Abstract
Objectives
Microarray analyses of sinus mucosa in pediatric patients with chronic rhinosinusitis (CRS) have recently demonstrated increased messenger RNA expression of the inflammatory chemokines CXCL5 and CXCL13 and of the innate immune mediators β-defensin 1 (DEFB1), serum amyloid A2 (SAA2), and serpin B4. The objectives of this study were to determine whether these gene products were expressed at the protein level in pediatric sinus mucosa and to determine their localization.
Design
Immunohistochemical analysis was used to identify protein expression and cellular localization of CXCL5, CXCL13, DEFB1, SAA2, and serpin B4. Coimmunofluorescence staining of inflammatory cells was performed to further evaluate expression of CXCL5 and CXCL13.
Setting
Pediatric tertiary care facility.
Patients
Fifteen children with CRS who underwent endoscopic sinus surgery and 8 children who underwent craniofacial or neurosurgical procedures for abnormalities other than sinusitis.
Main Outcome Measures
Protein expression and cellular localization of CXCL5, CXCL13, DEFB1, SAA2, and serpin B4 in pediatric sinus mucosa.
Results
Ciliated and basal cells in the pseudostratified epithelium stained positively for the 5 mediators examined in both cohorts. Except for serpin B4, goblet cells did not stain for any mediators in either cohort. Glandular cells stained positively for all 5 mediators in both cohorts. Coimmunofluorescence staining of inflammatory cells showed that CXCL13 was expressed in macrophages, T and B cells but not in neutrophils. CXCL5 was detected only in T cells.
Conclusions
CXCL5, CXCL13, DEFB1, SAA2, and serpin B4 were expressed at the protein level in the sinus mucosa of controls and pediatric patients with CRS and exhibited cell-specific localization. These mediators, not typically associated with pediatric CRS, may be involved in the inflammatory response and mucus hypersecretion seen in pediatric CRS.
The causes of chronic rhinosinusitis (CRS) are not well understood, but it is generally accepted that various proinflammatory mediators and immunoreactive products play a significant role in initiating and sustaining the inflammatory response seen in patients with CRS. These mediators include proinflammatory molecules from both the innate immune system, which is the first-line defense of sinonasal respiratory epithelium, and the more evolved and specialized adaptive immune system. The innate immune system, in addition to providing physical barriers like mucus that traps particles and pathogens removing them via mucociliary clearance, produces broad-spectrum antimicrobial products with nonspecific tissue responses. Adaptive immune mediators, on the other hand, rely on specificity and memory to exert their effects on pathogens. Both inflammatory and immune mediators have been studied extensively and are important to the pathogenesis of adult CRS. However, the relationships of these mediators to one another and to the pathogenesis of CRS are difficult to ascertain.1,2 The challenges are even greater in pediatric CRS, which is understudied compared with adult CRS.
With the advent of microarray technology, it has become possible to identify differentially expressed genes that may impact on the pathogenesis of pediatric CRS. Recently, our research group3 reported on microarray analyses of sinus mucosa from children and adolescents with CRS and from control patients. In that study, a greater than 5-fold upregulation in messenger RNA (mRNA) of 2 adaptive immune mediators, CXCL13 and CXCL5, was observed and validated by real-time quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) in the sinus mucosa of children with CRS. These mediators belong to the CXC family of chemokines that function as chemoattractants. The CXCL13 mediator, also known as B-lymphocyte chemoattractant, plays a critical role in lymphocyte homing to secondary lymphoid organs and is localized to the follicular dendritic cells of lymph node germinal centers.4 It is more highly expressed in adult patients with CRS and nasal polyps than in adult patients with CRS without polyps and in controls.2 The CXCL5 mediator, or epithelial-derived neutrophil-activating peptide 78 (ENA-78), stimulates the chemotaxis of neutrophils and has been localized to colonic epithelial cells5 as well as to the epithelium of sinus mucosa.6 It is intriguing that CXCL13 and CXCL5 mRNA are upregulated in pediatric CRS mucosa because this pattern differs from the adaptive mediators often reported in adult CRS, which include sargramostim (GM-CSF), interleukin 3 (IL-3), IL-4, IL-5, IL-6, IL-8, and RANTES (regulated on activation, normal T-cell expressed and secreted).7,8
In addition to CXCL5 and CXCL13, 3 innate immune response genes—β-defensin 1 (DEFB1), serum amyloid A2 (SAA2) and serpin B4—were also identified and shown by microarray analyses in pediatric CRS sinus mucosa to be upregulated by a 5-fold increase in mRNA.3 These results were validated by qRT-PCR in an independent set of pediatric CRS sinus mucosa. The DEFB1 mediator shows antimicrobial potency against a number of gram-positive and gram-negative bacteria as well as yeasts and viruses.9 The SAA2 mediator is a major acute-phase protein that recruits immune cells to inflammatory sites and is increased in adult CRS.1,10 Serpin B4 belongs to the superfamily of serine proteinase inhibitors involved in the suppression of squamous cell carcinoma via cellular apoptosis.11 However, a role for these mediators in the pathophysiologic development of pediatric CRS is unclear.
Expression of inflammatory and/or immune response mediators was long thought to be restricted to immune and/or inflammatory cells. However, epithelial cells are now known to also express these mediators.12,13 There are markedly increased numbers of epithelial cells in patients with CRS because glandular hyperplasia in the sinus mucosa is a characteristic phenotype in both adult14 and pediatric15 CRS. To distinguish whether the increased mRNA expression observed for the 5 inflammatory mediators and immune reactive products in pediatric CRS tissue3 originates in the epithelial cells or is merely a reflection of the increased presence of inflammatory cells in sinusitis, we sought to identify the precise histologic localization of these 5 mediators in separate but representative sinus mucosal specimens from children with CRS and from controls. The location of the increased mRNA signals, whether coming from inflammatory cells or epithelial cells, or both, may have implications for their causal role in pediatric CRS.
