Abstract
[Ca2 +]i signalling is a key regulatory mechanism in sperm function. In mammalian sperm the Ca2 +-permeable plasma membrane ion channel CatSper is central to [Ca2 +]i signalling, but there is good evidence that Ca2 + stored in intracellular organelles is also functionally important. Here we briefly review the current understanding of the diversity of Ca2 + stores and the mechanisms for the regulation of their activity. We then consider the evidence for the involvement of these stores in [Ca2 +]i signalling in mammalian (primarily human) sperm, the agonists that may activate these stores and their role in control of sperm function. Finally we consider the evidence that membrane Ca2 + channels and stored Ca2 + may play discrete roles in the regulation of sperm activities and propose a mechanism by which these different components of the sperm Ca2 +-signalling apparatus may interact to generate complex and spatially diverse [Ca2 +]i signals.
Ca2 + signalling in sperm
Cellular activity is constantly regulated by environmental cues and signals from other cells. Long-term regulation of cell function is normally achieved by control of gene expression, changing the complement and levels of proteins in the cell, but rapid or short-term changes are achieved by ‘post-translational’ protein modification, such as phosphorylation, sumoylation and nitrosylation, which alter the function/activity of proteins already present. Ca2 +-signalling is a key regulator of such post-translational modifications, with changes in cytoplasmic Ca2 + concentration ([Ca2 +]i) controlling the activities of key enzymes and proteins. Large changes in [Ca2 +]i can be achieved ‘instantaneously’ by flux of Ca2 + into the cytoplasm from the extracellular fluid or from storage organelles (primarily the endoplasmic reticulum) within the cell (Fig. 1a). The rapidity with which [Ca2 +]i-signals can be generated is crucial for ‘instantaneous’ cellular responses such as activation of muscle contraction and secretion of neurotransmitters that are achieved by rapid post-translational modification of protein function.
Figure 1.
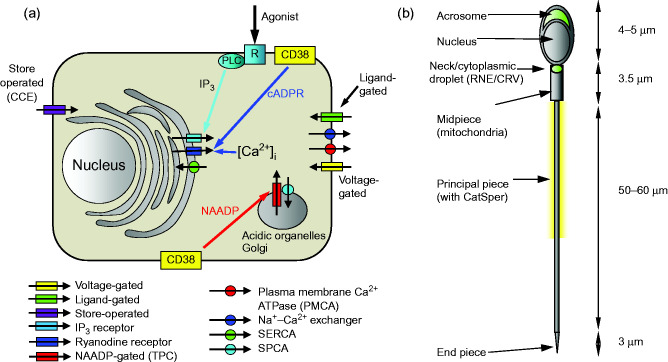
(a) Simplified diagrammatic summary of [Ca2 +]i signalling toolkit in a somatic cell. Ion channels are shown as rectangles with arrow indicating normal direction of Ca2 + flow (yellow, voltage-gated; green, ligand-gated; purple, store-operated; light blue, IP3 receptor; dark blue, ryanodine receptor; red, NAADP-gated). Pumps are shown as circles with arrows indicating normal direction of Ca2 + movement (red, PMCA'; blue, Na+–Ca2 + exchanger; green, SERCA; blue, SPCA). Activation of IP3 receptors by membrane receptor activation and phospholipase C is shown in light blue. Generation of cADPR and NAADP by CD38 and possibly other enzymes (leading to mobilisation of Ca2 + from intracellular stores) is shown by yellow boxes. (b) Structure of human sperm showing positions of CatSper channels (yellow shading around anterior flagellum) and Ca2 + stores in the acrosome and at the sperm neck (redundant nuclear envelope and calreticulin-containing vesicles) (shown in green).
The highly condensed nucleus of sperm is transcriptionally silent (Miller et al. 2005, Miller & Ostermeier 2006) and translational activity is also negligible (though evidence has been presented for translation occurring at mitochondrial ribosomes; Gur & Breitbart 2008, Zhao et al. 2009, Chandrashekran et al. 2014a , b ). Regulation of sperm function is therefore dependent primarily on post-translational processes. [Ca2 +]i signalling is pivotal to this regulation, and in mammalian sperm it plays a central role in controlling the cell's behaviour (motility type and potentially chemotaxis), the induction of acrosome reaction (AR) and the process of capacitation (Publicover et al. 2007, Darszon et al. 2007, 2011). The importance for sperm function of membrane Ca2 +-channels and Ca2 +-influx is well established (Darszon et al. 2011) but there is also good evidence for the existence and functional importance of intracellular Ca2 +-storage organelles in sperm (Darszon et al. 2007, Publicover et al. 2007). Previously we reviewed the identities and functions of Ca2 + stores in sperm, focussing on the evidence for the existence of such stores, their components (pumps and channels) and their possible roles in the regulation of function in the mature sperm cell (Costello et al. 2009). Since then considerable progress has been made in understanding the central role of Ca2 + signalling in the regulation of mammalian and non-mammalian sperm function and the mechanisms by which sperm [Ca2 +]i signals are generated. In particular successful application of whole cell patch clamp technique, in human as well as mouse sperm, has revealed the central importance of Ca2 + influx through CatSper, a sperm specific, Ca2 +-permeable channel in the membrane of the flagellar principal piece. Male mice null for CatSper are infertile (Ren et al. 2001) and their sperm show defective motility (Carlson et al. 2003). Here we review recent progress in understanding the diversity of mechanisms for the regulation of Ca2 + store activity and the evidence for their involvement in controlling sperm function.
Ca2 + stores and their regulation
The importance of Ca2 + stores in generating complex Ca2 + signals in somatic cells has long been recognized. Until relatively recently the endoplasmic reticulum Ca2 + store has been the major focus for research as this was the first organelle to show controllable mobilization of Ca2 + through second messengers acting upon intracellular Ca2 + channels, as well as being able to be refilled via Ca2 + pumps. Additionally, these Ca2 + signals could also be re-modelled through the regulation of these Ca2 + transporters to generate complex spatial and temporal Ca2 + transients (Berridge et al. 2003). It has now become clear that many other organelles such as mitochondria, endosomes, lysosomes and Golgi complexes also contribute to the generation and propagation of these complex Ca2 + signals within cells (Michelangeli et al. 2005). Furthermore, novel Ca2 + transporters have also been identified within these other organelles and several have recently been identified in sperm (Costello et al. 2009).
Intracellular Ca2 + channels
The major intracellular Ca2 + channels that have been identified and appear to be almost ubiquitously distributed within mammalian cells, especially on the endoplasmic reticulum, include the inositol-1,4,5-trisphosphate-(IP3)-sensitive Ca2 + channel (or IP3 receptor; IP3R) and the ryanodine receptor (RyR) (Michelangeli et al. 2005) (Fig. 1a). The IP3 receptor, as the name implies, is activated by the second messenger IP3 that is generated through the hydrolysis of phosphatidylinositol-4,5-bisphosphate. This channel has a specific IP3 binding site that is located towards the N-terminus of the protein (Seo et al. 2012) and also has a requirement for Ca2 + which acts as a co-agonist in order for the channel to open (Bezprozvanny et al. 1991). The activation of RyR is likely to be through a mechanism involving Ca2 + induced Ca2 + release (CICR) and by the action of the putative second messenger cyclic-adenosine diphospho-ribose (cADPR) (Ogunbayo et al. 2011) (Fig. 1a). cADPR is made from NAD by the action of an ADP-ribosyl cyclase enzyme such as CD38 (Cosker et al. 2010), although other as yet unidentified enzymes may also be involved in catalysing this reaction (Guse 2015). It is as yet unclear whether, unlike the IP3R, cADPR binds directly to RyR or whether it binds to accessory proteins such as calmodulin or FK506-binding protein, that then interact with the RyR (Guse 2015).
Another metabolite of NAD which is believed to have Ca2 + mobilizing ability is nicotinic acid adenine dinucleotide phosphate (NAADP; Genazzani et al. 1997). NAADP is made from NADP through the action of either CD38 acting as a base-exchanger, swapping the nicotinamide group for nicotinic acid or via an unidentified NADP-deaminase (Guse 2015). NAADP is believed specifically to mobilize Ca2 + from acidic stores such as lysosomes (Churchill et al. 2002, Menteyne et al. 2006), which can then induce CICR at RyRs and IP3Rs in mammalian cells (Cancela et al. 1999; Fig. 1a). Results initially presented by Calcraft et al. (2009), indicated that NAADP specifically activates Ca2 +-specific two-pore channels (TPC) within the acidic organelles, these channels being first described in plants (Peiter et al. 2005). However, in kinetic studies there is a prominent lag between addition of NAADP and Ca2 + mobilization (Genazzani et al. 1997). Combined with the observation that photo-affinity labelling with azido-NAADP (Lin-Moshier et al. 2012) showed labelling of only low molecular weight proteins, not consistent with TPCs, it suggests that NAADP might function by binding to accessory proteins rather than directly to the channel. Recently there has been considerable controversy as to whether the NAADP-sensitive Ca2 + channel is a TPC (Morgan & Galione 2014). Data from two studies (Wang et al. 2012, Cang et al. 2013) suggested that TPCs are in fact Na+-specific channels with very low Ca2 + selectivity that are activated by phosphoinositide lipids and modulated by mTOR, but not by NAADP. However, recently published work with cells from mice null for TPC1 and TPC2 provided strong evidence that TPCs are similarly permeable to Ca2 + and Na+ and are NAADP-gated through binding to an accessory protein (Ruas et al. 2015).
Numerous kinases have been shown to modulate the activity of both the IP3Rs and RyRs, including several ubiquitous ser/thr kinases such as PKA, PKG and CaMKII (Yule et al. 2010, Camors & Valdivia 2014). Indeed, some of these kinases such as PKA appear to have both stimulatory and inhibitory effects on the IP3R, dependent upon isoform subtype and the presence of multiple kinase-dependent phosphorylation sites on the same receptor (Dyer et al. 2003). Less ubiquitous ser/thr kinases such as Akt and polo kinases as well as tyrosine kinases such as fyn kinase have also been shown to affect these channels (Yule et al. 2010, Camors & Valdivia 2014).
