Abstract
Background
Indications for implantable cardioverter-defibrillators (ICDs) in heart failure (HF) are expanding and may include more than 1 million patients. This study examined patient expectations from ICDs for primary prevention of sudden death in HF.
Methods and Results
Study participants (n = 105) had an EF <35% and symptomatic HF, without history of ventricular tachycardia/fibrillation or syncope. Subjects completed a written survey about perceived ICD benefits, survival expectations, and circumstances under which they might deactivate defibrillation. Mean age was 58, LVEF 21%, 40% were New York Heart Association Class III-IV, and 65% already had a primary prevention ICD. Most patients anticipated more than10 years survival despite symptomatic HF. Nearly 54% expected an ICD to save ≥50 lives per 100 during 5 years. ICD recipients expressed more confidence that the device would save their own lives compared with those without an ICD (P < .001). Despite understanding the ease of deactivation, 70% of ICD recipients indicated they would keep the ICD on even if dying of cancer, 55% even if having daily shocks, and none would inactivate defibrillation even if suffering constant dyspnea at rest.
Conclusions
HF patients anticipate long survival, overestimate survival benefits conferred by ICDs, and express reluctance to deactivate their devices even for end-stage disease.
Keywords: Implantable defibrillators, heart failure, sudden death, cardiomyopathy
Indications have been expanding in recent years for implantable cardioverter-defibrillators (ICDs) for the primary prevention of sudden cardiac death.1,2 ICDs have mortality benefits in heart failure patients with a reduced ejection fraction from either ischemic or nonischemic cardiomyopathies.3–7 This potentially lifesaving therapy may affect more than 1 million heart failure patients, a high-risk group 6 to 9 times more likely to experience sudden death than the general population.8,9 There are approximately 10,000 ICDs implanted each month in the United States.10
Even as enthusiasm grows for ICDs, it is imperative that patients be informed effectively of the likelihood of benefit and the risks from ICD implantation.11 This should take into account the complete trajectory of their illness, because many heart failure patients will die of causes not preventable by ICDs.12,13 Sudden death and non-sudden hemodynamic death are both more common in patients with advanced heart disease in New York Heart Association Class III-IV. Although a potentially lifesaving technology, ICDs are expensive and not without risk.14,15 In recent clinical trials of ICDs in heart failure, the rate of major complications related to implantation was 4% to 9%.3,5,16 Other complications of ICDs can occur after successful implantation.17 Although timely defibrillation can save lives, as many as 31% to 50% of all shocks delivered by ICDs may be inappropriate and may reduce quality of life.3,16,18–26
Selecting an appropriate heart failure patient for ICD implantation is crucial to ensure the benefit of the primary prevention strategy. Initial triage of potential ICD candidates will be guided by the latest American Heart Association/American College of Cardiology guidelines for management of devices in heart failure.27 The discussion about placing an ICD must be further informed by the expectations and preferences of heart failure patients themselves. To better understand patient perceptions of the ICD and their illness, we surveyed patients with and without primary prevention ICDs in terms of lifesaving capacity, potential complications, and conditions for which inactivation of the defibrillator would be considered.
Methods
Study Population
Subjects were enrolled from 2 heart failure referral centers in Boston, Massachusetts, between February 2005 and January 2006. The study protocol was approved by an institutional review committee. Participants were both inpatients and outpatients, and could already have an ICD in place for primary prevention. Inclusion criteria were left ventricular ejection fraction (LVEF) <35% and symptomatic heart failure. To collect a primary prevention cohort, patients were excluded if they had a history of ventricular tachycardia, ventricular fibrillation, cardiac arrest, or syncope occurring without an ICD. These entry criteria established a group of 105 heart failure patients similar to the Sudden Cardiac Death in Heart Failure Trial participants in whom ICDs saved 7.2 lives per 100 patients over 5 years.3
Patients signed informed consent before being given the questionnaire. Baseline clinical data from within 3 months before study enrollment were compiled from the electronic medical record to determine the overall level of illness and to evaluate any differences between patients with or without an ICD. These data included ejection fraction, duration and etiology of heart failure, peak oxygen consumption with exercise, and NYHA functional class as determined by a cardiologist. Serum markers of renal and cardiac function were collected, including serum sodium, serum creatinine, blood urea nitrogen, and B-type natriuretic peptide. The medical record and ICD interrogation reports were reviewed to determine frequency and nature of previous ICD discharges for patients with ICDs. Patients without any information about previous device activity were presumed to have had no ICD shocks. For purposes of this analysis, antitachycardia pacing was not considered device activity.
Survey Instrument
After providing informed consent, study subjects completed a written survey with 14 items about their perception of ICD survival benefit and device operation, including defibrillator inactivation. The survey has not been independently validated outside this patient cohort, so this report should be considered a pilot study of the instrument. At the beginning of the survey, patients read that an implantable defibrillator was designed to prevent only sudden death. All items were multiple choice and some allowed multiple responses to be selected. Survey items were similar for subjects with and without ICD. However, ICD recipients were also asked if they had experienced any device activity and how long their ICD had been in place. The survey instrument could be completed either at the clinical encounter or at home to be returned by mail.
At enrollment, a heart failure nurse interviewed each patient and administered a time tradeoff questionnaire. Time tradeoff is a utility to offers direct assessment of relative value placed by patients on survival time versus perceived symptomatic health and has been used in multiple illnesses, including heart failure.28,29 Patients iteratively chose between different set amounts of life in a compromised health state (heart failure) and a shorter time in perfect health. This survey employed a time horizon of 2 years. Time tradeoff is reported as the ratio of the shorter life expectancy in perfect health divided by 2 years in their current state of heart failure. This derives a utility from 0 to 1, with 1 representing patients not wishing to trade away any time in their current state of health.