METHODS
PATIENT POPULATION
Sinus tissues from children who underwent craniofacial and/or neurosurgical procedures for abnormalities other than sinusitis served as controls.16 For this study, persons aged from 1 to 21 years were considered children, per NIH definitions and guidelines.17 Eight patients (6 boys), mean age 218 months (age range, 161–238 months), were included in the control group. Exclusion criteria for the control population included a history of sinonasal surgery, current sinonasal infection, sinonasal or allergic symptoms within the previous 3 months, and/or treatment with topical nasal corticosteroids within 1 month or antihistamines within 3 months before surgery. A combination of computed tomographic (CT) scans and/or magnetic resonance images with sinonasal sections was obtained. There was no radiographic evidence of sinusitis at the time of surgery for children in the control group. Six control specimens were analyzed by immunohistochemical analysis, and 2 were examined using confocal analysis.
Sinus tissue was obtained from 15 patients with CRS (4 boys), mean age 104 months (age range, 23–189 months), who underwent functional endoscopic sinus surgery for CRS refractory to medical management. Chronic rhinosinusitis was defined as persistent symptoms for more than 3 months, despite antimicrobial and topical nasal corticosteroid therapies. Patients with sinusitis had at least 2 of the major signs and symptoms of chronic sinusitis: nasal congestion, rhinorrhea, headache, facial pain and/or pressure, or change in olfaction.18,19 In addition, all of these children had at least 1 of the following minor signs or symptoms in conjunction with a minimum of 2 major signs and symptoms: fever, halitosis, cough, and/or irritability.18,19 All patients with CRS were receiving antibiotics and/or topical nasal corticosteroids at the time of surgery. Patients with cystic fibrosis, immunodeficient states, ciliary dyskinesias, and/or craniofacial abnormalities were excluded. In all patients with CRS, CT scans of the sinuses were obtained, evaluated, and scored by means of the Lund-Mackay system.19 All CT scans received a minimum score of 8 in this cohort. Twelve samples were used for immunohistochemical analyses, and 3 specimens were evaluated with confocal analyses.
All patients were entered consecutively into the study after appropriate surgical and research consents (and assents when applicable) were obtained. This study was reviewed and approved by the institutional review board of Children’s National Medical Center.
SPECIMEN COLLECTION
Using standard endoscopic instruments (ie, Blakesely forceps), mucosal specimens from the right and left paranasal sinuses were collected separately at the time of surgery. All patients with CRS had either maxillary or ethmoid tissues collected in the vicinity of the osteomeatal complex. When clinically indicated, mucosa from the frontal and sphenoid sinuses was also collected. In control patients, the mucosa of the paranasal sinuses entered as a result of the craniofacial and/or neurosurgical procedure performed was collected in an identical manner to that used in the sinusitis population.16 Whenever feasible, sinus mucosa from the control population was collected as close as possible to the osteomeatal complex. Specimens were immediately fixed with 10% neutral buffered formalin (pH, 6.8–7.2) (Richard-Allan Scientific) for paraffin embedding and sectioning.
IMMUNOHISTOCHEMICAL ANALYSIS
Five-micron paraffin tissue sections were deparaffinized in xylenes and rehydrated in 100% and 95% ethanol. Endogenous peroxidase activity was blocked by treating with 0.3% hydrogen peroxide in methyl alcohol for 30 minutes followed by phosphate-buffered saline (PBS) washing for 5 minutes. Sections were incubated overnight at 4°C with primary antibodies diluted in blocking buffer (PBS with 2% serum and 0.05% polysorbate 20). The following primary antibodies were used: CXCL5 (ENA-78, 1:150, goat IgG; R&D Systems); CXCL13 (1:6, goat IgG; R&D Systems); DEFB 1 (1:100, rabbit polyclonal; Santa Cruz Biotechnology); SAA2 (1:1000, mouse polyclonal; Abnova); serpin B4 (SCCA2, 1:10, mouse monoclonal Santa Cruz Biotechnology). Omission of primary antibody was used as a negative control. After being washed with PBS, each section was incubated with 1:250 diluted biotinylated secondary antibodies (Vector Laboratories Inc) for 1 hour at room temperature. Secondary antibody was detected with Vectastain ABC reagent (ABC Elite Kit; Vector Laboratories), and 3′3′diaminobenzidine substrate (Sigma). Slides were washed in tap water for 5 minutes and then counterstained with hematoxylin (Sigma). Following counterstaining, slides were washed in tap water for 5 minutes, dehydrated (95%, 100% ethanol), transferred to 100% xylene, and mounted. Brightfield immunohistochemical images were acquired with a Nikon Eclipse E800 microscope (Nikon Corp). Control mucosa typically contains far fewer submucosal glands (SMG) than CRS mucosa,15 so areas of control mucosa with SMG were selected for these studies.
