Abstract
Background and Purpose
Patients with chronic obstructive pulmonary disease (COPD) are at higher risk of stroke than those without COPD. This study aims to explore the impact of inhaled pharmacotherapy on stroke risk in COPD patients during a three-year follow-up, using a nationwide, population-based study and a matched cohort design.
Methods
The study cohort comprised 10,413 patients who had received COPD treatment between 2004 and 2006; 41,652 randomly selected subjects comprised the comparison cohort. Cox proportional hazard regressions and two-stage propensity score calibration were performed to determine the impact of various inhaled therapies including short-acting muscarinic antagonists, long-acting muscarinic antagonists, short-acting β-agonists (SABAs), long-acting β-agonists (LABAs), and LABA plus inhaled corticosteroid (ICS), on the risk after adjustment for patient demographic characteristics and comorbid disorders.
Results
Of the 52,065 sampled patients, 2,689 (5.2%) developed stroke during follow-up, including 727 (7.0%) from the COPD cohort and 1,962 (4.7%) from the comparison cohort (p < 0.001). Treatment with SABA was associated with 1.67-fold (95% CI 1.45–1.91; p < 0.001) increased risk of stroke in COPD patients. By contrast, the cumulative incidence of stroke was significantly lower in those treated with LABA plus ICS than those treated without (adjusted hazard ratio 0.75, 95% CI 0.60–0.94, p = 0.014).
Conclusions
Among COPD patients, the use of inhaled SABA is associated with an increased risk of stroke, and combination treatment with inhaled LABA and ICS relates to a risk reduction. Further prospective research is needed to verify whether LABA plus ICS confers protection against stroke in patients with COPD.
Introduction
Chronic obstructive pulmonary disease (COPD), characterized by enhanced airway inflammatory response to noxious gas and persistent airflow limitation [1], is a leading cause of morbidity and mortality globally and leads to a growing and substantial socioeconomic burden [2]. Systemic inflammation is often present in COPD patients and links between COPD and cardiovascular diseases that greatly influence the prognosis, including hypertension, ischemic heart disease and stroke [3, 4]. Moreover, patients diagnosed with COPD are at higher risk of subsequent stroke than those without [5], and the incidence rate of stroke increased significantly following acute exacerbations of COPD [6].
Current COPD guideline advocates that inhaled bronchodilators, including short-acting muscarinic antagonists (SAMAs), short-acting β-agonists (SABAs), long-acting muscarinic antagonists (LAMAs) or long-acting β-agonists (LABAs) are used first for treatment of COPD, and inhaled corticosteroids (ICSs) are added for patients with frequent exacerbations [1]. These agents, either alone or in combination, were reported to have modifying effects on COPD inflammation [7]. Recently, two multi-center, randomized, double-blind, placebo-controlled trials reported that administration of LAMA or LABA plus ICS could provide symptomatic relief, improve lung function and reduce acute exacerbations in patients with COPD [8, 9]. However, to date, the impact of these pharmacological agents on the risk of comorbidities associated with COPD remains unknown.
We thus used a nationwide, population-based dataset to evaluate the effect of various inhaled pharmacotherapy on the risk of stroke among COPD patients during a 3-year follow-up.
Materials and Methods
Main Study
A dataset “Longitudinal Health Insurance Database 2005 (LHID2005)” released by the Taiwan National Health Research Institute (NHRI) was used in this study. In order to provide affordable health care for all residents in Taiwan, Taiwan government initiated its National Health Insurance (NHI) program on March 1, 1995. Currently, the program covers over 25 million enrollees, representing around 98% of the population [10]. In cooperation with the Bureau of NHI, NHRI of Taiwan provides all the medical claim data of 1,000,000 beneficiaries, randomly sampled from all 25 million NHI enrollees [10]. As the data set used in this analysis comprised of de-identified secondary data released to the public for investigation purposes, this study was exempt from full audit by the TMU-Joint Institutional Review Board of Taipei Medical University.
Validation Study
The National Health Interview Survey sampled from the entire Taiwan population was used as an external validation study. The National Health Interview Survey 2005 (NHIS2005) assessed health behaviors and quality of life, and the details of NHIS2005 can be referred to Guo et al [11].
Study Design and Samples
Study cohort and comparison cohort were both selected from the LHID and subjects aged less than or equal to 50 years were excluded. The study cohort comprised all patients who had visited ambulatory care centers for the treatment of COPD between January 1, 2004 and December 31, 2004. Patients were included in the cohort if they fulfilled all the following criteria: (1) at least 3 consensus COPD diagnoses (ICD-9-CM codes 491, 492, or 496) in the year before treatment initiation (i.e., the date of the first claim for a COPD pharmacotherapy on or after January 1, 2004); (2) no prior diagnosis of stroke in the year before treatment initiation; (3) more than three claims for a COPD pharmacotherapy between January 1, 2004, and December 31, 2004, recorded in the LHID databases; (4) no claim for a COPD pharmacotherapy ≥1 year before treatment initiation to assess the effect of a new use of therapy; (5) no change between COPD pharmacotherapies in the year after treatment initiation. The NIH program covered all the cost of prescribed COPD pharmacotherapy. As a result, the study cohort comprised 10,413 subjects.