Statistical Analysis
Baseline data in subjects with and without ICD were compared using a t-test for differences in the means of continuous variables and a Fisher’s exact test for differences in proportions. Subjects without an ICD were used as a control group to describe the ambient impressions and assumptions such patients would bring to a discussion of ICD therapy, then they were compared with ICD recipients to measure indirectly how impressions of heart failure and ICD therapy might change with device implantation. Responses to the questionnaire are reported as percent of subjects answering each item. Survey responses were stratified by ICD presence or absence, patient location, prior shocks if ICD in place, NYHA Class, and time tradeoff. For purposes of analysis, the mean time tradeoff score (TTO) was used, along with the following TTO strata: >0.875 (trading 3 months or less), >0.75 (trading 6 months or less), or >0.5 (trading less than 12 months, half of theoretical time remaining). Item responses between these different groups were compared using Fisher’s exact test for differences in proportions. Statistical analysis was performed using SPSS (v.17). The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
Baseline Characteristics
Baseline clinical characteristics are reported in Table 1 and have been stratified by the presence or absence of an ICD for primary prevention at the time of survey. The survey population (n = 105) had a mean age of 58 years, 70% were male, mean LVEF was 21%, 52% had heart failure more than 5 years, 40% were NYHA Class III or IV, and 35% had an ischemic etiology. In all, 65% of patients surveyed already had an ICD placed for primary prevention of sudden death. In aggregate, patients with or without an ICD for primary prevention had a similar symptom profile and quality of life. ICD recipients surveyed were more likely to be male and had a slightly lower mean ejection fraction. Most ICD recipients (70%) said that their primary source of ICD information was their physician. Of patients without an ICD, 78% had heard of the device before the survey and 47% had previously discussed an ICD with their physician.
Table 1.
Baseline Characteristics
| Total (n = 105) | With Implantable Cardioverter-defibrillator (n = 67) | Without Implantable Cardioverter-defibrillator (n = 38) | P Value | |
|---|---|---|---|---|
| Age (y) | 58 (13) | 58 (12) | 58 (15) | .889 |
| Male (%) | 70 | 78 | 58 | .045 |
| New York Heart Association Class (%) | .696 | |||
| I | 18 | 15 | 24 | |
| II | 42 | 42 | 41 | |
| III | 37 | 39 | 32 | |
| IV | 3 | 3 | 3 | |
| Duration >5 y (%) | 52 | 55 | 47 | .542 |
| Ischemic etiology (%) | 35 | 40 | 24 | .133 |
| Left ventricular ejection fraction (%) | 21 (7) | 20 (6) | 23 (7) | .017 |
| Peak VO2 (mL/kg/min) | 14.8 (4.7) | 14.5 (4.4) | 16.1 (5.8) | .368 |
| Serum sodium (mEq/L) | 139 (3) | 138 (3) | 139 (3) | .572 |
| Blood urea nitrogen (mg/dL) | 34 (21) | 35 (22) | 33 (19) | .688 |
| Serum creatinine (mg/dL) | 1.6 (1.0) | 1.4 (0.5) | 1.8 (1.6) | .270 |
| B-type natriuretic peptide (pg/mL) | 809 (1303) | 622 (954) | 1043 (1646) | .374 |
| Time tradeoff utility | 0.81 (0.32) | 0.83 (0.31) | 0.78 (0.34) | .472 |
P values derived by t-test for continuous variables and Fisher’s exact test of categorical variables.
Continuous variables presented as mean ± SD.
Estimating the Lifesaving Capacity of ICDs
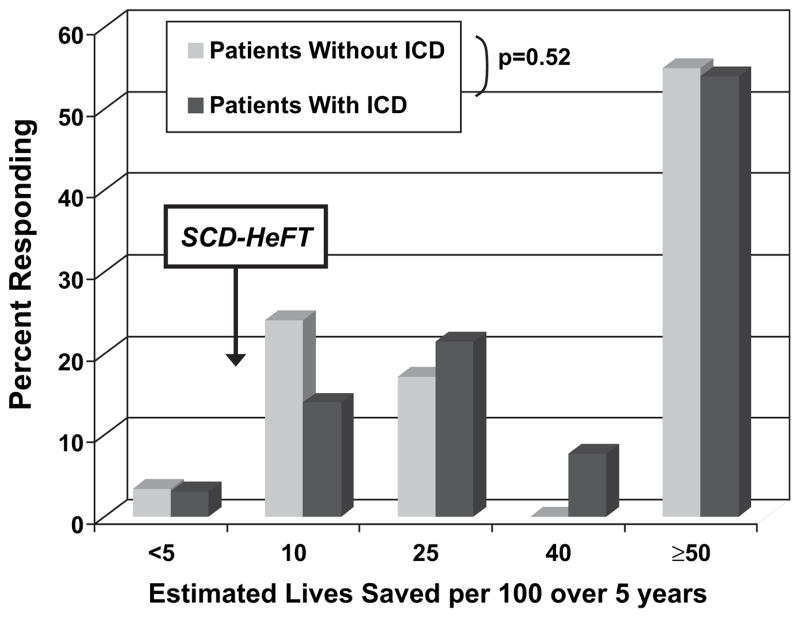
When asked how many lives they expected ICDs to save over 5 years, 77% surveyed thought >10 lives per 100 would be saved, and 54% thought that an ICD would save more than 50 lives per 100 over 5 years (Fig. 1). Patient estimates of numbers of lives saved by an ICD for primary prevention were similar whether or not they themselves had ICDs (P = .52). There were also no significant differences in the overall estimate of lives saved when patients surveyed were stratified by inpatient/outpatient status, NYHA functional class, or time tradeoff utility.
