Abstract
Small cell lung cancer (SCLC) is an aggressive neuroendocrine malignancy with a unique natural history characterized by a short doubling time, high growth fraction, and early development of widespread metastases. Although a chemotherapy- and radiation-sensitive disease, SCLC typically recurs rapidly after primary treatment, with only 6% of patients surviving five years from diagnosis. This disease has been notable for the absence of major improvements in its treatment: nearly four decades after the introduction of a platinum-etoposide doublet, therapeutic options have remained virtually unchanged, with correspondingly little improvement in survival rates. Here, we summarize specific barriers and challenges inherent to SCLC research and care that have limited progress in novel therapeutic development to date. We discuss recent progress in basic and translational research, especially in the development of mouse models, which will provide insights into the patterns of metastasis and resistance in SCLC. Opportunities in clinical research aimed at exploiting SCLC biology are reviewed, with an emphasis on ongoing trials. SCLC has been described as a recalcitrant cancer, for which there is an urgent need for accelerated progress. The NCI convened a panel of laboratory and clinical investigators interested in SCLC with a goal of defining consensus recommendations to accelerate progress in the treatment of SCLC, which we summarize here.
Introduction
Small cell lung cancer (SCLC) remains a worldwide public health problem. In the United States, the decrease in prevalence of tobacco use has resulted in a gradual decrease in SCLC incidence over the past decade; nonetheless, SCLC remains a major cause of cancer mortality, currently accounting for 14% of all lung cancers, or approximately 30,000 patients annually (1, 2). Tobacco exposure is strongly associated with the development of SCLC, with only 2 to 3% of patients being never-smokers (3, 4). Outcomes for SCLC have not changed dramatically as the majority of patients, including those with limited-stage disease and those initially responsive to chemotherapy and radiation, develop chemoresistance. As a result, overall five year survival rates are a dismal 6% (1, 2).
Few improvements have been made in the fundamentals of SCLC treatment in the past few decades, with most advances being restricted to improved radiation approaches. Notably, the standard chemotherapy regimen of cisplatin or carboplatin plus etoposide used for the first-line treatment of limited stage (LS-SCLC) and extensive stage (ES-SCLC) disease has not changed over the last four decades. Radiation therapy is administered to those patients with LS-SCLC, whose cancer is confined to the chest in a single tolerable radiation field. The superiority of hyperfractionated radiation therapy and early initiation of radiation, either during the first or second cycle, has been suggested in numerous clinical trials (5-12), although the question of standard hyperfractionation vs. a higher total dose radiation is being revisited in a large national cooperative group study using modern radiation techniques (NCT00632853). Those patients with LS-SCLC and ES-SCLC demonstrating a response to front-line platinum-based therapy generally are offered prophylactic cranial irradiation (PCI), which has been shown to decrease the risk of intracranial recurrence and improve overall survival (13, 14).
First-line treatment for SCLC yields optimal tumor response rates as high as 60-80%, which unfortunately, translates to cure in only approximately 20% of patients with LS-SCLC (15). Essentially all patients with ES-SCLC, and the majority of patients with LS-SCLC, suffer relapse within months of completing initial therapy. The strongest predictor of outcome for patients with relapsed SCLC is the duration of remission. Patients with sensitive disease who maintain a response to initial treatment for 3 months or greater have approximately a 25% response rate to additional chemotherapy and a median survival from the time of relapse of approximately 6 months. In contrast, those patients with refractory disease who either have no response to initial therapy, or progress within 3 months, rarely benefit from additional treatment, with response rates less than 10% and median survival of 4 months.
Topotecan is the only FDA-approved agent for recurrent or progressive SCLC, based on the results of three phase III trials (16-18). There are no accepted regimens for patients whose disease has progressed after first- and second-line treatments for SCLC. This is in stark contrast to the progress that has been made in NSCLC, and there is a critical need for more effective therapies in SCLC.
The starting point for considering new approaches is that the large majority of SCLC patients show dramatic tumor responses to initial therapy; however, in nearly all cases the tumors become resistant to this treatment.
Barriers to Progress in SCLC
There have been numerous barriers to progress in the care and treatment of SCLC patients.
Lack of early detection methods
First, there is a lack of early detection methods predominantly due to the natural history of the disease, characterized by rapid growth and early metastatic spread. While the National Lung Screening Trial demonstrated that screening high risk patients with low dose CT scans found higher numbers of early stage adenocarcinomas compared to chest x-ray, and led to a reduction in lung cancer specific mortality, there was no evidence of a similar stage shift, or mortality improvement, for SCLC (19). An effective method for early detection or screening of SCLC has not been defined.