Both the RyRs and the IP3Rs are modulated by changes in their oxidation states caused by reactive oxygen species (ROS) and reactive nitrogen species (RNS), and this occurs mainly through modification of specific cysteine (cys) amino acid residues. Oxidation of these cys residues in RyRs occurs both by S-glutathionylation as well as S-nitrosylation by the second messenger nitric oxide (NO; Csordas & Hajnoczky 2009) and promotes the activity of the channel by enhancing RyR subunit interactions and also by reducing the efficacy of inhibitory modulators (Hamilton & Reid 2000). In IP3Rs the effects of oxidative stress are complex: low levels of cys oxidation caused by low concentrations of thimerosal (a cys–modifying mercuric compound) and naturally generated ROS cause sensitization of this channel, while higher concentrations of thimerosal inhibit channel activity (Missiaen et al. 1991, Sayers et al. 1993). Currently, however, there is little evidence that NO can affect the activity of the IP3Rs.
Intracellular Ca2 + pumps
The major transporter involved in refilling Ca2 + stores within the endoplasmic reticulum is the sarcoplasmic/endoplasmic reticulum Ca2 + ATPase (SERCA; Fig. 1a), and these pumps occur abundantly in all somatic cells. Their role is to pump Ca2 + back into the storage organelles to help terminate Ca2 + signals (Michelangeli et al. 2005, Michelangeli & East 2011). There are three isoforms of this Ca2 + ATPase, each encoded by a different gene and each isoform can exist in a variety of spliced variants that differ in size and regulatory properties (Michelangeli & East 2011). SERCA1 is mainly confined to skeletal muscle, while SERCA2 is widely distributed in most other tissues and organs and type 3 has a limited expression. Another related Ca2 + ATPase that is also found ubiquitously within somatic cells is the secretory pathway Ca2 + ATPase (SPCA), which is localized to the Golgi apparatus (Wootton et al. 2004). SPCA exists in two isoforms with the expression of type 1 being far more widespread than type 2, which appears to be mainly located within glandular tissues (Vanoevelen et al. 2005). Recently there has been evidence to suggest that SPCA2 can interact with and regulate the plasma membrane located ORAI Ca2 + channels that are implicated in store-operated Ca2 + entry (Feng et al. 2010), which may indicate a dual function for this Ca2 + ATPase in cells that express it.
There is currently some debate as to which type of intracellular Ca2 + ATPase is expressed in mature sperm. We have highlighted that SPCA1 is present in human sperm, where it appears to be mainly localized to the neck region of the cell where the redundant nuclear envelope (RNE) and calreticulin-containing vesicles are situated (Harper et al. 2005). This study also found no evidence for expression of SERCA in human sperm as no cross-reactivity was observed with a pan-isoform SERCA antibody and no effects on [Ca2 +]i were observed with specific but saturating concentrations of the SERCA-inhibitor thapsigargin. However, a more recent study (Lawson et al. 2007) detected SERCA2, mainly localized to the acrosome and mid-piece, using a SERCA2-specific antibody.
Unlike the intracellular Ca2 + channels, there is no strong evidence to suggest that either SERCA or SPCA can be directly phosphorylated and regulated by protein kinases, although some Ca2 + ATPase modulatory proteins like phospholamban (that is found almost exclusively in heart) are regulated through phosphorylation by PKA, PKG and CamKII (Colyer 1998). There is considerable evidence indicating that oxidative stress can modulate SERCA activity (although no studies have yet been undertaken on SPCA). Again a number of critical cys residues such as cys674 can be S-glutathionylated to cause an increase in SERCA pump activity (Adachi et al. 2004). Modifications of other cys residues on the Ca2 + ATPase, however, can have inhibitory effects (Sayers et al. 1993, Sharov et al. 2006, Csordas & Hajnoczky 2009).
Ca2 + stores, mechanisms for store mobilisation and store-operated Ca2 + channels in sperm
During the later stages of their development spermatozoa shed much of their cytoplasm including intracellular organelles. Thus mammalian sperm contain no organised endoplasmic reticulum. However, studies on the expression of Ca2 + store components and on the generation [Ca2 +]i signals suggest that the remaining intracellular organelles function as Ca2 +-stores and play a significant role in the regulation of cellular function (Costello et al. 2009). In particular, the acrosomal vesicle at the apex of the head and the collection of vesicular membranous structures that occur at the sperm neck and anterior midpiece (including the cytoplasmic droplet of human sperm) appear to be functionally important Ca2 +-stores (Fig. 1b; shown in green). At both these locations IP3Rs have been detected in human and in bovine sperm by immuno-staining (Dragileva et al. 1999, Kuroda et al. 1999, Ho & Suarez 2001, 2003, Naaby-Hansen et al. 2001). RyRs have also been detected in human and rodent sperm (Trevino et al. 1998, Lefievre et al. 2007). Staining of human sperm with anti-RyR1, anti-RyR2, pan-RyR and BODIPY-FLX ryanodine is localised primarily to the neck region, though some acrosomal staining was also observed (Harper et al. 2004, Lefievre et al. 2007, Park et al. 2011). In contrast, other authors (Ho & Suarez 2001) have reported no staining of bovine sperm with BODIPY-FLX ryanodine (see Costello et al. (2009) for further discussion). Thus mobilisation of stored Ca2 + in mammalian sperm may occur in response to generation of IP3 by activity of phospholipase C and by CICR at IP3Rs or RyRs. These processes can be sensitised by effects such as oxidative stress and S-nitrosylation (see ‘Ca2 + stores and their regulation’). For instance, exposure of human sperm to NO at levels equivalent to those produced by explants of reproductive tract lining mobilises stored Ca2 + and modifies flagellar activity (Lefievre et al. 2007, Machado-Oliveira et al. 2008).
In addition to generation of IP3 in sperm, there is evidence that other Ca2 + mobilising messengers (NAADP and cADPR) are synthesised in sperm and/or produced in response to stimulation. Sea urchin sperm contain significant levels of both cADPR and NAADP, which may contribute to oocyte activation (Chini et al. 1997, Billington et al. 2002). Human sperm have been shown to contain cADPR at micromolar concentrations but NAADP was not detected (Billington et al. 2006). Interestingly, this study also demonstrated synthesis of cADPR by human sperm but the ecto-enzyme CD38 (an enzyme present on mammalian cells that synthesises both cADPR and NAADP; see ‘Ca2 + stores and their regulation’) could not be detected by western blotting. In contrast, Park et al. (2011), reported detection of CD38 in human sperm after co-incubation with prostasomes (prostate-derived membrane vesicles; see below). Furthermore, the presence of a novel NAADP synthase, which lacks the cyclase activity of CD38, has been described both in sea urchin (Vasudevan et al. 2008) and human sperm (Sanchez-Tusie et al. 2014). In sea urchin sperm this enzyme is strongly Ca2 +-regulated and most active at acid pH whereas the human enzyme shows only weak Ca2 +-regulation and activity is maximal at pH 7–8 (Vasudevan et al. 2008, Sanchez-Tusie et al. 2014).
Recent findings have supported the idea that NAADP is functional in human sperm. Sanchez-Tusie et al. (2014) investigated the effects of cell-permeant (AM-ester) derivatives of NAAPD and cADPR. No effects were observed with cADPR, consistent with previous pharmacological investigation by Billington et al. (2006), but NAADP caused elevation of [Ca2 +]i both in cells incubated under standard conditions and also when [Ca2 +]o was buffered to 100 nM, conditions under which Ca2 + influx is negligible and [Ca2 +]i signalling depends solely on mobilisation of stored Ca2 +. Staining of NAADP receptors using the fluorescent NAADP receptor ligand Ned-19 and identification of acidic organelles using lysotracker highlighted both an anterior store (potentially the acrosome) and a store at the sperm neck (Fig. 1b). Consistent with these findings, Arndt et al. (2014), studying AR (see below), provided evidence for involvement in this process of NAADP and TPCs, which have been proposed to be the NAADP receptor/Ca2 + channel of acidic Ca2 + storage organelles (Calcraft et al. 2009; Fig 1a; see ‘Ca2 + stores and their regulation’).
Park et al. (2011) investigated the incorporation into human sperm of proteins from prostasomes (prostate-derived vesicles which are normally added to sperm during ejaculation) and their effects on [Ca2 +]i signalling. They concluded that CatSper channel proteins were present in the differentiated sperm, but other Ca2 + signalling ‘tools’ including RyRs and CD38 were added to the freshly-ejaculated sperm upon mixing with prostasomes, by fusion with the membrane of the midpiece. They also examined the effects of stimulation with progesterone on [Ca2 +]i and motility of sperm exposed to prostasomes and sperm that had been rapidly removed from semen to minimise mixing with prostasomes. Their data suggest that the generation of sustained [Ca2 +]i signals (such as the second component of the biphasic progesterone-induced [Ca2 +]i signal) and consequent effects on motility may depend, at least partly, upon generation of cADPR by prostasome-derived enzymes. Interestingly, CD38-null mice proved to be fertile, but analysis showed that 20% of normal ADPR cyclase activity remained in prostasomes from these animals, indicating the presence of a non-CD38 ADPR-cyclase, potentially that described by Sanchez-Tusie et al. (2014). Thus both NAADP and cADPR are potentially synthesised by sperm and involved in regulation of sperm Ca2 + store activity but their roles are not yet clear.
