Abstract
B cells differentiate from pluripotent hematopoietic stem cells (pHSCs) in a series of distinct stages. During early embryonic development, pHSCs migrate into the fetal liver, where they develop and mature to B cells in a transient wave, which preferentially populates epithelia and lung as well as gut-associated lymphoid tissues. This is followed by continuous B cell development throughout life in the bone marrow to immature B cells that migrate to secondary lymphoid tissues, where they mature. At early stages of development, before B cell maturation, the gene loci encoding the heavy and light chains of immunoglobulin that determine the B cell receptor composition undergo stepwise rearrangements of variable region-encoding gene segments. Throughout life, these gene rearrangements continuously generate B cell repertoires capable of recognizing a plethora of self-antigens and non–self-antigens. The microenvironment in which these B cell repertoires develop provide signaling molecules that play critical roles in promoting gene rearrangements, proliferation, survival, or apoptosis, and that help to distinguish self-reactive from non–self-reactive B cells at four distinct checkpoints. This refinement of the B cell repertoire directly contributes to immunity, and defects in the process contribute to autoimmune disease.
Introduction
Non-hematopoietic microenvironments allow multipotent hematopoietic progenitors to migrate first into fetal liver and later into bone marrow, where they become resident in new non-hematopoietic microenvironments to develop along the B lineage pathway. There, stepwise V(D)J rearrangements of Ig genes first generate IgH chain–expressing precursors. At a first checkpoint, the surrogate light chain (SLC) probes IgH fitness to pair with an IgL chain, and a pre–B cell receptor (pre-BCR) is formed. A second checkpoint interrogates the pre-BCR for autoreactivity of the IgH chain. Subsequently, if IgL chains with light-chain variable (VL) regions are expressed that fit the pre-expressed heavy-chain variable (VH) region of the IgH chain, then IgM is displayed as a BCR on immature B cells, with each B cell expressing only one BCR.
The newly generated VH/VL-repertoires of immature B cells then enter the third checkpoint, where autoantigens are presented. B cells expressing high-affinity autoreactive BCRs are deleted. B cells expressing low-affinity autoreactive BCRs are positively selected to exit the bone marrow and enter the peripheral pools as BI-type B cells, especially of the gut- and lung-associated lymphoid tissues. B cells unable to recognize autoantigens, which are ignored by the repertoire-selecting, autoantigen-presenting microenvironment, also enter the peripheral mature B cell pools to become organized as conventional, BII-type cells in B cell follicles of the spleen and lymph nodes. Over 85% of the newly formed immature B cells die in bone marrow, probably as a consequence of this autoantigen recognition. The cells of the microenvironment that generate “central tolerance” to autoantigens in bone marrow at the last two checkpoints, and their molecular modes of autoantigen presentation still need more detailed characterization.
In the spleen, a fourth checkpoint monitors B cells in transition from immature to mature cells. Only mature B cells that appear in the peripheral pools can be probed for their capacity to recognize foreign antigens. The responding B cells are propagated by an antigen-presenting microenvironment, which drives proliferation, hypermutation to induce a better fit for the foreign antigen, and longevity of the fully developed, foreign antigen–specific memory B cells. Any B cells that become autoreactive through hypermutation may instigate autoimmune disease, and they must be eliminated or suppressed by the microenvironments. The mechanisms whereby these microenvironments promote elimination of autoreactive B cells need further characterization. This Review describes the major steps in the molecular and cellular development of antigen-recognizing B lymphocytes in the environments of fetal liver and adult bone marrow.
In the immune system, pools of nearly 109 B lymphocytes in a mouse (nearly 1012 in a human adult) have half-lives that can vary from a few days for newly generated, antigen-sensitive but inexperienced B cells to the lifetime of the organism for memory B cells (1–3). B cells are continuously generated from pluripotent HSCs (pHSCs), multipotent myeloid/lymphoid progenitors (MPPs), common lymphoid progenitors (CLPs), and pro-B and pre-B cells (4). pHSCs are self-renewing, can differentiate to all lineages of blood cells, including B cells, and can migrate back to their specialized microenvironment or niche in the bone marrow. Upon transplantation into a genetically or experimentally immunodeficient recipient, one pHSC can reconstitute all functional B cell pools and serve as a long-term repopulating HSC (LT-HSC) in subsequent transplantations. B cells develop at different sites in the body, which implies that different microenvironments influence different hematopoietic and lymphopoietic stages of this development. The developing pHSCs must be mobile, because they have to migrate from one site to the next, while their microenvironments are sessile. Residence at a given site determines their capacity to continue their differentiation. In an inappropriate microenvironment, B lineage cells will not develop further, while a microenvironment that presents autoantigens can inhibit autoreactive B cells through central deletion, select autoreactive B cells through positive selection, or ignore non-autoreactive B cells. Hence, all microenvironments that select B cell repertoires should have the capacity to decide whether a B cell is to survive or to die.
Embryonic development of the first B cell repertoires
The mouse embryo is colonized by waves of hematopoietic cell development (5–7). The first wave, called primitive hematopoiesis, begins at E7.5 in the extra-embryonic environment of the yolk sac (Figure 1). Almost nothing is known about this environment, which generates fetal-type hemoglobin-expressing erythrocytes, megakaryocytes, platelets, and special types of myeloid cells, the latter of which have unusual longevity (e.g., glial cells in the brain) (8, 9). Although these cells have T- and B-lymphoid developmental capacities (10) and can be found in embryonic primary lymphoid organs (11), it is not known whether or how they contribute to early B cell development in the mouse. The second and third wave of now-definitive hematopoiesis originate intra-embryonically at E10.5 from the aorta-gonad-mesonephros area (5, 12), where undifferentiated pHSCs (13–15) migrate through embryonic blood to the developing rudiments of fetal liver, omentum, thymus, and bone marrow. In fact, pHSCs and other hematopoietic progenitors have been seen in close contact with endothelium of the inside of embryonic blood vessels (15). Embryonic endothelium has been found to produce c-kit ligand (KIT-L), an essential cytokine for pHSCs and progenitors, as well as CXCL10, a chemokine that from E12.5 onward might attract these hematopoietic progenitors to transmigrate from blood into the developing primary organ, notably as the second B-lymphopoietic wave in fetal liver (11) and bone marrow.