Limited SCLC tumor tissue is available for diagnosis and study
Second, limited SCLC tumor tissue is available for translational research because of how the disease is typically diagnosed and treated. First, the diagnosis of SCLC readily is made on small specimens such as bronchoscopic biopsies, fine-needle aspirates, core biopsies, and cytology due to its characteristic appearance of dense sheets of small cells with scant cytoplasm, finely granular nuclear chromatin, inconspicuous or absent nucleoli, and frequent mitoses. Second, there are few surgically resected SCLC samples, as the majority of patients present with advanced, metastatic disease and as the treatment of this malignancy hinges primarily on chemotherapy, with or without radiation, rather than surgery. Only 4% of solitary pulmonary nodules are diagnosed as SCLC (20, 21). The ability to perform comprehensive molecular profiling such as in-depth whole genome and exome sequencing and comprehensive expression analyses requires more robust material than what has traditionally been available, and such studies have lagged behind those in NSCLC. This absence of extensive banked tumor was one factor contributing to exclusion of SCLC from The Cancer Genome Atlas (TCGA) efforts, which thus far has comprehensively evaluated hundreds of squamous carcinomas and adenocarcinomas of the lung (22, 23).
Decreased research attention to SCLC
The lack of clinically meaningful progress, the scarcity of readily available tissues to study, and the relative paucity of animal models may have all contributed to decreased research attention directed toward this important and lethal disease. In the 2012 fiscal year, the National Cancer Institute (NCI) research portfolio contained 745 projects that included lung cancer research, but only 17 (approximately 2%) of those had a focus on SCLC (24).
Challenges in SCLC
The inherent biology of SCLC presents numerous challenges, further hindering potential advancements.
SCLC has a complex molecular biologic pathogenesis with many mutations but few obvious therapeutic targets
First, the molecular pathology of SCLC is particularly complex. SCLC is most strongly linked to long term, high exposure to tobacco carcinogens, leading to an exceptionally high degree of genomic alterations, including mutations, insertions, deletions, large scale copy number alterations, and gross inter- and intra-chromosomal rearrangements (25-27). With approximately 8.88 mutations per megabyte, the only other malignancy with a higher mutational burden than SCLC is melanoma, caused by ultraviolet light, another potent carcinogen (26-28). Most of the mutations observed in SCLC tumors are passengers, that is, those that do not meaningfully contribute to growth, progression or invasion of disease. Further, the most commonly recurrent mutations that are seen in this disease are inactivating mutations in the tumor suppressor genes TP53 (75 - 90%) (29) and RB1 (60 - 90%) (30, 31), which cannot be targeted directly.
Two independent, comprehensive genomic studies, which included exome, whole genome, transcriptome, and copy number alteration data from primary SCLC patient samples (together over 100 samples) have provided some initial insights into the fuller landscape of genetic alterations in this disease (26, 27). They confirm TP53 and RB1 inactivation and the exceptionally high degree of genomic alteration in this tumor type. The two studies emphasize that, unlike lung adenocarcinoma, the genomic landscape of SCLC is not broadly characterized by a set of mutually exclusive, targetable driver oncogenes involved in activation of kinase signaling. Other processes such as transcriptional deregulation, histone modification (e.g., mutations in CREBBP, EP300 and MLL), and dysregulation of the cytoskeleton (e.g., mutations in SLIT2 and EPHA7) are implicated by mutational data. Additional alterations of interest in SCLC defined by the two studies include amplification of MYC, MYCN, and MYCL1; a recurrent fusion involving MYCL1 (9%); inactivation of PTEN (10%) and mutations of other factors in the same signaling pathway; and amplification of the tyrosine kinase FGFR1 (6%) and of the developmental regulator and transcription factor SOX2 (27%) (26, 27). It is important to note that the less common genomic alterations detected in each of the reports differed, highlighting that these current studies have been insufficiently powered to reliably identify recurrent mutations present in <10% of SCLC patients, and that such efforts should be expanded further. Importantly, both the functional and therapeutic implications of the large majority of the genetic alterations documented to date in SCLC have not been defined.