The comparison cohort was sampled from the remaining subjects in the LHID2005 with the exclusion of subjects who had been diagnosed as having stroke before 2004 and having COPD diagnosis (ICD-9-CM codes 491, 492, or 496) during 2004 to 2006. There were 41,652 randomly selected subjects (four for each subject in the study cohort) matched with those in the study cohort in terms of gender and age (51–60, 61–70 and > 70 year-old).
Inhaled Pharmacotherapy
Inhaled COPD medications that had been prescribed to COPD patients were recorded in the LHID databases in five categories: (1) SAMA: Ipratropium bromide metered-dose inhaler (MDI) (Atrovent, Boehringer Ingelheim, Germany); (2) SABA: Fenoterol hydrobromide MDI (Berotec, Boehringer Ingelheim, Germany), Terbutaline sulfate Turbuhaler (Bricanyl, GlaxoSmithKline, UK) or Salbutamol sulfate MDI (Ventolin, GlaxoSmithKline, UK); (3) LAMA: Tiotropium HandiHaler (Spiriva, Boehringer Ingelheim, Germany); (4) LABA: Formoterol Turbuhaler (Oxis, AstraZeneca, Sweden) or Salmeterol MDI (Serevent, GlaxoSmithKline, UK); (5) LABA/ICS: Formoterol/Budesonide Turbuhaler (Symbicort, AstraZeneca, Sweden), or Salmeterol/Fluticasone MDI (Seretide, GlaxoSmithKline, UK). The accumulated prescription for each claim of these inhaled pharmacotherapies must last > 14 days and the use of these inhalers were not mutually exclusive. For patients treated with regular long-acting inhalers (LAMA, LABA or LABA/ICS), a brief use of SABA or SAMA as an on demand rescue for acute exacerbation was allowed. Accordingly, the treatments for COPD patients were categorized as using (1) SAMA, (2) SABA, (3) LAMA, (4) LABA, and (5) LABA/ICS. To assess the precise impact of a particular class of inhaled pharmacotherapy, the switch from the initial inhaler to other category of inhalers during the follow-up was precluded from these categorizations. Moreover, in order to assess the independent contribution of each agent to the stroke risk, patients who used combination inhaler SABA/SAMA: salbutamol/ipratropium bromide MDI (Combivent, Boehringer Ingelheim, Germany) were not included in the analysis.
Survival Analysis
To avoid the immortal time bias [12], the cohort entry for every COPD patient was taken as the date of the first claim for any of the five inhaled COPD pharmacotherapy, as described in the inclusion criteria. Each subject was then individually followed up to 3 years or until they developed stroke (ICD-9-CM codes 430~432 (hemorrhagic) and 433~435 (ischemic)). The date of final follow-up is December 31, 2006. The median durations of follow-up were 963 days (IQR 709–1077 days) for total subjects, 520 days (IQR 299–725 days) for subjects developing stroke, and 990 days (IQR 713–1077 days) for stroke-free subjects. There are 2,689 first strokes recorded during the follow-up time.
Statistical Analysis
All statistical analyses were performed using the matched cohort study design and applying the SAS statistical package (SAS System for Windows, Version 9.3) and SPSS. Pearson Chi-square test was used to compare demographic differences between the COPD and comparison cohorts in main and validation studies. Cox model was performed to determine whether subjects with COPD have a higher risk of stroke and whether various inhaled COPD pharmacotherapy affect the risk after adjusting for selected comorbid medical disorders of the sampled patients. The propensity score calibration method was proposed to correct the bias of estimator after adjusted unmeasured confounders. For detail, please refer to next paragraph. The three-year stroke-free survival rate was evaluated by using the Kaplan-Meier method, and the log-rank test was also applied to examine the differences in the risk of stroke between with SABA use and without SABA use, and with LABA plus ICS use and without LABA plus ICS use in COPD cohort. A p-value < 0.05 was used to determine the significance of predictors in the models.
Two-stage Propensity Score Calibration
In order to deal with the unmeasured confounding problem, we applied a two-stage approach. The method of combining samples from primary and validation data for adjusting biases caused by unmeasured confounders has been studied in the literature of two-stage designs [13–15]. We used inclusion criteria consistent to those for the primary study (LHID cohort) to identify 471 patients with COPD and 1896 age- and gender-matched subjects in the comparison cohort between 2004 and 2006 from NHIS2005, including smoking, drinking and BMI variables, which were not observed in our primary study. We then proposed a two-stage method which has been studied by Stürmer et al., to combine with the propensity score to adjust the unmeasured high-dimensional confounders (such as smoking, drinking and BMI) by using the information of NHIS2005 [14].
Let T denote an indicator interesting variable for COPD or medication, take value 1 if patients have COPD, and 0 otherwise. Let C be a vector of observed confounding variables such as patient’s age, sex, diabetes mellitus, hypertension, coronary artery disease, and hyperlipidemia. Let U denote an indicator for unmeasured confounders in our primary study. These include unmeasured confounders for smoking, drinking, and BMI. Let propensity score PS = Pr(T = 1|C), we will consider modeling of the estimated PS-stroke association in a Cox proportional hazards model h(t | T, ps) = h0 (t) exp {β TT + β Cps} we would define propensity score P S ep = Pr(T = 1|C) as the error-prone variable and PS GS = pr(T = 1|C, U) as the gold-standard in the validation study. The measurement error model then is E(PS GS | T, PS ep) = γ + γ T T + γ C PS ep, then the regression cerebration [16] adjusted C and U confounding factors estimator for the effect of T is .