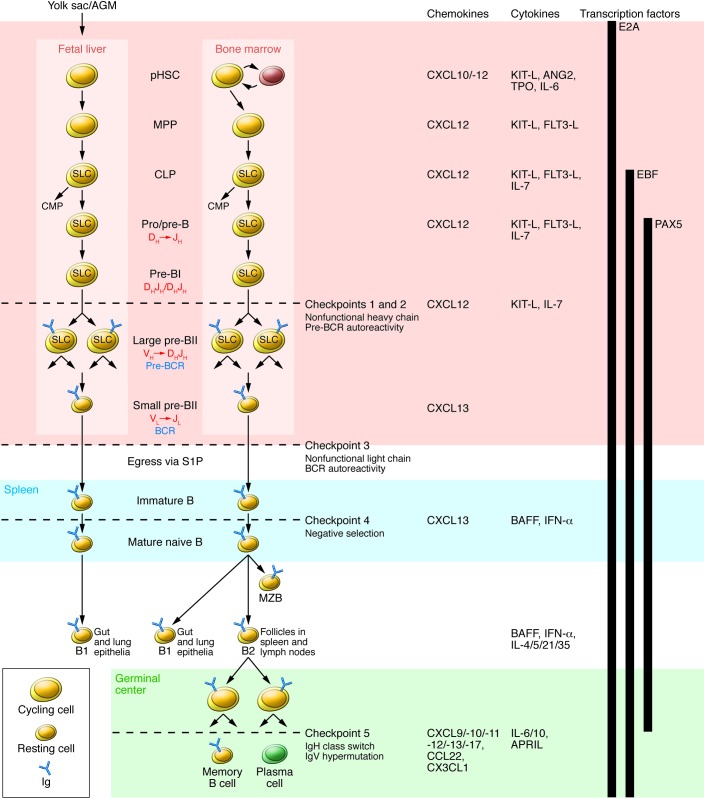
Figure 1. B cell development in fetal liver and bone marrow.
pHSC progenitors originate extraembyronically from yolk sac and, at later stages of development, intraembyronically from the aorta-gonad-mesonephros (AGM) region. pHSCs develop in the fetal liver prenatally and in bone marrow postnatally; both of these environments (pink region) provide crucial transcription factors, chemokines, cytokines, and cell contacts that regulate differentiation. Additionally, pHSCs localize to specialized niches that allow for their long-term survival in bone marrow but not in fetal liver. Expression of the transcription factor E2A and the V(D)J rearrangement machinery RAG1/2 restricts CLPs from developing into B lineage cells. BCRs are generated by stepwise rearrangements of Ig segments. Pre-B cells that have undergone productive VHDHJH rearrangement express an Igμ chain that can pair with SLC to form a pre-BCR, which stimulates large pre-B cell proliferation. Lastly, VL-to-JL rearrangement occurs in small pre-BII cells, which become surface BCR–expressing immature B cells. They then enter the spleen (blue region), where fetal liver–derived BI cells predominately populate the gut and lung epithelia as mature B cells. Bone marrow–derived B cells mature in spleen to BI cells, which populate the lung and epithelia; MZB cells, which populate the marginal zone; and BII cells, which are organized in B cell-rich follicles where T cell-dependent antigenic stimulation promotes development of germinal centers (green region). Antigen-specific follicular helper T cells induce B cell Ig class switching and IgV-region hypermutation and help to develop memory B cells and plasma cells. The developing B cell repertoires are monitored for structural fitness and autoreactivity at five checkpoints. CMP, common myeloid progenitor.
B cell development in fetal liver
Once inside fetal liver, MPPs and CLPs express cytokine receptors, the M-CSF receptor C-FMS for myeloid-directed differentiation and IL-7Rα for lymphoid-directed differentiation. The progenitors establish contact with mesenchymal and epithelial microenvironments, which produce the chemokines CXCL10, CXCL12, CCL7, CCL9, and CX3CL1 to attract the hematopoietic progenitors. They also produce KITLG, M-CSF, and IL-7 (Figure 1 and ref. 11). Early FLT3+ c-kit+ IL-7Rα+ CD19– progenitors also require Fms-related tyrosine kinase 3 ligand (FLT3LG) to differentiate to CD19+ progenitors (16), but it remains to be determined which cell type contributes to this part of B cell development, as mesenchymal stromal cells do not participate (11).
In these fetal liver microenvironments, commitment to B-lymphoid cell differentiation begins with the expression of the transcription factor E2A and of the V(D)J-rearrangement machinery RAG1 and RAG2. It continues with the expression of the transcription factor EBF, the pre-BCR– and BCR-anchoring molecules Igα and Igβ, and the SLC components VpreB and λ5, followed by the opening of the two IgH-chain alleles for DH-to-JH rearrangements. Final commitment to B lineage differentiation is induced by the expression of the transcription factor Pax5 (17, 18). Proliferation of CD19+ DH/JH-rearranged c-kit+ IL-7Rα+ pre-BI cells is stimulated by KITLG and IL-7, which are provided by the microenvironment of mesenchymal stromal cells. IL-7 inhibits apoptosis and further differentiation (19). Proliferation and differentiation of CLP, pro-B, and pre-B cells is seen between E14.5 and E16.5, when these cells have become resident in the mesenchymal microenvironment (11).
B cell development in the bone marrow
From E17.5 onward, bone marrow becomes the major site of the third wave of continuous hematopoiesis, which generates B cells throughout life. Again, early V(D)J-rearranging B cell progenitors are found in close proximity to IL-7–producing mesenchymal stromal cells, and CXCR4/CXCL12-guided chemoattraction is an important molecular mode of interaction between B cell progenitors and their inductive microenvironment (20). Less is known about the additional chemokines that might participate in this process.
Development of B cells in fetal liver and in bone marrow differs by at least three properties that influence the generation and selection of B cell repertoires. First, unlike the fetal liver, the bone marrow supports life-long generation of B cells from pHSCs. Second, the bone marrow supports the generation of more diverse antigen-binding complementarity determining region 3 (CDR3) VH regions. In humans, N-region insertions contribute to VL domain diversity. Third, the bone marrow supports the development of peripheral pools of B cells (Figure 1). Each of these points are discussed in detail below.
B cell development in bone marrow is continuous throughout life (21) because pHSCs find niches in which they can reside for the life span of the organism. In contrast, B lymphopoiesis in fetal liver is transient, and there are not niches that provide long-term residence. In fact, bone marrow appears to provide niches in which not only pHSCs, but also long-lived memory T lymphocytes and plasma cells, can survive for extended periods of time without cell proliferation or cell death (22, 23).
