Abstract
Numerous studies have reported the beneficial effects of antioxidants in human diseases. Among their biological effects, a majority of antioxidants scavenge reactive radicals in the body, thereby reducing oxidative stress that is associated with the pathogenesis of many diseases. Antioxidant dendrimers are a new class of potent antioxidant compounds reported recently. In this study, six polyphenol-based antioxidant dendrimers with or without electron donating groups (methoxy group) were synthesized in order to elucidate the influence of electron donating groups (EDG) on their antioxidant activities. Syringaldehyde (2 ortho methoxy groups), vanillin (1 ortho methoxy group), and 4-hydroxybenzaldehyde (0 methoxy group) were derivatized with propargylamine to form building blocks for the dendrimers. All the six dendrimers contain polyether cores, which were synthesized by attaching pentaerythritol and methyl α-D-glucopyranoside to in-house prepared spacer units. To prepare generation 1 antioxidant dendrimers, microwave energy and granulated metallic copper catalyst were used to link the cores and building blocks together via alkyne-azide 1,3-cycloaddition click chemistry. These reaction conditions resulted in high yields of the target dendrimers that were free from copper contamination. Based on DPPH antioxidant assay, antioxidant dendrimers decorated with syringaldehyde and vanillin exhibited over 70- and 170-fold increase in antioxidant activity compared to syringaldehyde and vanillin, respectively. The antioxidant activity of dendrimers increased with increasing number of EDG groups. Similar results were obtained when the dendrimers were used to protect DNA and human LDL against organic carbon and nitrogen-based free radicals. In addition, the antioxidant dendrimers did not show any pro-oxidant activity on DNA in the presence of physiological amounts of copper. Although the dendrimers showed potent antioxidant activities against carbon and nitrogen free radicals, EPR and DNA protection studies revealed lack of effectiveness of these dendrimers against hydroxyl radicals. The dendrimers were not cytotoxic to CHO-K1 cells.
Keywords: Polyphenol, dendrimer, antioxidant, pro-oxidant, electron donating group
1. Introduction
Free radicals are produced during metabolism and inflammation. These highly reactive species are neutralized by antioxidant enzymes or endogenous/dietary antioxidants. Within cells, antioxidant enzymes such as glutathione peroxidase, superoxide dismutase and catalase play an important role in preventing oxidation of biomolecules. However, in extracellular fluids, key antioxidants include proteins that bind metal ions such as ceruloplasmin, transferrin and albumin and exogenous small molecules such as vitamins C and E and dietary polyphenols [1]. Excess free radicals are often associated with oxidative stress, which has been implicated in various diseases such as cancer, cardiovascular and neurological disorders [2,3,4]. Antioxidants work in many different ways such as quenching singlet oxygen, binding metal ions, and scavenging free radicals [1]. The majority of antioxidants including polyphenols scavenge free radicals. In this process, the antioxidant transfers hydrogen atom (HAT) or electron (ET) to neutralize the free radical [5,6], thereby preventing chain reactions such as lipid peroxidation. Other antioxidants prevent free radical formation by chelating transition metal ions such as Fe2+ and Cu+, which are known to generate free radicals by reaction with hydrogen peroxide [7,8]. Unfortunately, many antioxidants are associated with deleterious pro-oxidant effects during which transition metal ions, such as Cu2+ and Fe3+ are reduced by the antioxidants to lower oxidation states (Cu+ and Fe2+) [9,10,11,12]. The reduced metal ions subsequently react with H2O2 to form powerful hydroxyl radicals, which are capable of damaging cellular components including proteins, lipids, and nucleic acids.
There are a variety of free radicals with different aqueous solubilities and atomic make-ups that are found in hydrophilic or lipophilic compartments of the human body. Therefore, it is not surprising that a given antioxidant is unable to quench all types of free radicals. A desirable antioxidant should show strong radical scavenging but no pro-oxidant activities. To achieve this goal of designing an ideal antioxidant, we reported synthesis of a new class of antioxidants termed as antioxidant dendrimers [13,14]. The surface of the antioxidant dendrimer is composed of free radical scavenging moieties such as phenolic groups while the core of the dendrimer contains groups that sequester metal ions. Our initial success with antioxidant dendrimers exhibiting potent antioxidant activities and reduced pro-oxidant effects inspired us to synthesize and evaluate other dendritic antioxidants with different cores. The construction of a library of such novel compounds will help us understand their structure-activity relationships. In this study we have synthesized dendrimers with varying amounts of surface electron donating groups (EDG) in order to evaluate the influence of EDG on their antioxidant properties. A pair of dendrimers with polyether cores was prepared from syringaldehyde (two methoxy groups), another pair from vanillin (one methoxy group) and a third pair from 4-hydroxybenzaldehyde (no methoxy group). In the syringaldehyde and vanillin dendrimers, the methoxy groups were ortho to the hydroxyl group. Natural and synthetic phenolic compounds with EDG at ortho or para position, were reported to be better antioxidants than those with meta-EDG [15,16]. Synthesis was performed via microwave-assisted 1,3-dipolar cycloaddition utilizing copper metal as a catalyst. The antioxidant ability of these dendrimers to scavenge organic carbon and nitrogen radicals as well as hydroxyl radicals was examined by DPPH assay, DNA and lipoprotein protection studies, and electron spin resonance. Their pro-oxidant effects on copper-mediated DNA damage and cell toxicity were also evaluated.
2. Materials and Methods
Syringaldehyde, vanillin, 4-hydroxybenzaldehyde, pentaerythritol (97%), methyl-α-D-glucopyranoside, sodium ascorbate, quercetin, sodium triacetoxyborohydride (Na(OAc)3BH), 1,1-diphenyl-2-picrylhydrazyl (DPPH), Fat Red 7B, phosphate-buffered saline (PBS), potassium persulfate, glacial acetic acid, sodium acetate and methanol were purchased from Sigma Aldrich and were used without further purification. 2,2'-Azobis(2-amidinopropane) dihydrochloride (AAPH) was obtained from Cayman Chemical (Ann Arbor, MI, USA). Human low-density lipoprotein (LDL) was obtained from Kalen Biomedical (Montgomery Village, MD, USA). The lipoprotein solution (protein = 5 mg/mL) contained 154 mM NaCl and 0.34 mM EDTA.
1H-NMR spectra were recorded with a Varian Mercury spectrometer operating at 500 MHz. 13C-NMR spectra were recorded using a Varian Mercury spectrometer operating at 125 MHz.
ESI mass spectra were obtained using a Waters LCT Premier XE mass spectrometer. The source capillary voltage was 3000 V, cone voltage was 10 V and source temperature was 80 °C. Samples analyzed by flow injection had a desolvation gas temperature of 250 °C and a gas flow rate of 200 L/hr. The mobile phase was water (50%)-acetonitrile (50%) containing 0.1% formic acid and its flow rate was 50 µL/min. The injection volume was 10 µL with a sample concentration of approximately 10 ng/uL.
Hitachi HPLC, consisting of L-7200 autosampler, L-7100 pump, L-7400 UV detector and D-7000 interface, was used to analyze the purity of the final dendrimers by reversed phase chromatography. Mobile phase was an acetonitrile-H2O gradient system (5→95 % acetonitrile) with 0.1% trifluoroacetic acid. The sample was detected at 214 nm. Flow rate was 1 mL/min. Separation was performed on a Varian C18 RP column.
All spectrophotometric data were obtained using Perkin Elmer UV/Vis spectrometer (Lambda 20) and Molecular Devices Corp. Spectra Max (M2e).
The microwave used for the click chemistry was a CEM brand Discover SP v 2.15.
2.1. General procedures for the synthesis of building block
Building block 1a and 1b were synthesized as previously described [17].
2.1.1. Building block 1c
TTo synthesize the building block, 250 mL of distilled THF was added to a 500 mL round bottom flask. 4-Hydroxybenzaldehyde (3.56 g, 29.15 mmol) was then dissolved into the THF. Propargylamine (3.027 g, 54.96 mmol) was then added to the solution dropwise via a syringe. The solution was then heated at 40–45 °C. Sodium tri-acetoxyborohydride (Na(OAc)3BH) (6.10 g, 28.78 mmol) was then added to the reaction mixture after the reaction was cooled to room temperature. After 24 h, a second equivalent of 4- hydroxybenzaldehyde (3.56 g, 29.15 mmol) was added to the reaction mixture without heating. After 36 h the last equivalent of reducing agent was added (Na(OAc)3BH) (6.10 g, 28.78 mmol) and the reaction was left to proceed for two days.
The reaction mixture was then filtered under reduced pressure and the filtrate was dried on the rotovap. The resulting residue was re-dissolved in 200 mL chloroform and then washed with 50 mL of water three times. The aqueous layer was extracted with 50 mL of methylene chloride twice. The combined organic layer was dried over magnesium sulfate. After evaporating the organic layer on a rotovap, the residue was purified using flash column chromatography equipped with a 40 g pre-packed silica gel column using a hexane-ethyl acetate gradient solvent system (6:1→3:1).
Yield 80% (11.73 g); Rf = 0.25 in hexane–ethyl acetate (2:1); 1H NMR (500 MHz, CDCl3) δ 6.95 (d, J = 8.5 Hz, 4H), 6.78 (d, J = 8.5 Hz, 4H), 5.02 (s, 2H), 3.58 (s, 4H), 3.22 (d, J = 2.3 Hz, 2H), 2.26 (s, 1H); 13C NMR (126 MHz, CDCl3) δ 191.1, 161.7, 154.9, 132.1, 78.9, 73.5, 57.0, 41.0; HRMS (ESI-TOF) m/z: Calcd for C17H18NO2 [M+H]+ 268.34, Found 268.53.