Mechanisms of primary and acquired resistance to chemotherapy are unknown
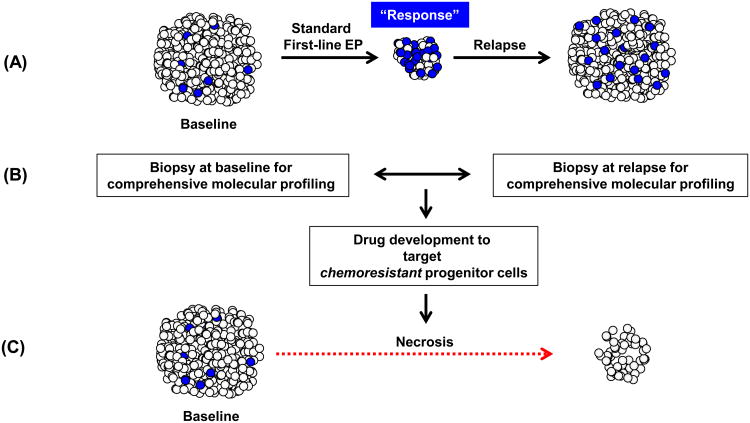
Second, while all patients with ES-SCLC, and the majority of patients with LS-SCLC, suffer relapse within months of completing initial therapy, the mechanisms of resistance and properties of the chemoresistant cell population remain unknown. In standard clinical practice, SCLC patients are not re-biopsied upon recurrence, given that disease progression is expected and often symptomatic, necessitating urgent treatment. In the context of limited treatment options, such biopsies have been considered unwarranted. However, following upon the experience of molecularly-driven NSCLC, where we have an increasingly clear understanding of the mechanisms of resistance for EGFR-mutated and ALK-rearranged tumors as discussed elsewhere in this CCR Focus section (32, 33), research programs to obtain acquired resistance biopsies should be considered for SCLC. Identifying specific molecular aberrations in SCLC tumors upon recurrence may help us understand the mechanisms of acquired resistance to first-line chemotherapy, may identify factors that may contribute to the variable responses observed with standard treatment, and may define opportunities to tailor effective treatment options for patients with SCLC (Fig. 1).
Figure 1.
Chemoresistance and potential for research and change in treatment. A, SCLC is very sensitive to first-line chemotherapy, with 60-80% response rate. However, there is almost uniform relapse or progression of disease.Such relapse likely is due to the behavior of the chemoresistant cell population, which may also have enhanced tumorigenic potential (blue-colored cells). B, Opportunities for research and drug development.Patients with newly diagnosed advanced SCLC could be enrolled onto tissue acquisition protocols and their tumors biopsied prior to initiating treatment, facilitating comprehensive molecular studies, including but not limited to genome, transcriptome, proteomic and methylome profiling. Further, these samples can be available for creation of patient derived xenografts (not shown).At the time of progressive or recurrent disease, patients could be approached to undergo repeat biopsy. Evaluation and comparisons of molecular features of paired samples from the same patient could identify pathways of resistance to standard first line therapy, define new biomarkers, and provide opportunities for targeted drug development. Pathways of interest can be evaluated further in genetically engineered mice models (not shown). C,Once agents are found to be of benefit against chemoresistant cells, these can be incorporated into clinical trials and potentially lead to responses and importantly, more durable outcomes. EP, etoposide/platinum.
Improved Research Strategies in SCLC
Mouse models of SCLC
Given the complexity of SCLC and the relatively limited number of available patient samples, animal models of this disease play a key role in translational research. These include both genetically engineered mouse models and patient-derived xenograft models.
Genetically engineered mouse models (GEMMs)
Many GEMMs have been developed that recapitulate the spectrum of human SCLC and other high-grade neuroendocrine cancers (34). Loss of function mutations in RB1 and TP53 genes are hallmarks of SCLC and are therefore the “backbone” of most GEMM models, to which additional alterations can be added to hasten tumor development or to study the contribution of a specific gene alteration. Mice with conditional loss of Trp53 and Rb1 in the lung develop spontaneous SCLC tumors that behave similarly to human tumors (such as the development of metastatic disease and the sites of those metastases). Genomically, these models have lower mutational burdens overall (presumably due to the absence of tobacco exposure), but still undergo both genetic and clonal progression (including the development of Mycl1 amplification and Pten loss) (35, 36). Therefore, while GEMMs do not fully capture the genetic complexity of human SCLC tumors, they nevertheless provide an important tool for studying the contribution of key genes while minimizing “noise” from passenger mutations characteristic of human SCLC. Finally, from a therapeutic standpoint, the use of GEMMs to test therapeutic interventions can be highly informative (37), despite being labor-intensive and costly given the time required to develop cancer (e.g., ≥9 months in models with Rb1 and Trp53 inactivation). In particular, GEMMs (unlike xenografts) allow for the investigation of SCLC within an immune-competent context--a valuable feature for studying the role of the immune system and immunotherapies in SCLC.