Chemoattraction via CXCR4 expressed on hematopoietic cells and CXCL12 produced by the microenvironmental stromal cells is important in the formation of these niches. Long-term resting pHSCs are found at hypoxic, sub-endosteal sites near intra-bone surfaces, in close contact with non-hematopoietic stromal cells (21, 24–28). This secures continuous, life-long generation of new MPPs, CLPs, and common myeloid progenitors (CMP), and hence also B lymphocytes (21). Since only a fraction of the pool of LT-pHSCs is mobilized to participate in hematopoiesis at any given time, the pool of pHSCs might be protected from adverse somatic mutations that could affect normal B lymphopoiesis and its repertoire development. When mobilized, cycling pHSCs repair most of the somatically acquired mutations. As activated pHSCs are able to revert back to a long-term resting state, their somatically mutated genetic constitution might increase the chances for abnormal hematopoiesis, lymphopoiesis, and B cell repertoire generation with age (29).
pHSCs and MPPs might find the appropriate niches to persist throughout life in bone marrow, in compartments composed of RANKL-producing osteoblasts (30, 31) and of high CXCL12-expressing VCAM1+ PECAM1-reticular cells (20). Environments that induce B lymphopoiesis from CLP and pro-/pre-B cells are expected to provide CXCL12 for attraction to sites that produce the cytokine IL-7. In contrast to ALCAM+ mesenchymal fetal liver cells, which have been found to produce both IL-7 and CXCL12, in bone marrow IL-7 is not produced by reticular cells expressing high levels of CXCL12, but by reticular cells expression low levels of CXCL12. (20). Therefore, it is possible that B cell development from CLP and pro-/pre-B cells is supported by a different environment in fetal liver than in bone marrow. Nevertheless, in the end, they respond to the same proliferation-inducing stimuli, as DH-JH-/DH-JH-rearranged pre-BI cells from fetal liver as well as bone marrow can be established as long-term proliferating cell lines on mesenchymal stromal cells in the presence of IL-7 (32).
IL-7–deficient and IL-7 receptor–deficient mice are severely deficient in B cell development, which supports the notion that IL-7 has a central role in B cell development at the transition from MPP to CLP and pro-/pre-B and pre-BI cells (33, 34). On the other hand, human B cells develop without IL-7, which suggests that thymic stromal lymphopoietin or another yet unknown cytokine fulfills this role in B cell development of humans (35).
Checkpoint 1 monitors VH SLC fitness
Once pre-BI cells lose contact with the KITLG- and IL-7–providing microenvironment, they are induced to differentiate: c-KIT and IL-7Rα are downregulated, KITLG/IL-7–dependent proliferation is terminated, and apoptosis is induced (32). This happens in fetal liver as well as in bone marrow. At the same time, VH- to DHJH-rearrangements are induced on one IgH chain allele. One of three such V(D)J rearrangements occur “in frame” and generate an IgH locus that can not only be transcribed, but also translated into an IgH chain. Each rearrangement in a single pre-BII cell is unique, combining one given DH-JH segment with one of many VH segments, thereby generating diverse repertoires of antigen-binding VH domains on μH chains in the developing repertoire of pre-B cells. In fetal liver, rearrangements do not generate N-region sequences at the V(D)J joints because the enzyme terminal desoxynucleotidyl transferase (TdT) is not expressed. Thus, the generated pre-BCR, and later BCR repertoires, will be diverse, autoreactive, and even polyreactive (36–39), but probably less so than the N-region–containing pre-BCRs and BCRs generated later in bone marrow, where TdT is expressed.
The μH chains — even without forming a pre-BCR with SLC — can be deposited in cell membranes. They signal the pre-B cell to close the second allele, which is often DH-JH rearranged (as both alleles in μH-chain transgenic mice typically are) to avoid expression of two IgH chains by one B lineage cell (40–42). This allelic exclusion is very efficient: at least 98.5% B lineage cells express only one IgH chain. Furthermore, to avoid VH- to DHJH-rearrangements at the second allele, RAG expression is downregulated (43, 44). The newly generated VH domains of the IgH chains are probed by SLC for fitness, to form a pre-BCR on the surface of pre-B cells in apparent anticipation of the later need to form a BCR with IgL chains.
The role of the pre-BCR has been studied in more detail in bone marrow. Depending on the relative fitness of the pre-BCR, pre-B cells enter between two and seven cell divisions stimulated by the pre-BCR, thereby counteracting apoptosis (45). As pre-BCR+, large, cycling pre-BII cells are formed, c-kit and IL-7Rα are downregulated and CD25 is upregulated. The pre-BCR also signals the cell to downregulate SLC (46, 47). Therefore, SLC becomes limiting as pre-B cells divide, so that sufficient numbers of pre-BCRs can no longer be formed, and the pre-B cell ceases to proliferate and becomes a resting, small pre-BII cell. Ultimately, one original pre-B cell expresses one unique IgH chain. Thereafter, a fitting pre-BCR may expand these unique IgH chain–expressing cells up to 100-fold, but each cell resulting from such a pre-B cell clone will then enter a separate, unique VL-to-JL rearrangement on the κ-IGL– or λ-IgL–chain locus. Surprisingly, as many as 50% to 70% of all newly formed IgμH chains do not pair and thus cannot form a pre-BCR on the cell surface. Hence, pre-B cells that express such μH chains do not proliferate, instead entering apoptosis. A subset of pre-B cells differentiates to resting, small pre-B cells, as all of pre-B cells do when they cannot form a pre-BCR, as in SLC-deficient mice (40, 48). It follows, then, that the contribution of the pre-BCR– cells to the total repertoire of small pre-B cells should be at least 20- to 40-fold lower than that of pre-BCR+ cells. In humans, lack of SLC expression results in severe B cell deficiencies (49). On the other hand, some species do not express SLC. It follows that SLC improves B cell development in mice and humans, but it is not mandatory, as its absence reduces but does not fully abrogate the generation of B cells.
Checkpoint 2: recognition of autoantigens by pre-BCRs?
A special, as yet unidentified microenvironment is expected to provide contacts, possibly via the non-Ig parts of the V pre-B and λ5 subunits of SLC, which are expected to bind to heparan sulfate–containing (50) or galectin-1–containing (51, 52) molecules within the microenvironment. This binding is likely to be modulated by the non-Ig parts of SLC, since pre-BCR surface deposition depends on these subunits (53). The non-Ig parts contain arginines, which in the case of the λ5-subunit of SLC have been shown to signal pre-B cells (54). This result suggests that most pre-BcRs do not recognize autoantigens via the CDRs of the VH domains of their μH chains. However, this does not rule out that some μH chains might have arginine residues in longer CDR3 regions, which in addition to or instead of the non-Ig part of λ5 could make contact with heparan sulfates or similarly charged molecules (e.g., nucleic acids) presented on stromal cells of the microenvironment. The recognition of such nuclear antigens results in positive or negative selection of the corresponding pre-B cells (55, 56), which in turn may influence the repertoire of antinuclear antigen–specific antibodies in the periphery (57).
Rearrangements at the IgL chain loci in autoreactive B cell development
As large pre-B cells exit the cell cycle and become small, resting pre-BII cells, the expression of RAG1/2 is reactivated, the IgL chain gene loci are opened, and VL segments are rearranged to JL segments (58). The first soluble IgM+ B cells appear in fetal liver between E16.5 and E17.5. Many of them are later found in the periphery, mostly as BIa cells in intraepithelial sites and in gut-associated lymphoid tissues. They secrete IgM, IgA, and IgG, sometimes called “natural” antibodies, which circulate in blood as a first line of humoral defense. It is not known whether the newly generated repertoire of B cells in fetal liver is screened for autoreactivity as it is in bone marrow at the third checkpoint (see below).