IMMUNOFLUORESCENCE
Five-micron paraffin tissue sections were deparaffinized in xylenes and rehydrated in 100% and 95% ethanol. For blocking, tissues were incubated with 5% normal serum in PBS with 0.3% Triton X-100 (hereinafter “PBS/Triton”; Sigma) for 1 hour at room temperature and then incubated with primary antibody in PBS/Triton overnight at 4°C. In addition to the listed antibodies, the following mouse monoclonal antibodies against immune cell markers were used for colocalization: neutrophil elastase (neutrophil marker, 1:50, mouse monoclonal IgG; Dako Cytomation), CD20cy (L26, B-cell marker, 1:50, mouse monoclonal IgG; Dako Cytomation), PGP9.5 (T-cell marker, 1:40, mouse monoclonal IgG; AbCam), and MAC387 (macrophage marker, 1:200, mouse monoclonal IgG; AbCam). Tissues were washed in PBS 3 times for 5 minutes each. A 2-minute wash with 0.4M high-salt PBS was added between the second and third PBS wash to reduce background. Tissues were then incubated with species-appropriate fluorochrome-conjugated secondary antibody at 1:250 dilution (Jackson Immunoresearch) in PBS/Triton for 1 hour at room temperature. CXCL5 or CXCL13 antibodies were detected with an Alexa 568–labeled secondary antibody (Invitrogen), and inflammatory cell markers (MAC387, PGP 9.5, LG26, or neutrophil elastase) were detected with an Alexa 488–labeled secondary antibody. The tissues were washed again in PBS 3 times for 5 minutes. To counterstain nuclei, tissue was incubated with PBS containing DAPI (1:1000; Sigma) for 15 minutes and rinsed once with PBS for 5 minutes, mounted with Prolong Gold Antifade Reagent (Molecular Probes), and stored at 4°C. Immunofluorescent images were acquired with a Zeiss LSM 510 confocal microscope (Carl Zeiss MicroImaging) using Zen 2008 Light Edition acquisition software, version 5 and an oil objective, magnification ×60.
RESULTS
LOCALIZATION OF CXCL5, CXCL13, DEFB1, SAA2, AND SERPIN B4 IN THE SINUS MUCOSA FROM PATIENTS WITH CRS AND CONTROLS
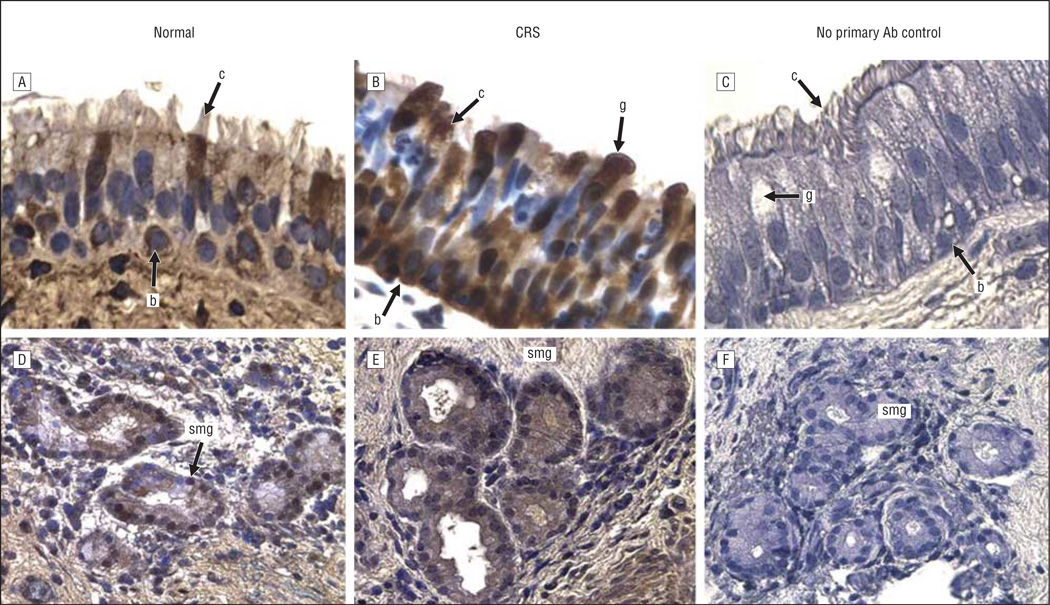
The expression and localization of 5 inflammatory and/or immune response gene products that exhibit increased levels of expression at the mRNA level in pediatric sinus mucosal samples3 were evaluated by immunohistochemical analysis. Localization in paranasal sinus mucosal specimens from 6 control patients and 12 patients with CRS was evaluated in 2 distinct microanatomical compartments—the surface epithelium and the submucosa. Control sinus mucosa specimens were selected that exhibited SMG so that expression in control SMG could be evaluated. Immunohistochemical data for localization of CXCL5, CXCL13, DEFB1, SAA2, and serpin B4 are shown in representative photomicrographs in Figures 1, 2, 3, 4, and 5. Immunohistochemical analysis showed that all 5 mediators were strongly expressed in the SMG and were also expressed in ciliated and basal cells in the epithelium of the sinus mucosa. Serpin B4 was also expressed in a few goblet cells (Figure 5). The expression patterns were very similar in both CRS and control sinus mucosa specimens, although control pediatric sinus mucosa typically contains far fewer SMG than CRS sinus mucosa.15
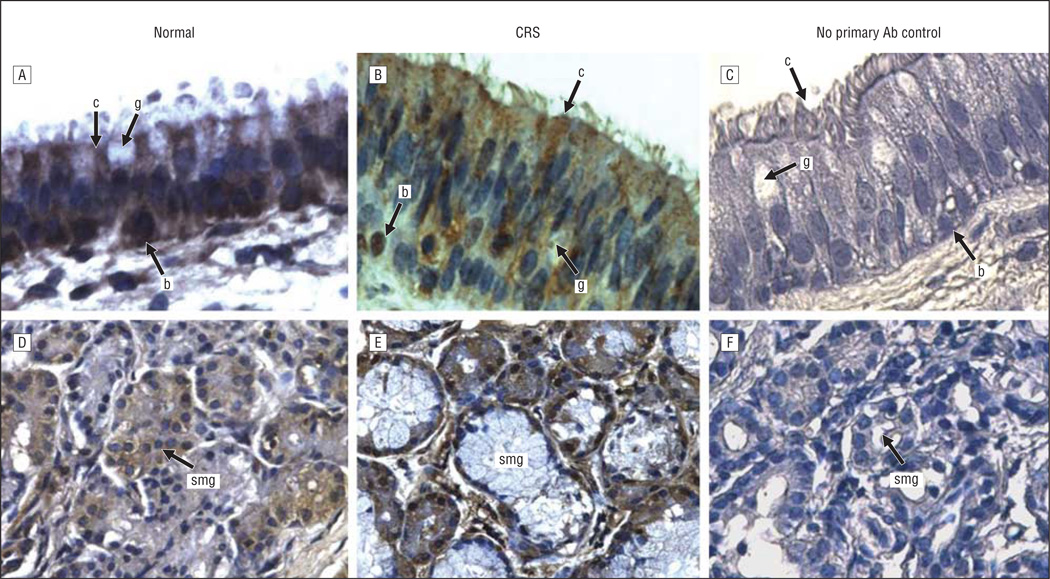
Figure 1.