GEMMs: transgenic mouse models of SCLC
Initial genetic mouse models of SCLC utilized tissue/cell type-specific promoters to drive expression of oncogenes or proto-oncogenes, such as Simian virus 40 (SV40) large T antigen (Tag) (a transforming oncogene that disrupts several key functions of Rb1 and Trp53) (38, 39) and Myc (38). Limitations of early transgenic models included a lack of an efficient method for gene deletion (but rather for addition or misexpression) and an absence of inducible systems. As such, oncogene expression depends upon the onset of promoter expression, regardless of age or stage of development of the mouse. The inability for these transgenic models to target neuroendocrine cells may, therefore, be due to promiscuity of transgene expression during early lung development (40).
GEMMs: conditional mouse models of SCLC
Genes can be conditionally deleted in mice utilizing specific methods, such as administering cell type-specific adenoviral vectors or by crossing to recombinant mice. Loss of function of both RB1 and TP53 occurs in almost all human SCLC and were the first targeted alleles to generate more histologically representative mouse models of SCLC. Deletion of Rb1 and Trp53 resulted in tumors expressing neuroendocrine markers that have morphological similarities to SCLC within six to nine months (41-43).
The addition of any of several third alleles, such as p130 (Rbl2) (44), Rbl2 and Smo or SmoM2 (45), and Pten (36, 42), to Rb1 and Trp53 loss can accelerate tumor formation and metastasis. Mice with loss of Rb1; Trp53; Rbl2 develop SCLC tumors and liver metastases within five to six months and do not survive to nine months (44). While this triple model results in accelerated SCLC formation, Rbl2 mutation is not commonly found in human SCLC. Mice with constitutively active Hedgehog signaling (Rb1; Trp53; SmoM2) form SCLC tumors with greater volume and higher mitotic index. Conversely, attenuation of Hedgehog signaling combined with the accelerated, triple model described by Schaffer et al., (Rb1; Trp53; Rbl2; Smo) (44) resulted in fewer and smaller SCLC tumors (45), supporting the idea that Hedgehog signaling is essential for SCLC tumor formation and progression. One of the most aggressive mouse models of SCLC incorporates deletion of the tumor suppressor Pten together with Trp53 and Rb1 loss. These mice develop hyperplastic lesions within two to four weeks, display neuroendocrine hyperplasia, tumor invasiveness and large cell tumors within two to three months, and do not survive beyond three months (42).
Primary benefits of these genetic models of SCLC include the ability to study the malignancy in a tightly manipulable system, to evaluate the characteristics of metastases that arise endogenously, and, in particular, the opportunity to assess the contribution of specific genetic lesions (ex., Smo, Pten) in the context of Rb1 and Trp53 loss (without the large burden of passenger mutations typical of human tumors). Mouse SCLC shares many characteristics with human SCLC, including cell morphology, gene expression profiling, and metastatic patterns. Interestingly, tumors arising in these mouse models are heterogeneous (46), a feature they share with human SCLC.
Patient derived xenografts (PDX)
PDX models, which depend on the immediate transfer of human SCLC from patients to recipient immuno-deficient mice without intervening tissue culture or cell line derivation ex vivo (47), provide an opportunity to study the fuller extent of human tumor heterogeneity, to expand original biopsies into a larger tumors that can then be used more successfully for molecular profiling (ex., DNA sequencing, proteomics), and to investigate response to drugs and other therapeutic approaches. Recently, the feasibility of using circulating tumor cells (CTCs) from the blood of SCLC patients to establish animal models (CTC-derived xenografts, CDXs) was demonstrated (48). These models may prove to be particularly transformative for the field, as they do not rely on actual invasive biopsies to obtain tissue, but rather a “liquid biopsy”, and further, allow for studying mechanisms of drug resistance and SCLC biology though sequential sampling of blood from the same patient at the time of initial diagnosis and relapse.
