Abstract
Autophagy is an important protein quality control mechanism for cells under stress conditions to promote cell survival. Modulation of autophagy on biomaterial substrates is rarely reported. In this study, the autophagy of adipose-derived stem cells (ADSCs) cultured on chitosan (CS) substrates was examined. Compared to the traditional monolayer culture, ADSCs cultured on CS substrates showed spheroid formation as well as a prolonged upregulation of autophagosomal marker-microtubule-associated protein 1 light chain 3 (LC3) II protein expression. In addition, the green fluorescent protein tagged-LC3 (GFP-LC3) expressing ADSCs also revealed more GFP-LC3 puncta on CS substrates. The enhanced autophagy on CS substrates was associated with Ca2+, while ethylene glycol tetraacetic acid (EGTA), a Ca2+ chelator, repressed the autophagy in a dose-dependent manner. Moreover, ADSC spheroids on CS substrates demonstrated a higher survival rate and autophagy response upon H2O2 treatment. The upstream components of autophagy signal pathway-UNC51-like kinase 1 (Ulk1), autophagy-related protein 13 (Atg13), and autophagy/beclin-1 regulator 1 (Ambra1) genes were more highly expressed in ADSC spheroids before and after adding H2O2 than those in the conventional culture. EGTA also decreased the cell viability and autophagy-associated gene expression for ADSC spheroids on CS substrates after H2O2 treatment. Therefore, we suggest that three-dimensional (3D) cell culture on CS may confer ADSCs the ability to increase the autophagic flux in response to stimulations in a Ca2+-dependent manner.
Key words: : autophagy, mesenchymal stem cells, oxidative stress, spheroid
Introduction
Autophagy is a conserved catabolic mechanism for degradation of nonessential or dysfunctional cellular organelles and proteins.1,2 Upon induction, the Ulk1 protein kinase complex (Ulk1, Atg13, FIP200, and Atg101) initiates autophagosome formation3 and the autophagy/beclin-1 regulator 1 (Ambra1) enhances Ulk1 kinase activity.4 Microtubule- associated protein 1 light chain 3-II (LC3-II) localized in the double-membranes of autophagosome is considered a proper marker to monitor autophagy.5 Besides, cytosolic Ca2+ is important for autophagy induction.6,7
Mesenchymal stem cells (MSCs) from different adult tissues have recently become a potential cell source for cell therapy because they can differentiate into a variety of cell types.8 The environmental conditions of host tissues, however, may not be suitable for MSC growth (e.g., inflammation, hypoxia, oxidative stress, or lack of nutrients). The poor cell viability may further lead to the limited therapeutic efficacy.9 Recent works have shown that autophagy protects the bone marrow-derived MSCs from apoptosis under hypoxia and serum deprivation.10–12
Chitosan (CS) is a polymer obtained from deacetylation of chitin, a nitrogen-containing polysaccharide abundant in nature. The thin membranes made of CS can facilitate cell–cell interaction and induce the self-assembly of MSCs to form three-dimensional (3D) spheroids on the membranes.13 These 3D spheroids express a greater level of stemness marker genes (Oct4, Sox2, and Nanog) and have higher differentiation and engrafting potentials.14,15 CS-derived MSC spheroids showed higher cell survival rates in an inflammatory milieu of a sciatic nerve gap16 or an infarcted myocardium in rats.17 The enhanced cell survival may suggest a protection mechanism from apoptosis.
The purpose of this study is to investigate the regulation of autophagy in adipose-derived stem cells (ADSCs) grown on CS substrates. Here, we demonstrated that culturing on CS substrates increased autophagy in ADSCs and this process required Ca2+. Finally, we suggested that enhanced autophagy may contribute to the cell survival under oxidative stress and nutrient starvation in CS-derived ADSC spheroids.