Checkpoint 3 monitors BCRs produced after IgL VL-JL gene rearrangements
Rearrangements of the IgL chain loci occur after pre-BCR expression has been terminated by downregulation of SLC expression and pre-BII cells have undergone either proliferative expansion or differentiation into resting, small pre-BII cells. The IgL-chain loci are opened for sterile transcription, the RAG loci are re-expressed, and VL-JL segments are rearranged (58, 59). DH-JH–rearranged IgH chain loci remain closed in order to maintain allelic exclusion at this locus. Deletions of genes controlling the expression or the function of the adaptor protein SLP65 abolishes SLC downregulation, leads to continued pre-BCR expression, and results in pre-B cell hyperplasia (60, 61). Such mutations affecting the function of the pre-BCR are expected to influence the emerging IgH chain repertoires and, thus, the presence and maintenance of autoreactive pre-BCRs.
Whenever the VL- JL rearrangement is productive and results in the expression of an IgL chain fit for BCR formation and the BcR is not autoreactive, the cell turns off RAG expression and its access to the IgL chain loci, possibly by “tonic” signaling (62). These immature B cells remain in a resting state and are thought to egress from the bone marrow via blood to the spleen.
In pre-B cells with non-productively rearranged IgL chain loci, the IgL chain loci remain accessible for secondary VL-JL rearrangements. These arrangements can occur on both alleles, and also on both κ- and λL-chain loci, until a productive rearrangement has led to the expression of an IgL chain that pairs with the pre-expressed IgH chain to form a BcR on the surface of the B cell. RAG expression and accessibility to IgL chain loci are also maintained in immature B cells, which express an autoreactive BCR that recognizes autoantigen that is supposedly present in the developmental microenvironment. The autoreactivity induces further VL-JL rearrangements as long as this editing of the IgL chain does not lead to the loss of autoreactivity (38, 59, 63–65). VH replacements on V(D)J-rearranged IgH chain loci may occur at this stage of B cell development, so that an autoreactive BCR becomes capable of recognizing a non–self-antigen. If editing does not change the specificity of the BCR, the immature B cell will be deleted. Continued, secondary rearrangements at the IgL chain loci can lead to two or more different IgL chains in one B cell (66–68). As a result, a B cell can express one non-autoreactive, one autoreactive, and even one polyreactive BCR (69). In the pools of peripheral B cells, up to 5% of all cells have been found to express more than one IgL chain.
Binding to autoantigens such as insulin and DNA, and to foreign antigens such as viruses and bacteria, has been used to probe antibody repertoires for the existence of autoreactive and non-autoreactive antibodies (39). This analysis has reinforced previous findings that the newly generated B cell repertoire consists not only of foreign antigen–reactive, non-autoreactive, and autoantigen-reactive antibodies, but also of polyreactive antibodies (70). It has been estimated that more than half of the newly generated antibodies can bind autoantigens (71). More than half of all BI cells appear to be in a stimulated, G1-like stage of the cell cycle; autoreactive and polyreactive antibodies are found in this BI population (72). Such B cells may be more easily activated in autoimmune diseases.
Many polyreactive, but not all autoreactive, human B cells are lost when immature sIgM+ cells develop from pre-BII cells. However, they are not lost in patients with SLE and RA (39), suggesting that the formation of the pre-BCR at checkpoint 2 and of the BCR at checkpoint 3 strongly influences the development of B cell repertoires. The use of VH families in repertoires expressed by immature B cells in bone marrow and by immature and mature B cells in spleen are not significantly different, indicating that polyreactive and autoreactive BCRs do not use special subsets of VH domains (73, 74). It is surprising how few of all the newly, continuously generated B cells — as little as 15% — manage to leave the bone marrow via the blood (75). Hence, negative selection, defined as the establishment of central B cell tolerance, extinguishes much of the diversity of the original B cell repertoire made in bone marrow.
Egress of the remaining B cell repertoire from bone marrow through blood into spleen is directed by sphingosine-1-phosphate–mediated (S1P-mediated) chemoattraction (76, 77), which overcomes the CXCR4/CXCL12-mediated retention that is effective during earlier stages of B cell development. Two chemokine receptors, CXCR5 and CCR7, are essential to organize B cells into follicular regions (78).
Checkpoint 4 monitors immature B cells in spleen
The newly arrived B cells in spleen are not yet mature cells that respond to antigenic stimulation with proliferation and differentiation to memory B cells and plasma cells. Instead, antigen can induce their anergy and apoptosis (75). Hence, a fourth checkpoint can screen potentially autoreactive B cells and eliminate them before they become mature. However, in mice, very little cell loss is detected during the transition from immature transitional T1 and T2 cells to mature B cells (73). In contrast, a further reduction from 40% to 20% of autoreactive B cells has been observed in humans (39). Again, patients with SLE or RA fail to reduce the presence of autoreactive B cells at this peripheral, fourth checkpoint (79). This failure to eliminate autoreactive B cells at checkpoints 2, 3, and 4 is likely to contribute to the development of SLE and RA.
Microenvironments that control checkpoints 2 and 3 in bone marrow
Little is known about the microenvironments in bone marrow that present autoantigens to the developing B cell repertoires at checkpoints 2 and 3. Genetic deficiencies that result in the development of SLE, as in the complement components C1q, C4, and CR2, serum IgM, or serum amyloid protein, have been used to formulate two models to describe the development of systemic autoimmune diseases characterized by the production of autoantibodies to nuclear antigens such as DNA. One model (80) proposes that macrophages, expressing C1q, CR1, and CR2 remove apoptotic cells complexed with serum IgM, C1q, and C4b, thereby preventing the accumulation of nuclear antigen and the subsequent activation of mature B cells. However, this model does not explain the observed deletions in poly- and autoreactive B cell repertoires. The other model (81) assumes that autoantigens (e.g., from apoptotic cells) are bound to natural antibodies, i.e., in immune complexes with serum IgM, C1q, and C4b on cells present in the microenvironment, which are expected to express the appropriate receptors C1qR, CR1, CR2, and FcRμ. In this model, autoantigen recognition by pre-BCRs and BCRs on pre-BII and immature B cells depends on the avidity of the autoantigen to a given pre-BCR or BCR. High avidity induces pre-B or B cell apoptosis (negative selection), while low avidity increases survival of pre-B and B cells (positive selection). No avidity, or lack of recognition, allows the ignored cells to migrate to the spleen, at least in part to organize the follicular regions of the BII compartments. The second model expects molecular modes of B cell/microenvironmental cell interactions to influence the survival of B cells. This influence can be positive (stimulated by the survival cytokine BAFF; ref. 82) or negative (mediated in an IFN-dependent manner; refs. 83, 84). The microenvironment of checkpoint 4 presenting autoantigens in the spleen is largely unknown. The cooperation of developing B cells with their microenvironments to express and select the pre-BCR and BCR repertoires at three checkpoints predicts a multitude of genetic abnormalities, expressed in either the B-lymphopoietic cells or in their microenvironment, which will contribute to systemic autoimmune disease.