Comprehensive molecular profiling
As summarized above, two recent independent, studies focused primarily on comprehensive genomic analyses of human SCLC (26, 27). However, beyond alterations in DNA, analysis of additional layers of cancer-specific dysregulation, including epigenetic alterations, changes in gene and miRNA expression profiles, and, ultimately, changes in the proteome will be instrumental in the understanding of the malignant transformation, clonogenic potential, tumor growth and metastatic spread of SCLC, and have already begun to yield potentially clinically relevant insights. For example, proteomic profiling of a large panel of SCLC cell lines led to the identification of increased expression of the DNA repair proteins, Poly (ADP-Ribose) Polymerase (PARP)-1 and checkpoint kinase 1 (Chk1), as well as the chromatin modulator, enhancer of zest 2 polycomb repressive complex 2 subunit (EZH2) (49). Utilizing high numbers of patient samples to perform these studies in real time will be necessary to more comprehensively characterize the landscape of potential targets.
Drug screening and bioinformatics
The Developmental Therapeutics Program at the NCI has been investigating drug sensitivity of >400 targeted drugs and >100 FDA-approved oncology agents in a panel of >60 SCLC cell lines. Results from this drug screen, as well as others, coupled with intense analyses of the pathways affected by the indicated agents utilizing the comprehensive methods indicated above may provide indications for future clinical trials (50).
An attractive modality of therapeutic discovery is drug repositioning utilizing novel bioinformatic approaches (51). An advantage of repurposed candidate drugs is that they can often enter clinical trials much more rapidly than drugs in preclinical development. Recently, a computational drug repositioning approach identified agents that can be repurposed to treat SCLC. Top candidates were validated in a comprehensive series of assays with SCLC cells, in culture and in vivo (37, 51). This approach identified tri-cyclic antidepressants (TCAs) as potent inhibitors of SCLC growth, including imipramine and desipramine, and led to a clinical trial evaluating the latter drug in SCLC patients (NCT01719861).
Novel Therapeutic Strategies in SCLC
In light of the therapeutic plateau achieved with chemotherapy, investigators have studied a wide range of novel therapies in the hopes of improving outcomes (see Table 1). Unfortunately, although often rationally designed based on existing data at the time, in general the outcomes of these trials have not been favorable. The genomic studies highlighted above, as well as additional proteomic, high throughput drug screening and pathway specific investigations, have yielded new insights and new potential therapeutic targets for this aggressive disease. Building upon these findings and continued focus on the biology of the disease to design future studies may lead to improved outcomes for SCLC patients.
Table 1. Agents that have undergone testing in small cell lung cancer.
| Agent (Ref.) | Mechanism of action | Study design | Result |
|---|---|---|---|
| Interferon-alfa (106-109) | Immunomodulator | Phase 3 (multiple) | Two studies with improved survival in limited-stage patients, two studies with no survival benefit |
| Interferon-gamma (110, 111) | Immunomodulator | Phase 3 (multiple) | No improvement in survival |
| Interleukin-2 (112) | Immunomodulator | Phase 2 | 21% response rate but excessive toxicity |
| Ipilimumab (104) | Humanized anti-CTLA4 antibody | Randomized Phase 2 | Improved immune-related PFS when administered with carboplatin/paclitaxel in phased dosing schedule in chemo-naïve extensive stage SCLC |
| Marimastat (113) | Matrix metalloproteinase inhibitor | Phase 3 | No improvement in progression-free or overall survival |
| Tanomastat (114) | Matrix metalloproteinae inhibitor | Phase 3 | No improvement in progression-free or overall survival |
| Imatinib (115-117) | c-kit tyrosine kinase inhibitor | Phase 2 (multiple) | No responses |
| Temsirolimus (118) | Mammalian target of rapamycin (mTOR) inhibitor | Randomized Phase 2 | Higher dose level demonstrated improved survival compared to lower dose level when given post-first line therapy; both doses showed improvement in outcome compared with historical control |
| Everolimus (119) | Mammalian target of rapamycin (mTOR) inhibitor | Phase 2 | Limited anti-tumor activity in relapsed SCLC. |
| Tipifarnib (120) | Farnesyl transferase inhibitor | Phase 2 | No responses |