In somatic cells mobilisation of stored Ca2 + induces secondary Ca2 + influx through channels at the cell membrane (store-operated channels, SOCs) by the process of capacitative Ca2 + entry (CCE) (Fig. 1a). CCE both prolongs Ca2 + signals that are induced by store mobilisation and provides Ca2 + for re-charging of the storage organelles. Recently great progress has been made in elucidating the key players and mechanisms in this process. Stromal interaction molecule (STIM) has been identified as the sensor molecule present in the membrane of the Ca2 + store. The intraluminal part of STIM includes a Ca2 +-binding EF hand that detects depletion of stored Ca2 +. STIM then redistributes, moving to a position adjacent to the plasma membrane where it activates channel proteins (ORAI and possibly members of the TRPC (transient receptor potential canonical) family (Cahalan 2009)). [Ca2 +]i signals in human and other mammalian sperm induced by agonists and by treatments designed to mobilise stored-Ca2 + show characteristics consistent with the occurrence of CCE (Blackmore 1993, Dragileva et al. 1999, O'Toole et al. 2000, Park et al. 2011, Lefievre et al. 2012). STIM1, ORAI and TRPC proteins have been detected in human sperm (Castellano et al. 2003, Darszon et al. 2012, Lefievre et al. 2012), STIM1 being localised primarily to the neck region/midpiece and the acrosome where Ca2 + stores are present (Lefievre et al. 2012). To date the application of whole-cell patch clamp has not provided evidence for the occurrence of CCE in human sperm (Lefievre et al. 2012) so these findings must be interpreted cautiously, but [Ca2 +]i signals generated by mobilisation of Ca2 + stores in sperm may be amplified by activation of CCE. Induction of CCE in somatic cells can have a latency of tens of seconds due to the need for STIM to migrate to the peripheral portions of the endoplasmic reticulum where it can interact with SOC proteins (Luik et al. 2006, Wu et al. 2006), but in sperm the storage organelles are close to the plasma membrane and STIM proteins are localised here, such that CCE could be near ‘instantaneous’. Pre-treatment of human sperm with low concentrations of 2-aminoethoxydiphenyl borate, which potentiates CCE by promoting the interaction of STIM with SOCs (Navarro-Borelly et al. 2008, Wang et al. 2009, Yamashita et al. 2011) significantly enhanced the amplitude of the progesterone-induced Ca2 + transient at the sperm neck (where secondary release of stored Ca2 + may occur; Fig. 1b; see ‘Model for interaction of CatSper channels and Ca2 +-stores’) but did not affect the response in the flagellum, where progesterone activates CatSper channels (Fig. 1b), or the kinetics of the signal at either location (Lefievre et al. 2012). Conversely, when sperm were pre-treated with a cell-penetrating peptide that mimics part of the key SOAR region of STIM1 (potentially preventing auto-inhibitory folding of STIM upon store-refilling) there was a marked prolongation of the progesterone-induced [Ca2 +]i transient in a subset of cells (Morris et al. 2015).
Mobilisation of sperm Ca2 + stores by agonists
In the majority of somatic cells mobilisation of stored Ca2 + occurs upon agonist-induced synthesis of Ca2 + mobilising intracellular messengers. Thus agonist-induced synthesis of inositol trisphosphate, cADPR and NAADP can lead to rapid release of stored Ca2 + and generation of local, global and complex spatio-temporal signals (Fig. 1a). Is there evidence that such processes occur and are functionally significant in responses to agonist stimulation of sperm?
The best-characterised agonist-induced [Ca2 +]i signals in sperm are responses to solubilised zona pellucida/zona proteins in mouse cells and progesterone in human. Application of patch clamp has clearly shown that the primary action of progesterone in human sperm is to activate CatSper channels, leading to Ca2 +-influx (Lishko et al. 2011, Strunker et al. 2011). Strunker et al. (2011) investigated the [Ca2 +]o dependence of progesterone-induced [Ca2 +]i signals in rapid-mixing experiments on human sperm and reported that buffering of [Ca2 +]o to ≤100 nM abolished the response (though see Espino et al. (2009)), suggesting that any mobilisation of stored Ca2 + is a secondary response. Synthesis of IP3 is reported to occur downstream of progesterone-induced Ca2 + influx (Thomas & Meizel 1989), an important observation that should be pursued. Stimulation of mouse sperm with zona proteins induces AR, which requires elevation of [Ca2 +]i in the sperm head (Florman et al. 2008) and is dependent on mobilisation of Ca2 + from the acrosomal store (De Blas et al. 2002; see below). The nature of the Ca2 + influx following stimulation is not clear and several channels may be involved (Florman et al. 2008, Xia & Ren 2009, Cohen et al. 2014), but Ca2 + signals are sensitive to inhibition of G-protein signalling (using pertussis toxin) and inhibition of PLC (Florman et al. 2008, Ren & Xia 2010). Furthermore, in sperm from mice null for PLCδ4 (in which males' fertility is severely impaired) the [Ca2 +]i response is reduced and zona-induced AR does not occur (Fukami et al. 2001, 2003). Thus conventional IP3-induced mobilisation of stored Ca2 + is apparently central to this essential aspect of mammalian sperm physiology.
Evidence for the existence of other store-mobilising agonists is largely preliminary, but there are a number of candidates, of which the best-studied is vitamin D (Blomberg Jensen 2014). Human sperm have been shown to express vitamin D receptor (VDR; Aquila et al. 2009, Blomberg Jensen et al. 2010, 2011), the enzymes CYP2R1 and CYP27B (which produce the active compound (1,25(OH)2D3) cholecalciferol) and the inactivating enzyme CYP24A1 (Blomberg Jensen et al. 2010, 2011). All are expressed in the neck region of the sperm and staining of cells for VDR and CYP24A1 shows a strong association. In sub-fertile patients the proportion of cells expressing CYP24A1 varies greatly and is significantly correlated with semen quality (sperm count, concentration, morphology and motility; Blomberg Jensen et al. 2011, 2012). Stimulation of human sperm with 1,25(OH)2D3 (100 pM–1 μM) induced a [Ca2 +]i response, including a transient and plateau, that was blocked by pre-treatment with the non-genomic VDR antagonist 1β,25(OH)2D3 but was insensitive to the nuclear VDR antagonist ZK159222 (Blomberg Jensen et al. 2011). This effect was greatly reduced by pre-treatment with the phospholipase C inhibitor U73122 (2 μM) but was also inhibited by incubation in EGTA-buffered medium for up to 20 min prior to stimulation. Both motility and AR were significantly increased upon stimulation with 1,25(OH)2D3 (Blomberg Jensen et al. 2011).
Kisspeptin, a peptide agonist of the G-protein coupled receptor GPR54/KISS1R, has also been shown to cause sustained, dose-dependent elevation of [Ca2 +]i in human and in mouse sperm (Pinto et al. 2012, Hsu et al. 2014). In neurons binding of kisspeptin to its receptor activates PLC and results in generation of IP3 and diacyglycerol, leading to mobilisation of stored Ca2 + and also depolarisation (Liu et al. 2008, Pielecka-Fortuna et al. 2008, Beltramo et al. 2014). In human sperm the effect of kisspeptin on [Ca2 +]i did not occlude the response to stimulation with the CatSper agonist progesterone and was not reduced when applied in the presence of progesterone (Pinto et al. 2012). Both KISS1R and kisspeptin itself were detected in the head of human sperm, suggesting that an autocrine action of the peptide may occur. Motility parameters of kisspeptin-treated cells were significantly altered, including an increase in lateral movement of the head and a decrease in linearity of the sperm path, characteristics of hyperactivated sperm (Pinto et al. 2012). Ghrelin, another peptide hormone that also acts through mobilisation of stored Ca2 + (Camina et al. 2003), has also been detected in human sperm (Moretti et al. 2014). Micromolar concentrations of ghrelin have been shown to increase [Ca2 +]i and motility in rat sperm (Lukaszyk et al. 2012), but expression of ghrelin receptors or effect of ghrelin on human sperm [Ca2 +]i have not been investigated.
Functional significance of Ca2 +-stores
The acrosome
AR is the fusion between the outer acrosomal membrane and the overlying plasma membrane. Fusion occurs at multiple points, resulting in vesiculation and loss of the fused outer acrosomal membrane/plasmalemma so that the acrosomal content is released and the inner acrosomal membrane becomes the new cell surface. Membrane fusion proteins from the SNARE family are present in the acrosomal region and may be integrated into microdomains that facilitate Ca2 +-regulated membrane fusion in a manner that has been compared with events at presynaptic terminals (De Blas et al. 2005, Mayorga et al. 2007, Zitranski et al. 2010). Zona pellucida proteins interact with sperm surface receptors to activate a signalling cascade leading to AR (Florman et al. 2008) and release of acrosomal content at the surface of the zona may, in combination with hyperactivated motility, facilitate zona penetration. However, observation of mouse IVF using sperm with GFP-labelled acrosomes showed that, in addition to cells that undergo AR at the surface of the zona, sperm which arrive having already lost their acrosome (probably within the cumulus) may go on to penetrate the zona and fertilise (Jin et al. 2011). Physiological inducers of AR that have been studied (primarily mouse ZP3 and progesterone) induce Ca2 + influx across the plasma membrane and a sustained rise in [Ca2 +]i. O'Toole et al. (2000) provided pharmacological evidence that ZP3-induced AR in mouse sperm involved activation of store-operated Ca2 + influx downstream of Ca2 + store mobilisation. De Blas et al. (2002) showed that in streptolysin-permeabilised human sperm, mobilisation of the acrosomal Ca2 + store was a requirement for AR even when it was directly induced by introduction of Rab3A into the cytoplasm. Further studies using this permeabilised sperm model have provided information about the mechanisms by which fusion of the plasma and outer acrosomal membranes is regulated, resulting in a detailed model in which mobilisation of the acrosomal store is a central and necessary event (Ruete et al. 2014). Stimulation of PLC, leading to generation of IP3 and activation of IP3Rs in the outer acrosomal membrane may be key to this process (Fukami et al. 2001, 2003), but there is also evidence that the acrosomal membrane contains the NAADP-sensitive, Ca2 +-permeable TPC (Calcraft et al. 2009) and that NAADP mobilises acrosomal Ca2 + in mouse sperm (Arndt et al. 2014).