Establishment of peripheral pools of mature B cells
The third difference between B cell development in fetal liver and bone marrow is the destination of the emerging B cell repertoires, which populate different peripheral sites of the immune system. Fetal liver predominantly generates BIa B cells, which are found in epithelia and in lung- and gut-associated lymphoid tissues. These B cells persist after birth, are mostly resting, and are radio resistant. The precursors of BIa B cells disappear early in life (10). Precursors of later B cell development, generated from pHSCs in bone marrow and used in the clinical setting of bone marrow transplantation to restore hematopoiesis and lymphopoiesis, can no longer develop these long-lived BIa cells. By contrast, bone marrow–derived pHSCs give rise to shorter-lived BIb cells, which have homing properties that are similar to BIa cells. Most importantly, bone marrow develops BII-type B lymphocytes, which organize the B cell follicles in spleen and lymph nodes (85, 86). Thus, transplantation of E13.5 pHSC-like cells from fetal liver preferentially repopulates the host with BIa cells. In contrast, transplantation of pHSCs and progenitors from adult bone marrow preferentially repopulates the host with BII cells. Bone marrow retains the capacity to repopulate the host, though the numbers of pHSCs decrease with increasing age by at least 100-fold in one year of the life of a mouse. Bone marrow also generates B cells that can be found in the marginal zone of spleen as marginal zone B (MZB) cells.
Checkpoint 5: improving antibodies and eliminating autoantibodies
Activated follicular BII cells can form germinal centers with the help of follicular helper T cells. Germinal centers generate B cell memory (i.e., B cells with an improved affinity for the stimulating antigen) with a changed effector activity and an extended life expectancy (87, 88). In the dark zone of germinal centers, these B cells are stimulated by antigen to proliferate. In proliferating B cells, activation-induced cytidine deaminase (AID) expression is induced. AID mediates IgH chain gene class switching to IgG, IgA, and IgE and induces hypermutation in VH and VL regions of IgH and IgL chain genes. Hence, B cells change their effector class of antibodies and their antigen recognition capacity. Better antigen recognition is selected by antigen-antibody complexes presented on follicular dendritic cells in the light zone of the germinal center, possibly by induction of longevity in the hypermutated, better-fitting B cells.
Any high-affinity, autoreactive B cells that emerge as accidental products of the hypermutation process during this affinity maturation of better-fitting antibodies should be silenced and eliminated to avoid autoimmune disease (89–92). Again, the microenvironments mediating this postulated negative selection of autoantibodies need further investigation.
Memory B cells can be generated inside as well as outside of the germinal centers (93).
BI and BII B cell compartments can also develop memory with B cells and antibody-secreting plasma cells for an antigen. BI B cells may need continued stimulation, hence the persistence of an antigen, while BII B cells appear to be long lived in the absence of antigen.
Diversity of recognition — an evolutionary accident with advantages and disadvantages
The RAG1 and RAG2 subunits of the rearrangement machinery are the generators of diversity (GODs). Up to the point of hematopoietic development of an MPP, these subunits are not yet activated and the IgH and IgL chain gene loci, with their V(D)J-segmented V region, are not yet open for RAG action. This closed and inactive state allows for the formation of innate lymphocytes (94). It must have been an evolutionary catastrophe when, during phylogeny, the three elements of GODs (the RAG genes, picked up from microbes; a gene encoding a IgV-like domain, broken at the CDR3 site; and sequences at the edges of the broken V[D]J segments, which allowed rejoining of the broken segments by RAG) evolved to be co-expressed together in MPPs. Thus, when the RAG machinery became expressed in CLP-like progenitors and the evolutionary predecessors of either the T cell receptor gene loci or the BCR loci became accessible for this machinery, the joining of the segmented V gene created variability for contacts at the CDR3 region so that it no longer bound only the inherited ligand but, by chance, a foreign or a self-antigen. The reaction of the new lymphocytes might still have been “innate,” but the recognition had become diverse and adaptive to both self and non-self. Subsequently, this heritable potential for variability was greatly expanded by V segment multiplications for binding capacities to CDR1 and CDR2 regions of the V domains and for binding to CDR3 by the generation of multiple heritable D segments, as well as by the action of TdT. Today, it is clear that the signaling functions of the families of V region–containing molecules (BCRs and TCRs) determine proliferation, survival, and apoptosis of lymphocytes. Moreover, the fate of these cells is determined by the avidities of interactions of each BCR and TCR, not only with the original interaction partner, but to the universe of available antigens.
Acknowledgments
The author thanks the Max Planck Society for support as a Max Planck Fellow and Senior Group Leader at the Max Planck Institute for Infection Biology, and the DFG for research support in the Kosellek program (grant ME-2464/1-1). He also thanks Tobias Haendel for his help in preparing the manuscript.
Footnotes
Conflict of interest: The author has declared that no conflict of interest exists.
Reference information:J Clin Invest. 2015;125(6):2203–2210. doi:10.1172/JCI78083.