2.2. Synthesis of compound 4
Sodium azide (5.886 g, 90.55 mmol) and 2-(2-chloroethoxy) ethanol (compound 2; 11.28 g, 90.55 mmol) were dissolved in a mixture of DMF (200mL) and water (5mL). The reaction was stirred for 48 h at 70–80 °C. After the reaction was complete, the reaction mixture was filtered and the filtrate was dried at 50 °C under reduced pressure. The resulting residue was re-suspended in acetone, followed by vacuum filtration. The filtrate was dried on the rotovap and the residue (compound 3; 11.86 g, 90.53 mmol) was then dissolved in 200 mL of chloroform. Triethylamine (21.05 g, 208.02 mmol) was added to the reaction vessel at 0 °C. Para-toluenesulfonyl chloride (18.10 g, 94.94 mmol) dissolved in chloroform was added dropwise. Reaction was stirred for 24 h and then washed with deionized water. Chloroform layer was dried with MgSO4. After filtering off MgSO4, chloroform was removed under reduced pressure. Before loading the dried oily residue on a 40 g pre-packed silica gel column, the column was pretreated with 250 mL of hexane-ethyl acetate (3:1) solvent system containing triethylamine (3%, V/V), followed by hexane (100 mL).
Then, the aliquot of dried oily reaction mixture (5 mL) was loaded on the column and purified with flash column chromatography using a hexane-ethyl acetate gradient system, 1:0 (100 mL), 5:1 (600 mL), and 3:1 (800 mL).
Yield 61%; (15.73 g); Rf= 0.44 in hexane–acetone (2:1); 1H NMR (300 MHz, CDCl3) δ 7.80 (d, J = 8.70 Hz, 2H), 7.34 (d, J = 9.01 Hz, 2H), 4.16 (t, J = 4.52 Hz, 2H), 3.69 (t, J = 4.65 Hz, 2H), 3.60 (t, J = 4.81 Hz, 2H), 3.33 (t, J = 4.80 Hz, 2H), 2.45 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 146.1, 134.0, 130.8, 128.2, 70.2, 69.5, 69.2, 50.3, 22.2; HRMS (ESI-TOF) m/z: Calcd for C11H15N3O4SNa [M+Na]+ 308.31, Found 308.21.
2.3. Synthesis of compound 5a (1,15-diazido-8,8-bis((2-(2-azidoethoxy)ethoxy)methyl)-3,6,10,13-tetraoxapentadecane)
Pentaerythritol (1.055 g, 7.70 mmol) was dissolved in anhydrous DMF (80 mL). NaH (0.80 g, 33.33 mmol) in powder form was added portion by portion to the reaction under argon. The reaction was stirred for 30 min and then compound 4 (8.843 g, 31.04 mmol) was added dropwise. After reaction was complete, the reaction mixture was filtered and the filtrate was dried at 50 °C under reduced pressure. The resulting residue was re-dissolved in chloroform and washed with water three times. The chloroform layer was dried with MgSO4. After chloroform was removed, the oily residue was purified using silica gel column chromatography in a gradient hexane-ethyl acetate system (10:1→2:1).
Yield 48% (2.170 g); Rf= 0.172 in hexane–acetone (2:1); 1H NMR (300 MHz, CDCl3) δ 3.70 – 3.65 (m, 8H), 3.64 – 3.60 (m, 8H), 3.57 (ddd, J = 5.8, 3.5, 1.1 Hz, 8H), 3.46 (s, 8H), 3.41 – 3.34 (m, 8H); 13C NMR (75 MHz, CDCl3) δ 71.3, 70.7, 70.3, 70.2, 51.0, 45.8; HRMS (ESITOF) m/z: Calcd for C21H41N12O8 [M+H]+ 589.62, Found 589.23.
2.4. Synthesis of compound 5b ((2R,3R,4S,5R,6R)-3,4,5-tris(2-(2-azidoethoxy)ethoxy)-2-((2-(2-azidoethoxy)ethoxy)methyl)-6-methoxytetrahydro-2H-pyran)
Methyl α-D-glucopyranoside (4.00 g, 20.60 mmol) was dissolved in anhydrous DMF (300 mL). NaH (2.00 g, 83.33 mmol) was added under argon and the reaction mixture was stirred for 30 min. Compound 4 (24.07 g, 84.46 mmol) was added and the reaction mixture was stirred for 48 h. The reaction mixture was filtered. The DMF filtrate was removed under reduced pressure at 50 °C. The residue was re-dissolved in chloroform and washed with water three times. Chloroform layer was dried with MgSO4. After filtering off MgSO4, the chloroform filtrate was dried under reduced pressure. The resulting yellow oily residue was purified with silica-based flash column chromatography using a gradient hexane-acetone solvent system (10:1→3:1).
Yield 38% (5.062 g); Rf = 0.35 in hexane–acetone (1:1) system; 1H NMR (500 MHz, CDCl3) δ 4.80 (d, J = 3.5 Hz, 1H), 4.09 – 3.94 (m, 2H), 3.91 (dd, J = 10.4, 5.0 Hz, 1H), 3.88 – 3.82 (m, 1H), 3.82 – 3.74 (m, 2H), 3.65 (qdd, J = 10.2, 4.0, 2.8 Hz, 19H), 3.38 (dt, J = 8.3, 3.3 Hz, 16H); 13C NMR (126 MHz, CDCl3) δ 97.6, 81.8, 80.5, 77.5, 72.0, 71.7, 70.4, 70.2, 69.7, 54.6, 50.4; HRMS (ESI-TOF) m/z: Calcd for C23H42N12O10Na [M+Na]+ 669.65, Found 669.44.
2.5. General procedures for the formation of dendrimers 6a–c and 7a–c
Core 5a (0.2174 g, 0.370 mmol) and building block 1a (0.6475 g, 1.673 mol) were dissolved in ultrapure THF (20 mL) in a microwave reactor vessel. Granulated copper (1.5 g, 0.2–0.6 mm grain size, 99.8%) was pre-treated in the order of 25% aqueous NaOH (30 min), deionized H2O, 20% aqueous H2SO4 (30 min) and rinsed with copious amount of water, followed by acetone. Immediately after they were dried with argon, they were added to the microwave reactor vessel. The reaction was set at a maximum temperature of 85 °C, pressure of 250 psi, and microwave energy of 150 W (1 h), 200 W (1 h), and 250 W (5 h). The maximum temperature reached was 71 °C, 79 °C, and 83 °C, respectively. The reaction was filtered to remove the copper spheres. The filtrate was condensed on the rotary evaporator. The reaction mixture was purified with flash chromatography with a 30 g pre-packed silica gel column. The solvent systems were hexane-ethyl acetate (1:1), ethyl acetate, ethyl acetate-methanol (9:1), and ethyl acetate-methanol (8:2).
2.5.1. Compound 6a
Yield 67% (0.531 g); Rf = 0.67 in ethyl acetate-methanol (1:1) system; 1H NMR (500 MHz, CDCl3) δ 7.54 (s, 4H), 6.64 (s, 16H), 4.92 (s, 8H), 4.46 (t, J = 5.2 Hz, 8H), 3.84 (s, 48H), 3.82 – 3.73 (m, 16H), 3.56 (s, 8H), 3.46 (dd, J = 5.9, 4.0 Hz, 16H), 3.35 (dd, J = 5.7, 3.9 Hz, 8H), 3.21 (s, 8H); 13C NMR (126 MHz, CDCl3) δ 147.2, 147.1, 133.8, 130.0, 123.8, 105.6, 70.8, 70.4, 69.8, 69.6, 57.7, 56.3, 50.9, 50.3, 47.8, 45.3; HRMS (ESI-TOF) m/z: Calcd for C105H142N16O32 [M+2H]+2 1069.66, Found 1070.22.
2.5.2. Compound 6b
Yield 50 %; Rf= 0.56 in ethyl acetate–methanol (4:1); 1H NMR (500 MHz, acetone-d6) δ 7.86 (s, 4H), 7.05 (d, J = 1.7 Hz, 8H), 6.85 (dd, J = 8.0, 1.8 Hz, 8H), 6.78 (d, J = 8.0 Hz, 8H), 4.94 (s, 8H), 4.52 (t, J = 5.3 Hz, 8H), 3.88 – 3.84 (m, 8H), 3.83 (s, 24H), 3.70 (s, 8H), 3.50 (s, 16H), 3.38 (dd, J = 5.5, 3.9 Hz, 8H), 3.31 (s, 8H), 3.23 (s, 8H); 13C NMR (126 MHz, acetone-d6) δ 148.2, 146.4, 145.2, 131.6, 124.6, 122.2, 115.4, 113.0, 71.7, 70.8, 70.2, 70.2, 57.6, 56.2, 50.7, 48.2, 46.2; HRMS (ESI-TOF) m/z: Calcd for C97H125N16O24[M+H]+ 1899.12, Found 1899.20.