| Cixutumumab (83) | Monoclonal IGF-1R antibody | Randomized Phase 2 | No improvement in progression free survival when added to cisplatin/etoposide in chemo-naïve extensive stage SCLC |
| Vismodegib (83) | Hedgehog pathway inhibitor | Randomized Phase 2 | No improvement in progression free survival when added to cisplatin/etoposide in chemo-naïve extensive stage SCLC |
| Oblimersen (121) | Bcl-2 antisense | Randomized Phase 2 | No improvement in response rate |
| Navitoclax (122) | Bcl-2 and bcl-xL inhibitor | Phase 2 | Limited activity in recurrent and progressive disease |
| Obatoclax mesylate (123, 124) | BH3-mimetic exhibits binding affinity for bcl-2 family members, including bcl-2, bcl-XL, and mcl-1 | Phase 2 | No increased response rate when added to topotecan in relapsed SCLC |
| Randomized Phase 2 | Trend toward improved response rate, PFS and OS in chemo-naïve extensive stage SCLC | ||
| Bortezomib (125) | Proteosome inhibitor | Phase 2 | One response in refractory patient (2% overall response rate) |
| BEC-2 + BCG adjuvant (126) | Ganglioside (GD3) anti-idiotype vaccine | Phase 3 | No improvement in progression-free or overall survival |
| Thalidomide (127, 128) | Multiple immunomodulatory effects, also inhibits vascular endothelial growth factor (VEGF) | Phase 3 | Improved survival from 8.7 to 11.7 months but not significant (hazard ratio 0.74; P =.16) |
| Phase 3 | No improvement in any parameters | ||
| Vandetanib (129) | Tyrosine kinase inhibitor of VEGFR-2 and EGFR | Randomized Phase 2 | No improvement in progression-free survival |
| Sorafenib (130) | RAF, VEGFR-2,VEGFR-3, PDGFRα Inhibitor | Phase 2 | 5% response rate in relapsed disease |
| Cediranib(131, 132) | VEGFR-1, VEGFR-2,VEGFR-3, PDGFRβ, c-KIT Inhibitor | Phase 2 | Minimal activity as a single agent in relapsed disease |
| Phase 1 | 8-month progression-free survival with cisplatin and etoposide | ||
| Bevacizumab (133-136) | Monoclonal antibody to VEGF | Phase 2 (multiple) | No increased risk of hemorrhage. Favorable survival compared with historical control. |
| Randomized Phase 2 | Improved progression free survival for bevacizumab, but not in overall survival | ||
| Sunitinib (137) | VEGFR-1, VEGFR-2,VEGFR-3, PDGFRα, PDGFRβ, RET, c-KIT, FLT3 | Phase 3 | Improved progression free survival as maintenance after etoposide/platinum compared to placebo (P = 0.037) |
Reprinted from ref. 138: DeVita VT Jr, Lawrence TS, Rosenberg SA, editors. DeVita, Hellman, and Rosenberg's cancer: principles & practice of oncology. 10th ed. Philadelphia: Wolters Kluwer; 2014.
Harnessing known molecular alterations in SCLC
Approximately 20% of SCLC patient tumors harbor alterations in the MYC gene family members of transcription factors, which are contributors to oncogenesis (52). Previous efforts to inhibit MYC activity were disappointing, yet utilizing the newer Aurora Kinase or bromodomain inhibitors may prove to be promising (53-55). MYC is a transcriptional regulator of Aurora Kinases A and B, which, in the absence of p53, provides a growth advantage (56-59). Preclinical models of SCLC suggest that tumor with MYC alterations may be most sensitive to Aurora Kinase inhibitors (56, 60). The Aurora Kinase A inhibitor, alisertib, was evaluated in a phase II clinical trial of patients with recurrent or progressive SCLC and demonstrated a response rate of 21% (61). Notably, patients with refractory disease were found to have the highest response rates. Further, these drugs may be active when administered with taxanes, as Aurora Kinase A has a key role in mitotic spindle assembly. There is an ongoing clinical trial evaluating paclitaxel with or without alisertib for the second line treatment of SCLC patients (NCT02038647). If the activity of the Aurora Kinase inhibitors is preferentially restricted to MYC-amplified tumors, MYC-amplification may represent the first genotypically defined subset of SCLC of clinical relevance.
As noted above, FGFR1 is amplified in 6% of SCLC, and sensitivity to FGFR inhibitors has been described in some, but not all, SCLC tumors (54). Although the extent to which this subset of SCLC is dependent on the FGFR pathway is not known, there are clinical studies evaluating drugs targeting the FGFR family members for SCLC patients, including JNJ 42756493 (a pan-FGFR inhibitor) (NCT01703481) and BIBF1120 (a multi-targeted drug that inhibits FGFR, vascular endothelial growth factor receptor and platelet-derived growth factor receptor) (NCT01441297).