Results
Study Population
Table 1 showed the distributions of socio-demographic characteristics and selected comorbid medical disorders between COPD and comparison cohorts in LHID main Database and in NHIS validation Database. In the main study, 62.4%, 25.8%, 36.9% and 25.6% of the 10,413 COPD patients had comorbidities of hypertension, hyperlipidemia, coronary artery disease and diabetes, respectively. Compared with subjects in the comparison cohort, COPD patients had significantly higher chances to coexist with all these comorbidities. Unmeasured confounders in the main study, including smoking, drinking and body mass index, were provided in the validation database.
Table 1. Demographic Characteristics and Comorbid Medical Disorders for Subjects with COPD and in the Comparison Cohort in LHID main Database and NHIS validation Database, 2004–2006.
| Main study | Validation study | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Subjects with COPD | Comparison Subjects | Subjects with COPD | Comparison Subjects | ||||
| N = 10,413 | N = 41,652 | N = 471 | N = 1896 | |||||
| No. | % | No. | % | No. | % | No. | % | |
| Gender | ||||||||
| Male | 6848 | 65.8 | 27392 | 65.8 | 288 | 61.1 | 1164 | 61.4 |
| Female | 3565 | 34.2 | 14260 | 34.2 | 183 | 38.9 | 732 | 38.9 |
| Age (years-old) | ||||||||
| 51–60 | 1868 | 17.9 | 7472 | 17.9 | 116 | 24.6 | 464 | 24.5 |
| 61–70 | 2853 | 27.4 | 11412 | 27.4 | 141 | 29.9 | 564 | 29.7 |
| > 70 | 5692 | 54.7 | 22768 | 54.7 | 214 | 45.4 | 868 | 45.8 |
| Hypertension | ||||||||
| Yes | 6500 | 62.4 | 22789 | 54.7 | 208 | 44.2 | 782 | 41.2 |
| No | 3913 | 37.6 | 18863 | 45.3 | 263 | 55.8 | 1114 | 58.8 |
| Hyperlipidemia | ||||||||
| Yes | 2689 | 25.8 | 10121 | 24.3 | 89 | 18.9 | 301 | 15.9 |
| No | 7724 | 74.2 | 31531 | 75.7 | 382 | 81.1 | 1595 | 84.1 |
| Coronary heart disease | ||||||||
| Yes | 3840 | 36.9 | 10374 | 24.9 | 99 | 21.0 | 251 | 13.2 |
| No | 6573 | 63.1 | 31278 | 75.1 | 372 | 79.0 | 1645 | 86.8 |
| Diabetes | ||||||||
| Yes | 2668 | 25.6 | 9990 | 24.0 | 83 | 17.6 | 298 | 15.7 |
| No | 7745 | 74.4 | 31662 | 76.0 | 388 | 82.4 | 1598 | 84.3 |
| Smoking | ||||||||
| Yes | 163 | 34.6 | 584 | 30.8 | ||||
| No | 308 | 65.4 | 1312 | 69.2 | ||||
| Drinking | ||||||||
| Yes | 132 | 28.0 | 526 | 27.7 | ||||
| No | 339 | 72.0 | 1370 | 72.3 | ||||
| BMI (SD) | 23.9 (3.8) | 24.1 (3.6) | ||||||
Abbreviation: COPD = chronic obstructive pulmonary disease
Survival Analysis
During the three-year follow-up period, there were 2,689 (5.2%) of the 52,065 sampled subjects developed stroke, including 727 (7.0%) of the COPD cohort and 1,962 (4.7%) of the comparison cohort, which were equal to 343, and 192 per 10,000 person-year, respectively (Table 2).
Table 2. The Crude and Adjusted Hazard Ratios for Stroke among the Sample Subjects during the Three-year Follow-up Period Starting from the Index Ambulatory Care Visits (N = 52,065).
| Total sample N = 52,065 | Comparison subjects N = 41,652 | Subjects with COPD N = 10,413 | |
|---|---|---|---|
| Occurrence of stroke, N (%) | 2,689 (5.2%) | 1962 (4.7%) | 727 (7.0%) |
| Incidence per 10000 person-year | 218 | 192 | 343 |
| Crude HR (95% CI) | – | 1.00 | 1.82* (1.67–1.99) |
| Adjusted HR (95% CI) a | – | 1.00 | 1.65* (1.51–1.79) |
| Propensity score calibration adjusted HR (95% CI) b | – | 1.00 | 1.62* (1.49–1.77) |
| Adjusted HR (95% CI) a for ischemic stroke | 1.00 | 1.64* (1.49–1.82) | |
| Adjusted HR (95% CI) a for hemorrhagic stroke | 1.00 | 1.18 (0.89–1.57) |
Abbreviation: COPD = chronic obstructive pulmonary disease; HR = hazard ratio; CI = confidence interval.
aAdjustment for patient’s age, gender, hypertension, coronary heart disease, hyperlipidemia and diabetes.
bAdjustment for patient’s age, gender, hypertension, coronary heart disease, hyperlipidemia and diabetes and unmeasured confounders including smoking, drinking and body mass index.
*p < 0.001.