Materials and Methods
Cell culture and transfection
All procedures followed the ethics guidelines and were approved by the Animal Care and Use Committee of the University. ADSCs were isolated from the adipose tissue of Sprague-Dawley rats (body weight from 250 to 500 g).13 ADSCs were cultured in Dulbecco's modified Eagle's medium–low glucose/F12 (1:1) supplemented with 10% fetal bovine serum (FBS), 100 μg/ml streptomycin, and 100 U/ml penicillin (all from Gibco) at 37°C in a 5% CO2 atmosphere. Cells of the third to the fifth passages were used in this study. Rat LC3 cDNA was inserted to pEGFP-C1 vector (Clontech) to create a green fluorescent protein tagged-LC3 protein expressing vector.5 ADSCs were seeded in a 6-well culture plate for 24 h before transfection. Two micrograms of GFP-LC3 plasmids was transfected into ADSCs using the K2® Transfection System (Biontex) according to the manufacturer's instructions.
Preparation of CS substrates and spheroid formation
To prepare 1% CS solution, CS powder (Sigma; molecular weight 510 kDa and degree of deacetylation 77%) was dissolved and stirred in 1% aqueous acetic acid solution at room temperature for 16 h. After filtration by a 100 μm mesh, the 1% CS solution was casted on a 24-well tissue culture plate (300 μl/well) or 6-well tissue culture plate (1.5 ml/well) and air-dried in a laminar cabinet for 24 h. Then 0.5 N NaOH in 75% ethanol was added to the CS substrates for 5 min and washed with distilled water for three times. For spheroid formation, 5×104 or 1×104 cells were seeded in each well of the CS-coated 24- or 96-well tissue culture plate.
H2O2 treatment and WST-1 assay
ADSCs (1×104) were seeded in the blank culture well (tissue culture polystyrene, TCPS) or CS-coated 96-well culture plate. Cells were cultured for 24 h on TCPS or 72 h on CS substrates. The medium was replaced in two groups for ADSCs grown on TCPS: a control group, and a H2O2-treated group (incubation with 600 μM H2O2 in 2% FBS medium). The medium was replaced in four groups for ADSCs grown on CS substrates: a control group, a H2O2 treated-group (incubation with 600 μM H2O2 in 2% FBS medium), a H2O2+ethylene glycol tetraacetic acid (EGTA)-treated group (incubation with 600 μM H2O2 and 2 mM EGTA in 2% FBS medium); and an EGTA-treated group (incubation with 2 mM EGTA in 2% FBS medium). After 2 h incubation, cell viability was detected by the cell proliferation reagent WST-1 kit (Roche Applied Science) according to the manufacturer's instructions.
Western blots analysis
ADSCs were seeded on TCPS or CS substrates at a density of 2.5×105 cells per well in a 6-well culture plate and cultured for 0, 2, 4, 8, 16, 24, and 48 h. ADSCs were harvested at the indicated times. For H2O2 treatment, ADSCs were cultured for 24 h on TCPS or 72 h on CS substrates. After 2 h incubation, ADSCs were harvested. Cells were lysed in lysis buffer containing 20 mM Hepes (pH 7.5), 420 mM NaCl, 1.5 mM MgCl2, 0.1% NP-40, and protease inhibitor cocktail (Sigma). Proteins were separated by 15% SDS-PAGE and the endogenous LC3 proteins were detected by rabbit polyclonal anti-LC3 antibody (Genetex). For internal control, GAPDH proteins were detected by rabbit monoclonal anti-GAPDH antibody (Cell Signaling). The intensities of protein bands were quantified by Labwork software (UVP).
RNA extraction and quantitative RT-PCR analysis
ADSCs were seeded in the TCPS or CS substrates at a density of 5×104 cells per well in a 24-well culture plate and were cultured 24 h for TCPS or 72 h for CS substrates. After 2 h incubation with H2O2, total RNAs were isolated with Trizol reagent (Invitrogen) and cDNA was synthesized using the RevertAid First Strand cDNA Synthesis Kit (MBI Fermentas) according to the manufacturer's instruction. RT-PCR was performed with DyNAmo Flash SYBR Green qPCR Kit (Finnzymes) on a Chromo 4 PTC200 Thermal Cycler (MJ Research). The primers used were Ulk1 forward 5′- TGGAGAACCTAGCCAGCAGT-3′ and reverse 5′-GTGCTTCACACACGACGACT-3′; Atg13 forward 5′-AGACCAAGCAAGTCGAAGGA-3′ and reverse 5′- CCTTTGGGAGATGATGGCTA-3′; Ambra1 forward 5′-GCAGCTTTCATCCCGAGTAG-3′ and reverse 5′-AAGACCTGGGCTACCATGTG-3′; and GAPDH forward 5′- AGGTCGGTGTGAACGGATTTG-3′ and reverse 5′-GGGGTCGTTGATGGCAACA-3′. The relative quantification in gene expression was determined using the 2-ΔΔ-CT method.18 Using this method, the fold of changes in gene expression normalized to the GAPDH gene was obtained relative to TCPS.