Exploiting the epigenome
Epigenetic alterations encompass somatically heritable differences in gene expression not attributable to alterations in the primary sequence of DNA, but rather to alterations in chromatin and other associated factors that modify the ability of genes to be transcribed (62). Aberrancies in gene promoter methylation patterns and histone acetylation are two of the many epigenetic processes dysregulated in cancer. Histone acetylation, which leads to increased accessibility of promoter regions and increased transcription of genes, is controlled by the interplay of acetyltransferases and deacetylases (HDACs) (63, 64). The histone deacetylase inhibitors vorinostat and belinostat have been found to have synergistic activity when added to topotecan and cisplatin/etoposide, respectively (65, 66). We are awaiting results of two clinical trials investigating the combination of vorinostat (NCT00702962) and belinostat (NCT00926640) with platinum and etoposide in the first-line treatment of patients with ES-SCLC. Notably HDAC inhibitors have been shown to downregulate expression of c-Myc (67-69). GSK525762 is a small molecule inhibitor of the BET (bromodomain and extra-terminal) family of bromodomain-containing proteins, which prevents interaction of BET proteins with acetylated histones, leading to focal chromatin remodeling and altered expression of a number of potential target genes of interest, including MYC, as noted above. This agent is being evaluated in a phase I clinical trial that includes SCLC patients (NCT01587703).
DNA repair
SCLC has been characterized by aberrant expression of a number of genes implicated in DNA damage repair. Frequent aberrant methylation and epigenetic silencing of the MGMT gene, which encodes the DNA-repair protein O6 alkyl-guanine (O6-AG) DNA alkyltransferase (MGMT) (70-72) has been demonstrated. Proteomic profiling of a large panel of SCLC cell lines has shown increased expression of PARP-1 and Chk1 (49). Altered expression of additional DNA repair proteins has been noted in SCLC when compared to NSCLC, including high levels of BRCA-1 and RAD51, with known roles in DNA double strand break repair (49). Multiple DNA repair pathways may represent attractive targets in SCLC.
Epigenetic silencing of MGMT via hypermethylation of specific CpG islands of its promoter leads to loss of MGMT activity and increased sensitivity to alkylating agents (70, 72). Left unrepaired, chemotherapy-induced lesions trigger apoptosis. Temozolomide, an oral alkylating agent that crosses the blood-brain barrier, demonstrated an overall response rate of 20% in a phase II clinical trial of patients with relapsed sensitive or refractory SCLC. Responses also were noted in patients receiving temozolomide as third-line treatment and in those with brain metastases. Based on these data, temozolomide has been added to compendia of agents recommended for use in the treatment of SCLC (73).
Subsequent to the observation that PARP is overexpressed in SCLC, PARP inhibitors were investigated preclinically and exhibited single agent activity in cell lines and/or animal models (49, 74). There are active studies evaluating the PARP inhibitors, BMN673 and veliparib either alone or in combination with chemotherapy for the treatment of SCLC (NCT01286987, NCT01642251, NCT02289690, NCT01638546). BMN673 has shown single agent activity in sensitive relapsed SCLC patients (75). An ongoing multi-center randomized phase II study is comparing veliparib plus temozolomide to temozolomide alone in patients with relapsed SCLC (NCT01638546).
Developmental pathways: the Hedgehog and Notch pathways
SCLC is a relatively undifferentiated airway epithelial tumor that may recapitulate aspects of early lung development (76, 77). Hedgehog and Notch pathways have been noted to be essential in early lung development and to regulate stem cell self-renewal; thus, when abnormally activated, can cause neoplastic proliferation, representing an early event in tumorigenesis (78-80). These pathways are being explored as potential targets in SCLC. These are hypothesized to be of particular interest in the clonogenic subset of SCLC cells that persistently gives rise to disease recurrence and metastatic spread (81, 82).
In vitro and in vivo studies have suggested that Hedgehog antagonists can inhibit SCLC growth, and when administered following chemotherapy, may delay or prevent recurrence of residual disease (77). The ECOG 1508 phase II randomized trial in patients with ES-SCLC included an arm evaluating the addition of vismodegib, a Hedgehog inhibitor, to cisplatin and etoposide, which unfortunately did not lead to an improvement in PFS (83). There are two ongoing studies evaluating other Hedgehog inhibitors, the results of which have not yet been reported (NCT01579929, NCT01722292).