Fig. 1.
Subjects were asked how many lives per 100 they would expect an implantable cardioverter-defibrillator (ICD) to save during the first 5 years after implantation. Heart failure patients surveyed overestimated the impact of ICDs on survival compared with Sudden Cardiac Death in Heart Failure Trial patients (7.2 lives per 100 over 5 years). Estimates were similar between ICD recipients and those without an ICD in place (P = .52).
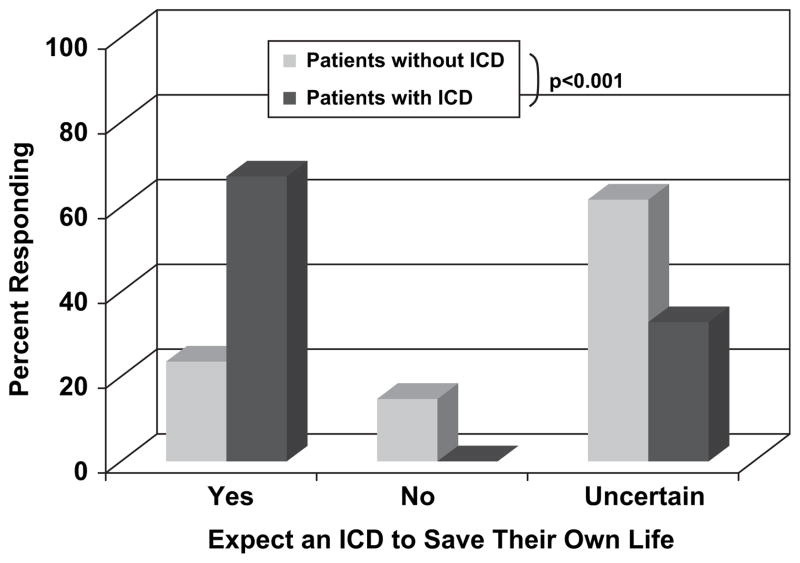
Patients were then asked whether or not they believed an ICD would save their own life (Fig. 2). ICD recipients were more confident of the device’s lifesaving capacity, with 67% believing the ICD would definitely save their own lives compared with only 19% of those without an ICD (P < .001). Those without an ICD were less certain about the device, with 66% reporting they did not know if such a device would save their lives. Not a single ICD recipient surveyed expressed doubt that the device would save their life if they should develop a life-threatening heart rhythm. In fact, 48% of ICD recipients surveyed said they would want the device even if it saved fewer than 5 lives per 100 overall patients during 5 years.
Fig. 2.
Subjects were asked whether or not they expected an implantable cardioverter-defibrillator (ICD) to save their own lives. ICD recipients were more confident that the device would save their lives (P < .001).
Information on previous ICD activity was available on 60 of 67 ICD recipients—the other 7 patients were presumed to have had no ICD shocks. In all, 8 (12%) patients had appropriate defibrillation in the past compared with 4 (6%) who only had an inappropriate shock. However, 55 ICD recipients (82%) had no device shocks. Each patient with a previous shock thought their device would save their life in the future. Of those without a history of any device shocks, 69% thought the ICD would eventually save their lives. Two of the 4 patients who received only an inappropriate shock thought the device had saved their lives. Meanwhile, 21% of those without any reported device activity thought the ICD had already saved their life.
Expected and Preferred Survival with Heart Failure
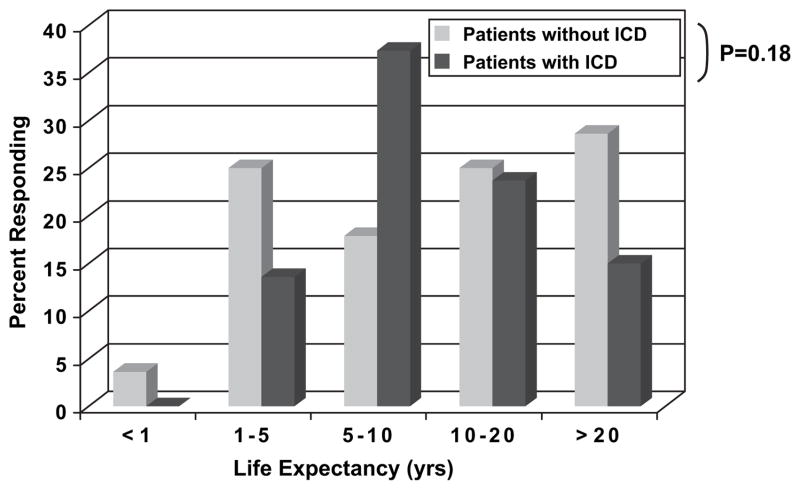
This group of symptomatic heart failure patients surveyed also had optimistic estimates of their own life expectancy (Fig. 3). Of those surveyed, 65% thought they would live more than 10 years and 34% believed they would be alive for at least 20 years. Patients without an ICD had an insignificant trend to predict reduced life expectancy compared with patients with an ICD. Inpatients and outpatients had similar estimates of their survival, but patients with NYHA Class III-IV symptoms anticipated somewhat shorter survival than NYHA Class I-II subjects (P < .01). Patient survival expectations had no relationship to their estimates of the number of lives saved by ICD therapy (P = .18).
Fig. 3.