2.5.3. Compound 6c
Yield 73%; Rf = 0.77 in ethyl acetate–methanol (4:1); 1H NMR (500 MHz, acetone-d6) δ 7.85 (s, 4H), 7.23 (d, J = 8.5 Hz, 16H), 6.80 (d, J = 8.5 Hz, 16H), 4.93 (s, 8H), 4.52 (t, J = 5.2 Hz, 8H), 3.85 (t, J = 5.2 Hz, 8H), 3.67 (s, 8H), 3.46 (s, 16H), 3.38 (dd, J = 5.5, 3.7 Hz, 8H), 3.32 (s, 8H), 3.24 (s, 8H); 13C NMR (126 MHz, acetone-d6) δ 157.2, 145.3, 130.9, 130.8, 124.6, 115.8, 71.7, 70.8, 70.1, 57.3, 50.8, 48.1, 46.2; HRMS (ESI-TOF) m/z: Calcd for C89H109N16O16 [M+H]+ 1658.91, Found 1658.15.
2.5.4. Compound 7a
Yield 59%; Rf = 0.27 in ethyl acetate–methanol (7:3); 1H NMR (500 MHz, CDCl3) δ 7.58 – 7.42 (m, 4H), 6.64 (s, 16H), 4.56 – 4.37 (m, 10H), 3.94 – 3.82 (m, 51H), 3.82 – 3.69 (m, 32H), 3.55 (s, 16H), 3.52 – 3.41 (m, 2H), 3.33 – 3.27 (m, 1H), 3.25 (s, 8H), 3.16 – 3.07 (m, 2H); 13C NMR (126 MHz, CDCl3) δ 147.1, 145.0, 133.7, 130.1, 123.7, 105.6, 97.7, 82.1, 80.8, 78.0, 72.0, 71.8, 70.8, 69.6, 57.8, 56.4, 55.1, 53.9, 50.1, 48.0; HRMS (ESI-TOF) m/z: Calcd for C107H144N16O34 [M+2H]+2 1098.68, Found 1099.17.
2.5.5. Compound 7b
Yield 43%; Rf = 0.33 in ethyl acetate–methanol (4:1); 1H NMR (500 MHz, acetone-d6) δ 7.98 – 7.77 (m, 4H), 7.06 (s, 8H), 6.86 (d, J = 7.9 Hz, 8H), 6.78 (dd, J = 8.0, 1.0 Hz, 8H), 4.71 – 4.41 (m, 10H), 3.90 – 3.75 (m, 40H), 3.71 (d, J = 4.0 Hz, 3H), 3.65 – 3.43 (m, 24H), 3.37–3.42 (m, 1H), 3.31 (s, 16H), 3.20 (s, 2H), 3.12 – 3.00 (m, 2H); 13C NMR (126 MHz, CD3OD) δ 148.2, 146.3, 145.1, 131.6, 124.6, 122.2, 115.4, 113.1, 98.5, 82.9, 81.5, 78.7, 72.6, 72.4, 71.4, 70.0, 57.6, 56.2, 55.0, 50.6, 49.6, 48.2; HRMS (ESI-TOF) m/z: Calcd for C99H127N16O26 [M+H]+ 1957.15, Found 1957.17.
2.5.6. Compound 7c
Yield 76%; Rf = 0.67 in ethyl acetate–methanol (4:1); 1H NMR (500 MHz, acetone-d6) δ 7.95 – 7.78 (m, 4H), 7.23 (d, J = 7.2 Hz, 16H), 6.81 (d, J = 8.5 Hz, 16H), 4.61 (d, J = 3 Hz, 1H), 4.55 – 4.51 (m, 8H), 3.88 – 3.78 (m, 8H), 3.71 – 3.67 (m, 8H), 3.61 – 3.42 (m, 34H), 3.33 (s, 8H), 3.19 (s, 4H), 3.14 – 3.06 (m, 3H); 13C NMR (126 MHz, CD3OD) δ 157.2, 145.3, 145.3, 130.8, 124.7, 115.8, 98.4, 82.8, 81.4, 78.6, 72.6, 72.4, 71.2, 70.1, 57.2, 55.1, 50.7, 49.7, 48.1; HRMS (ESI-TOF) m/z: Calcd for C91H111N16O18 [M + H]+ 1716.94, Found 1716.05.
2.6. DPPH assay
Reduction of DPPH radical was determined for dendrimers as previously reported [18]. DPPH and antioxidants were dissolved in methanol. Antioxidant sample (10 µL) was added to 1.0 mL of DPPH reagent and the absorbance was monitored at 515 nm after one hour. All samples were run in triplicates at room temperature. The within-run coefficient of variation of the % inhibition values was less than 6%.
2.7. LDL oxidation-electrophoresis
Low-density lipoprotein was incubated with 20 mM AAPH in PBS and 70 µM antioxidant (made in methanol) at 37 °C for 21 h. The mixture was then subjected to electrophoresis on 1% agarose gels (Helena Labs, Beaumont, TX) using the Ciba Corning Clinical Electrophoresis System. The gels were stained with Fat Red 7B.
2.8. Antioxidant effects on DNA
DNA electrophoresis was performed on pBR 322 after 4 h incubation at 37 °C in the presence of 10 mM AAPH in PBS and various concentrations (12–90 µM) of antioxidants (made in methanol).
2.9. Hydroxyl radical (OH•) assay by Electron Spin Resonance (ESR) spectrometer
Hydroxyl radicals were generated by reaction between FeSO4 (final concentration 0.25 mM) and H2O2 (final concentration 0.25 mM) in PBS and in the presence of spin trap agent 5,5-Dimethyl-1-pyrroline N-oxide (DMPO; final concentration 63 mM) and antioxidant (final concentration, 16 µM in acetonitrile) [19,20]. The DMPO-OH 1:2:2:1 signal was detected with Bruker ESR spectrometer at 5 min. The antioxidant potency was determined from suppression of the DMPO-OH adduct signal.
2.10. Pro-oxidant effects on DNA
DNA (pBR 322) was incubated with 10 µM CuCl2 in the presence of 12–90 µM antioxidants (made in methanol) at 37 °C for 1 h. DNA damage was monitored by agarose electrophoresis.
2.11. Cell Viability Assay
Chinese Hamster Ovary (CHO-K1) cells were cultured in F-12K medium supplemented with 10% fetal bovine serum in a 5% CO2 humidified incubator. The cells (2.5 × 105/ml) were seeded in 100 µL volumes in 96-well culture plates and incubated with either 100 µL solvent (cell culture grade DMSO, 0.15%) or dendrimer for 1, 3, or 5 days. The dendrimer concentrations tested include 1000, 100, or 10 nM of dendrimers 6a and 7a and 250, 25, and 2.5 nM dendrimers 6b, 6c, 7b, and 7c. Dendrimer concentrations selected for this study were determined by their solubility in DMSO. Two hours before termination of the experiment, 20 µL of a 5 mg/ml 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution in 0.01 M phosphate buffered saline was added to each well. The culture plates were centrifuged at 450 × g for 10 minutes and supernatant was removed from each well. The resultant formazan crystals were dissolved in 100 µL DMSO. Absorbance was measured using a Biolog microplate reader (Biotek Instruments) at dual wavelengths, 590 and 650 nm. Percent control response was calculated as follows:
(Absorbance of treatment /Absorbance of control) × 100
All experiments were performed in triplicate and repeated. The data were analyzed using Systat 12 for Windows. Multiple groups were compared using a one-way analysis of variance and a Tukey test for mean separation. A p value < 0.05 was considered statistically significant.
3. Results and Discussion
3.1. Synthesis
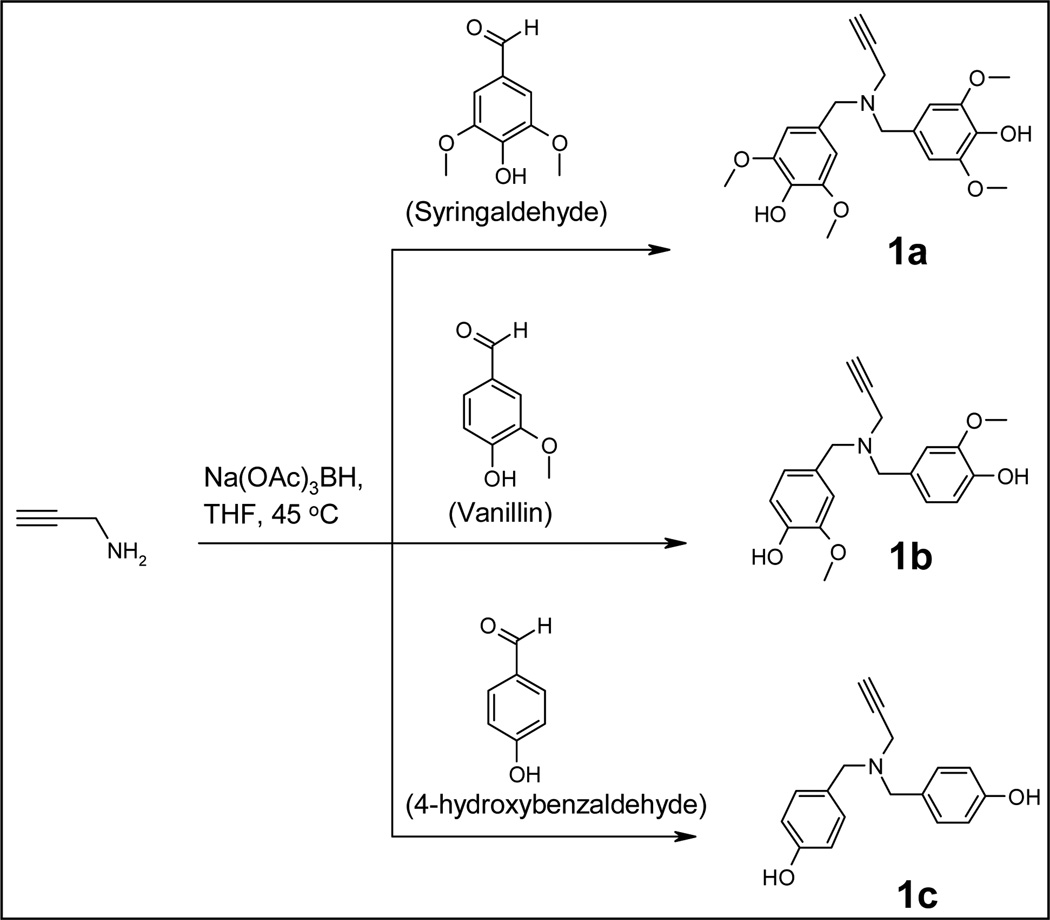
Dendrimers were synthesized using copper metal catalyzed alkyne-azide1,3-dipolar cycloaddition, click chemistry. In order to perform the click reactions, alkyne-derivatized building blocks and azide-derivatized core were first synthesized. Alkyne derivatives 1a, 1b, and 1c were prepared by reacting propargylamine with syringaldehyde, vanillin and 4-hydroxybenzaldehyde via reductive amination, respectively (Scheme 1).