Table 2 also showed the crude and adjusted hazard ratios (HR) for stroke between these two cohorts. For COPD subjects, the hazard of stroke was 1.82 times greater than that of the subjects in the comparison cohort (95% CI 1.67–1.99, p < 0.001). After adjusting for age, gender, hypertension, hyperlipidemia, coronary artery disease and diabetes, the hazard ratio was 1.65 (95% CI1.51–1.79, p < 0.001), comparing to that of subjects in the comparison cohort. In addition, using the NHIS2005 as an external validation study, the two-stage calibration for the association between COPD and stroke demonstrated adjusted HR 1.62 (95% CI 1.49–1.77, p < 0.001). Moreover, the adjusted HR of ischemic stroke was 1.64 times (95% CI, 1.49–1.82, p < 0.001) greater for COPD patients than for patients in comparison cohort. However, there was no significant difference in the hazards of hemorrhagic stroke between the two groups.
Inhaled Pharmacotherapy and the Risk of Stroke
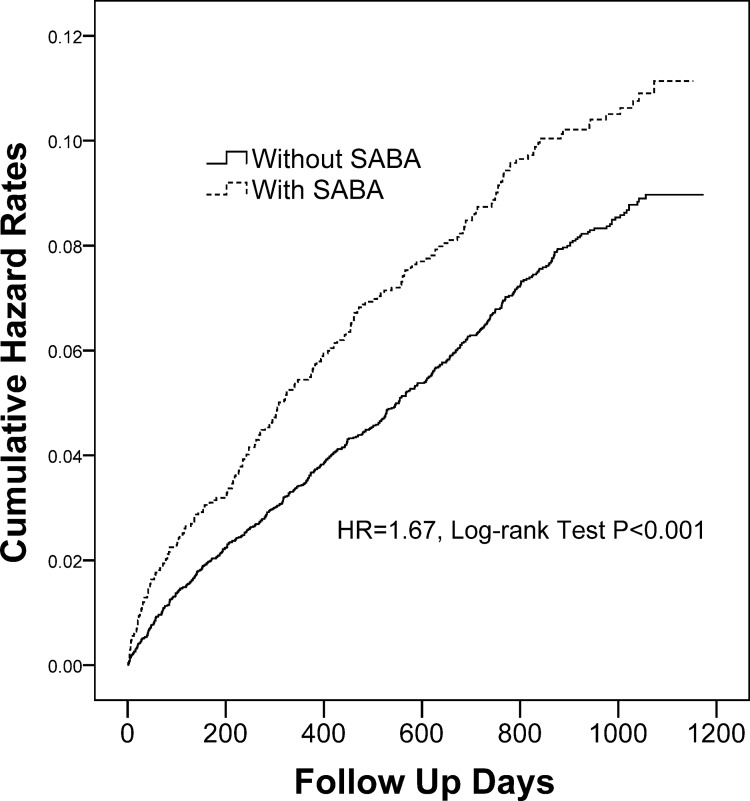
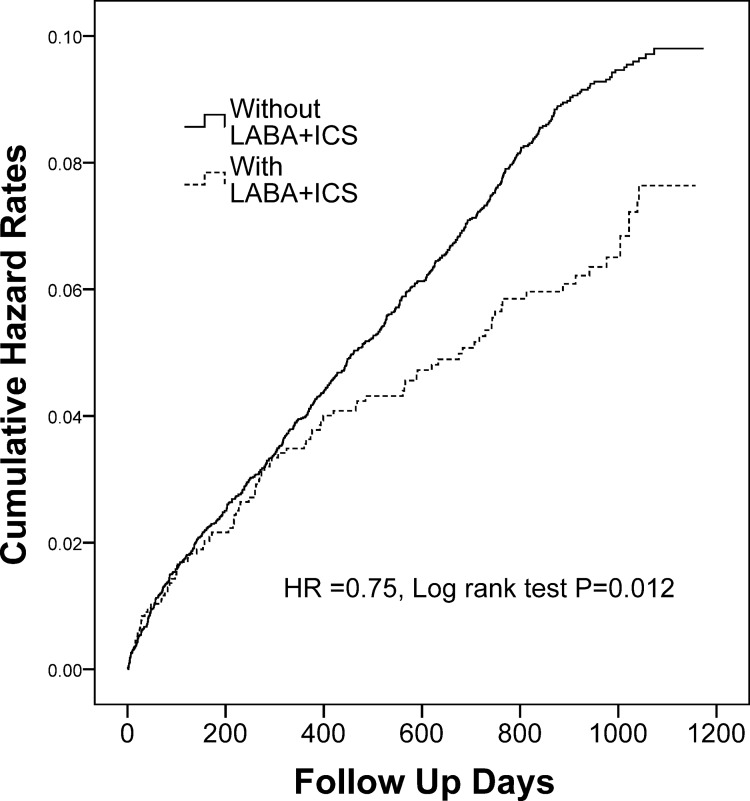
The adjusted HR for stroke between COPD patients with and without using one of the 5 categories of inhaled pharmacotherapy during three-year follow-up period are shown in Table 3. After adjustment of age, gender, hypertension, hyperlipidemia, coronary artery disease and diabetes, the stroke risk was significantly lower in patients treated with SAMA and higher in patients treated with SABA than those treated without, respectively. However, after two-stage propensity score calibration (Table 4), the impact of SAMA on stroke risk did not reach statistical significance, while the adjusted HR for stroke was increased to 1.67 (95% CI 1.45–1.91, p < 0.001) with the use of SABA. Moreover, Table 3 also showed that the stroke risk was significantly decreased in patients treated with LABA plus ICS, as compared to those treated without (adjusted HR 0.77, 95% CI 0.61–0.97; p = 0.028), which was further validated by two-stage propensity score calibration (adjusted HR 0.75, 95% CI 0.60–0.94; p = 0.014) (Table 4). Consistently, the cumulative incidence of stroke was significantly higher in patients treated with SABA (Fig 1) and less in patients treated with LABA/ICS (Fig 2) than those treated without, respectively. On the other hand, administration with either LAMA or LABA alone has no significant effect on the risk of subsequent stroke (Table 3).