Heart failure patients surveyed anticipated a long life when asked to estimate their life expectancy. There was no significant difference in estimates between patients with and without an implantable cardioverter-defibrillator (ICD) (P = .18).
Most heart failure patients surveyed were reluctant to trade away significant time in their current state of health to improve their symptoms. The mean time tradeoff utility was 0.81 (TTO of 1 would be a preference to trade no time for better health). Using the hypothetical time horizon of 2 years, 71% expressed preference to trade fewer than 3 months of time away to have increased health during the remaining time. Only 16% expressed preference to trade away more than 12 months to have a better state of health. Patient estimates of the number lives saved by ICD therapy were consistent across TTO tertiles and when separated by willingness to trade either 3, 6, or 12 months. Their preference for survival did not affect the confidence patients had that an ICD would save their own life. Patients willing to trade more time expected shorter survival than those unwilling to trade time (P = .02); 46% of the patients willing to trade away at least 12 months (TTO <0.5) anticipated that they would not survive 5 years.
Understanding ICD Complications
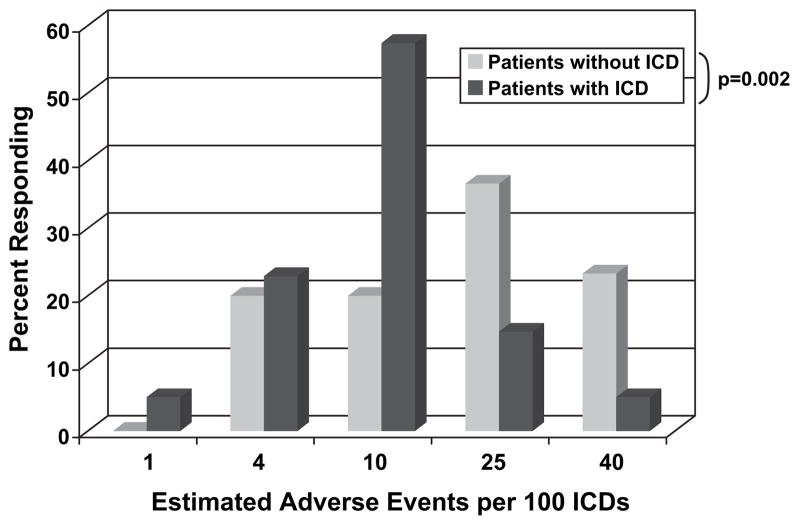
Subjects were told to estimate the number of ICD complications or unnecessary shocks that might occur. ICD recipients estimated a lower number of complications or unnecessary shocks compared with patients without ICDs (Fig. 4). Sixty percent of patients without an ICD thought there would be 25 or more complications or unnecessary shocks per 100 patients over 5 years, compared with only 20% of ICD recipients (P = .002). More than half (52%) of subjects surveyed said they would want an ICD in place regardless of the number of unnecessary shocks or complications.
Fig. 4.
Subjects were asked to estimate the number of inappropriate implantable cardioverter-defibrillator (ICD) shocks or complications that would occur per 100 ICD implanted. ICD recipients estimated fewer adverse events compared to patients without an ICD (P = .002).
ICDs at the End of Life
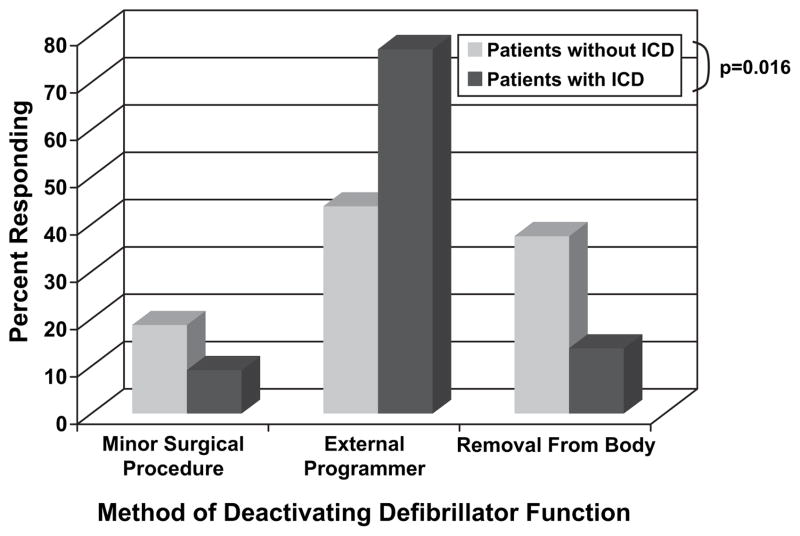
Subjects were asked to consider circumstances under which they would consider turning off the defibrillator function of their ICD. ICD recipients had a better understanding of how the ICD could be deactivated compared to patients without one in place, with 73% of recipients vs. 42% of those without an ICD (P = .016) understanding that the defibrillator could be easily programmed off with an external device (Fig. 5). More than half of patients without an ICD thought a minor surgical procedure or full device extraction would be necessary to deactivate defibrillation.
Fig. 5.
Asked how the defibrillator feature of an implantable cardioverter-defibrillator (ICD) could be deactivated, most heart failure patients surveyed understood that an external programmer could be used. Subjects already with an in place ICD exhibited a better understanding of the simplicity of inactivating defibrillation. (P = .016).