The RNE and calreticulin-containing vesicles
A second area where Ca2 + storage organelles have been identified in mammalian sperm is at the sperm neck and midpiece (Fig. 1b). Mitochondria have mechanisms for accumulation and release of Ca2 + (Drago et al. 2011, Pizzo et al. 2012) and therefore may contribute to Ca2 + buffering and signalling in this part of the sperm. Inhibition of mitochondrial function in sea urchin sperm, using respiratory inhibitors or uncouplers, causes a rise in [Ca2 +]i and leads to activation of Ca2 + influx that has characteristics consistent with SOCs (Ardon et al. 2009). Treatment with mitochondrial uncouplers (2,4-dinitrophenol, carbonyl cyanide-4-(trifluoromethoxy)-phenyl-hydrazone) also increases [Ca2 +]i in human sperm (J Morris and S Publicover, unpublished observations). Mitochondria may thus contribute to shaping of Ca2 + signals in sperm. However, the primary stimulus-regulated Ca2 + storage in this part of the sperm is in the RNE and/or a second, apparently separate group of calreticulin-containing vesicular structures, both of which are sited at the sperm neck region and cytoplasmic droplet (Ho & Suarez 2001, 2003, Naaby-Hansen et al. 2001). Mobilisation of Ca2 + stored in these compartments regulates flagellar activity and treatment of mouse sperm with thimerosal stimulates hyperactivated motility by activating Ca2 + release from these organelles (Ho & Suarez 2001, Marquez et al. 2007). This effect occurs in the absence of extracellular Ca2 + and can be induced in sperm that are null for CatSper (Marquez et al. 2007). In mouse sperm the direction of the major, high-amplitude flagellar bend of hyperactivated sperm can be clearly characterised by reference to the hooked acrosomal cap (pro-hook or anti-hook). Sperm that became hyperactivated during capacitation in vitro (due to activation of CatSper) show pro-hook bends whereas those activated by store mobilisation (using thimerosal) show anti-hook bends (Chang & Suarez 2011). When sperm were observed interacting with the lining of isolated mouse oviducts, most hyperactivated cells showed anti-hook bending of the type that is elicited by store mobilisation (Chang & Suarez 2012).
In human sperm a similar effect of store mobilisation is observed. Thimerosal greatly increases the proportion of cells showing hyperactivated motility and 4-aminopyridine, which both alkalinises the cytoplasm (thus activating CatSper) and mobilises stored Ca2 +, is similarly potent (Alasmari et al. 2013a , b ). In contrast, manipulations that should activate CatSper (elevation of pHi, stimulation with progesterone or prostaglandin E1) elevate [Ca2 +]i but have only minor stimulatory effects on the proportion of hyperactivated cells. Instead, these manipulations significantly increase penetration into viscous media (Alasmari et al. 2013a , b , Luo et al. 2014).
Model for interaction of CatSper channels and Ca2 +-stores
Patch clamp recordings have provided no evidence that conventional voltage-operated Ca2 + channels contribute to Ca2 + influx in mature mammalian sperm. In mouse sperm null for CatSper1 and the K+ channel Slo3, only a small leak current was recorded even at high intracellular pH and strong depolarisation (Zeng et al. 2013). CatSper channels in mouse and human sperm are pH- and (weakly) voltage-sensitive, but in human sperm the channel is also ligand-sensitive. Established Ca2 +-mobilising agonists of human sperm such as progesterone and prostaglandin E1 have been shown to activate CatSper but also a range of other small molecules including environmental pollutants such as 1,1,1-trichloro-2,2-bis(4-chlorophenyl)ethane, 1,1-bis(4-chlorophenyl)-2,2,2-trichloroethane (4,4′-DDT), p,p′-dichlorodiphenyldichloroethylene and 4-methylbenzylidene camphor are potent agonists (Tavares et al. 2013, Schiffer et al. 2014). In addition, agents used to demonstrate cyclic-nucleotide-activated Ca2 + influx (such as 8-Br-AMP) have been shown directly to activate CatSper by binding at the extracellular surface (Brenker et al. 2012). Thus it is possible that a significant proportion of the pharmacological data that apparently support the existence of multiple Ca2 + influx pathways in sperm are misleading and in fact reflect actions of the drugs on Ca2 + flux through CatSper channels (Brenker et al. 2012). Furthermore, experiments using CatSper null mice provide strong evidence that [Ca2 +]i elevation induced by solubilised ZP is dependent on Ca2 + influx through the CatSper channel in the flagellum, which then propagates to the head (Xia & Ren 2009; though see Cohen et al. (2014)). Interestingly, the ability of solubilised zona to induce AR was not diminished in CatSper-null sperm. These findings not only suggest that CatSper is the primary Ca2 + influx pathway in mammalian sperm, but also that in human sperm it may act as a Ca2 +-signalling ‘hub’ or ‘node’, such that the effects of diverse agonists are summated/integrated in the rate of Ca2 + influx into the flagellum (Brenker et al. 2012). This is an elegant and simple model for which there is already a significant body of data, but in its basic form it does not address the question of how a sperm can generate and use diverse [Ca2 +]i signals to control diverse Ca2 +-sensitive functions.
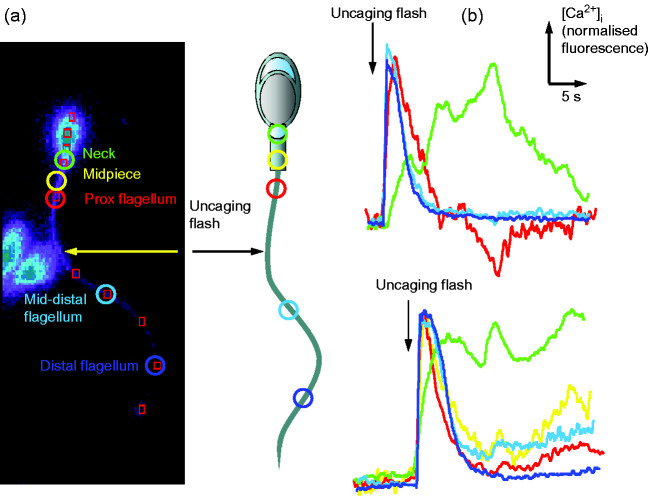
Mouse sperm null for CatSper are unable to hyperactivate (Carlson et al. 2003) and evidence from clinical cases suggests that CatSper is also required for normal levels of motility in human sperm (Avenarius et al. 2009, Smith et al. 2013). Why, then, is manipulation of Ca2 + stores more effective in inducing hyperactivated motility than treatments targeted to CatSper (Alasmari et al. 2013b )? We have proposed that CatSper activation acts as a trigger and consequent elevation of flagellar [Ca2 +]i stimulates secondary release of stored Ca2 + at the sperm neck, either by stimulating synthesis of IP3 or by CICR, leading to hyperactivation (Alasmari et al. 2013b ). Mathematical modelling of the Ca2 + signals induced by CatSper activation in mouse sperm suggests that diffusion of Ca2 + from the flagellum cannot explain the [Ca2 +]i increase that occurs at the sperm head upon activation of CatSper and that such a secondary Ca2 + release at the neck region must occut (Olson et al. 2010, 2011, Li et al. 2014). Recently we have investigated the occurrence of such secondary responses in human sperm by uncaging Ca2 + in the principal piece of the flagellum. Uncaging induces a clear [Ca2 +]i transient in the flagellum that decays within 5–10 s. At the neck region of the sperm the transient is truncated and rises more slowly, consistent with diffusion of Ca2 + from the uncaged pool, but in a small proportion of cells (∼10%) we have observed a late [Ca2 +]i response at the neck region, often including multiple peaks (Fig. 2). The low incidence of this secondary Ca2 +-mobilisation is consistent with our observation that, though direct release of stored Ca2 + can induce hyperactivated motility in the majority of human sperm, only a small proportion of cells hyperactivate upon activation of CatSper (Alasmari et al. 2013a , b ).
Figure 2.
Ca2 + responses evoked in human sperm by uncaging of Ca2 + in the flagellum. Cells were labelled with fluo-4 and loaded with caged Ca2 + (NP-EGTA), then stimulated by an uncaging flash (360 nm laser) at the central flagellum (shown by arrow) while collecting images at 33 Hz. Changes in fluorescence, assessed at each of the positions shown by coloured circles in panel ‘a’, are plotted (normalised to minimum and maximum) in panel ‘b’ using the same colour code. Green, neck; yellow-midpiece; red, proximal flagellum; light blue, mid-distal flagellum; dark blue, distal flagellum.
Ca2 +-store-mediated [Ca2 +]i oscillations occur more readily in sperm incubated for a prolonged period (>24 h) under capacitating conditions (Kirkman-Brown et al. 2004). Capacitation involves generation of ROS and RNS (Herrero et al. 1999, 2001, Aitken & Nixon 2013) and we have observed that store mobilisation is sensitised and induced by low concentrations of NO donors, through a mechanism that involves protein S-nitrosylation (Machado-Oliveira et al. 2008). RyRs were detected in the human sperm nitrosoproteome (Lefievre et al. 2007) and it is well-established that IP3Rs and RyRs are sensitised by oxidative stress (Bootman et al. 1992, Sayers et al. 1993, Stoyanovsky et al. 1997, Meissner 2004, Bansaghi et al. 2014) (see ‘Ca2 + stores and their regulation’). We propose that CICR from the sperm neck Ca2 +-store is regulated during capacitation, perhaps through the effects of oxidative stress on Ca2 + release channels (Alasmari et al. 2013b ) (Fig. 3).
Figure 3.
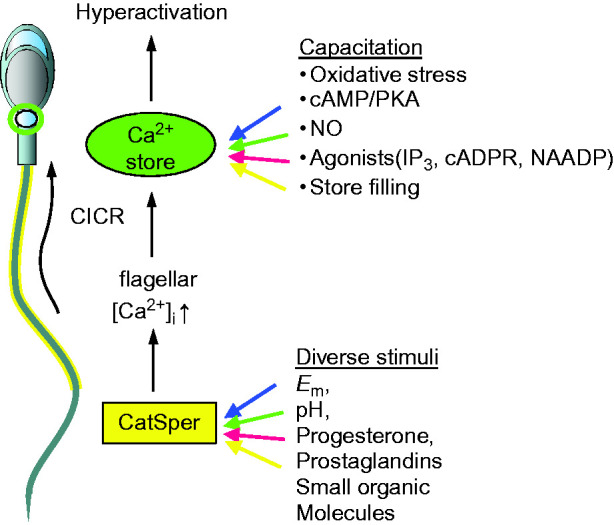
Model for triggering/regulation of CatSper-activated hyperactivation. CatSper channels in the flagellum (yellow box; shown by yellow shading on sperm flagellum) are activated by diverse stimuli including intracellular pH (pHi), membrane potential (E m), progesterone, prostaglandins and other organic molecules. Ca2 + from the flagellum diffuses forward, raising [Ca2 +]i at the sperm neck and can mobilise stored Ca2 + by Ca2 +-induced Ca2 + release (CICR). Susceptibility of the store to CICR is potentially regulated/sensitised by processes occurring during capacitation, including cAMP signalling, oxidative stress and S-nitrosylation as well as Ca2 + store filling and effects of agonists on Ca2 +-store release channels.