Table 3. Adjusted Hazard Ratios for Stroke (Included Ischemic Stroke and Hemorrhagic Stroke) between COPD Patients with or without Using the Five Categories of Inhaled Pharmacotherapy during Three-Year Follow-up (N = 10,413).
| Medication | N | Adjusted HR b | 95% CI | p-value |
|---|---|---|---|---|
| SAMA | 2019 | 0.80 | 0.64–0.99 | 0.045 |
| SABA | 2345 | 1.32 | 1.08–1.62 | 0.007 |
| LAMA a | 607 | 0.92 | 0.66–1.28 | 0.640 |
| LABA a | 118 | 0.90 | 0.44–1.82 | 0.773 |
| LABA plus ICS a | 1559 | 0.77 | 0.61–0.97 | 0.028 |
Abbreviation: COPD = chronic obstructive pulmonary disease; SAMA = shorting-acting muscarinic antagonist; SABA = short-acting β-agonists; LAMA = long-acting muscarinic antagonist; LABA = long-acting β-agonists; ICS = inhaled corticosteroid; HR = hazard ratio.
aA brief use of SABA or SAMA on demand for acute exacerbation is allowed.
bAdjustments for patient’s age, gender, hypertension, hyperlipidemia, coronary heart disease and diabetes.
Table 4. Adjusted Hazard Ratios for Stroke between COPD Patients with or without Using the Inhaled Pharmacotherapy by Propensity Score Calibration Method.
| Medication | N | Adjusted HR b | 95% CI | p-value |
|---|---|---|---|---|
| SAMA | 2019 | 1.09 | 0.89–1.33 | 0.374 |
| SABA | 2345 | 1.67 | 1.45–1.91 | <0.001 |
| LABA plus ICS a | 1559 | 0.75 | 0.60–0.94 | 0.014 |
Abbreviation: COPD = chronic obstructive pulmonary disease; SAMA = shorting-acting muscarinic antagonist; SABA = short-acting β-agonists; LAMA = long-acting muscarinic antagonist; LABA = long-acting β-agonists; ICS = inhaled corticosteroid; HR = hazard ratio.
aA brief use of SABA or SAMA on demand for acute exacerbation is allowed.
bAdjustment for patient’s age, gender, hypertension, coronary heart disease, hyperlipidemia and diabetes and unmeasured confounders including smoking, drinking and body mass index.
Fig 1. Cumulative incidence of stroke in COPD patients treated with and without inhaled SABA.
COPD = chronic obstructive pulmonary disease; SABA = short-acting β-agonist.
Fig 2. Cumulative incidence of stroke in COPD patients treated with and without inhaled LABA plus ICS.
COPD = chronic obstructive pulmonary disease; LABA = long-acting β-agonist; ICS = inhaled corticosteroid.
Discussion
Our study showed that in age- and gender-matched subjects, the likelihood of any type of stroke and ischemic stroke were 1.62- and 1.64-times, respectively, greater among patients with COPD than those without COPD during the 3-year follow-up after adjustment of other risk factors and two-stage propensity score calibration. Treatment with SABA was associated with 1.67-fold increased risk of stroke, and LABA plus ICS therapy related to reduced stroke hazard by 25%. To the best of our knowledge, this is the first study to investigate the effect of inhaled pharmacotherapy on risk of stroke among COPD patients in the 3 years following a COPD diagnosis, adjusting for demographic features and comorbid medical disorders.
COPD is a persistent airway inflammatory process involving neutrophils, macrophages and elevated levels of interleukin (IL)-1β, IL-6, IL-8 and tumor necrosis factor (TNF)-α [17]. It has been shown that inflammation may spill-over from the lungs to the blood stream, inducing a systemic inflammatory response [17]. Human studies also demonstrated significantly increased serum levels of TNF-α, C-reactive protein (CRP), fibrinogen and alteration of circulating inflammatory cells in COPD patients compared with controls [18]. The spill-over of lung inflammation may cause endothelial dysfunction and generate a procoagulation state, leading to atherothrombosis which could provide a mechanistic link among COPD, atherosclerosis and acute vascular event [17].
Previous reports has demonstrated increased prevalence of stroke among COPD patients, which may be due to shared risk factors such as smoking and low socioeconomic status [3, 4]. In contrast, few studies were identified describing the risk of subsequent stroke in patients with COPD. A recent longitudinal analysis revealed that COPD was associated with 2.8-fold increase in the rate of acute stroke, independent of other risk factors such as sex and smoking status [5]. Another study reported a 1.25-fold increased risk of stroke 1 to 49 days following COPD acute exacerbation [6]. In line with the previous reports [5, 6], our data demonstrated a significant increase in the risk of developing stroke among COPD patients during a 3-year follow-up, after adjusting for age, gender and other comorbidies associated with stroke. All these findings suggest that the COPD-associated systemic inflammation may predispose to atherosclerosis and cerebrovascular events, or that the coexistence of COPD and stroke in individuals may represent different aspects of a single inflammatory syndrome [17]. Nevertheless, the impact of COPD treatment on the risk of acute vascular event has not yet been investigated.