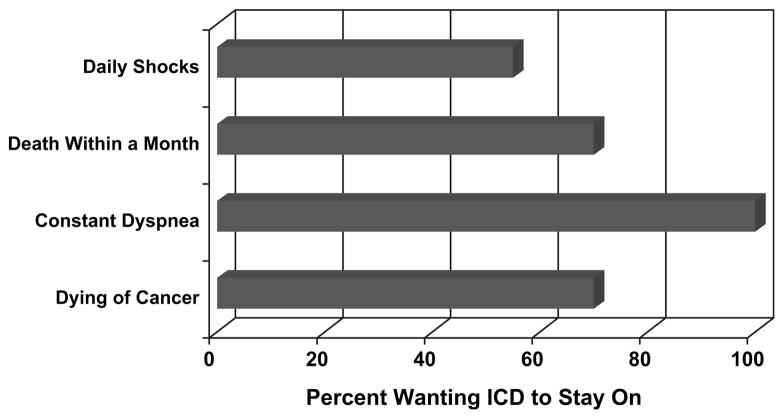
ICD recipients anticipated great reluctance in turning off their device (Fig. 6). When faced with the description of possible terminal disease, during which death could occur suddenly or slowly, 39% of subjects with ICDs said that they would never turn the defibrillation function of the device off, although most realized that this could easily be done. Nearly 55% said they would keep their ICD on if receiving daily shocks and 70% would keep it on if they were dying of cancer or knew they would be dead within a month from another noncardiac cause. None surveyed would want the defibrillator function turned off if they were experiencing constant dyspnea at rest. Patient reluctance to deactivate the defibrillator was consistent across inpatient/outpatient status, NYHA functional class, and time tradeoff utility strata.
Fig. 6.
Implantable cardioverter-defibrillator (ICD) recipients were asked to consider circumstances that might prompt ICD deactivation. ICD recipients anticipated reluctance to turn off defibrillator function.
Discussion
This study shows that symptomatic heart failure patients anticipate long survival and overestimate the survival benefits of ICDs for primary prevention of sudden death. Confidence in ICDs was robust and unrelated to the presence or absence of an existing ICD, NYHA functional class, or the value each subject placed on quality versus length of life as assessed by time tradeoff. Although patients with ICDs in place understood the ease of reprogramming, most indicated that they would not consider inactivating defibrillation even in the presence of end-stage heart failure or other disease.
Optimizing Use of Primary Prevention ICDs
Though the development and basis of evidence for ICDs has been a milestone in cardiac care, the absolute magnitude of ICD benefit for primary prevention in heart failure may often be lost in translation to patients. The Multicenter Automatic Defibrillator Implantation Trial of patients with LVEF ≤30% late after myocardial infarction detected an absolute improvement in survival of 6% with defibrillator therapy with an average follow-up of 20 months.5,30 More recently, the Sudden Cardiac Death in Heart Failure Trial included patients with Class II or III heart failure from both ischemic and nonischemic cardiomyopathy and demonstrated an absolute mortality benefit with ICD of 7.2% at 5 years, the longest demonstrated benefit for primary prevention.3 Neither of these observed a net benefit from ICD therapy during the first year after implantation.31 Furthermore, cost-benefit calculations suggest that a life expectancy of 7 to 8 years is required to meet the cost-effectiveness threshold of $40,000 per life-year saved.14,15,32,33 This contrasts with the larger benefit observed in the more selected group receiving ICDs for secondary prevention after previous life-threatening arrhythmic event.34
Finding the rational balance between optimizing ICD benefit in the individual patient and addressing the broader resource needs of the large eligible heart failure population requires appropriate selection of primary prevention candidates.35 The heart failure patients with reduced systolic function most likely to survive long enough to benefit from ICD implantation include those without renal dysfunction or other major comorbidities, with fewer than 3 heart failure hospitalizations, and without limitation of routine daily activities.36,37 After 3 heart failure hospitalizations, patients older than age 70 have an estimated median survival of only 1.5 years, even if all sudden deaths could be prevented.12
Attitudes toward ICDs at the End of Life
In this survey, most ICD recipients understood that their device could be easily programmed off. Despite this, patients anticipated a reluctance to turn off their ICDs if dying of cancer or receiving daily shocks. Surprisingly, no recipients surveyed would turn their ICD off even if suffering from constant dyspnea at rest even though only 21% of deaths among such patients are considered unexpected.38 Death in late-stage heart failure often occurs with bradycardia, asystole, or pulseless electrical activity, and not the tachyarrhythmias the ICD was designed to prevent. Nonetheless, inappropriate and futile defibrillations near the end can cause significant distress for both the patient and his or her family, which may occur more often in those with frequent previous device activity.39 Even before an ICD is implanted, patients should be told that situations may arise leading some of them to request to have the defibrillation function turned off to allow a natural death.40 For some patients and families, the discussion about ICD inactivation can be an important milestone along the path of preparation for the end.41–43
Improving the Dialogue with Patients
Patient perceptions of ICD therapy are influenced by the inherent difficulties physicians have communicating risk and prognosis in heart failure. The presence of an ICD did not alter patient estimates of overall life expectancy or lives saved by the ICD. Rather than making the ICD discussion a teaching moment for patients to educate them about their illness, health care providers may be contributing to or at least reinforcing patient misunderstandings. These survey data highlight the importance of delivering clear information to our patients about the likely outcomes with heart failure with and without an ICD is placed for primary prevention.44 The message at the time of informed consent must emphasize that by far the most common event after ICD implantation is that patients will not experience their device at all, even in appropriately selected patients. Enthusiasm for ICDs among heart failure patients must not cloud decision-making about the selection of patients for the ICD discussion. One contributor to patient enthusiasm for ICDs may be patient misunderstanding of device risk and benefit. This may reflect the challenge of communicating uncertain risks and benefits to the individual patient translated from population-based clinical trials.45 Communication about ICD therapy may become more effective using absolute risk, such as events per 100 patients or number needed to treat, rather than percentages or relative risks.