Immunocytochemistry
After transfection with GFP-LC3 plasmids for 24 h, ADSCs were recovered for another 24 h before seeding on CS substrates. After 24 h the spheroids were then carefully collected and washed with PBS. The spheroids were trypsinized into single cells and seeded on a 1% gelatin-coated cover slide for 4 h. After washing with PBS and fixation in 4% paraformaldehyde at room temperature for 10 min, and two washes with 1% Tween/PBS, the slide was mounted by 90% glycerol (Merk). Puncta of GFP-LC3 were analyzed using a fluorescence microscope.
Statistical analysis
Numerical values were expressed as the mean±standard deviation (SD). Differences between two groups were assessed by unpaired two-tailed t-tests. Results involving more than two groups were assessed by one-way ANOVA and Tukey's multiple comparison test. A p-value of less than 0.05 was considered to be statistically significant. Independent experiments were performed for each type of experiments.
Results
Induction of autophagy in ADSCs on CS substrates
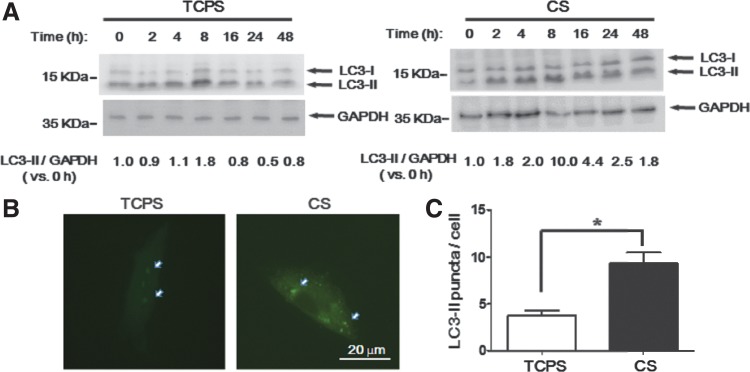
To examine whether cell culture on CS substrates increased autophagy, the expression of endogenous LC3-II, an autophagosomal marker, was detected by Western blots. ADSCs on TCPS or CS substrates were analyzed at different time points. When grown on CS substrates, the LC3-II protein expression increased from 2 h and reached a high level at 8 h. Although the LC3-II protein expression decreased from 16 to 48 h, it was still higher than the starting point (Fig. 1A, right panel). The LC3-II protein expression on TCPS from 4 to 8 h also slightly increased, but the fold of increase was much lower than that on CS substrates (Fig. 1A, left panel). The formation of LC3 puncta was further examined at 24 h. Formation of GFP-LC3 puncta in ADSCs on CS substrates was clearly observed and the number of GFP-LC3 puncta was more than that on TCPS (Fig. 1B,C). These results indicated that autophagy was more pronounced and prolonged in ADSCs when cultured on CS substrates.
FIG. 1.
Upregulation of autophagy in ADSC spheroids. (A) ADSCs were seeded on CS substrates in a 24-well plate or a blank well (TCPS) and harvested at 0, 2, 4, 8, 16, 24, and 48 h. The LC3 protein expression was detected by Western blots at the indicated time points. GAPDH was used as an internal control. Band intensities were quantified, and the LC3-II expression was normalized to GAPDH. (B) GFP-LC3 plasmids were transfected to ADSCs and cells were seeded to CS substrates for 24 h. Cells were then transferred to a 1% gelatin-coated cover slide and fixed. The GFP-LC3 puncta were analyzed with 100×magnification using a fluorescence microscope. (C) Quantitative analyses of GFP-LC3 puncta per cell. *p<0.05 versus TCPS. ADSCs, adipose-derived stem cells; CS, chitosan; TCPS, tissue culture polystyrene.