Inhaled β-agonist, either SABA or LABA, relaxes airway smooth muscle by stimulating β2-receptors and improve airflow limitation and symptoms in patients with obstructive airway diseases [19]. However, concerns have been raised about the pro-arrhythmic effect of Inhaled β-agonists through adrenergic stimulation [20, 21], which may precipitate cerebrovascular thrombosis and ischemic stroke. Actually, the use of SABA has been reported to increase the risk of cardiovascular event and stroke associated with bronchial asthma [22]. Similar to the previous report [22], our analysis disclosed that SABA did, while LABA did not, increase the risk of subsequent stroke. The differential impact on stroke hazard can be attributed to the fact that LABA imparts greater lung function improvement and less acute exacerbation [8, 9], which may keep COPD patients from stroke-precipitating arrhythmias. However, as patients with severe stages of COPD more frequently develop acute exacerbation that contributes to the risk of stroke [6], whether the increased risk was linked to the use of SABA or to the disease severity remains to be determined. In the present study, two scenarios are possible for patients treated with SABA: (1) SABA alone was the main treatment of a specific group of COPD patients who might have milder severity and less risk of stroke than those treated with other categories (e.g. LAMA, LABA or LABA/ICS), suggesting that SABA directly contributed to the risk of stroke; and (2) SABA was used as a rescue medicine for patients frequently suffering from acute exacerbation, who might have greater disease severity and exacerbation risk, indicating the association between disease severity and the odds of developing stroke. Both conditions might exist in the current study and further studies incorporating the data of disease severity are needed to verify the virtual impact of SABA on stroke risk in COPD patients.
COPD guidelines advocated the use of inhaled long-acting bronchodilators with or without inhaled corticosteroids to treat moderate to severe COPD patients, depending on the combined assessment including airflow limitation, symptoms, and exacerbation history [1]. Two recent large, randomized, double-blind trial demonstrated that inhaled long-acting bronchodilators with or without inhaled corticosteroids decrease exacerbations and enhance health status in COPD patients [8–9], suggesting that inhaled therapies may modulate COPD inflammation. However, the results of previous reports are conflicting regarding whether inhaled pharmacotherapy can diminish systemic inflammation by reducing the spill-over of airway inflammation [19–21]. Pinto-Plata et al. found that the circulating CRP level was 20% lower in COPD patients with inhaled corticosteroid treatment than those without [23]. Furthermore, a double-blind placebo-controlled trial demonstrated that withdrawing inhaled steroids increased serum CRP levels and reintroduction of inhaled fluticasone resulted in 50% and 26% decrease in CRP and IL-6 levels, respectively [24]. However, when repeated as a multicenter, randomized trial, no significant changes in CRP or IL-6 were identified in COPD patients treated with inhaled fluticasone or combination of fluticasone/salmeterol [25]. Moreover, the effect of inhaled pharmacotherapy on the risk of inflammation-related vascular events remains unknown. Our study showed by means of Cox regression and considering influential covariates that addition of ICS to LABA correlated with less incidence of stroke in patients with COPD, whereas treatment with SAMA or long-acting bronchodilator (LAMA or LABA) did not affect the occurrence of cerebrovascular events. This data suggests that LABA/ICS combined treatment may modulate systemic inflammation and alter the risk of stroke in patients with COPD. Accordingly, further large-scaled, randomized control trials are mandatory to validate the beneficial effect of inhaled pharmacotherapy on the risk of cerebrovascular comorbidities.
The strength of this study is the setting of population-based data, which facilitates monitoring all cases of COPD and stroke during the follow up. Additionally, the large sample size confers substantial statistical power for recognizing the virtual distinctions between 2 cohorts and the effect of inhaled pharmacotherapy on stroke risk in the 3 years following a COPD diagnosis. Finally, as this study was centered on actual use of drugs, the results are more likely to reflect usual community care. However, this study has a few limitations. First, this study was not a randomized controlled trial, and the levels of COPD severity were not recorded in the data set, which may confound the measured risk of developing stroke between patients using different inhaled therapy. Nevertheless, the important confounding factors including smoking, drinking, and BMI were adjusted by using two-stage calibration approach. Second, the utilization of COPD medication was estimated on prescription claims, which did not entirely reveal how patients virtually use these medications. However, this study cohort included patients who had at least three prescription claims and no switch of inhaled pharmacotherapy within one year, which may imply a substantial adherence with COPD medications in these patients. Finally, in all cases, the diagnoses of COPD were made by physician but not verified by spirometry because of the lack of this element in administrative claims databases.
Conclusions
In conclusion, this study demonstrated that patients who have a COPD diagnosis were at an increased risk of stroke in the subsequent 3 years. The use of inhaled SABA was associated with an increased risk of stroke, and combination treatment with inhaled LABA and ICS related to a risk reduction. Further prospective research is needed to verify whether LABA plus ICS treatment confers protection against stroke in patients with COPD.