Heart failure patients, including those in this survey, demonstrate a poor understanding of mortality in heart failure and overestimate their own anticipated survival, as recently documented by Allen at al.46 Patient misunderstanding about the heart failure disease process and expected mortality has the potential to impact their impression of any therapy, not just ICDs. Yet patients with heart failure can express a meaningful preferences for therapies by weighing quality of life and survival.47 More precise information about prognosis in heart failure will inform patient decision-making about ICD implantation and may influence when they might considering turning off their defibrillator.48 Specific discussion should also include discussion of anticipated prognosis after a life is saved by an ICD placed for primary prevention, which for some patients may not extend beyond the next year.49 More focused conversation about patient priorities will inform physician strategies about ICD and other devices that can alter quality of life and modes of death. New strategies must be developed and implemented for improving 2-way communication about overall prognosis, device therapies, and the patient preferences.50,51
Limitations
These survey data on ICDs have several limitations. The survey instrument was only administered to patients in two heart failure referral programs, both located at quaternary-care referral centers. Patients at referral centers may be more willing to embrace invasive or device therapies such as ICDs. These patients may be different from heart failure patients in the community, who are older and have more comorbidities. ICD perceptions among the more typical heart failure patient seen in the community may be even more important because most of these older patients have a higher chance of death from causes other than sudden cardiac death. The survey instrument was novel and developed only for purposes of this study, and all results should be interpreted in the context of a pilot study. It was not validated for stability of responses over time or for variability in answers based on question and answer sequence. As such, the ability to generalize patient responses beyond the population studied at two referral centers may be limited. Patients were given hypothetical scenarios that might prompt ICD inactivation. Their decision about turning off an ICD may well be quite different when actually facing severe illness or the end of life. There is a recognized difficulty in patient perception of competing risks and benefits. We cannot comment on the details of physician communication of the ICD data, which provides a crucial link between clinical trials and heart failure patient perceptions.
Implications
Heart failure patient education must include direct discussion about ICD benefits, limitations, general prognosis, and modes of death in end-stage heart failure. Continuous revisions of postimplantation data about ICDs are crucial to identify patients who will derive the maximum benefit from these costly devices and to inform patient as well as physician decisions. Ongoing critical appraisal of the risks and benefits of ICD therapy should guide the discussion between physician and patient.11 A web-based ICD registry has recently collected data on more than 200,000 device implants, including the approximately 10,000 ICDs implanted each month in the United States.10 The longitudinal component of this registry can guide efforts to identify appropriate candidates, and educate both physicians and patients about the natural history of ICDs after implantation. Further effort should be invested into the development of patient education tools. In the meantime, the focus should remain on heart failure therapies that prevent disease progression, and decrease both sudden and anticipated mortality. Diligent application of these therapies will enhance both the quality and duration of lives after lifesaving ICD therapies have been delivered.
Footnotes
Conflict of interests: None.
References
- 1.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 2.HFSA. 2006 Comprehensive Heart Failure Practice Guideline. J Cardiac Failure. 2006;12:e1–2. doi: 10.1016/j.cardfail.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 4.Hohnloser SH, Kuck KH, Dorian P, Roberts RS, Hampton JR, Hatala R, et al. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med. 2004;351:2481–8. doi: 10.1056/NEJMoa041489. [DOI] [PubMed] [Google Scholar]
- 5.Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–83. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 6.Kadish A, Dyer A, Daubert JP, Quigg R, Estes NA, Anderson KP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151–8. doi: 10.1056/NEJMoa033088. [DOI] [PubMed] [Google Scholar]
- 7.Desai AS, Fang JC, Maisel WH, Baughman KL. Implantable defibrillators for the prevention of mortality in patients with nonischemic cardiomyopathy: a meta-analysis of randomized controlled trials. JAMA. 2004;292:2874–9. doi: 10.1001/jama.292.23.2874. [DOI] [PubMed] [Google Scholar]
- 8.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, et al. Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 9.Kannel WB, Plehn JF, Cupples LA. Cardiac failure and sudden death in the Framingham Study. Am Heart J. 1988;115:869–75. doi: 10.1016/0002-8703(88)90891-5. [DOI] [PubMed] [Google Scholar]