CXCL5 immunoreactivity in the sinus mucosa followed by hematoxylin counterstaining. A and D, Control tissues. B and E, Chronic rhinosinusitis (CRS) tissues. C and F, Negative controls. A-C, Epithelia. D-F, Glands. Original magnification ×400 for all panels. Ab indicates antibody; b, basal cell; c, ciliated cell; g, goblet cell; and smg, submucosal gland.
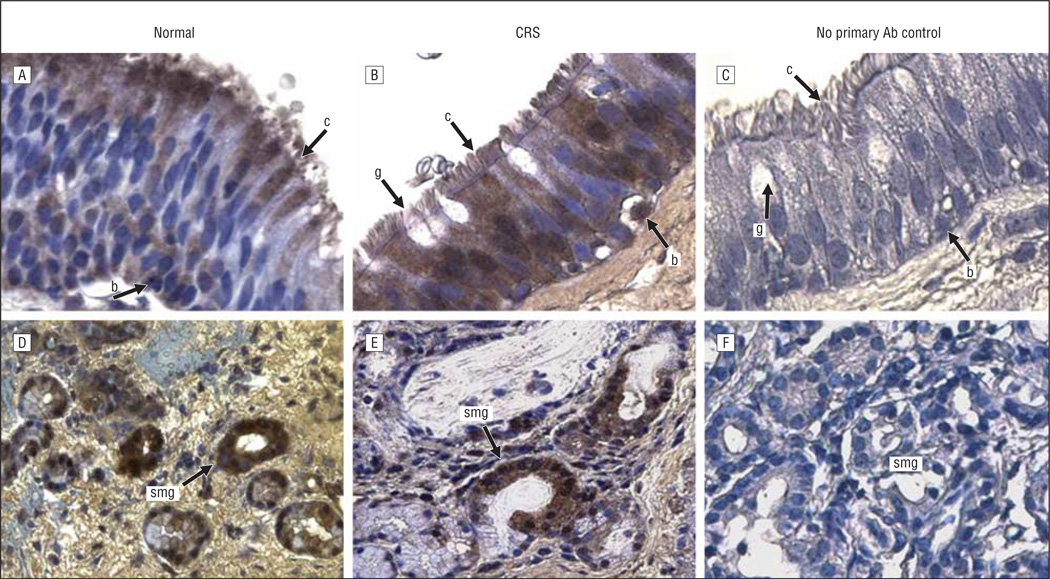
Figure 2.
CXCL13 immunoreactivity in the sinus mucosa followed by hematoxylin counterstaining. A and D, Control tissues. B and E, Chronic rhinosinusitis (CRS) tissues. C and F, Negative controls. A-C, Epithelia. D-F, Glands. Original magnification ×400 for all panels. Ab indicates antibody; b, basal cell; c, ciliated cell; g, goblet cell; and smg, submucosal gland.
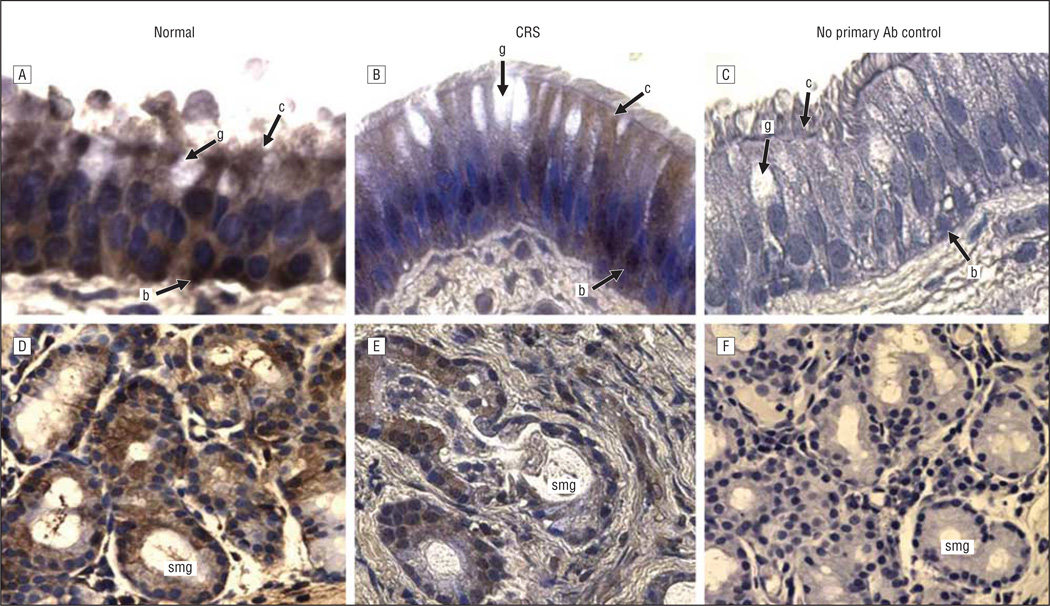
Figure 3.
β-Defensin 1 immunoreactivity in the sinus mucosa followed by hematoxylin counterstaining. A and D, Control tissues. B and E, Chronic rhinosinusitis (CRS) tissues. C and F, Negative controls. A-C, Epithelia. D-F, Glands. Original magnification ×400 for all panels. Ab indicates antibody; b, basal cell; c, ciliated cell; g, goblet cell; and smg, submucosal gland.