Scheme 1.
Building block synthesis.
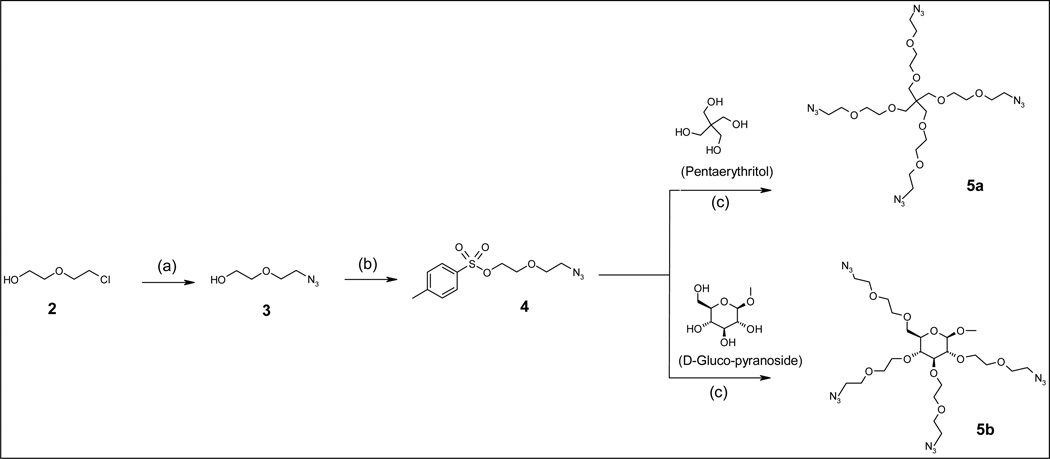
Since our goal was to synthesize potent dendritic antioxidants with metal ion chelation capability, the interior branches were designed to contain metal binding sites. Polyether units are well known for binding metal ions as exemplified by 18-crown-6-ether that coordinates potassium and 12-crown-4-ether that chelates sodium. We hypothesized that incorporation of multiple ether units and nitrogen atoms within the dendrimers would be beneficial for binding copper and iron ions, which are involved pro-oxidant effects. Compound 3 is the branch that constitutes the interior of the dendrimer and provides ethylene glycolic ether units (Scheme 2). This compound was synthesized using 2-(2-chloroethoxy) ethanol (2) and sodium azide (NaN3). The reaction was carried out in DMF and H2O as a co-solvent (40:1) at 70–85 °C. The use of high temperatures (>100 °C) resulted in formation of several unknown side products and gave a poor yield (<10%). The compound was an oily substance that was soluble in water and chloroform (Scheme 2) but not in dichloromethane. In order to attach 3 to the polyol core, its hydroxyl terminal was tosylated (compound 4) in chloroform.
Scheme 2.
Polyol-based core synthesis (a) NaN3, DMF/H2O= 40:1, 70 – 80 °C; (b) CHCl3, Tosyl chloride, TEA, 0 °C; (c) NaH, anhydrous DMF
Core compound 5a with four extended arms carrying ethylene glycolic ether units and azide group on each terminal, was formed by reacting pentaerythritol (PETN) with compound 4. Similarly, core 5b was synthesized from methyl α-D-glucopyranoside (pyranoside). Pyranoside with hydroxyl groups in a staggered conformation was selected to reduce the steric hindrance between branches and ultimately between surface building blocks. The extended arms with multiple ethylene glycol units were incorporated to enhance the polarity of dendrimer’s interior compartment as well as its metal binding capacity.
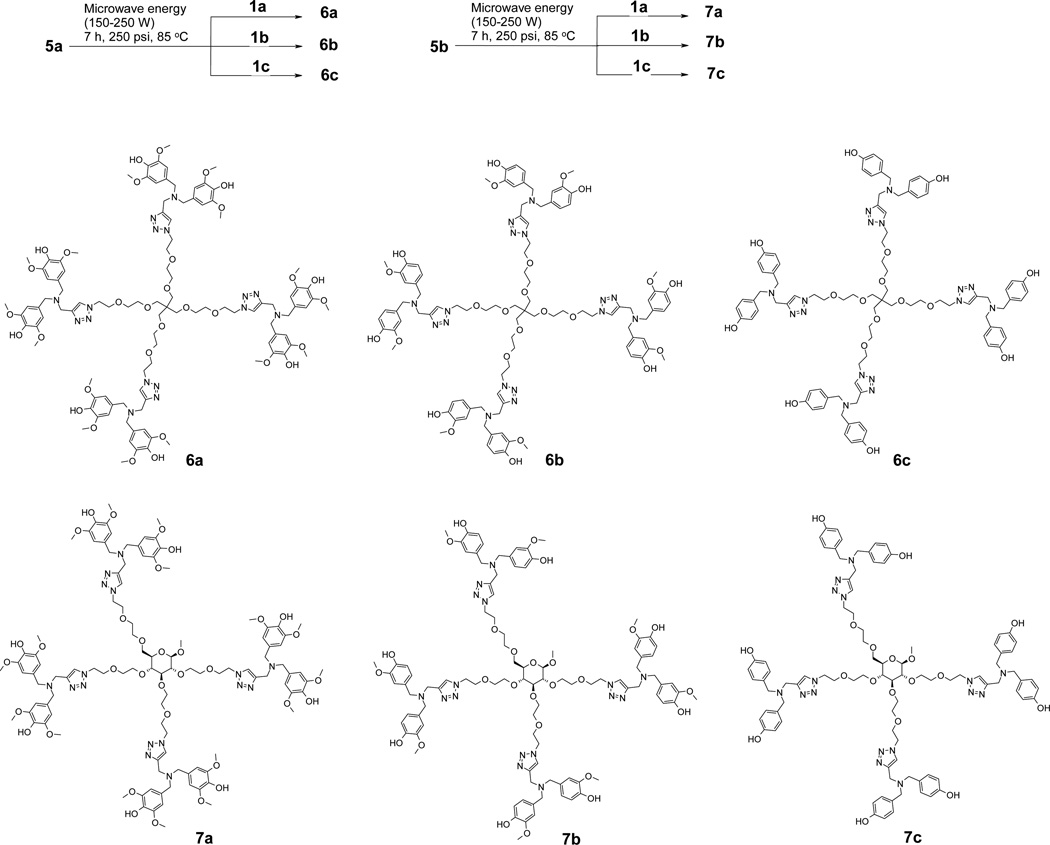
To synthesize six generation 1 (G1) antioxidant dendrimers (Scheme 3), in-house prepared building blocks (compound 1a–c) were attached to the PETN-based core (compound 5a) and pyranoside-based core (compound 5b) via our previously reported granulated copper metal-assisted 1,3-dipolar cycloaddition [17].
Scheme 3.
Dendrimer synthesis
Alkyne-azide 1,3-dipolar cycloaddition was pioneered by Huisgen but produces a mixture of 1,4-disubstituted and 1,5-disubstituted 1,2,3-triazoles. In comparison, the same alkyne-azide 1,3-dipolar cycloaddition but, using Cu(I) as a catalyst, produces 1,4-disubstituted 1,2,3-triazole as a sole product in high yield and is called Sharpless click chemistry. Due to the sterospecificity and high efficiency, the Sharpless click reaction has been widely adopted in modern organic synthesis. We also initially attempted dendrimer synthesis using Cu(I) in the form of copper iodide (CuI) or copper sulfate/ascorbate. However, the dendrimer products we obtained were contaminated with copper ions (detected by ESI). Although the copper contaminated antioxidant dendrimers could be isolated, they could not be used as antioxidants due to the presence of toxic copper. Therefore, alternative copper free-click chemistry methods were attempted. Although the use of highly strained cyclooctyne ring derivative and azide is reported to be highly efficient, the ring might decrease solubility of the target dendrimers in biologically compatible solvents. We carried out the reaction with copper metal spheres (0.2–0.6mm, 99.8%) in place of Cu(I). To enhance the reaction rate and yield, microwave energy was applied to the reaction. Microwave energy-assisted click reaction produced the target dendrimers (6a, 6b, 6c, 7a, 7b, and 7c) in very good yields (50–76%) in a relatively short reaction time (7 h). Based on HPLC analysis, the purified fraction consists of mostly the target compound (>98%) with a minor amount of defective species such as missing phenol rings.