- 10.Hammill SC, Stevenson LW, Kadish AH, Kremers MS, Heidenreich P, Lindsay BD, et al. Review of the registry’s first year, data collected, and future plans. Heart Rhythm. 2007;4:1260–3. doi: 10.1016/j.hrthm.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 11.Tung R, Zimetbaum P, Josephson ME. A critical appraisal of implantable cardioverter-defibrillator therapy for the prevention of sudden cardiac death. J Am Coll Cardiol. 2008;52:1111–21. doi: 10.1016/j.jacc.2008.05.058. [DOI] [PubMed] [Google Scholar]
- 12.Setoguchi S, Stevenson LW, Schneeweiss S. Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J. 2007;154:260–6. doi: 10.1016/j.ahj.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 13.Carson P, Anand I, O’Connor C, Jaski B, Steinberg J, Lwin A, et al. Mode of death in advanced heart failure: the Comparison of Medical, Pacing, and Defibrillation Therapies in Heart Failure (COMPANION) trial. J Am Coll Cardiol. 2005;46:2329–34. doi: 10.1016/j.jacc.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 14.Sanders GD, Hlatky MA, Owens DK. Cost-effectiveness of implantable cardioverter-defibrillators. N Engl J Med. 2005;353:1471–80. doi: 10.1056/NEJMsa051989. [DOI] [PubMed] [Google Scholar]
- 15.Mark DB, Nelson CL, Anstrom KJ, Al-Khatib SM, Tsiatis AA, Cowper PA, et al. Cost-effectiveness of defibrillator therapy or amiodarone in chronic stable heart failure: results from the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Circulation. 2006;114:135–42. doi: 10.1161/CIRCULATIONAHA.105.581884. [DOI] [PubMed] [Google Scholar]
- 16.Ellenbogen KA, Levine JH, Berger RD, Daubert JP, Winters SL, Greenstein E, et al. Are implantable cardioverter defibrillator shocks a surrogate for sudden cardiac death in patients with nonischemic cardiomyopathy? Circulation. 2006;113:776–82. doi: 10.1161/CIRCULATIONAHA.105.561571. [DOI] [PubMed] [Google Scholar]
- 17.Moss AJ, Greenberg H, Case RB, Zareba W, Hall WJ, Brown MW, et al. Long-term clinical course of patients after termination of ventricular tachyarrhythmia by an implanted defibrillator. Circulation. 2004;110:3760–5. doi: 10.1161/01.CIR.0000150390.04704.B7. [DOI] [PubMed] [Google Scholar]
- 18.Ahmad M, Bloomstein L, Roelke M, Bernstein AD, Parsonnet V. Patients’ attitudes toward implanted defibrillator shocks. Pacing Clin Electrophysiol. 2000;23:934–8. doi: 10.1111/j.1540-8159.2000.tb00877.x. [DOI] [PubMed] [Google Scholar]
- 19.Passman R, Subacius H, Ruo B, Schaechter A, Howard A, Sears SF, et al. Implantable cardioverter defibrillators and quality of life: results from the defibrillators in nonischemic cardiomyopathy treatment evaluation study. Arch Intern Med. 2007;167:2226–32. doi: 10.1001/archinte.167.20.2226. [DOI] [PubMed] [Google Scholar]
- 20.Daubert JP, Zareba W, Cannom DS, McNitt S, Rosero SZ, Wang P, et al. Inappropriate implantable cardioverter-defibrillator shocks in MADIT II: frequency, mechanisms, predictors, and survival impact. J Am Coll Cardiol. 2008;51:1357–65. doi: 10.1016/j.jacc.2007.09.073. [DOI] [PubMed] [Google Scholar]
- 21.Sola CL, Bostwick JM. Implantable cardioverter-defibrillators, induced anxiety, and quality of life. Mayo Clin Proc. 2005;80:232–7. doi: 10.4065/80.2.232. [DOI] [PubMed] [Google Scholar]
- 22.Dunbar SB, Warner CD, Purcell JA. Internal cardioverter defibrillator device discharge: experiences of patients and family members. Heart Lung. 1993;22:494–501. [PubMed] [Google Scholar]
- 23.Schron EB, Exner DV, Yao Q, Jenkins LS, Steinberg JS, Cook JR, et al. Quality of life in the antiarrhythmics versus implantable defibrillators trial: impact of therapy and influence of adverse symptoms and defibrillator shocks. Circulation. 2002;105:589–94. doi: 10.1161/hc0502.103330. [DOI] [PubMed] [Google Scholar]
- 24.Prudente LA, Reigle J, Bourguignon C, Haines DE, DiMarco JP. Psychological indices and phantom shocks in patients with ICD. J Interv Card Electrophysiol. 2006;15:185–90. doi: 10.1007/s10840-006-9010-z. [DOI] [PubMed] [Google Scholar]
- 25.Thomas SA, Friedmann E, Kao CW, Inguito P, Metcalf M, Kelley FJ, et al. Quality of life and psychological status of patients with implantable cardioverter defibrillators. Am J Crit Care. 2006;15:389–98. [PubMed] [Google Scholar]
- 26.Mark DB, Anstrom KJ, Sun JL, Clapp-Channing NE, Tsiatis AA, Davidson-Ray L, et al. Quality of life with defibrillator therapy or amiodarone in heart failure. N Engl J Med. 2008;359:999–1008. doi: 10.1056/NEJMoa0706719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Epstein AE, Dimarco JP, Ellenbogen KA, Estes NA, 3rd, Freedman RA, Gettes LS, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: Executive Summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices): developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation. 2008;117:2820–40. doi: 10.1161/CIRCUALTIONAHA.108.189742. [DOI] [PubMed] [Google Scholar]
- 28.Torrance GW. Utility approach to measuring health-related quality of life. J Chronic Dis. 1987;40:593–603. doi: 10.1016/0021-9681(87)90019-1. [DOI] [PubMed] [Google Scholar]
- 29.Stevenson LW, Hellkamp AS, Leier CV, Sopko G, Koelling T, Warnica JW, et al. Changing preferences for survival after hospitalization with advanced heart failure. J Am Coll Cardiol. 2008;52:1702–8. doi: 10.1016/j.jacc.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldenberg I, Moss AJ, Hall WJ, McNitt S, Zareba W, Andrews ML, et al. Causes and consequences of heart failure after prophylactic implantation of a defibrillator in the multicenter automatic defibrillator implantation trial II. Circulation. 2006;113:2810–7. doi: 10.1161/CIRCULATIONAHA.105.577262. [DOI] [PubMed] [Google Scholar]