References
- 1.Rolink AG, Schaniel C, Busslinger M, Nutt SL, Melchers F. Fidelity and infidelity in commitment to B-lymphocyte lineage development. Immunol Rev. 2000;175:104–111. [PubMed] [Google Scholar]
- 2.Rolink AG, Schaniel C, Andersson J, Melchers F. Selection events operating at various stages in B cell development. Curr Opin Immunol. 2001;13(2):202–207. doi: 10.1016/S0952-7915(00)00205-3. [DOI] [PubMed] [Google Scholar]
- 3.Melchers F, et al. Repertoire selection by pre-B-cell receptors and B-cell receptors, and genetic control of B-cell development from immature to mature B cells. Immunol Rev. 2000;175:33–46. [PubMed] [Google Scholar]
- 4.Akashi K, et al. Lymphoid development from stem cells and the common lymphocyte progenitors. Cold Spring Harb Symp Quant Biol. 1999;64:1–12. doi: 10.1101/sqb.1999.64.1. [DOI] [PubMed] [Google Scholar]
- 5.Cumano A, Ferraz JC, Klaine M, Di Santo JP, Godin I. Intraembryonic, but not yolk sac hematopoietic precursors, isolated before circulation, provide long-term multilineage reconstitution. Immunity. 2001;15(3):477–485. doi: 10.1016/S1074-7613(01)00190-X. [DOI] [PubMed] [Google Scholar]
- 6.Ling KW, Dzierzak E. Ontogeny and genetics of the hemato/lymphopoietic system. Curr Opin Immunol. 2002;14(2):186–191. doi: 10.1016/S0952-7915(02)00320-5. [DOI] [PubMed] [Google Scholar]
- 7.Godin I, Cumano A. The hare and the tortoise: an embryonic haematopoietic race. Nat Rev Immunol. 2002;2(8):593–604. doi: 10.1038/nri857. [DOI] [PubMed] [Google Scholar]
- 8.Irion S, et al. Temporal specification of blood progenitors from mouse embryonic stem cells and induced pluripotent stem cells. Development. 2010;137(17):2829–2839. doi: 10.1242/dev.042119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schulz C, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336(6077):86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 10.Böiers C, et al. Lymphomyeloid contribution of an immune-restricted progenitor emerging prior to definitive hematopoietic stem cells. Cell Stem Cell. 2013;13(5):535–548. doi: 10.1016/j.stem.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Tsuneto M, et al. B-cell progenitors and precursors change their microenvironment in fetal liver during early development. Stem Cells. 2013;31(12):2800–2812. doi: 10.1002/stem.1421. [DOI] [PubMed] [Google Scholar]
- 12.Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86(6):897–906. doi: 10.1016/S0092-8674(00)80165-8. [DOI] [PubMed] [Google Scholar]
- 13.Ohmura K, et al. Immature multipotent hemopoietic progenitors lacking long-term bone marrow-reconstituting activity in the aorta-gonad-mesonephros region of murine day 10 fetuses. J Immunol. 2001;166(5):3290–3296. doi: 10.4049/jimmunol.166.5.3290. [DOI] [PubMed] [Google Scholar]
- 14.Cumano A, Godin I. Ontogeny of the hematopoietic system. Annu Rev Immunol. 2007;25:745–785. doi: 10.1146/annurev.immunol.25.022106.141538. [DOI] [PubMed] [Google Scholar]
- 15.Yokomizo T, Ng CE, Osato M, Dzierzak E. Three-dimensional imaging of whole midgestation murine embryos shows an intravascular localization for all hematopoietic clusters. Blood. 2011;117(23):6132–6134. doi: 10.1182/blood-2011-02-334037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sitnicka E, et al. Complementary signaling through flt3 and interleukin-7 receptor α is indispensable for fetal and adult B cell genesis. J Exp Med. 2003;198(10):1495–1506. doi: 10.1084/jem.20031152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Decker T, et al. Stepwise activation of enhancer and promoter regions of the B cell commitment gene Pax5 in early lymphopoiesis. Immunity. 2009;30(4):508–520. doi: 10.1016/j.immuni.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 18.Revilla-I-Domingo R, et al. The B-cell identity factor Pax5 regulates distinct transcriptional programmes in early and late B lymphopoiesis. EMBO J. 2012;31(14):3130–3146. doi: 10.1038/emboj.2012.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grawunder U, et al. Down-regulation of RAG1 and RAG2 gene expression in preB cells after functional immunoglobulin heavy chain rearrangement. Immunity. 1995;3(5):601–668. doi: 10.1016/1074-7613(95)90131-0. [DOI] [PubMed] [Google Scholar]
- 20.Tokoyoda K, Egawa T, Sugiyama T, Choi BI, Nagasawa T. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity. 2004;20(6):707–718. doi: 10.1016/j.immuni.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Lu R, Neff NF, Quake SR, Weissman IL. Tracking single hematopoietic stem cells in vivo using high-throughput sequencing in conjunction with viral genetic barcoding. Nat Biotechnol. 2011;29(10):928–933. doi: 10.1038/nbt.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tokoyoda K, Zehentmeier S, Chang HD, Radbruch A. Organization and maintenance of immunological memory by stroma niches. Eur J Immunol. 2009;39(8):2095–2099. doi: 10.1002/eji.200939500. [DOI] [PubMed] [Google Scholar]
- 23.Tokoyoda K, Hauser AE, Nakayama T, Radbruch A. Organization of immunological memory by bone marrow stroma. Nat Rev Immunol. 2010;10(3):193–200. doi: 10.1038/nri2727. [DOI] [PubMed] [Google Scholar]
- 24.Spangrude GJ, et al. Mouse hematopoietic stem cells. Blood. 1991;78(6):1395–1402. [PubMed] [Google Scholar]
- 25.Smith LG, Weissman IL, Heimfeld S. Clonal analysis of hematopoietic stem-cell differentiation in vivo. Proc Natl Acad Sci U S A. 1991;88(7):2788–2792. doi: 10.1073/pnas.88.7.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morrison SJ, Uchida N, Weissman IL. The biology ofhematopoietic stem cells. Annu Rev Cell Dev Biol. 1995;11:35–71. doi: 10.1146/annurev.cb.11.110195.000343. [DOI] [PubMed] [Google Scholar]
- 27.Simsek T, et al. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell. 2010;7(3):380–390. doi: 10.1016/j.stem.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takubo K, et al. Regulation of the HIF-1α level is essential for hematopoietic stem cells. Cell Stem Cell. 2010;7(3):391–402. doi: 10.1016/j.stem.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 29.Rossi DJ, et al. Hematopoietic stem cell quiescence attenuates DNA damage response and permits DNA damage accumulation during aging. Cell Cycle. 2007;6(19):2371–2376. doi: 10.4161/cc.6.19.4759. [DOI] [PubMed] [Google Scholar]
- 30.Visnijk D, et al. Hematopoiesis is severely altered with an induced osteoblast deficiency. Blood. 2004;103(9):3258–3264. doi: 10.1182/blood-2003-11-4011. [DOI] [PubMed] [Google Scholar]