Data Availability
For the main study, a dataset “Longitudinal Health Insurance Database 2005 (LHID2005)” released by the Taiwan National Health Research Institute (NHRI) was used. For the validation study, The National Health Interview Survey sampled from the entire Taiwan population was used as an external validation study. The National Health Interview Survey 2005 (NHIS2005) assessed health behaviors and quality of life, and the details of NHIS2005 can be referred to Guo et al. (Self-reported myopia in Taiwan: 2005 Taiwan National Health Interview Survey. Eye 2012; 26: 684–689). The datasets “Longitudinal Health Insurance Database 2005 (LHID2005)” and “National Health Interview Survey 2005 (NHIS2005)” were released by the NHRI to the specific applicants who planned to perform the research related to the national health care issues. The applicant must have a study protocol to pass the application procedure, and the data can only be used by the applicant for this specific investigation protocol. Data cannot be released to the general public. The permit number of LHID2005 is 98274 Lin Wui-Wen and the permit date of NHIS2005 is 2010/8/24 Lin Wui-Wen (no number). LHID2005 is available on http://nhird.nhri.org.tw/en/Data_Subsets.html#S3. But it is under the following regulation (from NHIRD, Taiwan. http://nhird.nhri.org.tw/en/index.htm). Contact e-mail: nhird@nhri.org.tw. All researchers who wish to use the NHIRD and its data subsets are required to sign a written agreement declaring that they have no intention of attempting to obtain information that could potentially violate the privacy of patients or care providers. Only citizens of the Republic of China who fulfill the requirements of conducting research projects are eligible to apply to the NHIRD. Applicants must follow the Computer-Processed Personal Data Protection Law (http://law.moj.gov.tw/Eng/LawClass/LawAll.aspx?PCode=I0050021) and related regulations of BNHI (Bureau of National Health Insurance) and NHRI, and an agreement must be signed by the applicant and his/her supervisor upon application submission. National Health Interview Survey 2005 is available at http://nhis.nhri.org.tw/2005nhis.html (in Chinese). You can download the detailed information about the questionnaire and the results at http://nhis.nhri.org.tw/2005download.html.
Funding Statement
The work was supported by the following: NSC96-2314-B-038-029 from the National Science Council of Taiwan to Bien MY; 96TMU-WFH-16 from Wan Fang Hospital, Taipei, Taiwan to Bien MY; and NSC101-2314-B-038-044-MY3 from the National Science Council of Taiwan to Chung CL.
References
- 1. Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, et al. (2013) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 187: 347–365. 10.1164/rccm.201204-0596PP [DOI] [PubMed] [Google Scholar]
- 2. Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS, Mannino DM. (2006) Global burden of COPD: systematic review and meta-analysis. Eur Respir J 28: 523–532. [DOI] [PubMed] [Google Scholar]
- 3. Man SF, Van Eeden S, Sin DD. (2012) Vascular risk in chronic obstructive pulmonary disease: role of inflammation and other mediators. Can J Cardiol 28: 653–661. 10.1016/j.cjca.2012.06.013 [DOI] [PubMed] [Google Scholar]
- 4. Schnell K, Weiss CO, Lee T, Krishnan JA, Leff B, Wolff JL, et al. (2012) The prevalence of clinically-relevant comorbid conditions in patients with physician-diagnosed COPD: a cross-sectional study using data from NHANES 1999–2008. BMC Pulm Med 12: 26 10.1186/1471-2466-12-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Feary JR, Rodrigues LC, Smith CJ, Hubbard RB, Gibson JE. (2010) Prevalence of major comorbidities in subjects with COPD and incidence of myocardial infarction and stroke: a comprehensive analysis using data from primary care. Thorax 65: 956–962. 10.1136/thx.2009.128082 [DOI] [PubMed] [Google Scholar]
- 6. Donaldson GC, Hurst JR, Smith CJ, Hubbard RB, Wedzicha JA. (2010) Increased risk of myocardial infarction and stroke following exacerbation of COPD. Chest 137: 1091–1097. 10.1378/chest.09-2029 [DOI] [PubMed] [Google Scholar]
- 7. Gross NJ. (2012) Novel antiinflammatory therapies for COPD. Chest 142: 1300–1307. 10.1378/chest.11-2766 [DOI] [PubMed] [Google Scholar]
- 8. Calverley PMA, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. (2007) Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 356: 775–789. [DOI] [PubMed] [Google Scholar]
- 9. Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, et al. (2008) A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med 359: 1543–1554. 10.1056/NEJMoa0805800 [DOI] [PubMed] [Google Scholar]
- 10.National Health Insurance Research Database, Taiwan. Available: http://nhird.nhri.org.tw/en/index.htm
- 11. Guo YH, Lin HY, Lin LL, Cheng CY. (2012) Self-reported myopia in Taiwan: 2005 Taiwan National Health Interview Survey. Eye 26: 684–689. 10.1038/eye.2012.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Suissa S. (2012) Randomized Trials Built on Sand: Examples from COPD, Hormone Therapy, and Cancer. Rambam Maimonides Med J. 31: e0014 10.5041/RMMJ.10082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lin HW, Chen YH. (2014) Adjustment for missing confounders in studies based on observational databases: Two-stage calibration combining propensity scores from primary and validation data. Am J of Epidemiol 180: 308–317. 10.1093/aje/kwu130 [DOI] [PubMed] [Google Scholar]