- 31.Moss AJ, Vyas A, Greenberg H, Case RB, Zareba W, Hall WJ, et al. Temporal aspects of improved survival with the implanted defibrillator (MADIT-II) Am J Cardiol. 2004;94:312–5. doi: 10.1016/j.amjcard.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 32.Stevenson LW, Stevenson WG. Cost-effectiveness of ICDs. N Engl J Med. 2006;354:205–7. author reply -7. [PubMed] [Google Scholar]
- 33.Zwanziger J, Hall WJ, Dick AW, Zhao H, Mushlin AI, Hahn RM, et al. The cost effectiveness of implantable cardioverter-defibrillators: results from the Multicenter Automatic Defibrillator Implantation Trial (MADIT)-II. J Am Coll Cardiol. 2006;47:2310–8. doi: 10.1016/j.jacc.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 34.A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. The Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. N Engl J Med. 1997;337:1576–83. doi: 10.1056/NEJM199711273372202. [DOI] [PubMed] [Google Scholar]
- 35.Goldenberg I, Vyas AK, Hall WJ, Moss AJ, Wang H, He H, et al. Risk stratification for primary implantation of a cardioverter-defibrillator in patients with ischemic left ventricular dysfunction. J Am Coll Cardiol. 2008;51:288–96. doi: 10.1016/j.jacc.2007.08.058. [DOI] [PubMed] [Google Scholar]
- 36.Zareba W, Piotrowicz K, McNitt S, Moss AJ. Implantable cardioverter-defibrillator efficacy in patients with heart failure and left ventricular dysfunction (from the MADIT II population) American J Cardiol. 2005;95:1487–91. doi: 10.1016/j.amjcard.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 37.Mozaffarian D, Anker SD, Anand I, Linker DT, Sullivan MD, Cleland JG, et al. Prediction of mode of death in heart failure: the Seattle Heart Failure Model. Circulation. 2007;116:392–8. doi: 10.1161/CIRCULATIONAHA.106.687103. [DOI] [PubMed] [Google Scholar]
- 38.Teuteberg JJ, Lewis EF, Nohria A, Tsang SW, Fang JC, Givertz MM, et al. Characteristics of patients who die with heart failure and a low ejection fraction in the new millennium. J Cardiac Failure. 2006;12:47–53. doi: 10.1016/j.cardfail.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 39.Sears SF, Jr, Conti JB. Quality of life and psychological functioning of ICD patients. Heart (Br Cardiac Soc) 2002;87:488–93. doi: 10.1136/heart.87.5.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lewis WR, Luebke DL, Johnson NJ, Harrington MD, Costantini O, Aulisio MP. Withdrawing implantable defibrillator shock therapy in terminally ill patients. Am J Med. 2006;119:892–6. doi: 10.1016/j.amjmed.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 41.Goldstein NE, Lampert R, Bradley E, Lynn J, Krumholz HM. Management of implantable cardioverter defibrillators in end-of-life care. Ann Intern Med. 2004;141:835–8. doi: 10.7326/0003-4819-141-11-200412070-00006. [DOI] [PubMed] [Google Scholar]
- 42.Berger JT. The ethics of deactivating implanted cardioverter defibrillators. Ann Intern Med. 2005;142:631–4. doi: 10.7326/0003-4819-142-8-200504190-00012. [DOI] [PubMed] [Google Scholar]
- 43.Mueller PS, Hook CC, Hayes DL. Ethical analysis of withdrawal of pacemaker or implantable cardioverter-defibrillator support at the end of life. Mayo Clin Proc. 2003;78:959–63. doi: 10.4065/78.8.959. [DOI] [PubMed] [Google Scholar]
- 44.Stevenson LW, Desai AS. Selecting patients for discussion of the ICD as primary prevention for sudden death in heart failure. J Cardiac Failure. 2006;12:407–12. doi: 10.1016/j.cardfail.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 45.Politi MC, Han PK, Col NF. Communicating the uncertainty of harms and benefits of medical interventions. Med Decis Making. 2007;27:681–95. doi: 10.1177/0272989X07307270. [DOI] [PubMed] [Google Scholar]
- 46.Allen LA, Yager JE, Funk MJ, Levy WC, Tulsky JA, Bowers MT, et al. Discordance between patient-predicted and model-predicted life expectancy among ambulatory patients with heart failure. JAMA. 2008;299:2533–42. doi: 10.1001/jama.299.21.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lewis EF, Johnson PA, Johnson W, Collins C, Griffin L, Stevenson LW. Preferences for quality of life or survival expressed by patients with heart failure. J Heart Lung Transplant. 2001;20:1016–24. doi: 10.1016/s1053-2498(01)00298-4. [DOI] [PubMed] [Google Scholar]
- 48.Yancy CW. Predicting life expectancy in heart failure. JAMA. 2008;299:2566–7. doi: 10.1001/jama.299.21.2566. [DOI] [PubMed] [Google Scholar]
- 49.Poole JE, Johnson GW, Hellkamp AS, Anderson J, Callans DJ, Raitt MH, et al. Prognostic importance of defibrillator shocks in patients with heart failure. New Engl J Med. 2008;359:1009–17. doi: 10.1056/NEJMoa071098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van der Weijden T, Bos LB, Koelewijn-van Loon MS. Primary care patients’ recognition of their own risk for cardiovascular disease: implications for risk communication in practice. Curr Opin Cardiol. 2008;23:471–6. doi: 10.1097/HCO.0b013e32830b35f6. [DOI] [PubMed] [Google Scholar]
- 51.Goodyear-Smith F, Arroll B, Chan L, Jackson R, Wells S, Kenealy T. Patients prefer pictures to numbers to express cardiovascular benefit from treatment. Ann Family Med. 2008;6:213–7. doi: 10.1370/afm.795. [DOI] [PMC free article] [PubMed] [Google Scholar]