Final remarks
The central role of [Ca2 +]i signalling in the physiology of mammalian sperm and the pivotal importance of CatSper in this process are well established – mice null for CatSper are infertile (Ren et al. 2001) and in men CatSper lesions are associated with impaired sperm function (Avidan et al. 2003, Avenarius et al. 2009, Zhang et al. 2009, Smith et al. 2013). The available evidence suggests that Ca2 +-stores also play important roles in both AR and the regulation of motility. Future studies should address the mechanisms by which store mobilisation is achieved (both by CICR and by agonist-induced generation of Ca2 +-mobilising 2nd messengers) and regulated, particularly the significance of capacitation in Ca2 +-store filling and in sensitising Ca2 + release mechanisms. Also, similarly to the important species differences in expression and function of sperm ion channels between human and mouse sperm (Brenker et al. 2014, Miller et al. 2015), there may be differences in store-regulation and/or function between species. An intriguing possibility is that, at least in human sperm, it may prove possible to bypass the effects on motility of lesions in the expression, function or regulation of CatSper channels by pharmacological activation of stored Ca2 + release.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the review.
Funding
J Correia was supported by the Wellcome Trust (grant number 086470).
References
- Adachi T, Weisbrod RM, Pimentel DR, Ying J, Sharov VS, Schoneich C, Cohen RA. S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nature Medicine. 2004;10:1200–1207. doi: 10.1038/nm1119. [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Nixon B. Sperm capacitation: a distant landscape glimpsed but unexplored. Molecular Human Reproduction. 2013;19:785–793. doi: 10.1093/molehr/gat067. [DOI] [PubMed] [Google Scholar]
- Alasmari W, Barratt C, Publicover S, Whalley K, Foster E, Kay V, Silva SMd, Oxenham S. The incidence and clinical significance of defects in calcium signalling pathways mediating human sperm hyperactivation in donors and sub fertile patients. Human Reproduction. 2013a;28:866–876. doi: 10.1093/humrep/des467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alasmari W, Costello S, Correia J, Oxenham SK, Morris J, Fernandes L, Ramalho-Santos J, Kirkman-Brown J, Michelangeli F, Publicover S, et al. Ca2+ signals generated by CatSper and Ca2+ regulate different behaviours in human sperm. Journal of Biological Chemistry. 2013b;288:6248–6258. doi: 10.1074/jbc.M112.439356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aquila S, Guido C, Middea E, Perrotta I, Bruno R, Pellegrino M, Ando S. Human male gamete endocrinology: 1α, 25-dihydroxyvitamin D3 (1,25(OH)2D3) regulates different aspects of human sperm biology and metabolism. Reproductive Biology and Endocrinology. 2009;7:140. doi: 10.1186/1477-7827-7-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardon F, Rodriguez-Miranda E, Beltran C, Hernandez-Cruz A, Darszon A. Mitochondrial inhibitors activate influx of external Ca2+ in sea urchin sperm. Biochimica et Biophysica Acta. 2009;1787:15–24. doi: 10.1016/j.bbabio.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Arndt L, Castonguay J, Arlt E, Meyer D, Hassan S, Borth H, Zierler S, Wennemuth G, Breit A, Biel M, et al. NAADP and the two-pore channel protein 1 participate in the acrosome reaction in mammalian spermatozoa. Molecular Biology of the Cell. 2014;25:948–964. doi: 10.1091/mbc.E13-09-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avenarius MR, Hildebrand MS, Zhang Y, Meyer NC, Smith LL, Kahrizi K, Najmabadi H, Smith RJ. Human male infertility caused by mutations in the CATSPER1 channel protein. American Journal of Human Genetics. 2009;84:505–510. doi: 10.1016/j.ajhg.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avidan N, Tamary H, Dgany O, Cattan D, Pariente A, Thulliez M, Borot N, Moati L, Barthelme A, Shalmon L, et al. CATSPER2, a human autosomal nonsyndromic male infertility gene. European Journal of Human Genetics. 2003;11:497–502. doi: 10.1038/sj.ejhg.5200991. [DOI] [PubMed] [Google Scholar]
- Bansaghi S, Golenar T, Madesh M, Csordas G, RamachandraRao S, Sharma K, Yule DI, Joseph SK, Hajnoczky G. Isoform- and species-specific control of inositol 1,4,5-trisphosphate (IP3) receptors by reactive oxygen species. Journal of Biological Chemistry. 2014;289:8170–8181. doi: 10.1074/jbc.M113.504159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltramo M, Dardente H, Cayla X, Caraty A. Cellular mechanisms and integrative timing of neuroendocrine control of GnRH secretion by kisspeptin. Molecular and Cellular Endocrinology. 2014;382:387–399. doi: 10.1016/j.mce.2013.10.015. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nature Review. Molecular and Cellular Biology. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Billington RA, Ho A, Genazzani AA. Nicotinic acid adenine dinucleotide phosphate (NAADP) is present at micromolar concentrations in sea urchin spermatozoa. Journal of Physiology. 2002;544:107–112. doi: 10.1113/jphysiol.2002.030098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billington RA, Harper C, Bellomo EA, Publicover S, Barratt CL, Genazzani AA. Characterization of cyclic adenine dinucleotide phosphate ribose levels in human spermatozoa. Fertility and Sterility. 2006;86:891–898. doi: 10.1016/j.fertnstert.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Blackmore PF. Thapsigargin elevates and potentiates the ability of progesterone to increase intracellular free calcium in human sperm: possible role of perinuclear calcium. Cell Calcium. 1993;14:53–60. doi: 10.1016/0143-4160(93)90018-2. [DOI] [PubMed] [Google Scholar]
- Blomberg Jensen M. Vitamin D and male reproduction. Nature Reviews. Endocrinology. 2014;10:175–186. doi: 10.1038/nrendo.2013.262. [DOI] [PubMed] [Google Scholar]
- Blomberg Jensen M, Nielsen JE, Jorgensen A, Rajpert-De Meyts E, Kristensen DM, Jorgensen N, Skakkebaek NE, Juul A, Leffers H. Vitamin D receptor and vitamin D metabolizing enzymes are expressed in the human male reproductive tract. Human Reproduction. 2010;25:1303–1311. doi: 10.1093/humrep/deq024. [DOI] [PubMed] [Google Scholar]
- Blomberg Jensen M, Bjerrum PJ, Jessen TE, Nielsen JE, Joensen UN, Olesen IA, Petersen JH, Juul A, Dissing S, Jorgensen N. Vitamin D is positively associated with sperm motility and increases intracellular calcium in human spermatozoa. Human Reproduction. 2011;26:1307–1317. doi: 10.1093/humrep/der059. [DOI] [PubMed] [Google Scholar]
- Blomberg Jensen M, Jorgensen A, Nielsen JE, Bjerrum PJ, Skalkam M, Petersen JH, Egeberg DL, Bangsboll S, Andersen AN, Skakkebaek NE, et al. Expression of the vitamin D metabolizing enzyme CYP24A1 at the annulus of human spermatozoa may serve as a novel marker of semen quality. International Journal of Andrology. 2012;35:499–510. doi: 10.1111/j.1365-2605.2012.01256.x. [DOI] [PubMed] [Google Scholar]
- Bootman MD, Taylor CW, Berridge MJ. The thiol reagent, thimerosal, evokes Ca2+ spikes in HeLa cells by sensitizing the inositol 1,4,5-trisphosphate receptor. Journal of Biological Chemistry. 1992;267:25113–25119. [PubMed] [Google Scholar]
- Brenker C, Goodwin N, Weyand I, Kashikar ND, Naruse M, Krahling M, Muller A, Kaupp UB, Strunker T. The Ca2+-activated K+ current of human sperm is mediated by Slo3. EMBO Journal. 2012;31:1654–1665. doi: 10.1038/emboj.2012.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenker C, Zhou Y, Muller A, Echeverry F, Trotschel C, Poetsch A, Xia X, Bonigk W, Lingle C, Kaupp U, et al. Slo3 in human sperm – a K+ channel activated by Ca2+ . eLife. 2014;3:e01438. doi: 10.7554/eLife.01438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan MD. STIMulating store-operated Ca2+ entry. Nature Cell Biology. 2009;11:669–677. doi: 10.1038/ncb0609-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcraft PJ, Ruas M, Pan Z, Cheng X, Arredouani A, Hao X, Tang J, Rietdorf K, Teboul L, Chuang KT, et al. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature. 2009;459:596–600. doi: 10.1038/nature08030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camina JP, Carreira MC, Micic D, Pombo M, Kelestimur F, Dieguez C, Casanueva FF. Regulation of ghrelin secretion and action. Endocrine. 2003;22:5–12. doi: 10.1385/ENDO:22:1:5. [DOI] [PubMed] [Google Scholar]
- Camors E, Valdivia HH. CaMKII regulation of cardiac ryanodine receptors and inositol triphosphate receptors. Frontiers in Pharmacology. 2014;5:101. doi: 10.3389/fphar.2014.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancela JM, Churchill GC, Galione A. Coordination of agonist-induced Ca2+-signalling patterns by NAADP in pancreatic acinar cells. Nature. 1999;399:74–77. doi: 10.1038/18032. [DOI] [PubMed] [Google Scholar]