Figure 4.
Serum amyloid A2 immunoreactivity in the sinus mucosa followed by hematoxylin counterstaining. A and D, Control tissues. B and E, Chronic rhinosinusitis (CRS) tissues. C and F, Negative controls. A-C, Epithelia. D-F, Glands. Original magnification ×400 for all panels. Ab indicates antibody; b, basal cell; c, ciliated cell; g, goblet cell; and smg, submucosal gland.
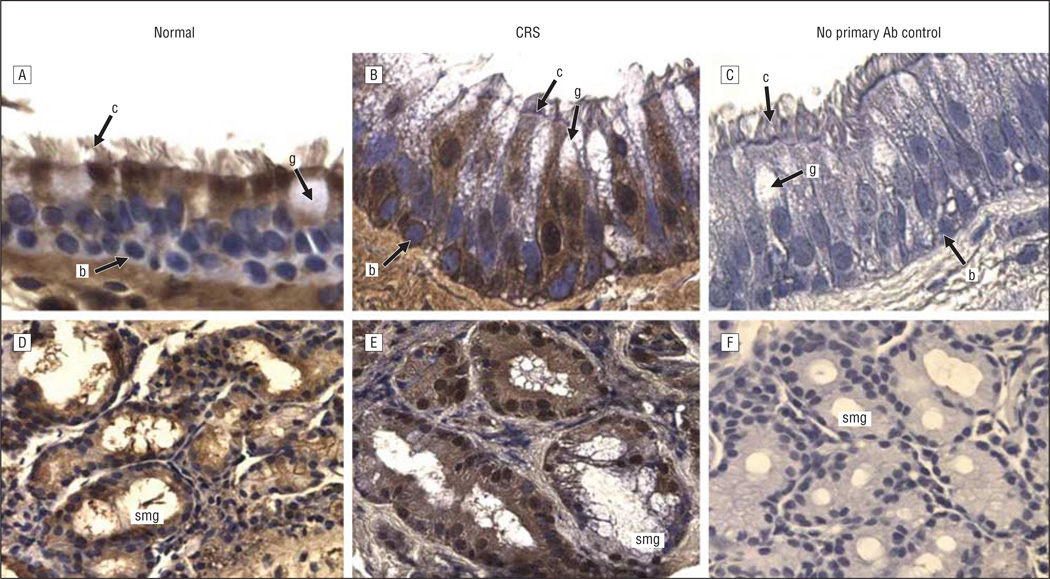
Figure 5.
Serpin B4 immunoreactivity in the sinus mucosa followed by hematoxylin counterstaining. A and D, Control tissues. B and E, Chronic rhinosinusitis (CRS) tissues. C and F, Negative controls. A-C, Epithelia. D-F, Glands. Original magnification ×400 for all panels. Ab indicates antibody; b, basal cell; c, ciliated cell; g, goblet cell; and smg, submucosal gland.
CELLULAR LOCALIZATION OF MEDIATORS IN INFLAMMATORY CELLS IN SINUS MUCOSA
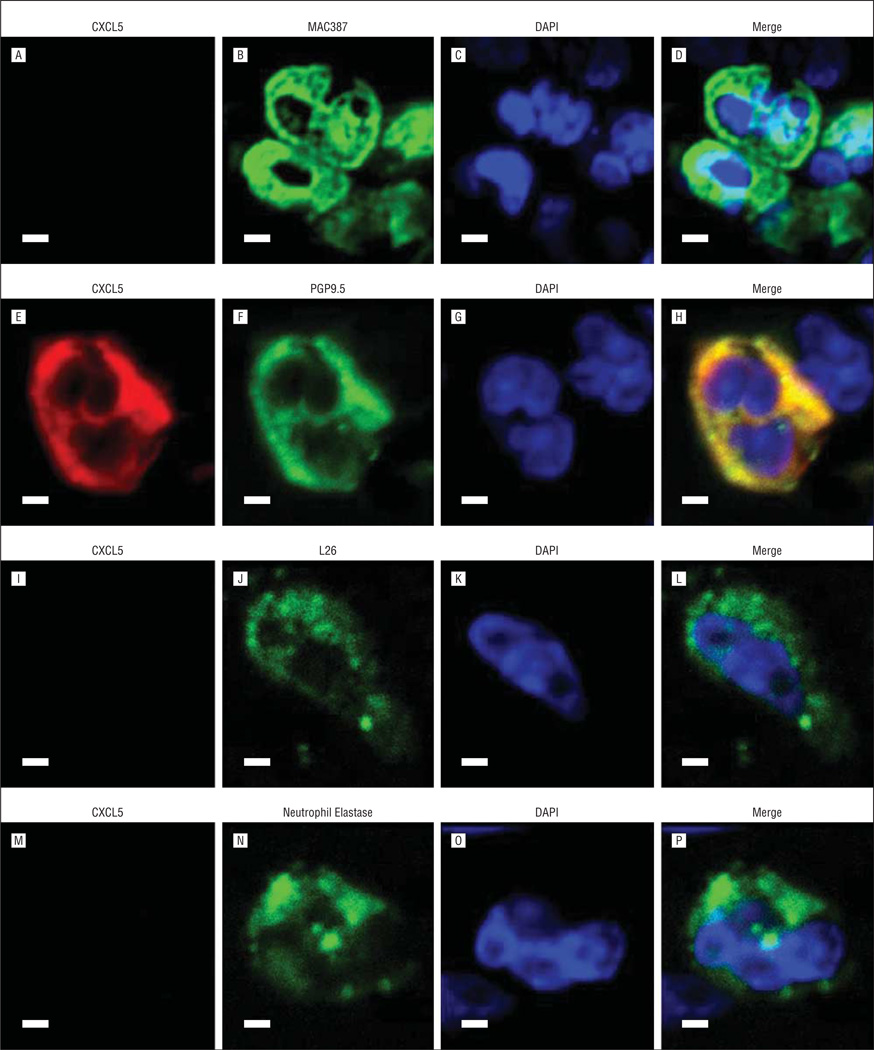
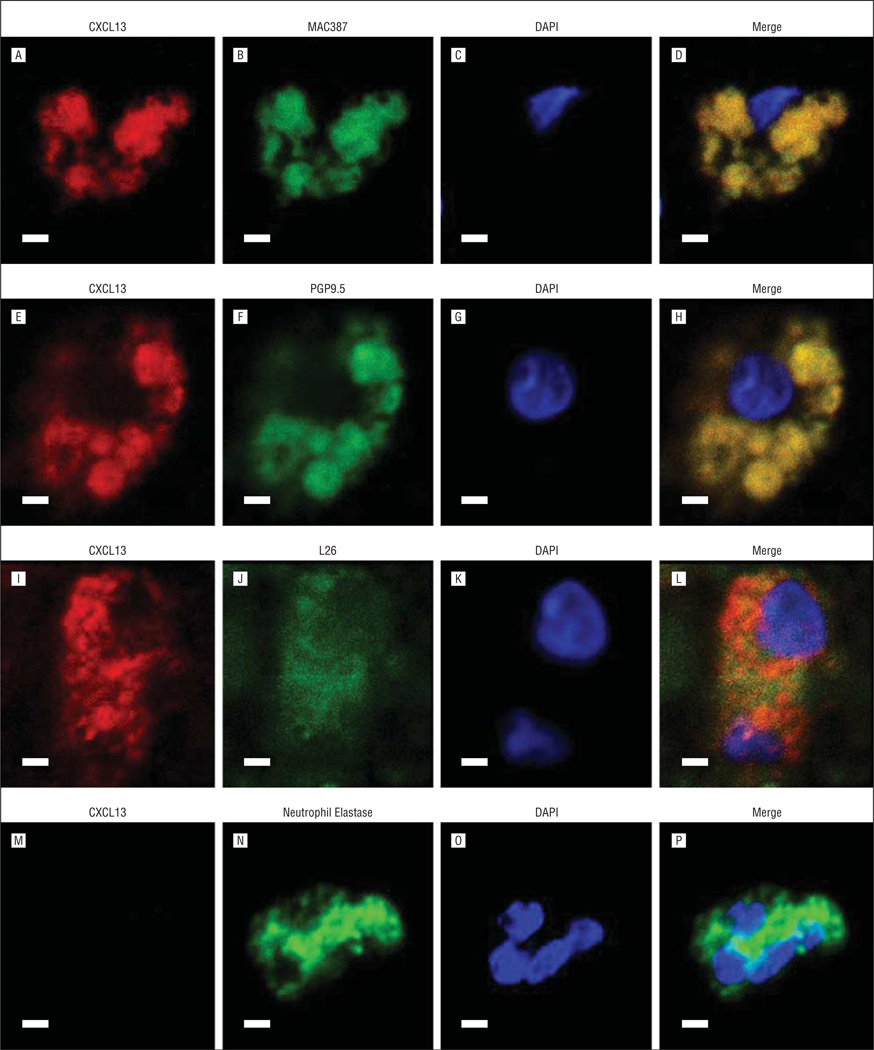
Chemokines are typically expressed by inflammatory cells. To determine whether the 2 chemokines (CXCL5 and CXCL13) that are highly upregulated in CRS sinus mucosa3 were expressed by inflammatory as well as epithelial cells in the sinus mucosa, their localization was evaluated by immunofluorescence staining and confocal microscopy (Figure 6 and Figure 7). Double labeling revealed that CXCL5 was colocalized only with PGP9.5 in T cells (Figure 6H, yellow areas). CXCL13 was colocalized with MAC387 in macrophages (Figure 7D, yellow areas), with PGP9.5 in T cells (Figure 7H, yellow areas), and with LG26 in B cells (Figure 7L, yellow areas). CXCL13 did not colocalize with neutrophils (Figure 7P).