3.2. Free radical scavenging
An important source of cell damage is from highly reactive chemical species called free radicals such as hydroxyl radicals and organic radicals. Free radicals are formed as a result of normal metabolism. Their high chemical reactivity leads to damage of any cellular structures in their vicinity. Oxidative damage to membranes, proteins and DNA are especially important in impairing cell function, accelerating aging and causing cell injury. The ability of the synthesized dendrimers to scavenge nitrogen-based free-radicals was evaluated by the DPPH assay. Syringaldehyde derivatized dendrimers (6a and 7a) had similar potency (IC50 mean of three experiments = 3.1 µM and 3.3 µM, respectively; SD = 1 µM at 60 min). The vanillin derivatized dendrimers (6b and 7b) had IC50 value of 5.4 µM and 5.7 µM respectively. In comparison, 4-hydroxybenzaldehyde derivatized dendrimers (6c and 7c) had no detectable DPPH quenching activity under the same assay conditions. These results clearly show that the antioxidant activity increases with increasing number of EDG groups. The lack of antioxidant activity of 4-hydroxybenzaldehyde dendrimer suggests the importance of the ortho-electron donating groups. Under identical experimental conditions, average IC50 value for naturally occurring antioxidants quercetin and vitamin C were 16 µM and 36 µM while the starting materials used for syntheses of the antioxidants displayed negligible DPPH activity. IC50 values of syringaldehyde and vanillin were 1.8 mM and 7.4 mM, respectively and were significantly higher than their corresponding dendrimers. The extremely weak antioxidant activities of syringaldehyde and vanillin may be partly due to the presence of aldehyde groups, which are electron-withdrawing. During synthesis of antioxidant dendrimers, these aldehyde groups are converted into electron donating benzylic moieties, resulting in formation of phenol rings with benzylic group and methoxy group at para and ortho position to the OH, respectively. The syringaldehyde dendrimer 6a (IC50 = 3.1 µM) showed 580-fold decrease IC50 value compared to syringaldehyde (IC50= 1.8 mM) while vanillin dendrimer 6b (IC50= 5.4 µM) showed 1370-fold decrease compared to vanillin (IC50= 7.4 mM). Even if we take into account that dendrimers 6a and 6b contain eight syringaldehyde and vanillin units, IC50 values of the dendrimers are still 73-fold and 171-fold lower than the IC50 values of syringaldehyde and vanillin, respectively. This extremely high antioxidant potency of dendrimers may be due to two factors. One is the replacement of electron withdrawing aldehyde with electron donating benzylic groups. The other may be a “dendritic effect”, whereby multiple phenolic units located within the same dendrimer molecule interact with one another to enhance its antioxidant properties. The dendritic effect of dendrimers is well known and involves the changes in their intrinsic physico-chemical properties with alterations in their size, shape, architecture, surface chemistry, flexibility and elemental composition [21]. These results clearly establish that formation of powerful macromolecular antioxidants from weak antioxidants is possible if they are appropriately assembled into dendritic architecture.
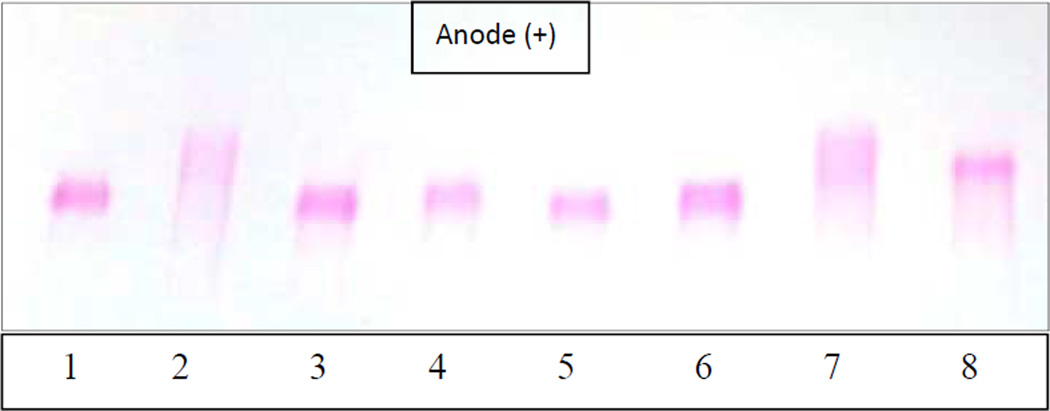
Scavenging of carbon radicals (AAPH) by the dendritic compounds was evaluated indirectly by monitoring the damage to two biomolecules, human low-density lipoproteins (LDL) and plasmid DNA (pBR 322) [22]. LDL was used as a model lipid for evaluating the ability of the dendrimers as chain-breaking antioxidants for lipids. When LDL is exposed to free radicals, both protein and lipid components of LDL may be oxidized. Since the integrity of multiple components may be affected during LDL damage by free radicals, the assessment of lipoprotein oxidation is not straightforward. The electrophoretic migration pattern of oxidized LDL is one of the best methods to evaluate the extent of lipoprotein oxidation [23]. We have successfully used a similar strategy for other antioxidants [13,14]. LDL was incubated with 20 mM AAPH and 70 µM dendrimers at 37 °C for 21 h. The lipoproteins were then subjected to agarose electrophoresis. As shown in Figure 1, native LDL showed as a sharp band (negative control, lane 1). Under the electrophoresis conditions, the LDL particles have a net negative charge that enables them to migrate towards the positive pole (anode). In comparison, LDL incubated with AAPH radical without any antioxidant (positive control) showed a lightly stained broad band, indicating that the sample is populated by heterogeneous species that were formed as a result of lipoprotein oxidation (lane 2, Fig. 1). The darkest part of this band even migrated faster than the native LDL, suggesting that some of the species in oxidized LDL had a higher negative charge density than native LDL. Under similar conditions, lipoprotein samples incubated with dendrimers 6a, 6b, 7a, or 7b showed similar migration rate and band intensity to that of native LDL, implying that the dendrimers protected LDL against AAPH free radical damage (lanes 3–6, Fig. 1). In contrast, LDL incubated with vitamin C showed a faster-moving smeared band (lane 7), similar to the positive control (lane 2), implying that vitamin C could not rescue most of the LDL under these conditions. Quercetin (lane 8) also showed a broad band like vitamin C; however, there was a sharper, more intense band with increased charge density. These results suggest that quercetin showed some protection of LDL, especially when compared to vitamin C. Nevertheless, its protective effect was far less than dendrimers 6a, 6b, 7a, and 7b. Under similar conditions, dendrimers derivatized with 4-hydroxybenzaldehyde (6c and 7c) were completely ineffective in protecting LDL (data not shown). These results once again confirm that the antioxidant properties increase with increasing number of EDGs.
Fig. 1.
Effect of antioxidants on LDL oxidation; lane 1 (native LDL); lanes 2 (AAPH-oxidized LDL with no antioxidant), lane 3–8 (6a, 6b, 7a, 7b, vitamin C, and quercetin at 70 µM, respectively).