- 14. Stürmer T, Schneeweiss S, Avorn J, Glynn RJ. (2005) Adjusting effect estimates for unmeasured confounding with validation data using propensity score calibration. Am J Epidemiol 162: 279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lin HW, Chen YH. (2010) Association analysis under population stratification: a two-stage procedure utilizing population- and family-based analyses. Hum Hered 69: 160–170. 10.1159/000267996 [DOI] [PubMed] [Google Scholar]
- 16. Rosner B, Spiegelman D, Willett WC. (1990) Correction of logistic regression relative risk estimates and confidence intervals for measurement error: The case of multiple covariates measured with error. Am J Epidemiol 132: 734–745. [DOI] [PubMed] [Google Scholar]
- 17. Van Eeden S, Leipsic J, Paul Man SF, Sin DD. (2012) The relationship between lung inflammation and cardiovascular disease. Am J Respir Crit Care Med 186: 11–16. 10.1164/rccm.201203-0455PP [DOI] [PubMed] [Google Scholar]
- 18. Sinden NJ, Stockley RA. (2010) Systemic inflammation and comorbidity in COPD: a result of 'overspill' of inflammatory mediators from the lungs? Review of the evidence. Thorax 65: 930–936. 10.1136/thx.2009.130260 [DOI] [PubMed] [Google Scholar]
- 19. Cook D, Guyatt G, Wong E, Goldstein R, Bedard M, Austin P, et al. (2001) Regular versus as-needed short-acting inhaled β-agonist therapy for chronic obstructive pulmonary disease. Am J Respir Crit Care Med 163: 85–90. [DOI] [PubMed] [Google Scholar]
- 20. Salpeter SR, Ormiston TM, Salpeter EE. (2004) Cardiovascular effects of beta-agonists in patients with asthma and COPD: a meta-analysis. Chest 125: 2309–2321. [DOI] [PubMed] [Google Scholar]
- 21. Wilchesky M, Ernst P, Brophy JM, Platt RW, Suissa S. (2012) Bronchodilator use and the risk of arrhythmia in COPD: part 2: reassessment in the larger Quebec cohort. Chest 142: 305–311. 10.1378/chest.11-1597 [DOI] [PubMed] [Google Scholar]
- 22. Varela S, Mendez J, González de la Cuesta C, Iglesias I, González C, et al. (2009) Cardiovascular disease risk associated with asthma and respiratory morbidity might be mediated by short-acting β2-agonists. J Investig Allergol Clin Immunol 123: 124–130. [DOI] [PubMed] [Google Scholar]
- 23. Pinto-Plata VM, Müllerova H, Toso JF, Feudjo-Tepie M, Soriano JB, Celli BR. (2006) C-reactive protein in patients with COPD, control smokers and non-smokers. Thorax 61: 23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Man SF, Sin DD. (2005) Effects of corticosteroids on systemic inflammation in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2: 78–82. [DOI] [PubMed] [Google Scholar]
- 25. Sin DD, Man SF, Marciniuk DD, Ford G, FitzGerald M, Wong E, et al. (2008) The effects of fluticasone with or without salmeterol on systemic biomarkers of inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 177: 1207–1214. 10.1164/rccm.200709-1356OC [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
For the main study, a dataset “Longitudinal Health Insurance Database 2005 (LHID2005)” released by the Taiwan National Health Research Institute (NHRI) was used. For the validation study, The National Health Interview Survey sampled from the entire Taiwan population was used as an external validation study. The National Health Interview Survey 2005 (NHIS2005) assessed health behaviors and quality of life, and the details of NHIS2005 can be referred to Guo et al. (Self-reported myopia in Taiwan: 2005 Taiwan National Health Interview Survey. Eye 2012; 26: 684–689). The datasets “Longitudinal Health Insurance Database 2005 (LHID2005)” and “National Health Interview Survey 2005 (NHIS2005)” were released by the NHRI to the specific applicants who planned to perform the research related to the national health care issues. The applicant must have a study protocol to pass the application procedure, and the data can only be used by the applicant for this specific investigation protocol. Data cannot be released to the general public. The permit number of LHID2005 is 98274 Lin Wui-Wen and the permit date of NHIS2005 is 2010/8/24 Lin Wui-Wen (no number). LHID2005 is available on http://nhird.nhri.org.tw/en/Data_Subsets.html#S3. But it is under the following regulation (from NHIRD, Taiwan. http://nhird.nhri.org.tw/en/index.htm). Contact e-mail: nhird@nhri.org.tw. All researchers who wish to use the NHIRD and its data subsets are required to sign a written agreement declaring that they have no intention of attempting to obtain information that could potentially violate the privacy of patients or care providers. Only citizens of the Republic of China who fulfill the requirements of conducting research projects are eligible to apply to the NHIRD. Applicants must follow the Computer-Processed Personal Data Protection Law (http://law.moj.gov.tw/Eng/LawClass/LawAll.aspx?PCode=I0050021) and related regulations of BNHI (Bureau of National Health Insurance) and NHRI, and an agreement must be signed by the applicant and his/her supervisor upon application submission. National Health Interview Survey 2005 is available at http://nhis.nhri.org.tw/2005nhis.html (in Chinese). You can download the detailed information about the questionnaire and the results at http://nhis.nhri.org.tw/2005download.html.