EGTA inhibited autophagy of ADSCs on CS substrates
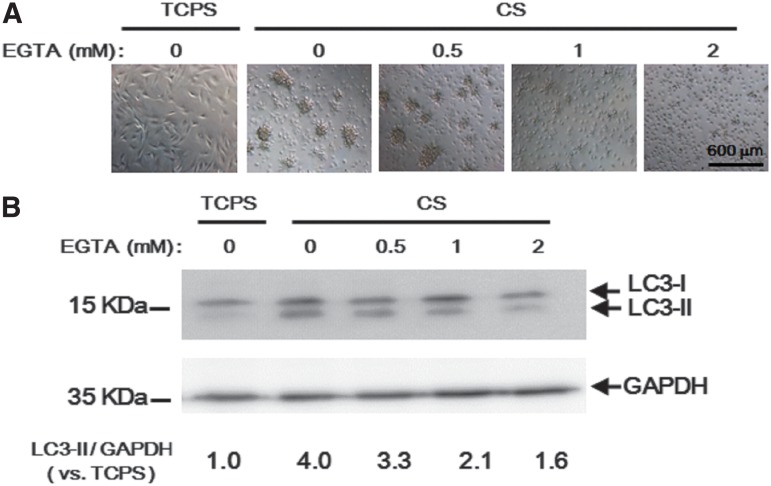
Since the increase in cytosolic Ca2+ could induce autophagy,6,7 we sought to investigate whether Ca2+ chelation affected the autophagy of ADSCs on CS substrates. To this end, ADSCs were cultured in the medium containing 0.5–2 mM EGTA (a Ca2+ chelator). After 8 h, the morphology of ADSCs and LC3-II protein expression level were examined. It was observed that 0.5 mM EGTA had no effect on sphere formation, but for concentrations greater than 1–2 mM EGTA the sphere formation was obviously inhibited (Fig. 2A). Western blots showed that when the EGTA concentration increased, the LC3-II protein expression gradually decreased for ADSCs on CS substrates (Fig. 2B). On CS, EGTA at 0.5 mM slightly decreased the LC3-II protein expression of ADSCs. The LC3-II protein expression of ADSCs on CS in the presence of 2 mM EGTA was similar to that on TCPS without EGTA. These results suggested that Ca2+ may be required for the upregulation of ADSC autophagy on CS substrates.
FIG. 2.
Autophagy inhibition by EGTA. (A) ADSCs were seeded on CS substrates in a 24-well plate with the medium containing 0, 0.5, 1, or 2 mM EGTA or in a blank well (TCPS) for 8 h. The morphologies of ADSCs were taken by the microscope. (B) Cell lysates were collected after treatment and subjected to Western blot analysis with anti-LC3 for LC3-II. GAPDH was used as an internal control. Band intensities were quantified and normalized to GAPDH. EGTA, ethylene glycol tetraacetic acid.