Figure 6.
Immunofluorescent costaining of CXCL5 and inflammatory cell markers. A, E, I, and M, CXCL5. Markers for macrophages (B), T cells (F), B cells (J), and neutrophils (N). DAPI (C, G, K, and O) and merged images (D, H, L, and P). Scale bars indicate 50 µm.
Figure 7.
Immunofluorescent costaining of CXCL13 and inflammatory cell markers. A, E, I, and M, CXCL13. Markers for macrophages (B), T cells (F), B cells (J), and neutrophils (N). DAPI (C, G, K, O) and merged images (D, H, L, and P). Scale bars indicate 50 µm.
COMMENT
Expression of adaptive (CXCL5 and CXCL13) and innate (serpin B4, SAA2, and DEFB1) immune mediators at the mRNA level and their marked upregulation in the sinus mucosa of patients with CRS has recently been reported.3 Herein, we undertook to establish whether these gene products were expressed at the protein level and to determine their cellular localization in pediatric sinus mucosa epithelium. CXCL5, CXCL13, serpin B4, SAA2, and DEFB1 were expressed both in the epithelium and in the SMG of sinus tissues of children with and without CRS. These data demonstrate that mediators from both the adaptive and innate immune systems are expressed in pediatric sinus mucosa, supporting the concept that the immune system’s innate and adaptive arms act in concert. Moreover, these mediators can be produced by epithelial cells, and their upregulation is not necessarily due to the increased presence of inflammatory cells.