- Cang C, Zhou Y, Navarro B, Seo YJ, Aranda K, Shi L, Battaglia-Hsu S, Nissim I, Clapham DE, Ren D. mTOR regulates lysosomal ATP-sensitive two-pore Na+ channels to adapt to metabolic state. Cell. 2013;152:778–790. doi: 10.1016/j.cell.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson AE, Westenbroek RE, Quill T, Ren D, Clapham DE, Hille B, Garbers DL, Babcock DF. CatSper1 required for evoked Ca2+ entry and control of flagellar function in sperm. PNAS. 2003;100:14864–14868. doi: 10.1073/pnas.2536658100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano LE, Trevino CL, Rodriguez D, Serrano CJ, Pacheco J, Tsutsumi V, Felix R, Darszon A. Transient receptor potential (TRPC) channels in human sperm: expression, cellular localization and involvement in the regulation of flagellar motility. FEBS Letters. 2003;541:69–74. doi: 10.1016/S0014-5793(03)00305-3. [DOI] [PubMed] [Google Scholar]
- Chandrashekran A, Isa I, Dudhia J, Thrasher AJ, Dibb N, Casimir C, Readhead C, Winston R. Lentiviral vector transduction of spermatozoa as a tool for the study of early development. FEBS Open Bio. 2014a;4:266–275. doi: 10.1016/j.fob.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekran A, Sarkar R, Thrasher A, Fraser SE, Dibb N, Casimir C, Winston R, Readhead C. Efficient generation of transgenic mice by lentivirus-mediated modification of spermatozoa. FASEB Journal. 2014b;28:569–576. doi: 10.1096/fj.13-233999. [DOI] [PubMed] [Google Scholar]
- Chang H, Suarez SS. Two distinct Ca2+ signaling pathways modulate sperm flagellar beating patterns in mice. Biological Reproduction. 2011;85:296–305. doi: 10.1095/biolreprod.110.089789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H, Suarez SS. Unexpected flagellar movement patterns and epithelial binding behavior of mouse sperm in the oviduct. Biological Reproduction. 2012;86:141–148. doi: 10.1095/biolreprod.111.096578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini EN, Thompson MA, Chini CC, Dousa TP. Cyclic ADP-ribose signaling in sea urchin gametes: metabolism in spermatozoa. American Journal of Physiology. 1997;272:C416–C420. doi: 10.1152/ajpcell.1997.272.2.C416. [DOI] [PubMed] [Google Scholar]
- Churchill GC, Okada Y, Thomas JM, Genazzani AA, Patel S, Galione A. NAADP mobilizes Ca2+ from reserve granules, lysosome-related organelles, in sea urchin eggs. Cell. 2002;111:703–708. doi: 10.1016/S0092-8674(02)01082-6. [DOI] [PubMed] [Google Scholar]
- Cohen R, Buttke DE, Asano A, Mukai C, Nelson JL, Ren D, Miller RJ, Cohen-Kutner M, Atlas D, Travis AJ. Lipid modulation of calcium flux through CaV2.3 regulates acrosome exocytosis and fertilization. Developmental Cell. 2014;28:310–321. doi: 10.1016/j.devcel.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colyer J. Phosphorylation states of phospholamban. Annals of New York Academy of Sciences. 1998;853:79–91. doi: 10.1111/j.1749-6632.1998.tb08258.x. [DOI] [PubMed] [Google Scholar]
- Cosker F, Cheviron N, Yamasaki M, Menteyne A, Lund FE, Moutin MJ, Galione A, Cancela JM. The ecto-enzyme CD38 is a mammalian NAADP synthase which couples receptor activation to Ca2+ mobilization from lysosomes. Journal of Biological Chemistry. 2010;285:38251–38259. doi: 10.1074/jbc.M110.125864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello S, Michelangeli F, Nash K, Lefievre L, Morris J, Machado-Oliveira G, Barratt C, Kirkman-Brown J, Publicover S. Ca2+ stores in sperm: their identities and functions. Reproduction. 2009;138:425–437. doi: 10.1530/REP-09-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csordas G, Hajnoczky G. SR/ER-mitochondrial local communication: calcium and ROS. Biochimica et Biophysica Acta. 2009;1787:1352–1362. doi: 10.1016/j.bbabio.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darszon A, Trevino CL, Wood C, Galindo B, Rodriguez-Miranda E, Acevedo JJ, Hernandez-Gonzalez EO, Beltran C, Martinez-Lopez P, Nishigaki T. Ion channels in sperm motility and capacitation. Society of Reproduction and Fertility Supplement. 2007;65:229–244. [PubMed] [Google Scholar]
- Darszon A, Nishigaki T, Beltran C, Trevino CL. Calcium channels in the development, maturation, and function of spermatozoa. Physiological Reviews. 2011;91:1305–1355. doi: 10.1152/physrev.00028.2010. [DOI] [PubMed] [Google Scholar]
- Darszon A, Sanchez-Cardenas C, Orta G, Sanchez-Tusie AA, Beltran C, Lopez-Gonzalez I, Granados-Gonzalez G, Trevino CL. Are TRP channels involved in sperm development and function? Cell and Tissue Research. 2012;349:749–764. doi: 10.1007/s00441-012-1397-5. [DOI] [PubMed] [Google Scholar]
- De Blas G, Michaut M, Trevino CL, Tomes CN, Yunes R, Darszon A, Mayorga LS. The intraacrosomal calcium pool plays a direct role in acrosomal exocytosis. Journal of Biological Chemistry. 2002;277:49326–49331. doi: 10.1074/jbc.M208587200. [DOI] [PubMed] [Google Scholar]
- De Blas GA, Roggero CM, Tomes CN, Mayorga LS. Dynamics of SNARE assembly and disassembly during sperm acrosomal exocytosis. PLoS Biology. 2005;3:e323. doi: 10.1371/journal.pbio.0030323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragileva E, Rubinstein S, Breitbart H. Intracellular Ca2+–Mg2+-ATPase regulates calcium influx and acrosomal exocytosis in bull and ram spermatozoa. Biological Reproduction. 1999;61:1226–1234. doi: 10.1095/biolreprod61.5.1226. [DOI] [PubMed] [Google Scholar]
- Drago I, Pizzo P, Pozzan T. After half a century mitochondrial calcium in- and efflux machineries reveal themselves. EMBO Journal. 2011;30:4119–4125. doi: 10.1038/emboj.2011.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer JL, Mobasheri H, Lea EJ, Dawson AP, Michelangeli F. Differential effect of PKA on the Ca2+ release kinetics of the type I and III InsP3 receptors. Biochemistry and Biophysics Research Communications. 2003;302:121–126. doi: 10.1016/S0006-291X(03)00120-7. [DOI] [PubMed] [Google Scholar]
- Espino J, Mediero M, Lozano GM, Bejarano I, Ortiz A, Garcia JF, Pariente JA, Rodriguez AB. Reduced levels of intracellular calcium releasing in spermatozoa from asthenozoospermic patients. Reproductive Biology and Endocrinology. 2009;7:11. doi: 10.1186/1477-7827-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng M, Grice DM, Faddy HM, Nguyen N, Leitch S, Wang Y, Muend S, Kenny PA, Sukumar S, Roberts-Thomson SJ, et al. Store-independent activation of Orai1 by SPCA2 in mammary tumors. Cell. 2010;143:84–98. doi: 10.1016/j.cell.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florman HM, Jungnickel MK, Sutton KA. Regulating the acrosome reaction. International Journal of Developmental Biology. 2008;52:503–510. doi: 10.1387/ijdb.082696hf. [DOI] [PubMed] [Google Scholar]
- Fukami K, Nakao K, Inoue T, Kataoka Y, Kurokawa M, Fissore RA, Nakamura K, Katsuki M, Mikoshiba K, Yoshida N, et al. Requirement of phospholipase Cdelta4 for the zona pellucida-induced acrosome reaction. Science. 2001;292:920–923. doi: 10.1126/science.1059042. [DOI] [PubMed] [Google Scholar]
- Fukami K, Yoshida M, Inoue T, Kurokawa M, Fissore RA, Yoshida N, Mikoshiba K, Takenawa T. Phospholipase Cdelta4 is required for Ca2+ mobilization essential for acrosome reaction in sperm. Journal of Cell Biology. 2003;161:79–88. doi: 10.1083/jcb.200210057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genazzani AA, Mezna M, Summerhill RJ, Galione A, Michelangeli F. Kinetic properties of nicotinic acid adenine dinucleotide phosphate-induced Ca2+ release. Journal of Biological Chemistry. 1997;272:7669–7675. doi: 10.1074/jbc.272.12.7669. [DOI] [PubMed] [Google Scholar]
- Gur Y, Breitbart H. Protein synthesis in sperm: dialog between mitochondria and cytoplasm. Molecular and Cellular Endocrinology. 2008;282:45–55. doi: 10.1074/jbc.272.12.7669. [DOI] [PubMed] [Google Scholar]
- Guse AH 2015 Calcium mobilizing second messengers derived from NAD. Biochimica et Biophysica Acta. In press. ( 10.1016/j.bbapap.2014.12.015) [DOI] [PubMed]
- Hamilton SL, Reid MB. RyR1 modulation by oxidation and calmodulin. Antioxidants & Redox Signaling. 2000;2:41–45. doi: 10.1089/ars.2000.2.1-41. [DOI] [PubMed] [Google Scholar]
- Harper CV, Barratt CL, Publicover SJ. Stimulation of human spermatozoa with progesterone gradients to simulate approach to the oocyte. Induction of [Ca2+]i oscillations and cyclical transitions in flagellar beating. Journal of Biological Chemistry. 2004;279:46315–46325. doi: 10.1074/jbc.M401194200. [DOI] [PubMed] [Google Scholar]
- Harper C, Wootton L, Michelangeli F, Lefievre L, Barratt C, Publicover S. Secretory pathway Ca2+-ATPase (SPCA1) Ca2+ pumps, not SERCAs, regulate complex [Ca2+]i signals in human spermatozoa. Journal of Cell Science. 2005;118:1673–1685. doi: 10.1242/jcs.02297. [DOI] [PubMed] [Google Scholar]
- Herrero MB, de Lamirande E, Gagnon C. Nitric oxide regulates human sperm capacitation and protein–tyrosine phosphorylation in vitro . Biological Reproduction. 1999;61:575–581. doi: 10.1095/biolreprod61.3.575. [DOI] [PubMed] [Google Scholar]
- Herrero MB, de Lamirande E, Gagnon C. Tyrosine nitration in human spermatozoa: a physiological function of peroxynitrite, the reaction product of nitric oxide and superoxide. Molecular Human Reproduction. 2001;7:913–921. doi: 10.1093/molehr/7.10.913. [DOI] [PubMed] [Google Scholar]