Enhanced cell survival of ADSCs on CS substrates under oxidative stress may be related to autophagy
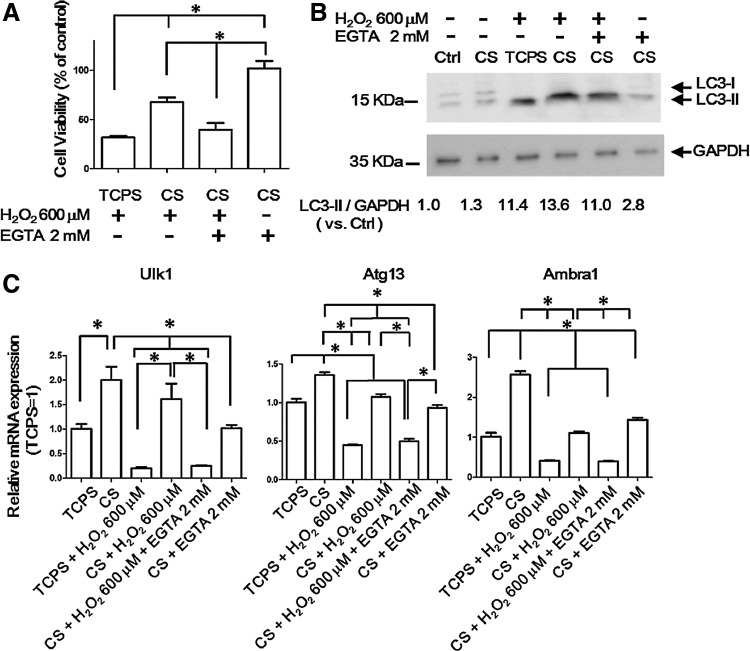
We hypothesized that the enhanced autophagy in CS-derived ADSC spheroids might reduce cell apoptosis under oxidative stress. To mimic the in vivo condition, following cell culture on TCPS for 24 h or on CS substrates for 72 h, the cells were treated with the medium containing 2% FBS and 600 μM H2O2 for 2 h before the cell viability was assessed. H2O2 treatment significantly reduced the cell survival of ADSCs grown on TCPS. On the other hand, ADSCs grown on CS substrates showed a higher percentage of cell survival (Fig. 3A). The LC3-II protein expression was also higher for ADSCs grown on CS substrates than on TCPS under the H2O2 treatment (Fig. 3B). Furthermore, adding 2 mM EGTA with H2O2 to the ADSCs grown on CS substrates decreased both the cell viability and LC3-II protein expression (Fig. 3A,B). EGTA treatment without H2O2 for 2 h presented minor effects to cell viability and LC3-II protein expression for ADSCs grown on CS substrates (Fig. 3A,B). These results implied that the autophagy of CS-derived ADSC spheroids increased and protected them from the oxidative stress and nutrient deprivation in a calcium-dependent manner.
FIG. 3.
Autophagy-protecting ADSCs against oxidative stress and low-nutrient condition. (A) Cell viability was estimated by WST-1 assay after 600 μM H2O2 or 2 mM EGTA treatment in 2% FBS medium for 2 h. *p<0.05. (B) ADSCs were cultured on TCPS for 24 h or CS 72 h. After treatment as described in (A) for 2 h, cells were harvested and subjected for Western blots with anti-LC3 antibodies. ADSCs grown on TCPS for 24 h without treatment were defined as the control group (ctrl). Band intensities were quantified and normalized to GAPDH (the internal control). (C) ULk1, Atg13, and Ambra1 mRNA levels were determined by quantitative RT-PCR after treatment as described in (A). *p<0.05.
To investigate the possible mechanisms behind the enhanced autophagy of CS-derived ADSC spheroids under the oxidative stress, the mRNA expression of autophagy upstream components was examined. It was noted that Ulk1, Atg13, and Ambra1 genes were all upregulated for ADSCs grown on CS substrates (Fig. 3C). After H2O2 treatment, the expression of Ulk1, Atg13, and Ambra1 mRNA was decreased for both ADSCs cultured on CS substrates and those cultured on TCPS. However, the mRNA expression was still much higher in CS-derived ADSC spheroids (Fig. 3C). EGTA inhibited the mRNA expression of these genes in CS-derived ADSC spheroids under H2O2 treatment. These results suggested that the enhanced autophagy in CS-derived ADSC spheroids may be associated with the upregulation of the autophagy upstream genes Ulk1, Atg13, and Ambra1.
Discussion
Various in vitro preconditioning methods have been developed to enhance the efficacy of transplanted MSCs.19 Hypoxia-induced autophagy can protect MSCs against apoptosis under oxidative stress.11,12 MSCs with hypoxic preconditioning significantly improve cell survival after transplantation.20 MSC spheroids derived from CS substrates were also shown to have better differentiation capacity and engrafting potential.16,17 Our previous study demonstrated that CS-derived ADSC spheroids once transplanted in a chronic myocardial infarction animal model had better therapeutic effects than single cells.17 The ischemic area usually exhibits oxidative stress and nutrient deprivation. In this study we demonstrated that autophagy was significantly enhanced in CS-derived ADSC spheroids. Inhibition of autophagy by EGTA treatment decreased the cell viability under the oxidative stress and nutrient deprivation. These results suggested that autophagy may be an important pathway against oxidative stress to enhance the engraftment efficiency for CS-derived MSC spheroids or hypoxia-preconditioned MSCs. Moreover, spermidine and resveratrol that could activate autophagy were used to extend longevity in various model organisms.21–23 CS-derived MSC spheroids maintained the expression of stemness marker genes.13 We thus further suggested that the enhanced autophagy in ADSC spheroids may not only protect cells form stress but also prevent early senescence during cell expansion in vitro.