Of the 3 innate immune mediators we identified in the sinus mucosa of children with CRS, SAA2 has previously been shown to be present in the sinonasal tissues of adults with CRS.1,10 This study showed that DEFB1 and serpin B4 are also expressed in the sinus mucosa. Mechanisms used during innate immunity include the secretion of broad-spectrum antimicrobial peptide products such as β-defensins9 and the secretion of acute-phase reactants and opsonins like SAA2.1,10 Inhibition of target enzymes in proteolytic cascades by serpin B4, a serine protease inhibitor, has also been described.11 It is likely that similar mechanisms are involved in pathogenic inflammatory responses characteristic of pediatric CRS.
CXCL5 and CXCL13 are chemokines typically expressed in inflammatory cells in various tissues. They belong to the CXC family of chemoattractant molecules that specialize in inducing the migration of leukocytes to areas of immunity challenges along a chemotactic gradient.5 They also modulate leukocyte adhesion and infiltration into tissues that are injured and infected. CXCL13 is a mediator in B-cell migration from primary follicles to the germinal centers of lymph nodes; it also creates feedback loops that further the development of other immune cells, especially naı¨ve T cells.20 CXCL13 was recently shown to have increased expression in the nasal polyps2 of adult patients with CRS. Patadia et al2 also report that there are areas of lymphoid neogenesis in the nasal polyps of these adult patients with CRS. Similar observations in nonpolypoid sinus mucosa have been made by us (data not shown), suggesting that CXCL13 also plays a role in attracting B cells to pediatric CRS sinus tissues. The present study, to the best of our knowledge, is the first report that demonstrates expression of CXCL13 at the protein level in ciliated and basal cells of the epithelial layer and in glandular cells in pediatric sinus mucosa as well as in T and B lymphocytes and macrophages in pediatric sinus mucosa. This suggests that CXCL13 may recruit T and B cells as well as macrophages to sustain the inflammatory response seen in pediatric CRS sinus tissues.
This investigation also supports an earlier report of CXCL5 expression in the sinus mucosa.6 CXCL5 has been identified in epithelial cells where it interacts with the CXCR2 receptor as a chemoattractant and angiogenic factor.21 CXCL5 expression was induced in an in vitro model of A549 cells exposed to staphylococcal proteases.6 We identified CXCL5 expression in ciliated and basal cells in the epithelium and in glandular cells, as well as in T cells, in the sinus mucosa. CXCL5, like CXCL13, was localized to T cells, suggesting that both chemokines may play a role in T-cell recruitment. This mechanism of action warrants further study.
Data from this study, like that of all investigations using human sinus mucosa tissues, are limited by a number of patient factors. Histologic and immunohistochemical analyses were performed on pediatric sinus tissues obtained at surgery and therefore reflect end-stage disease. Key steps in inflammatory and/or immune response pathways that drive the pathophysiologic development have already occurred and likely been modulated by the patients’ immune responses as well as by the effects of any antibiotic and intranasal steroid management, the length of exposure to these agents, and patient and/or family compliance with any prescribed perioperative regime. In addition, variability in the clinical profiles of children with CRS, their age at presentation, and the duration of disease prior to surgery likely also influenced the inflammatory response and study results. Since surgery itself is a traumatic event, it likely creates an inflammatory response, and although one would expect this to be similar for both healthy and diseased cohorts, the impact is unknown.
Despite the confounding factors and the fact that CXCL5, CXCL13, serpin B4, SAA2, and DEFB1 are similarly localized in CRS and control tissues, it is important to remember that there is a distinct difference in the histologic phenotype between these 2 cohorts and to interpret the data within this context. Chronic rhinosinusitis tissues have a significantly increased SMG area compared with nondiseased sinus mucosa.15 Since CXCL5, CXCL13, serpin B4, SAA2, and DEFB1 were localized to the SMG in all tissue samples, and CRS mucosa contains significantly more SMG, there is likely to be increased protein expression of 1 or more mediators in CRS sinus secretions if they impact on the pathophysiologic development of CRS. To assess this, we compared expression of these 5 mediators using Western blot or enzyme-linked immunosorbent assay. Western blot data showed that serpin B4 was significantly increased in the sinus mucosa of patients with CRS (data not shown). The other mediators are smaller than 10 kDa and were not amenable to Western blot analyses. Enzyme-linked immunosorbent assay showed that CXCL5 and CXCL13 were increased in patients with CRS, but the difference did not reach statistical significance, likely reflecting the high variability in CRS tissue and small sample size (data not shown).
To perform experiments to address this and other questions related to the pathophysiologic characteristics of pediatric CRS, the development of 3-dimensional in vitro models that mimic the in vivo histological features seen in the sinus tissues of these patients will be critical. With such models, it will be possible to begin to assess the relationship between the inflammatory response and mucous hypersecretion characteristics of pediatric CRS without being hampered by the limitations of using surgical mucosal specimens.
Acknowledgments
Funding/Support: This work was supported by a grant from the Cystic Fibrosis Foundation (Dr Peña) and National Institutes of Health grant R21 AI083995-01 (Drs Rose and Peña). The confocal microscopy imaging was supported by core grant 1P30HD40677 to the Children’s Mental Retardation and Developmental Disabilities Research Center.
Footnotes
Author Contributions: Drs Wu and Peñ a had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Wu, Rose, and Peñ a. Acquisition of data: Wu, Mimms, Lima, and Peters-Hall. Analysis and interpretation of data: Wu, Lima, Peters-Hall, Rose, and Peña. Drafting of the manuscript: Wu, Lima, and Peña. Critical revision of the manuscript for important intellectual content: Wu, Mimms, Lima, Peters-Hall, Rose, and Peña. Statistical analysis: Wu and Mimms. Obtained funding: Rose and Peñ a. Administrative, technical, and material support: Wu, Lima, and Peters-Hall. Study supervision: Wu and Peña.
Financial Disclosure: None reported.