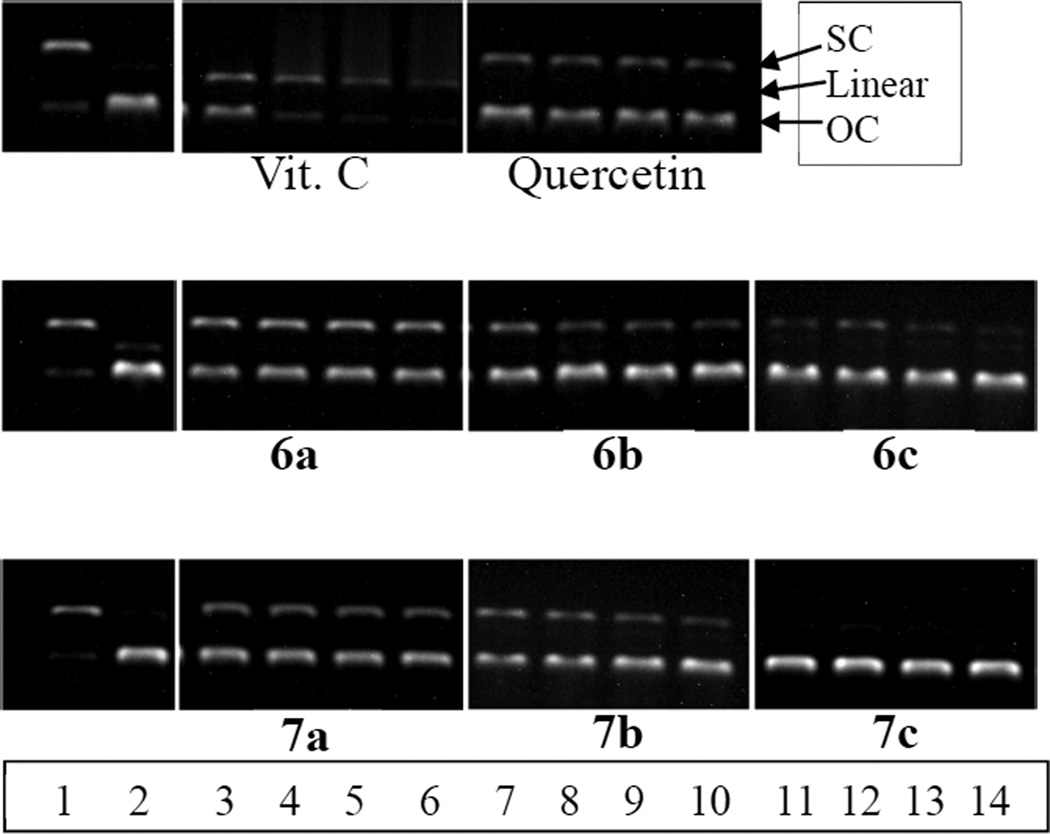
The ability of the antioxidant dendrimers to protect DNA against AAPH-derived carbon radicals was also evaluated. In these experiments plasmid DNA (pBR 322) was incubated with AAPH (final concentration, 10 mM) at 37 °C for 4 h with antioxidants (final concentrations, 12–90 µM). The extent of DNA damage was evaluated by agarose electrophoresis [24]. As shown in Figure 2, native DNA was mostly in its supercoiled (SC) form (lane 1, Fig. 2). In the presence of AAPH, the DNA was transformed almost entirely into its open circular (OC) form (lane 2, Fig. 2). Vitamin C did not protect DNA at all concentrations (12–90 µM) tested. The DNA incubated with AAPH and vitamin C showed not only the OC form but also the more damaged linear form (lanes 3–6, Fig. 2). Quercetin showed a slight protection of DNA at all concentrations but it was noticeable that the intensity of SC bands became weaker as the concentration of quercetin decreases. In comparison, the native SC bands of DNA incubated with syringaldehyde derivatized dendrimers 6a and 7a were much more intense than those of quercetin or vitamin C. Interestingly, the amount of DNA protected at all concentrations were the same; the increase in the concentrations of antioxidant did not result in enhanced protection. This might be due to the limited aqueous solubility of the antioxidant dendrimers, which limits antioxidant activity at higher concentrations. Vanillin-surfaced dendrimers (6b and 7b) showed slightly less effective protection than syringaldehyde derivatized dendrimers (6a and 7a). In case of 4-hydroxybenzaldehyde derivatized dendrimers, the PETN core-based dendrimer (6c) showed a minor amount of protection at 90, 45, and 23 µM but negligible protection at 12 µM. In comparison, pyranoside-core based dendrimer 7c did not show any protection. It should be noted that the PETN- and pyranoside-derivatized cores (5a and 5b) by themselves did not have antioxidant effects (data not shown). Overall, in each dendrimer family, syringaldehyde derivatized dendrimers showed superior DNA protective effects against the AAPH radical induced damage compared to vanillin derivatized dendrimers, followed by 4-hydroxybenzaldehyde-derivatized dendrimers. These DNA protection assay results also emphasize the importance of number of electron donating group(s).
Fig. 2.
Protection against AAPH-induced DNA oxidation by antioxidants. Lane 1(native DNA); lane 2 (AAPH-oxidized DNA, no antioxidant); lanes 3–6 (AAPH-oxidized DNA with 90, 45, 23, 12 µM syringaldehyde-derivatized dendrimers (6a, 7a), respectively); lanes 7–10 (AAPH-oxidized DNA with 90, 45, 23, 12 µM vanillin-derivatized dendrimers (6b, 7b), respectively); lanes 11–14 (AAPH-oxidized DNA with 90, 45, 23, 12 µM 4-HBAderivatized dendrimers (6c, 7c), respectively).
Similar studies were performed to determine the ability of dendritic antioxidants to protect DNA against damage by hydroxyl radicals. In these experiments, pBR 322 was incubated at 37 °C for 1 h with a hydroxyl radical generating system (Fenton reaction) composed of ferrous ions and hydrogen peroxide (final concentrations 0.06 µM ferrous ions and 22 µM hydrogen peroxide) in 2-(N-morpholino)ethane sulfonic acid (MES) buffer, pH 6.0. The damaged OC form of DNA was obtained in all cases (with or without antioxidants; data not shown), suggesting that the antioxidants do not protect against hydroxyl radicals. ESR was also used to study hydroxyl radical scavenging by the dendrimers. Hydroxyl radicals, generated via Fenton reaction (Fe2+/H2O2) were reacted with 5,5-dimethyl-1-pyrroline N-oxide (DMPO), a nitrone spin-trap agent in the absence (control) and presence of antioxidant. The DMPO-OH 1:2:2:1 signal suppression by the antioxidant derivatized dendrimers was similar to control (methanol without antioxidants; data not shown). The inability of the relatively hydrophobic dendritic antioxidants to scavenge hydroxyl radicals may be due to the polar nature of the hydroxyl radicals compared to organic carbon or nitrogen radicals.
3.3. Pro-oxidant effect
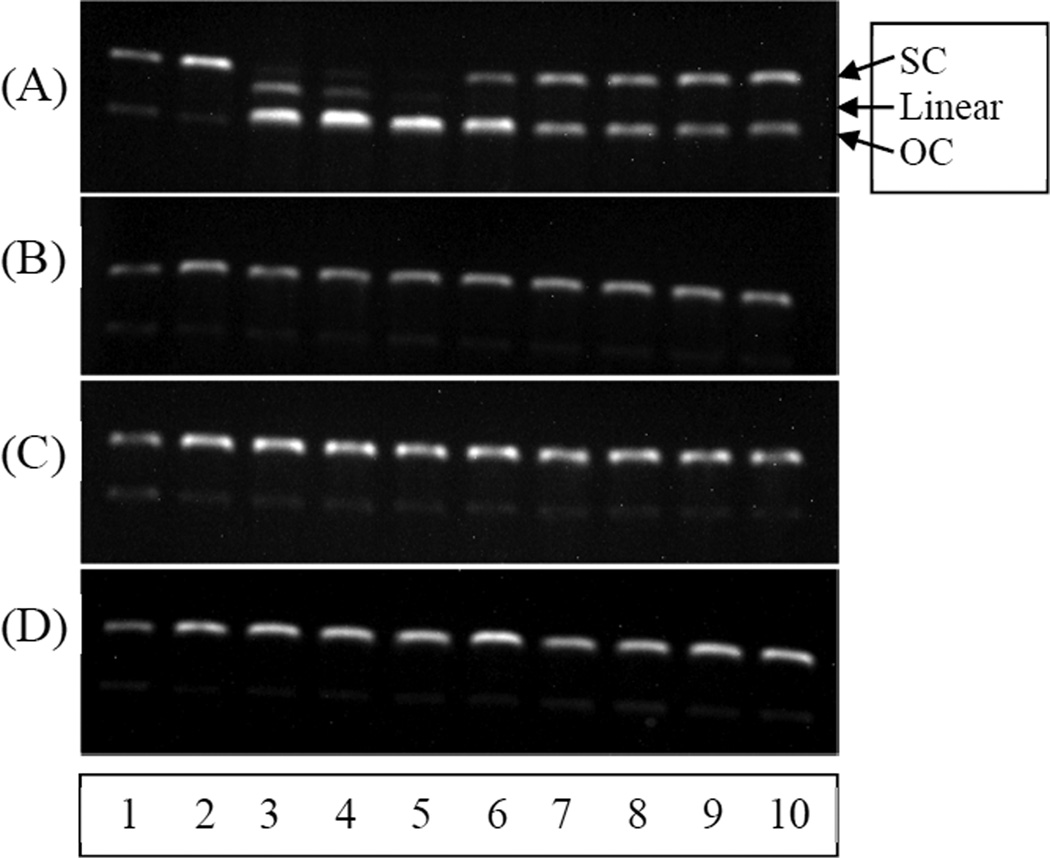
Many naturally available antioxidants including quercetin and vitamin C have been reported to exhibit pro-oxidant property in the presence of transition metals ions (e.g., Cu2+ and Fe3+) [25]. This antioxidant-induced production of free radicals may damage biomolecules such as DNA and cause oxidative stress. The damaging effects on DNA can pose mutagenic and carcinogenic health hazards [9–12]. Quercetin is one of the most abundantly obtained flavonoids from dietary source and produces many beneficial health effects. However, it has been reported to be associated with pro-oxidant effects [26]. Since our antioxidants were developed for potential use in biological applications, we evaluated their pro-oxidant activity compared to vitamin C and quercetin. This was performed by co-incubation of plasmid DNA (pBR 322) with physiological amounts of Cu2+ ion (~10 µM) and antioxidants at 90, 45, 23, 12 uM at 37 °C for 1 h. Figure 3 shows an agarose gel obtained with vitamin C and quercetin (A) and dendrimers (B–D). In each panel, lane 1 is native DNA (negative control), lane 2 is native DNA incubated with Cu2+ (positive control), lanes 3–6, native DNA incubated with Cu2+ and 90, 45, 23, and 12 µM antioxidant, respectively, and lanes 7–10, native DNA incubated with Cu2+ and 90, 45, 23, and 12 µM antioxidant, respectively. Native DNA was mostly in the supercoiled form but contained some open circular form (lane 1). DNA incubated with Cu(II) ions also displayed similar gels, indicating that Cu(II) ion by itself does not damage DNA under these conditions (lane 2). As shown in Figure 3A, vitamin C showed severe pro-oxidant effect. It generated the OC form at all concentrations tested (lanes 3–6, 90, 45, 23, and 12 µM of vitamin C, respectively). High concentrations even gave highly damaged linear DNA. Compared to vitamin C, quercetin showed less pro-oxidant activity under these conditions. The intensity of SC band was very similar to that of OC band at all concentrations (Fig. 3A, lanes 7–10; 90, 45, 23, and 12 µM quercetin, respectively). Unlike the natural antioxidants, neither dendrimers 6a (lanes 3–6) nor 6b (lanes 7–10) caused any damage to DNA, indicating that the dendrimers do not show pro-oxidant effects under these conditions (Fig. 3B). Dendrimers 6c (lanes 3–6) and 7b (lanes 7–10) in Figure 3C and dendrimers 7c (lanes 3–6) and 7a (lanes 7–10) in Figure 3D also gave similar gel patterns with no pro-oxidant effects. The PETN (5a) and pyranoside-derivatized core (5b) themselves did not show any pro-oxidant effect on DNA under these conditions (data not shown). In summary, all of the synthesized dendrimers did not produce the deleterious pro-oxidant effects regardless of surface functionality. The lack of pro-oxidant effects displayed by our dendrimers is probably due in part to their copper ion chelating capability. Copper chelation by dendrimers containing multiple oxygen and nitrogen atoms is well-known. For example, polyamidoamine dendrimers with multiple amide and tert-amino groups have been reported to possess strong copper chelation properties [27]. Another evidence of copper chelation by our dendrimers was observed during their click synthesis catalyzed by copper ions instead of granulated copper metal, which led to strongly bound copper ions (detected by mass spectrometry) that were difficult to decontaminate. These results demonstrate that chelation of copper ions by antioxidants can markedly reduce new free radical formation. This is especially important in the chromatin region where one copper ion is present per kilobase of DNA [28]. In addition, the phenols with bulky functional group(s) on the surface of the dendrimers did not allow copper ions to bind for subsequent reduction. The lack of pro-oxidant properties of 4-hydroxybenzaldehyde-derivatized dendrimers (6c and 7c) was probably due to their weak reducing properties. As shown by the DPPH data and other antioxidant activity tests, it is evident that these dendrimers (6c and 7c) do not quench radicals, which means that they are not able to reduce copper ions to sustain the pro-oxidant activity. These results indicate that 4-hydroxybenzaldehyde-derivatized dendrimers (6c and 7c) are neither antioxidants nor pro-oxidants.