In the current study, the autophagy was induced from 4 to 8 h when ADSCs were grown on TCPS. On CS substrates, ADSCs revealed a greater autophagy response at each same period, and in particular, the autophagy response was prolonged till 24 h (Fig. 1A). After 48 h, the autophagy in CS-derived ADSC spheroids returned to nearly the basal level (Fig. 1A). Therefore, it seemed that the CS substrate did not constitutively stimulate the autophagy by itself but rather enhanced the autophagy response. Before H2O2 treatment, LC3-II protein expression was not increased in ADSCs grown on CS substrates for 72 h (Fig. 3B). After H2O2 treatment, the LC3-II protein expression was higher in ADSCs grown on CS substrates than that on TCPS. This result also supported our hypothesis that CS substrates enhanced the autophagy response.
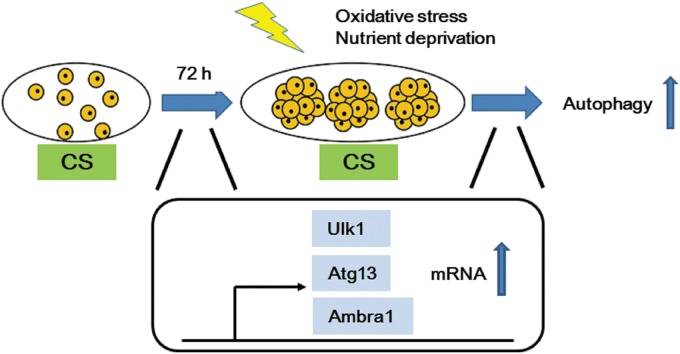
According to the literature, Ulk1, Atg13, and Ambra1 were kept in an inactive state by mammalian target of rapamycin complex 1 (mTORC1) phosphorylation before stimulation.3,4 Upon autophagy induction, mTORC1 is inhibited and Ulk1, Atg13, and Ambra1 are dephosphorylated and initiate the formation of autophagosomes.3,4 In this study, the mRNA expression of components in autophagy-initiating complex increased before H2O2 treatment in CS-derived ADSC spheroids (Fig. 3C). Even after H2O2 treatment, the mRNA expression of these genes was higher in ADSCs grown on CS substrates than that on TCPS. Based on the above findings, a possible mechanism is depicted (Fig. 4), where higher mRNA expressions of Ulk1, Atg13, and Ambra1 genes may provide more inactivated autophagy-initiating complex in CS-derived ADSC spheroids, which in turn enhances the autophagy activity upon stimulation (Fig. 4).
FIG. 4.
Possible mechanisms for the enhanced autophagy in ADSCs grown on CS substrates. CS substrates may upregulate Ulk1, Atg13, and Ambra1 mRNA expression. These proteins are inactivated in basal conditions, while upon H2O2 stimulation, the autophagy-initiating complex may be activated to enhance the autophagy of ADSCs to protect them against oxidative stress.
The sphere formation of ADSCs on CS substrates was impaired by EGTA treatment (Fig. 2A), as observed in our previous report.24 Here we demonstrated that EGTA treatment also inhibited autophagy (Fig. 2B). Autophagy was induced from 2 h (Fig. 2A), but at this time cells had not aggregated. In another study, inhibition of basal autophagy in glioblastoma stem cells reduced the number of spheres.25 Furthermore, we used the low-adherent bacterial Petri dish (Petri dish) to examine the correlation between autophagy and spheroid formation. The spheroid formation of ADSCs was observed on Petri dish (Supplementary Data and Supplementary Fig. S1A). The LC3-II level of ADSCs on Petri dish was upregulated 2.5-folds than that in TCPS (Supplementary Fig. S1B). However, the LC3-II level of ADSCs on CS increased fourfolds than that in TCPS (Fig. 2B), and the size of ADSC spheroids on CS was more than that on Perti dish.26 These findings indicated that the level of autophagy is associated with the efficiency of spheroid formation.