REFERENCES
- 1.Ramanathan M, Jr., Lane AP. Innate immunity of the sinonasal cavity and its role in chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2007;136(3):348–356. doi: 10.1016/j.otohns.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 2.Patadia M, Dixon J, Conley D, et al. Evaluation of the presence of B-cell attractant chemokines in chronic rhinosinusitis. Am J Rhinol Allergy. 2010;24(1):11–16. doi: 10.2500/ajra.2010.24.3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu X, Ghimbovschi S, Aujla PK, Rose MC, Peña MT. Expression profiling of inflammatory mediators in pediatric sinus mucosa. Arch Otolaryngol Head Neck Surg. 2009;135(1):65–72. doi: 10.1001/archoto.2008.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ansel KM, Harris RB, Cyster JG. CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity. 2002;16(1):67–76. doi: 10.1016/s1074-7613(01)00257-6. [DOI] [PubMed] [Google Scholar]
- 5.Keates S, Keates AC, Mizoguchi E, Bhan A, Kelly CP. Enterocytes are the primary source of the chemokine ENA-78 in normal colon and ulcerative colitis. Am J Physiol. 1997;273(1, pt 1):G75–G82. doi: 10.1152/ajpgi.1997.273.1.G75. [DOI] [PubMed] [Google Scholar]
- 6.Sachse F, von Eiff C, Stoll W, Becker K, Rudack C. Induction of CXC chemokines in A549 airway epithelial cells by trypsin and staphylococcal proteases: a possible route for neutrophilic inflammation in chronic rhinosinusitis. Clin Exp Immunol. 2006;144(3):534–542. doi: 10.1111/j.1365-2249.2006.03089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamilos DL, Leung DY, Huston DP, Kamil A, Wood R, Hamid Q. GM-CSF, IL-5 and RANTES immunoreactivity and mRNA expression in chronic hyperplastic sinusitis with nasal polyposis (NP) Clin Exp Allergy. 1998;28(9):1145–1152. doi: 10.1046/j.1365-2222.1998.00380.x. [DOI] [PubMed] [Google Scholar]
- 8.Lennard CM, Mann EA, Sun LL, Chang AS, Bolger WE. Interleukin-1 beta, interleukin-5, interleukin-6, interleukin-8, and tumor necrosis factor-alpha in chronic sinusitis: response to systemic corticosteroids. Am J Rhinol. 2000;14(6):367–373. doi: 10.2500/105065800779954329. [DOI] [PubMed] [Google Scholar]
- 9.Singh PK, Jia HP, Wiles K, et al. Production of beta-defensins by human airway epithelia. Proc Natl Acad Sci U S A. 1998;95(25):14961–14966. doi: 10.1073/pnas.95.25.14961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lane AP, Truong-Tran QA, Myers A, Bickel C, Schleimer RP. Serum amyloid A, properdin, complement 3, and toll-like receptors are expressed locally in human sinonasal tissue. Am J Rhinol. 2006;20(1):117–123. [PMC free article] [PubMed] [Google Scholar]
- 11.Schick C, Kamachi Y, Bartuski AJ, et al. Squamous cell carcinoma antigen 2 is a novel serpin that inhibits the chymotrypsin-like proteinases cathepsin G and mast cell chymase. J Biol Chem. 1997;272(3):1849–1855. doi: 10.1074/jbc.272.3.1849. [DOI] [PubMed] [Google Scholar]
- 12.Hippenstiel S, Opitz B, Schmeck B, Suttorp N. Lung epithelium as a sentinel and effector system in pneumonia: molecular mechanisms of pathogen recognition and signal transduction. Respir Res. 2006;7(1):97. doi: 10.1186/1465-9921-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bals R, Hiemstra PS. Innate immunity in the lung: how epithelial cells fight against respiratory pathogens. Eur Respir J. 2004;23(2):327–333. doi: 10.1183/09031936.03.00098803. [DOI] [PubMed] [Google Scholar]
- 14.Tos M, Mogensen C. Mucus production in chronic maxillary sinusitis: a quantitative histopathological study. Acta Otolaryngol. 1984;97(1–2):151–159. doi: 10.3109/00016488409130975. [DOI] [PubMed] [Google Scholar]
- 15.Peña MT, Aujla PK, Zudaire E, et al. Localization and expression of MUC5B and MUC7 mucins in pediatric sinus mucosa. Ann Otol Rhinol Laryngol. 2007;116(5):389–397. doi: 10.1177/000348940711600513. [DOI] [PubMed] [Google Scholar]
- 16.Peña MT, Aujla PK, Patel KM, Zalzal GH, Rose MC. Immunohistochemical analyses of MUC5AC mucin expression in sinus mucosa of children with sinusitis and controls. Ann Otol Rhinol Laryngol. 2005;114(12):958–965. doi: 10.1177/000348940511401212. [DOI] [PubMed] [Google Scholar]
- 17.National Institutes of Health. [Accessed February 6, 2012];NIH policy and guidelines on the inclusion of children as participants in research involving human subjects. http://grants.nih.gov/grants/guide/notice-files/not98-024.html.
- 18.Lusk RP, Stankiewicz JA. Pediatric rhinosinusitis. Otolaryngol Head Neck Surg. 1997;117(3, pt 2):S53–S57. doi: 10.1016/S0194-59989770008-1. [DOI] [PubMed] [Google Scholar]
- 19.Lund VJ, Kennedy DW. The Staging and Therapy Group. Quantification for staging sinusitis. Ann Otol Rhinol Laryngol Suppl. 1995;167:17–21. [PubMed] [Google Scholar]
- 20.Ansel KM, Ngo VN, Hyman PL, et al. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406(6793):309–314. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- 21.Bürkle A, Niedermeier M, Schmitt-Gräff A, Wierda WG, Keating MJ, Burger JA. Overexpression of the CXCR5 chemokine receptor, and its ligand, CXCL13 in B-cell chronic lymphocytic leukemia. Blood. 2007;110(9):3316–3325. doi: 10.1182/blood-2007-05-089409. [DOI] [PubMed] [Google Scholar]