- Ho HC, Suarez SS. An inositol 1,4,5-trisphosphate receptor-gated intracellular Ca2+ store is involved in regulating sperm hyperactivated motility. Biological Reproduction. 2001;65:1606–1615. doi: 10.1095/biolreprod65.5.1606. [DOI] [PubMed] [Google Scholar]
- Ho HC, Suarez SS. Characterization of the intracellular calcium store at the base of the sperm flagellum that regulates hyperactivated motility. Biological Reproduction. 2003;68:1590–1596. doi: 10.1095/biolreprod.102.011320. [DOI] [PubMed] [Google Scholar]
- Hsu MC, Wang JY, Lee YJ, Jong DS, Tsui KH, Chiu CH. Kisspeptin modulates fertilization capacity of mouse spermatozoa. Reproduction. 2014;147:835–845. doi: 10.1530/REP-13-0368. [DOI] [PubMed] [Google Scholar]
- Jin M, Fujiwara E, Kakiuchi Y, Okabe M, Satouh Y, Baba SA, Chiba K, Hirohashi N. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. PNAS. 2011;108:4892–4896. doi: 10.1073/pnas.1018202108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkman-Brown JC, Barratt CL, Publicover SJ. Slow calcium oscillations in human spermatozoa. Biochemistry Journal. 2004;378:827–832. doi: 10.1042/BJ20031368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda Y, Kaneko S, Yoshimura Y, Nozawa S, Mikoshiba K. Are there inositol 1,4,5-triphosphate (IP3) receptors in human sperm? Life Sciences. 1999;65:135–143. doi: 10.1016/S0024-3205(99)00230-1. [DOI] [PubMed] [Google Scholar]
- Lawson C, Dorval V, Goupil S, Leclerc P. Identification and localisation of SERCA 2 isoforms in mammalian sperm. Molecular Human Reproduction. 2007;13:307–316. doi: 10.1093/molehr/gam012. [DOI] [PubMed] [Google Scholar]
- Lefievre L, Chen Y, Conner SJ, Scott JL, Publicover SJ, Ford WC, Barratt CL. Human spermatozoa contain multiple targets for protein S-nitrosylation: an alternative mechanism of the modulation of sperm function by nitric oxide? Proteomics. 2007;7:3066–3084. doi: 10.1002/pmic.200700254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefievre L, Nash K, Mansell S, Costello S, Punt E, Correia J, Morris J, Kirkman-Brown J, Wilson SM, Barratt CL, et al. 2-APB-potentiated channels amplify CatSper-induced Ca2+ signals in human sperm. Biochemistry Journal. 2012;448:189–200. doi: 10.1042/BJ20120339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LF, Xiang C, Zhu YB, Qin KR. Modeling of progesterone-induced intracellular calcium signaling in human spermatozoa. Journal of Theoretical Biology. 2014;351:58–66. doi: 10.1016/j.jtbi.2014.02.026. [DOI] [PubMed] [Google Scholar]
- Lin-Moshier Y, Walseth TF, Churamani D, Davidson SM, Slama JT, Hooper R, Brailoiu E, Patel S, Marchant JS. Photoaffinity labeling of nicotinic acid adenine dinucleotide phosphate (NAADP) targets in mammalian cells. Journal of Biological Chemistry. 2012;287:2296–2307. doi: 10.1074/jbc.M111.305813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lishko PV, Botchkina IL, Kirichok Y. Progesterone activates the principal Ca2+ channel of human sperm. Nature. 2011;471:387–391. doi: 10.1038/nature09767. [DOI] [PubMed] [Google Scholar]
- Liu X, Lee K, Herbison AE. Kisspeptin excites gonadotropin-releasing hormone neurons through a phospholipase C/calcium-dependent pathway regulating multiple ion channels. Endocrinology. 2008;149:4605–4614. doi: 10.1210/en.2008-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. Journal of Cell Biology. 2006;174:815–825. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukaszyk A, Kotwicka M, Jankowska A, Kasprzak A, Rucinski M, Sterzynska K, Ziolkowska A, Sawinski P, Ruchala M. Expression of ghrelin receptor (GHSR-1a) in rat epididymal spermatozoa and the effects of its activation. Reproductive Biology. 2012;12:293–300. doi: 10.1016/j.repbio.2012.09.002. [DOI] [PubMed] [Google Scholar]
- Luo T, Li N, He YQ, Weng SQ, Wang T, Zou QX, Zeng XH. Emodin inhibits human sperm functions by reducing sperm [Ca] and tyrosine phosphorylation. Reproductive Toxicology. 2014;51C:14–21. doi: 10.1016/j.reprotox.2014.11.007. Epub 2014 Nov 22. [DOI] [PubMed] [Google Scholar]
- Machado-Oliveira G, Lefievre L, Ford C, Herrero MB, Barratt C, Connolly TJ, Nash K, Morales-Garcia A, Kirkman-Brown J, Publicover S. Mobilisation of Ca2+ stores and flagellar regulation in human sperm by S-nitrosylation: a role for NO synthesised in the female reproductive tract. Development. 2008;135:3677–3686. doi: 10.1242/dev.024521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez B, Ignotz G, Suarez SS. Contributions of extracellular and intracellular Ca2+ to regulation of sperm motility: release of intracellular stores can hyperactivate CatSper1 and CatSper2 null sperm. Developmental Biology. 2007;303:214–221. doi: 10.1016/j.ydbio.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayorga LS, Tomes CN, Belmonte SA. Acrosomal exocytosis, a special type of regulated secretion. IUBMB Life. 2007;59:286–292. doi: 10.1080/15216540701222872. [DOI] [PubMed] [Google Scholar]
- Meissner G. Molecular regulation of cardiac ryanodine receptor ion channel. Cell Calcium. 2004;35:621–628. doi: 10.1016/j.ceca.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Menteyne A, Burdakov A, Charpentier G, Petersen OH, Cancela JM. Generation of specific Ca2+ signals from Ca2+ stores and endocytosis by differential coupling to messengers. Current Biology. 2006;16:1931–1937. doi: 10.1016/j.cub.2006.07.070. [DOI] [PubMed] [Google Scholar]
- Michelangeli F, East JM. A diversity of SERCA Ca2+ pump inhibitors. Biochemical Society Transactions. 2011;39:789–797. doi: 10.1042/BST0390789. [DOI] [PubMed] [Google Scholar]
- Michelangeli F, Ogunbayo OA, Wootton LL. A plethora of interacting organellar Ca2+ stores. Current Opinion in Cell Biology. 2005;17:135–140. doi: 10.1016/j.ceb.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Miller D, Ostermeier GC. Spermatozoal RNA: why is it there and what does it do? Gynecologic and Obstetric Investigation. 2006;34:840–846. doi: 10.1016/j.gyobfe.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Miller D, Ostermeier GC, Krawetz SA. The controversy, potential and roles of spermatozoal RNA. Trends in Molecular Medicine. 2005;11:156–163. doi: 10.1016/j.molmed.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Miller MR, Mansell SA, Meyers SA & Lishko PV 2015 Flagellar ion channels of sperm: similarities and differences between species. Cell Calcium. In press. ( 10.1016/j.ceca.2014.10.009) [DOI] [PubMed]
- Missiaen L, Taylor CW, Berridge MJ. Spontaneous calcium release from inositol trisphosphate-sensitive calcium stores. Nature. 1991;352:241–244. doi: 10.1038/352241a0. [DOI] [PubMed] [Google Scholar]
- Moretti E, Vindigni C, Tripodi SA, Mazzi L, Nuti R, Figura N, Collodel G. Immunolocalisation of ghrelin and obestatin in human testis, seminal vesicles, prostate and spermatozoa. Andrologia. 2014;46:979–985. doi: 10.1111/and.12183. [DOI] [PubMed] [Google Scholar]
- Morgan AJ, Galione A. Two-pore channels (TPCs): current controversies. BioEssays. 2014;36:173–183. doi: 10.1002/bies.201300118. [DOI] [PubMed] [Google Scholar]
- Morris J, Jones S, Howl J, Lukanowska M, Lefievre L & Publicover S 2015 Cell penetrating peptides, targeting the regulation of store-operated channels, slow decay of the progesterone-induced [Ca2+]i signal in human sperm. Molecular Human Reproduction. In press. ( 10.1093/molehr/gav019) [DOI] [PMC free article] [PubMed]
- Naaby-Hansen S, Wolkowicz MJ, Klotz K, Bush LA, Westbrook VA, Shibahara H, Shetty J, Coonrod SA, Reddi PP, Shannon J, et al. Co-localization of the inositol 1,4,5-trisphosphate receptor and calreticulin in the equatorial segment and in membrane bounded vesicles in the cytoplasmic droplet of human spermatozoa. Molecular Human Reproduction. 2001;7:923–933. doi: 10.1093/molehr/7.10.923. [DOI] [PubMed] [Google Scholar]
- Navarro-Borelly L, Somasundaram A, Yamashita M, Ren D, Miller RJ, Prakriya M. STIM1–Orai1 interactions and Orai1 conformational changes revealed by live-cell FRET microscopy. Journal of Physiology. 2008;586:5383–5401. doi: 10.1113/jphysiol.2008.162503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogunbayo OA, Zhu Y, Rossi D, Sorrentino V, Ma J, Zhu MX, Evans AM. Cyclic adenosine diphosphate ribose activates ryanodine receptors, whereas NAADP activates two-pore domain channels. Journal of Biological Chemistry. 2011;286:9136–9140. doi: 10.1074/jbc.M110.202002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson SD, Suarez SS, Fauci LJ. A model of CatSper channel mediated calcium dynamics in mammalian spermatozoa. Bulletin of Mathematical Biology. 2010;72:1925–1946. doi: 10.1007/s11538-010-9516-5. [DOI] [PubMed] [Google Scholar]
- Olson SD, Fauci LJ, Suarez SS. Mathematical modeling of calcium signaling during sperm hyperactivation. Molecular Human Reproduction. 2011;17:500–510. doi: 10.1093/molehr/gar040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole CM, Arnoult C, Darszon A, Steinhardt RA, Florman HM. Ca2+ entry through store-operated channels in mouse sperm is initiated by egg ZP3 and drives the acrosome reaction. Molecular Biology of the Cell. 2000;11:1571–1584. doi: 10.1091/mbc.11.5.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KH, Kim BJ, Kang J, Nam TS, Lim JM, Kim HT, Park JK, Kim YG, Chae SW, Kim UH. Ca2+ signaling tools acquired from prostasomes are required for progesterone-induced sperm motility. Science Signaling. 2011;4:ra31. doi: 10.1126/scisignal.2001595. [DOI] [PubMed] [Google Scholar]