Mammary epithelial cells grown on poly(2-hydroxyethyl methacrylate) (poly-HEMA)-coated plates detached and aggregated because of extracellular matrix deprivation.27 In 3D epithelial cultures, autophagy was induced to promote epithelial cell survival, but how the loss of integrin engagement may induce autophagy remains unclear.27,28 Activation of AMP-activated protein kinase (AMPK) in detached cells was a possible pathway.27 LKB1 activation or increase in Ca2+ could activate AMPK and subsequently inhibit mTORC1 to induce autophagy.7 Our previous study showed an upregulation of calcium-associated genes in CS-derived MSC spheroids.29 CS bound more calcium than TCPS.24 Many reports demonstrated that 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′–tetraaceticacid (BAPTA-AM), a Ca2+ chelator, blocked autophagy.30 In the current study, EGTA also decreased autophagy in a dose-dependent manner (Fig. 2B). These results suggested that Ca2+ may play a positive role in the autophagy induction of substrate-derived spheroids.
In conclusion, we found a new method to enhance the stem cell autophagy by 3D spheroid formation on CS substrates. The induced autophagy may equip MSCs with better survival and therapeutic efficacy once they are transplanted.
Supplementary Material
Abbreviations Used
- 3D
three-dimensional
- ADSCs
adipose-derived stem cells
- Ambra1
autophagy/beclin-1 regulator 1
- AMPK
AMP-activated protein kinase
- Atg13
autophagy-related protein 13
- CS
chitosan
- EGTA
ethylene glycol tetraacetic acid
- FBS
fetal bovine serum
- LC3
microtubule-associated protein 1 light chain 3
- MSCs
mesenchymal stem cells
- mTORC1
mammalian target of rapamycin complex 1
- TCPS
tissue culture polystyrene
- Ulk1
UNC51-like kinase 1
Acknowledgments
We thank Dr. Keng-fu Hsu for providing the GFP-LC3 plasmid. This work was supported by the Program for Stem Cell and Regenerative Medicine Frontier Research (NSC101-2321-B-002-039) sponsored by the National Science Council, Taiwan, ROC.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004;306:990–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mizushima N, Levine B. Autophagy in mammalian development and differentiation. Nat Cell Biol. 2010;12:823–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanida I. Autophagy basics. Microbiol Immunol. 2011;55:1–11 [DOI] [PubMed] [Google Scholar]
- 4.Nazio F, Strappazzon F, Antonioli M, et al. . mTOR inhibits autophagy by controlling ULK1 ubiquitylation, self-association and function through AMBRA1 and TRAF6. Nat Cell Biol. 2013;15:406–416 [DOI] [PubMed] [Google Scholar]
- 5.Kabeya Y, Mizushima N, Ueno T, et al. . LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghislat G, Patron M, Rizzuto R, et al. . Withdrawal of essential amino acids increases autophagy by a pathway involving Ca2+/calmodulin-dependent kinase kinase-β (CaMKK-β). J Biol Chem. 2012;287:38625–38636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kondratskyi A, Yassine M, Kondratska K, et al. . Calcium-permeable ion channels in control of autophagy and cancer. Front Physiol. 2013;4:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lotfinegad P, Shamsasenjan K, Movassaghpour A, et al. . Immunomodulatory nature and site specific affinity of mesenchymal stem cells: a hope in cell therapy. Adv Pharm Bull. 2014;4:5–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mastri M, Lin H, Lee T. Enhancing the efficacy of mesenchymal stem cell therapy. World J Stem Cells. 2014;6:82–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herberg S, Shi X, Johnson MH, et al. . Stromal cell-derived factor-1β mediates cell survival through enhancing autophagy in bone marrow-derived mesenchymal stem cells. PLoS One. 2013;8:e58207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Q, Yang YJ, Wang H, et al. . Autophagy activation: a novel mechanism of atorvastatin to protect mesenchymal stem cells from hypoxia and serum deprivation via AMP-activated protein kinase/mammalian target of rapamycin pathway. Stem Cells Dev. 2012;21:1321–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L, Hu X, Zhu W, et al. . Increased leptin by hypoxic-preconditioning promotes autophagy of mesenchymal stem cells and protects them from apoptosis. Sci China Life Sci. 2014;57:171–180 [DOI] [PubMed] [Google Scholar]