- 31.Dougall WC, et al. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999;13(18):2412–2424. doi: 10.1101/gad.13.18.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rolink A, Kudo A, Karasuyama H, Kikuchi Y, Melchers F. Long-term proliferating early pre B cell lines and clones with the potential to develop to surface Ig-positive, mitogen reactive B cells in vitro and in vivo. EMBO J. 1991;10(2):327–336. doi: 10.1002/j.1460-2075.1991.tb07953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peschon JJ, et al. Early lymphocyte expansion is severely impaired at the transition from MPP to CLP in interleukin 7 receptor-deficient mice. J Exp Med. 1994;180(5):1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SE, Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL7 as a non-redundant cytokine. J Exp Med. 1995;181(4):1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scheeren FA, et al. Thymic stromal lymphopoietin induces early human B-cell proliferation and differentiation. Eur J Immunol. 2010;40(4):955–965. doi: 10.1002/eji.200939419. [DOI] [PubMed] [Google Scholar]
- 36.Haspel MV, et al. Multiple organ-reactive monoclonal autoantibodies. Nature. 1983;304(5921):73–76. doi: 10.1038/304073a0. [DOI] [PubMed] [Google Scholar]
- 37.Radic MZ, et al. Residues that mediate DNA binding of autoimmune antibodies. J Immunol. 1993;150(11):4966–4977. [PubMed] [Google Scholar]
- 38.Tiegs SL, Russell DM, Nemazee D. Receptor editing in self-reactive bone marrow B cells. J Exp Med. 1993;177(4):1009–1020. doi: 10.1084/jem.177.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wardemann H, Nussenzweig MC. B cell tolerance in humans. Adv Immunol. 2007;95:83–110. doi: 10.1016/S0065-2776(07)95003-8. [DOI] [PubMed] [Google Scholar]
- 40.Shimizu T, Mundt C, Licence S, Melchers F, Mårtensson IL. VpreB1/VpreB2/λ 5 triple-deficient mice show impaired B cell development but functional allelic exclusion of the IgH locus. J Immunol. 2002;168(12):6286–6293. doi: 10.4049/jimmunol.168.12.6286. [DOI] [PubMed] [Google Scholar]
- 41.Schuh W, Meister S, Roth E, Jäck HM. Cutting edge: signaling and cell surface expression of a mu H chain in the absence of λ 5: a paradigm revisited. J Immunol. 2003;171(7):3343–3347. doi: 10.4049/jimmunol.171.7.3343. [DOI] [PubMed] [Google Scholar]
- 42.Galler GR, et al. Surface mu heavy chain signals down-regulation of the V(D)J-recombinase machinery in the absence of surrogate light chain components. J Exp Med. 2004;199(11):1523–1532. doi: 10.1084/jem.20031523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grawunder U, et al. In-vitro analyses of mechanisms of B-cell development. Semin Immunol. 1995;7(3):155–167. doi: 10.1016/1044-5323(95)90043-8. [DOI] [PubMed] [Google Scholar]
- 44.Llorian M, Stamataki Z, Hill S, Turner M, Mårtensson IL. The PI3K p110Δ is required for down-regulation of RAG expression in immature B cells. J Immunol. 2007;178(4):1981–1985. doi: 10.4049/jimmunol.178.4.1981. [DOI] [PubMed] [Google Scholar]
- 45.Melchers F. The pre-B-cell receptor: selector of fitting immunoglobulin heavy chains for the B-cell repertoire. Nat Rev Immunol. 2005;5(7):578–584. doi: 10.1038/nri1649. [DOI] [PubMed] [Google Scholar]
- 46.Parker MJ, et al. The pre-B-cell receptor induces silencing of VpreB and λ5 transcription. EMBO J. 2005;24(22):3895–3905. doi: 10.1038/sj.emboj.7600850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karnowski A, et al. Silencing and nuclear repositioning of the λ5 gene locus at the pre-B cell stage requires Aiolos and OBF-1. PLoS One. 2008;3(10):e3568. doi: 10.1371/journal.pone.0003568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kitamura D, et al. A critical role of λ5 protein in B cell development. Cell. 1992;69(5):823–831. doi: 10.1016/0092-8674(92)90293-L. [DOI] [PubMed] [Google Scholar]
- 49.Conley ME. Genes required for B Cell development. J Clin Invest. 2003;112(11):1636–1638. doi: 10.1172/JCI20408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bradl H, Wittmann J, Milius D, Vettermann C, Jäck HM. Interaction of murine precursor B cell receptor with stroma cells is controlled by the unique tail of λ5 and stroma cell-associated heparan sulfate. J Immunol. 2003;171(5):2338–2348. doi: 10.4049/jimmunol.171.5.2338. [DOI] [PubMed] [Google Scholar]
- 51.Gauthier L, Rossi B, Roux F, Termine E, Schiff C. Galectin-1 is a stromal cell ligand of the pre-B cell receptor (BCR) implicated in synapse formation between pre-B and stromal cells and in pre-BCR triggering. Proc Natl Acad Sci U S A. 2002;99(20):13014–13019. doi: 10.1073/pnas.202323999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Espeli M1, Mancini SJ, Breton C, Poirier F, Schiff C. Impaired B-cell development at the pre-BII-cell stage in galectin-1-deficient mice due to inefficient pre-BII/stromal cell interactions. Blood. 2009;113(23):5878–5886. doi: 10.1182/blood-2009-01-198465. [DOI] [PubMed] [Google Scholar]
- 53.Knoll M, et al. The non-Ig parts of the VpreB and λ5 proteins of the surrogate light chain play opposite roles in the surface representation of the precursor B cell receptor. J Immunol. 2012;188(12):6010–6017. doi: 10.4049/jimmunol.1200071. [DOI] [PubMed] [Google Scholar]
- 54.Ohnishi K, Melchers F. The nonimmunoglobulin portion of λ5 mediates cell-autonomous pre-B cell receptor signaling. Nat Immunol. 2003;4(9):849–856. doi: 10.1038/ni959. [DOI] [PubMed] [Google Scholar]
- 55.Keenan RA, et al. Censoring of autoreactive B cell development by the pre-B cell receptor. Science. 2008;321(5889):696–699. doi: 10.1126/science.1157533. [DOI] [PubMed] [Google Scholar]
- 56.Almqvist N, Mårtensson IL. The pre-B cell receptor; selecting for or against autoreactivity. Scand J Immunol. 2012;76(3):256–262. doi: 10.1111/j.1365-3083.2012.02751.x. [DOI] [PubMed] [Google Scholar]
- 57.Herzog S, Jumaa H. Self-recognition and clonal selection: autoreactivity drives the generation of B cells. Curr Opin Immunol. 2012;24(2):166–172. doi: 10.1016/j.coi.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 58.Melchers F, Yamagami T, Rolink A, Andersson J. Rules for the rearrangement events at the L chain gene loci of the mouse. Adv Exp Med Biol. 2007;596:63–70. doi: 10.1007/0-387-46530-8_6. [DOI] [PubMed] [Google Scholar]
- 59.Yamagami T, ten Boekel E, Andersson J, Rolink A, Melchers F. Frequencies of multiple IgL chain gene rearrangements in single normal or kappaL chain-deficient B lineage cells. Immunity. 1999;11(3):317–322. doi: 10.1016/S1074-7613(00)80107-7. [DOI] [PubMed] [Google Scholar]
- 60.Flemming A, Brummer T, Reth M, Jumaa H. The adaptor protein SLP-65 acts as a tumor suppressor that limits preB cell expansion. Nat Immunol. 2003;4(1):38–43. doi: 10.1038/ni862. [DOI] [PubMed] [Google Scholar]
- 61.Jumaa H, et al. Deficiency of the adaptor SLP-65 in pre-B-cell acute lymphoblastic leukaemia. Nature. 2003;423(6938):452–456. doi: 10.1038/nature01608. [DOI] [PubMed] [Google Scholar]
- 62.Monroe JG. ITAM-mediated tonic signalling through pre-BCR and BCR complexes. Nat Rev Immunol. 2006;6(4):283–294. doi: 10.1038/nri1808. [DOI] [PubMed] [Google Scholar]