Fig. 3.
Pro-oxidant effects of antioxidants on DNA (pBR 322)
(A) Lane 1 (native DNA), lane 2 (native DNA + Cu2+ ion), vitamin C (lanes 3–6 contain 90, 45, 23, and 12 µM, respectively) and quercetin (lanes 7–10 contain 90, 45, 23, and 12 µM, respectively)
(B) Same as A, but with dendrimers 6a (lanes 3–6) and 6b (lanes 7–10)
(C) Same as A, but with dendrimers 6c (lanes 3–6) and 7b (lanes 7–10)
(D) Same as A, but with dendrimers 7c (lanes 3–6) and 7a (lanes 7–10).
3.4. Cell testing
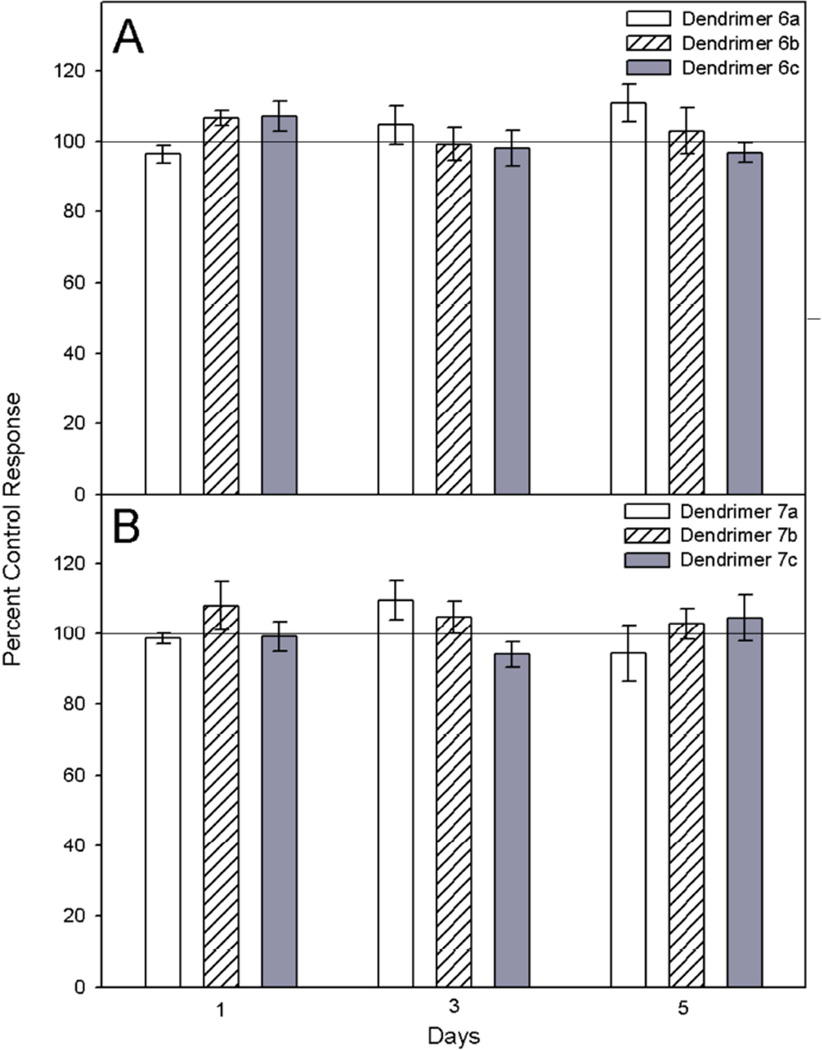
CHO-K1 cell cytotoxicity following exposure to dendrimers was evaluated using the 3-(4,5-di-methylthizol-2-yl)-2,5 diphenyltetrazolium bromide (MTT) conversion assay [29]. Exposure of cells (2.5 × 105/ml) to dendrimer 6a and 7a (1000 – 10 nM) and 6b, 6c, 7b, and 7c (250 – 2.5 nM) caused no statistically significant change in cell viability for up to 5 days (Fig. 4). This lack of toxicity results offer hope for the potential use of these novel compounds in biological systems.
Fig. 4.
Comparative effects of dendrimers on CHO-K1 cell viability. CHO-K1 cells were incubated with DMSO control, or dendrimer 6a or 7a (1000 nM), 6b, 6c, 7b, or 7c (250 nM) for 1, 3, or 5 days. Cytotoxicity was measured using the MTT conversion assay. Control values are represented as 100% viability. Data represents mean ± SEM. A p < 0.05 was considered statistically significant.
4. Conclusion
The aim of this study was to evaluate effect of EDGs on the antioxidant behavior of antioxidant dendrimers. We synthesized antioxidant dendrimers using PETN and pyranoside as the cores and di-ethylene glycolic unit as spacers to help chelate metal ions. Syringaldehyde, vanillin, and 4-hydroxybenzaldehyde were used as surface building blocks to furnish antioxidant properties. The difference between the various building blocks is the number of electron donating groups (methoxy groups) at ortho position with respect to the hydroxyl group on the phenol ring. Based on the antioxidant activity tests, syringaldehyde-based dendrimers (with two methoxy groups on each phenol ring) showed the best antioxidant activities, followed by vanillin-derivatized dendrimers (one methoxy on each ring). 4-Hydroxybenzaldehyde derivatized dendrimers (no methoxy group) showed virtually no antioxidant activity. The antioxidant activity testing data provide important information on the structure-activity relationship of the antioxidant dendrimers. Our results of this study clearly indicate that the existence of electron donating group ortho (possibly para as well) to phenolic OH group is more important than simply having multiple phenol rings. Although these dendrimers were effective in scavenging organic radicals, they did not show any effects in quenching polar hydroxyl radicals. These antioxidant dendrimers did not show any pro-oxidant side effects or cytotoxicity.
Highlights.
Formation of powerful polyphenolic macromolecular antioxidants by assembling various weak phenolic antioxidants into dendritic architecture.
Dendritic antioxidants showed strong free radical scavenging due to multiple phenolic units and no pro-oxidant effects due to their metal chelation properties.
Dendritic antioxidants with more electron donating groups showed stronger antioxidant effects
Effective against carbon and nitrogen-based radicals but not hydroxyl radicals.
Acknowledgements
This work was supported by Award Number 1R15GM087697-01 and 3R15GM087697-01S1 from the National Institute of General Medical Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health.