- 13.Huang GS, Dai LG, Yen BL, et al. . Spheroid formation of mesenchymal stem cells on chitosan and chitosan-hyaluronan membranes. Biomaterials. 2011;32:6929–6945 [DOI] [PubMed] [Google Scholar]
- 14.Hsu SH, Huang GS, Feng F. Isolation of the multipotent MSC subpopulation from human gingival fibroblasts by culturing on chitosan membranes. Biomaterials. 2012;33:2642–2655 [DOI] [PubMed] [Google Scholar]
- 15.Hsu SH, Huang GS. Substrate-dependent Wnt signaling in MSC differentiation within biomaterial-derived 3D spheroids. Biomaterials. 2013;34:4725–4738 [DOI] [PubMed] [Google Scholar]
- 16.Tseng TC, Hsu SH. Substrate-mediated nanoparticle/gene delivery to MSC spheroids and their applications in peripheral nerve regeneration. Biomaterials. 2014;35:2630–2641 [DOI] [PubMed] [Google Scholar]
- 17.Liu BH, Yeh HY, Lin YC, et al. . Spheroid formation and enhanced cardiomyogenic potential of adipose-derived stem cells grown on chitosan. Biores Open Access. 2013;2:28–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408 [DOI] [PubMed] [Google Scholar]
- 19.Sart S, Ma T, Li Y. Preconditioning stem cells for in vivo delivery. Biores Open Access. 2014;3:137–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haque N, Rahman MT, Abu Kasim NH, et al. . Hypoxic culture conditions as a solution for mesenchymal stem cell based regenerative therapy. ScientificWorldJournal. 2013:632972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eisenberg T, Knauer H, Schauer A, et al. . Induction of autophagy by spermidine promotes longevity. Nat Cell Biol. 2009;11:1305–1314 [DOI] [PubMed] [Google Scholar]
- 22.Howitz KT, Bitterman KJ, Cohen HY, et al. . Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196 [DOI] [PubMed] [Google Scholar]
- 23.Wood JG, Rogina B, Lavu S, et al. . Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689 [DOI] [PubMed] [Google Scholar]
- 24.Yeh HY, Liu BH, Hsu SH. The calcium-dependent regulation of spheroid formation and cardiomyogenic differentiation for MSCs on chitosan membranes. Biomaterials. 2012;33:8943–8954 [DOI] [PubMed] [Google Scholar]
- 25.Filippi-Chiela EC, Villodre ES, Zamin LL, et al. . Autophagy interplay with apoptosis and cell cycle regulation in the growth inhibiting effect of resveratrol in glioma cells. PLoS One. 2011;6:e20849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang GS, Hsieh PS, Tseng CS, et al. . The substrate-dependent regeneration capacity of mesenchymal stem cell spheroids derived on various biomaterial surfaces. Biomater Sci. 2014:2:1652–1660 [DOI] [PubMed] [Google Scholar]
- 27.Fung C, Lock R, Gao S, et al. . Induction of autophagy during extracellular matrix detachment promotes cell survival. Mol Biol Cell. 2008;19:797–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lock R, Debnath J. Extracellular matrix regulation of autophagy. Curr Opin Cell Biol. 2008;20:583–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yeh HY, Liu BH, Sieber M, et al. . Substrate-dependent gene regulation of self-assembled human MSC spheroids on chitosan membranes. BMC Genomics. 2014;15:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Decuypere JP, Bultynck G, Parys JB. A dual role for Ca(2+) in autophagy regulation. Cell Calcium. 2011;50:242–250 [DOI] [PubMed] [Google Scholar]
References
Cite this article as: Yang CM, Huang YJ, Hsu SH (2015) Enhanced autophagy of adipose-derived stem cells grown on chitosan substrates, BioResearch Open Access 4:1, 89–96, DOI: 10.1089/biores.2014.0032.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.