Combined effects of blood and temperature shift on Borrelia burgdorferi gene expression as determined by whole genome DNA array. Infect Immun. 2004;72(9):5419–32. doi: 10.1128/IAI.72.9.5419-5432.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang IN, Dykhuizen DE, Qiu W, Dunn JJ, Bosler EM, Luft BJ. Genetic diversity of ospC in a local population of Borrelia burgdorferi sensu stricto. Genetics. 1999;151(1):15–30. doi: 10.1093/genetics/151.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, Krause PJ, Bakken JS, Strle F, Stanek G, Bockenstedt L, Fish D, Dumler JS, Nadelman RB. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43(9):1089–134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- Xu Q, McShan K, Liang FT. Modification of Borrelia burgdorferi to overproduce OspA or VlsE alters its infectious behaviour. Microbiology. 2008;154(Pt 11):3420–9. doi: 10.1099/mic.0.2008/019737-0. [DOI] [PubMed] [Google Scholar]
- Zhang X, Yang X, Kumar M, Pal U. BB0323 function is essential for Borrelia burgdorferi virulence and persistence through tick-rodent transmission cycle. J Infect Dis. 2009;200(8):1318–30. doi: 10.1086/605846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckert WR, Kerentseva TA, Lawson CL, Barbour AG. Structural conservation of neurotropism-associated VspA within the variable Borrelia Vsp-OspC lipoprotein family. J Biol Chem. 2001;276(1):457–63. doi: 10.1074/jbc.M008449200. [DOI] [PubMed] [Google Scholar]