List of abbreviations
- AAPH
2,2'-azobis(2-amidinopropane) dihydrochloride
- CDCl3
Deuterated Chloroform
- CHO-K1
Chinese hamster ovary cells
- CuCl2
Cupric chloride
- DMSO
Dimethylsulfoxide
- DMF
Dimethylformamide
- DMPO
5,5-Dimethyl-1-pyrroline N-oxide
- DMPO-OH
5,5-Dimethyl-1-pyrroline N-oxide spin trap and hydroxyl radical adduct
- DNA
Deoxyribonucleic acid
- DPPH
2,2-diphenyl-1-picrylhydrazyl
- ESI-TOF
Electrospray ionization-time of flight
- ESR
Electron spin resonance spectrometer
- ET
Electron transfer
- FeSO4
Ferrous sulfate
- HAT
Hydrogen atom transfer
- H2O2
Hydrogen peroxide
- LDL
Low density lipoprotein
- MgSO4
Magnesium sulfate
- MTT
3-(4,5-Di-methylthizol-2-yl)-2,5-diphenyltetrazolium bromide
- NaH
Sodium hydride
- Na(OAc)3BH
Sodium triacetoxyborohydride
- NaN3
Sodium azide
- OC
Open circular form of DNA
- PAGE
Polyacrylamide gel electrophoresis
- pBR 322
Plasmid DNA
- PBS
Phosphate-buffered saline
- PETN
pentaerythritol
- TEA
Triethylamine
- SC
Supercoiled form of DNA
- V/V
Volume to volume
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare that there is no conflict of interest.
References
- 1.Halliwell B, Gutteridge JM. The antioxidants of human extracellular fluids. Arch. Biochem. Biophys. 1990;280:1–8. doi: 10.1016/0003-9861(90)90510-6. [DOI] [PubMed] [Google Scholar]
- 2.Halliwell B. Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging. 2001;18:685–716. doi: 10.2165/00002512-200118090-00004. [DOI] [PubMed] [Google Scholar]
- 3.Valko M, Leibfritz D, Moncola J, Cronin M, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Knight J. Free radicals: their history and current status in aging and disease. Ann. Clin. Lab. Sci. 1998;28:331–346. [PubMed] [Google Scholar]
- 5.Foti MC, Daquino C, Geraci C. Electron-transfer reaction of cinnamic acids and their methyl esters with the DPPH radical in alcoholic solutions. J. Org. Chem. 2004;69:2309–2314. doi: 10.1021/jo035758q. [DOI] [PubMed] [Google Scholar]
- 6.Litwinienko G, Ingold KU. Abnormal solvent effects on hydrogen atom abstraction. 2. Resolution of the curcumin antioxidant controversy. The role of sequential proton loss electron transfer. J. Org. Chem. 2004;69:5888–5896. doi: 10.1021/jo049254j. [DOI] [PubMed] [Google Scholar]
- 7.Verdan AM, Wang HC, García CR, Henry WP, Brumaghim JL. Iron binding of 3-hydroxychromone, 5-hydroxychromone, and sulfonated morin: Implications for the antioxidant activity of flavonols with competing metal binding sites. J. Inorg. Biochem. 2011;105:1314–1322. doi: 10.1016/j.jinorgbio.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 8.García CR, Angelé-Martínez C, Wilkes JA, Wang HC, Battin EB, Brumaghim JL. Prevention of iron- and copper-mediated DNA damage by catecholamine and amino acid neurotransmitters, L-DOPA, and curcumin: metal binding as a general antioxidant mechanism. Dalton Trans. 2012;41:6458–6467. doi: 10.1039/c2dt30060e. [DOI] [PubMed] [Google Scholar]
- 9.Azam S, Hadi N, Khan NU, Hadi SM. Prooxidant property of green tea polyphenols epicatechin and epigallocatechin-3-gallate: implications for anticancer properties. Toxicol. in Vitro. 2004;18:555–561. doi: 10.1016/j.tiv.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Kawanishi S, Oikawa S, Murata M. Evaluation for safety of antioxidant chemopreventive agents. Antiox. Redox. Signal. 2005;7:1728–1739. doi: 10.1089/ars.2005.7.1728. [DOI] [PubMed] [Google Scholar]
- 11.Rahman A, Shahabuddin, Hadi SM, Parish JH, Ainley K. Strand scission in DNA induced by quercetin and Cu(II): role of Cu(I) and oxygen free radicals. Carcinogenesis. 1989;10:1833–1839. doi: 10.1093/carcin/10.10.1833. [DOI] [PubMed] [Google Scholar]
- 12.Ahsan H, Hadi SM. Strand scission in DNA induced by curcumin in the presence of Cu(II) Cancer Lett. 1998;124:23–30. doi: 10.1016/s0304-3835(97)00442-4. [DOI] [PubMed] [Google Scholar]
- 13.Lee CY, Sharma A, Cheong JE, Nelson JL. Synthesis and antioxidant properties of dendritic polyphenols. Bioorg. Med. Chem. Lett. 2009;19:6326–6330. doi: 10.1016/j.bmcl.2009.09.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee CY, Sharma A, Uzarski RL, Cheong JE, Xu H, Held RA, Upadhaya SK, Nelson JL. Potent antioxidant dendrimers lacking pro-oxidant activity. Free Radic. Biol. Med. 2011;50:918–925. doi: 10.1016/j.freeradbiomed.2010.10.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cuvelier ME, Richard H, Berset C. Comparison of the antioxidative activity of some acid-phenols: Structure-activity relationship. Biosci. Biotech. Biochem. 1992;56:324–325. [Google Scholar]
- 16.Ali HM, Abo-Shady A, Eldeen HAS, Soror HA, Shousha WG, Abdel-Barry OA, Saleh AM. Structural features, kinetics and SAR study of radical scavenging and antioxidant activities of phenolic and anilinic compounds. Chem. Cent. J. 2013;7:53–62. doi: 10.1186/1752-153X-7-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee CY, Held R, Sharma A, Baral R, Nanah C, Dumas D, Jenkins S, Upadhaya S, Du W. Copper-granule-catalyzed microwave-assisted click synthesis of polyphenol dendrimers. J. Org. Chem. 2013;78:11221–11228. doi: 10.1021/jo401603d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brand-Williams W, Cuvelier ME, Berset C. Use of free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995;28:25–30. [Google Scholar]
- 19.Rosen GM, Rauckman EJ. Spin trapping of superoxide and hydroxyl radicals. In: Packer L, editor. Methods in Enzymology. Vol. 105. Orlando, FL: Academic Press; 1984. pp. 198–209. [DOI] [PubMed] [Google Scholar]
- 20.Park PJ, Je JY, Kim SE. Free radical scavenging activity of chitooligosaccharides by electron spin resonance spectrometry. J. Agric. Food Chem. 2003;51:4624–4627. doi: 10.1021/jf034039+. [DOI] [PubMed] [Google Scholar]
- 21.Tomalia DA. Dendritic effects: Dependency of dendritic nano-periodic property patterns on critical nanoscale design parameters (CNDPs) New J. Chem. 2012;36:264–281. [Google Scholar]
- 22.Salah N, Miller NJ, Paganga G, Tijburg L, Bolwell GP, Rice-Evans CA. Polyphenolic flavanols as scavengers of aqueous phase radicals and as chain-breaking antioxidants. Arch. Biochem. Biophys. 1995;322:339–346. doi: 10.1006/abbi.1995.1473. [DOI] [PubMed] [Google Scholar]
- 23.Foxx KK, Roberts RL, Waxdal MJ. Kalen Biomedical Technical Note 2: Effect of copper ion concentration on the oxidation of human LDL. [Accessed 2014 December 23]; Available: http://www.kalenbiomed.com/50tech_info.php via the INTERNET. [Google Scholar]
- 24.Sakihama Y, Cohen MF, Grace SC, Yamasaki H. Plant phenolic antioxidant and prooxidant activities: phenolics-induced oxidative damage mediated by metals in plants. Toxicol. 2002;177:67–80. doi: 10.1016/s0300-483x(02)00196-8. [DOI] [PubMed] [Google Scholar]
- 25.Rietjens IMCM, Boersma MG, de Haan L, Spenkelink B, Awad HM, Cnubben NHP, van Zanden JJ, van der Woude H, Alink GM, Koeman JH. The pro-oxidant chemistry of the natural antioxidants vitamin C, vitamin E, carotenoids and flavonoids. Environ. Toxicol. Phar. 2002;11:321–333. doi: 10.1016/s1382-6689(02)00003-0. [DOI] [PubMed] [Google Scholar]
- 26.Yamashita N, Tanemura H, Kawanishi S. Mechanism of oxidative DNA damage induced by quercetin in the presence of Cu(II) Mutat. Res. 1999;425:107–115. doi: 10.1016/s0027-5107(99)00029-9. [DOI] [PubMed] [Google Scholar]
- 27.Diallo MS, Christie S, Swaminathan P, Balogh L, Shi X, Um W, Papelis C, Goddard WA, Johnson JH., Jr Dendritic chelating agents. 1. Cu(II) binding to ethylene diamine core poly(amidoamine) dendrimers in aqueous solutions. Langmuir. 2004;20:2640–2651. doi: 10.1021/la036108k. [DOI] [PubMed] [Google Scholar]
- 28.Drouin R, Rodriquez H, Gao SW, Gebreyes Z, O'Connor TR, Holmquist GP. Cupric ion/ascorbate/hydrogen peroxide-induced DNA damage: DNA-bound copper ion primarily induces base modifications. Free Radic. Biol. Med. 1996;21:261–273. doi: 10.1016/0891-5849(96)00037-8. [DOI] [PubMed] [Google Scholar]
- 29.Pestka JJ, Uzarski RL, Islam Z. Induction of apoptosis and cytokine production in the Jurkat human T cells by deoxynivalenol: Role of mitogen-activated protein kinases and comparison to other 8-ketotrichothecenes. Toxicol. 2005;206:207–219. doi: 10.1016/j.tox.2004.08.020. [DOI] [PubMed] [Google Scholar]