- Peiter E, Maathuis FJ, Mills LN, Knight H, Pelloux J, Hetherington AM, Sanders D. The vacuolar Ca2+-activated channel TPC1 regulates germination and stomatal movement. Nature. 2005;434:404–408. doi: 10.1038/nature03381. [DOI] [PubMed] [Google Scholar]
- Pielecka-Fortuna J, Chu Z, Moenter SM. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology. 2008;149:1979–1986. doi: 10.1210/en.2007-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto FM, Cejudo-Roman A, Ravina CG, Fernandez-Sanchez M, Martin-Lozano D, Illanes M, Tena-Sempere M, Candenas ML. Characterization of the kisspeptin system in human spermatozoa. International Journal of Andrology. 2012;35:63–73. doi: 10.1111/j.1365-2605.2011.01177.x. [DOI] [PubMed] [Google Scholar]
- Pizzo P, Drago I, Filadi R, Pozzan T. Mitochondrial Ca2+ homeostasis: mechanism, role, and tissue specificities. Pflügers Archiv. 2012;464:3–17. doi: 10.1007/s00424-012-1122-y. [DOI] [PubMed] [Google Scholar]
- Publicover S, Harper CV, Barratt C. [Ca2+]i signalling in sperm-making the most of what you've got. Nature Cell Biology. 2007;9:235–242. doi: 10.1038/ncb0307-235. [DOI] [PubMed] [Google Scholar]
- Ren D, Xia J. Calcium signaling through CatSper channels in mammalian fertilization. Physiology. 2010;25:165–175. doi: 10.1152/physiol.00049.2009. [DOI] [PubMed] [Google Scholar]
- Ren D, Navarro B, Perez G, Jackson AC, Hsu S, Shi Q, Tilly JL, Clapham DE. A sperm ion channel required for sperm motility and male fertility. Nature. 2001;413:603–609. doi: 10.1038/35098027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruas M, Davis LC, Chen CC, Morgan AJ, Chuang KT, Walseth TF, Grimm C, Garnham C, Powell T, Platt N, et al. Expression of Ca2+-permeable two-pore channels rescues NAADP signalling in TPC-deficient cells. EMBO Journal. 2015 doi: 10.15252/embj.201490009. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruete MC, Lucchesi O, Bustos MA, Tomes CN. Epac, Rap and Rab3 act in concert to mobilize calcium from sperm's acrosome during exocytosis. Cell Communication and Signaling. 2014;12:43. doi: 10.1186/s12964-014-0043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Tusie AA, Vasudevan SR, Churchill GC, Nishigaki T, Trevino CL. Characterization of NAADP-mediated calcium signaling in human spermatozoa. Biochemistry and Biophysics Research Communications. 2014;443:531–536. doi: 10.1016/j.bbrc.2013.12.011. [DOI] [PubMed] [Google Scholar]
- Sayers LG, Brown GR, Michell RH, Michelangeli F. The effects of thimerosal on calcium uptake and inositol 1,4,5-trisphosphate-induced calcium release in cerebellar microsomes. Biochemistry Journal. 1993;289(Pt 3):883–887. doi: 10.1042/bj2890883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer C, Muller A, Egeberg DL, Alvarez L, Brenker C, Rehfeld A, Frederiksen H, Waschle B, Kaupp UB, Balbach M, et al. Direct action of endocrine disrupting chemicals on human sperm. EMBO Reports. 2014;15:758–765. doi: 10.15252/embr.201438869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo MD, Velamakanni S, Ishiyama N, Stathopulos PB, Rossi AM, Khan SA, Dale P, Li C, Ames JB, Ikura M, et al. Structural and functional conservation of key domains in InsP3 and ryanodine receptors. Nature. 2012;483:108–112. doi: 10.1038/nature10751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharov VS, Dremina ES, Galeva NA, Williams TD, Schoneich C. Quantitative mapping of oxidation-sensitive cysteine residues in SERCA in vivo and in vitro by HPLC-electrospray-tandem MS: selective protein oxidation during biological aging. Biochemistry Journal. 2006;394:605–615. doi: 10.1042/BJ20051214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JF, Syritsyna O, Fellous M, Serres C, Mannowetz N, Kirichok Y, Lishko PV. Disruption of the principal, progesterone-activated sperm Ca2+ channel in a CatSper2-deficient infertile patient. PNAS. 2013;110:6323–6328. doi: 10.1073/pnas.1216588110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoyanovsky D, Murphy T, Anno PR, Kim YM, Salama G. Nitric oxide activates skeletal and cardiac ryanodine receptors. Cell Calcium. 1997;21:19–29. doi: 10.1016/S0143-4160(97)90093-2. [DOI] [PubMed] [Google Scholar]
- Strunker T, Goodwin N, Brenker C, Kashikar ND, Weyand I, Seifert R, Kaupp UB. The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature. 2011;471:382–386. doi: 10.1038/nature09769. [DOI] [PubMed] [Google Scholar]
- Tavares RS, Mansell S, Barratt CL, Wilson SM, Publicover SJ, Ramalho-Santos J. p,p′-DDE activates CatSper and compromises human sperm function at environmentally relevant concentrations. Human Reproduction. 2013;28:3167–3177. doi: 10.1093/humrep/det372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Meizel S. Phosphatidylinositol 4,5-bisphosphate hydrolysis in human sperm stimulated with follicular fluid or progesterone is dependent upon Ca2+ influx. Biochemistry Journal. 1989;264:539–546. doi: 10.1042/bj2640539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevino CL, Santi CM, Beltran C, Hernandez-Cruz A, Darszon A, Lomeli H. Localisation of inositol trisphosphate and ryanodine receptors during mouse spermatogenesis: possible functional implications. Zygote. 1998;6:159–172. doi: 10.1017/S0967199498000094. [DOI] [PubMed] [Google Scholar]
- Vanoevelen J, Dode L, Van Baelen K, Fairclough RJ, Missiaen L, Raeymaekers L, Wuytack F. The secretory pathway Ca2+/Mn2+-ATPase 2 is a Golgi-localized pump with high affinity for Ca2+ ions. Journal of Biological Chemistry. 2005;280:22800–22808. doi: 10.1074/jbc.M501026200. [DOI] [PubMed] [Google Scholar]
- Vasudevan SR, Galione A, Churchill GC. Sperm express a Ca2+-regulated NAADP synthase. Biochemistry Journal. 2008;411:63–70. doi: 10.1042/BJ20071616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Deng X, Zhou Y, Hendron E, Mancarella S, Ritchie MF, Tang XD, Baba Y, Kurosaki T, Mori Y, et al. STIM protein coupling in the activation of Orai channels. PNAS. 2009;106:7391–7396. doi: 10.1073/pnas.0900293106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhang X, Dong XP, Samie M, Li X, Cheng X, Goschka A, Shen D, Zhou Y, Harlow J, et al. TPC proteins are phosphoinositide- activated sodium-selective ion channels in endosomes and lysosomes. Cell. 2012;151:372–383. doi: 10.1016/j.cell.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wootton LL, Argent CC, Wheatley M, Michelangeli F. The expression, activity and localisation of the secretory pathway Ca2+-ATPase (SPCA1) in different mammalian tissues. Biochimica et Biophysica Acta. 2004;1664:189–197. doi: 10.1016/j.bbamem.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. Journal of Cell Biology. 2006;174:803–813. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Ren D. Egg coat proteins activate calcium entry into mouse sperm via CATSPER channels. Biological Reproduction. 2009;80:1092–1098. doi: 10.1095/biolreprod.108.074039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M, Somasundaram A, Prakriya M. Competitive modulation of Ca2+ release-activated Ca2+ channel gating by STIM1 and 2-aminoethyldiphenyl borate. Journal of Biological Chemistry. 2011;286:9429–9442. doi: 10.1074/jbc.M110.189035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yule DI, Betzenhauser MJ, Joseph SK. Linking structure to function: recent lessons from inositol 1,4,5-trisphosphate receptor mutagenesis. Cell Calcium. 2010;47:469–479. doi: 10.1016/j.ceca.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng XH, Navarro B, Xia XM, Clapham DE, Lingle CJ. Simultaneous knockout of Slo3 and CatSper1 abolishes all alkalization- and voltage-activated current in mouse spermatozoa. Journal of General Physiology. 2013;142:305–313. doi: 10.1085/jgp.201311011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Malekpour M, Al-Madani N, Kahrizi K, Zanganeh M, Mohseni M, Mojahedi F, Daneshi A, Najmabadi H & Smith RJ 2009 Sensorineural deafness and male infertility: a contiguous gene deletion syndrome. BMJ Case Reports. pii: bcr08.2008.0645. ( 10.1136/bcr.08.2008.0645) [DOI] [PMC free article] [PubMed]
- Zhao C, Guo XJ, Shi ZH, Wang FQ, Huang XY, Huo R, Zhu H, Wang XR, Liu JY, Zhou ZM, et al. Role of translation by mitochondrial-type ribosomes during sperm capacitation: an analysis based on a proteomic approach. Proteomics. 2009;9:1385–1399. doi: 10.1002/pmic.200800353. [DOI] [PubMed] [Google Scholar]
- Zitranski N, Borth H, Ackermann F, Meyer D, Vieweg L, Breit A, Gudermann T, Boekhoff I. The “acrosomal synapse” subcellular organization by lipid rafts and scaffolding proteins exhibits high similarities in neurons and mammalian spermatozoa. Communicative & Integrative Biology. 2010;3:513–521. doi: 10.4161/cib.3.6.13137. [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a