- 63.Gay D, Saunders T, Camper S, Weigert M. Receptor editing: an approach by autoreactive B cells to escape tolerance. J Exp Med. 1993;177(4):999–1008. doi: 10.1084/jem.177.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nemazee D, Weigert M. Revising B cell receptors. J Exp Med. 2000;191(11):1813–1817. doi: 10.1084/jem.191.11.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rolink A, Grawunder U, Haasner D, Strasser A, Melchers F. Immature surface Ig+ B cells can continue to rearrange κ and λ L chain gene loci. J Exp Med. 1993;178(4):1263–1270. doi: 10.1084/jem.178.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gerdes T, Wabl M. Autoreactivity and allelic inclusion in a B-cell nuclear transfer mouse. Nat Immunol. 2004;5(12):1282–1287. doi: 10.1038/ni1133. [DOI] [PubMed] [Google Scholar]
- 67.Li H, Jiang Y, Prak EL, Radic M, Weigert M. Editors and editing of anti-DANN receptors. Immunity. 2001;15(6):947–957. doi: 10.1016/S1074-7613(01)00251-5. [DOI] [PubMed] [Google Scholar]
- 68.Doyle CM, Han J, Weigert MG, Prak ET. Consequences of receptor editing at the lambda locus: multireactivity and light chain secretion. Proc Natl Acad Sci U S A. 2006;103(30):11246–11269. doi: 10.1073/pnas.0604657103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Witsch EJ, Cao H, Fukuyama H, Weigert M. Light chain editing generates polyreactive antibodies in chronic graft-versus-host reaction. J Exp Med. 2006;203(7):1761–1772. doi: 10.1084/jem.20060075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leslie D, Lipsky P, Notkins AL. Autoantibodies as predictors of disease. J Clin Invest. 2001;108(10):1417–1422. doi: 10.1172/JCI14452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nemazee D. Does immunological tolerance explain the waste in the B-lymphocyte immune system? Experiment and theory. Ann N Y Acad Sci. 1995;764:397–401. doi: 10.1111/j.1749-6632.1995.tb55854.x. [DOI] [PubMed] [Google Scholar]
- 72.Rowley B, Tang L, Shinton S, Hayakawa K, Hardy RR. Autoreactive B-1 B cells: constraints on natural autoantibody B cell antigen receptors. J Autoimmun. 2007;29(4):236–245. doi: 10.1016/j.jaut.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.ten Boekel E, Melchers F, Rolink AG. Changes in the V(H) gene repertoire of developing precursor B lymphocytes in mouse bone marrow mediated by the pre-B cell receptor. Immunity. 1997;7(3):357–368. doi: 10.1016/S1074-7613(00)80357-X. [DOI] [PubMed] [Google Scholar]
- 74.Rolink AG, Brocker T, Bluethmann H, Kosco-Vilbois MH, Andersson J, Melchers F. Mutations affecting either generation or survival of cells influence the pool size of mature B cells. Immunity. 1999;10(5):619–628. doi: 10.1016/S1074-7613(00)80061-8. [DOI] [PubMed] [Google Scholar]
- 75.Rolink AG, Andersson J, Melchers F. Characterization of immature B cells by a novel monoclonal antibody, by turnover and by mitogen reactivity. Eur J Immunol. 1998;28(11):3738–3748. doi: 10.1002/(SICI)1521-4141(199811)28:11<3738::AID-IMMU3738>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 76.Allende ML, Tuymetova G, Lee BG, Bonifacino E, Wu YP, Proia RL. S1P1 receptor directs the release of immature B cells from bone marrow into blood. J Exp Med. 2010;207(5):1113–1124. doi: 10.1084/jem.20092210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Simmons S, Ishii M. Sphingosine-1-phosphate: a master regulator of lymphocyte egress and immunity. Arch Immunol Ther Exp (Warsz). 2014;62(2):103–115. doi: 10.1007/s00005-013-0264-8. [DOI] [PubMed] [Google Scholar]
- 78.Ohl L, et al. Cooperating mechanisms of CXCR5 and CCR7 in development and organization of secondary lymphoid organs. J Exp Med. 2003;197(9):1199–1204. doi: 10.1084/jem.20030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yurasov S, et al. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J Exp Med. 2005;201(5):703–711. doi: 10.1084/jem.20042251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Carroll MC. A protective role for innate immunity in systemic lupus erythematosus. Nat Rev Immunol. 2004;4(10):825–831. doi: 10.1038/nri1456. [DOI] [PubMed] [Google Scholar]
- 81.Melchers F, Rolink AR. B cell tolerance — how to make it and how to break it. Curr Top Microbiol Immunol. 2006;305:1–23. doi: 10.1007/3-540-29714-6_1. [DOI] [PubMed] [Google Scholar]
- 82.Rolink AG, Tschopp J, Schneider P, Melchers F. BAFF is a survival and maturation factor for mouse B cells. Eur J Immunol. 2002;32(7):2004–2010. doi: 10.1002/1521-4141(200207)32:7<2004::AID-IMMU2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 83.Shlomchik MJ. Sites and stages of autoreactive B cell activation and regulation. Immunity. 2008;28(1):18–28. doi: 10.1016/j.immuni.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 84.Lood C, et al. C1q inhibits immune complex-induced interferon-alpha production in plasmacytoid dendritic cells: a novel link between C1q deficiency and systemic lupus erythematosus pathogenesis. Arthritis Rheum. 2009;60(10):3081–3090. doi: 10.1002/art.24852. [DOI] [PubMed] [Google Scholar]
- 85.Hardy RR, Hayakawa K. B cell development pathways. Annu Rev Immunol. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- 86.Hardy RR, Hayakawa K. Positive and negative selection of natural autoreactive B cells. Adv Exp Med Biol. 2012;750:227–238. doi: 10.1007/978-1-4614-3461-0_17. [DOI] [PubMed] [Google Scholar]
- 87.Victora GD. SnapShot: the germinal center reaction. Cell. 2014;159(3):700–700.e1. doi: 10.1016/j.cell.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 88.Oropallo MA, Cerutti A. Germinal center reaction: antigen affinity and presentation explain it all. Trends Immunol. 2014;35(7):287–289. doi: 10.1016/j.it.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Königsberger S, et al. Suboptimal B-cell antigen receptor signaling activity in vivo elicits germinal center counterselection mechanisms. Eur J Immunol. 2015;45(2):603–611. doi: 10.1002/eji.201444538. [DOI] [PubMed] [Google Scholar]
- 90.Heise N, et al. Germinal center B cell maintenance and differentiation are controlled by distinct NF-κB transcription factor subunits. J Exp Med. 2014;211(10):2103–2118. doi: 10.1084/jem.20132613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brink R. The imperfect control of self-reactive germinal center B cells. Curr Opin Immunol. 2014;28:97–101. doi: 10.1016/j.coi.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 92.Chan TD, et al. Elimination of germinal-center-derived self-reactive B cells is governed by the location and concentration of self-antigen. Immunity. 2012;37(5):893–904. doi: 10.1016/j.immuni.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 93.Takemori T, Kaji T, Takahashi Y, Shimoda M, Rajewsky K. Generation of memory B cells inside and outside germinal centers. Eur J Immunol. 2014;44(5):1258–1264. doi: 10.1002/eji.201343716. [DOI] [PubMed] [Google Scholar]
- 94.Spits H, et al. Innate lymphoid cells — a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13(2):145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]