The Notch pathway is complex and multipartite: depending on the cellular content, Notch signaling can have oncogenic or tumor suppressive effects, and influences multiple other oncogenic pathways (84). Notch2 and Notch3 receptors and target genes have been noted to be overexpressed in SCLC. Tarextumab (OMP-59R5), a fully human monoclonal antibody that selectively inhibits the function of Notch2 and Notch3 receptors, has been shown to delay tumor recurrence following the discontinuation of chemotherapy in preclinical models of SCLC, and to decrease cancer stem cell frequency and tumorigenicity (85). A phase I study of tarextumab with etoposide/platinum in patients with ES-SCLC has been completed, and a randomized phase II study is ongoing (NCT01859741).
Achaete-scute homolog-1 as a lineage oncogene
A highly expressed gene in SCLC and other neuroendocrine lung cancers is the lineage-specific transcription factor achaete-scute homolog 1 (ASCL1) (86-90). ASCL1 is necessary to establish the lineage of pulmonary neuroendocrine cells and for the continued survival of the large fraction of SCLCs which express ASCL1 (86-89). ASCL1 is not amplified or mutated but remains over expressed in SCLCs (88, 89). Knockdown of ASCL1 or targeting some of its downstream regulated genes leads to SCLC death (87, 89, 90). Thus, an attractive strategy in SCLC may be to develop new therapeutics targeting ASCL1 pathways.
Immunotherapy
Several lines of evidence support modulating the immune response in SCLC as a treatment modality. The disease is associated with immunogenic effects, evidenced by the prolonged survival of patients with autoantibodies (i.e., anti-Hu) and neurologic paraneoplastic syndromes (91). The expression of major histocompatibility complex antigens is reduced in SCLC and this may play a role in this tumor's ability to escape immune surveillance (92, 93). Interestingly, effector T cells associated with cytolytic responses are significantly higher in the peripheral blood of patients with LS-SCLC compared to those with ES-SCLC and in long term disease-free survivors relative to those with recurrent disease (94). Most recently, the programmed death-1 (PD1) and programmed death ligand-1 (PD-L1) pathway, a major target of anti-tumor immunotherapy, has been interrogated in SCLC utilizing immunohistochemistry and RNA-expression (95). While there appears to be only low-level PDL1 expression in SCLC tumor cells, PDL1 is expressed in tumor infiltrating macrophages and correlates with the presence of tumor infiltrating lymphocytes (95).
Soria and colleagues detail studies suggesting that smokers with lung cancers are most likely to benefit from PD-1/PDL-1 blockade (96-99). The anti-PD-1 antibody, nivolumab, recently was approved for the second-line treatment of patients with squamous cell lung cancer, a subtype that, like SCLC, is tightly linked to tobacco use (100, 101). Mutational burden appears to be an important determinant of response to immune checkpoint inhibitors. In a recent study analyzing tumor mutational burden in NSCLC patients treated with the PD-1 antibody pembrolizumab, higher mutational burden was associated with improved objective response, durable clinical benefit and progression-free survival (102). The association between response to PD-1 inhibitors, mutation burden, and tobacco exposure may have important implications for SCLC as this disease is strongly associated with smoking and has a markedly elevated mutation burden, as highlighted previously.
Therefore, immune checkpoint blockade, either alone or in combination with chemotherapy, represents a potentially promising approach to treatment in this malignancy. Ipilimumab, a humanized IgG1 monoclonal antibody against cytotoxic T-lymphocyte antigen-4 (CTLA-4), was evaluated in a randomized, double-blind, three arm phase II trial in patients with untreated stage IIIB/IV NSCLC or ES-SCLC to evaluate its efficacy and safety with paclitaxel and carboplatin on two dosing schedules (103). Among the 130 patients with SCLC, the phased dosing schedule, in which ipilimumab was started in cycle three of paclitaxel and carboplatin, appeared to improve immune-related PFS (median 6.4 months for the phased ipilimumab arm versus 5.3 months for the control arm (P = 0.03)), immune-related best overall response rate (71% (95% CI, 55 – 84) versus 53% (95% CI, 38 – 68)) and OS (median 12.9 months versus 9.9 months (P = 0.13)), compared to paclitaxel and carboplatin, while the concurrent regimen did not lead to improved outcomes (104). Given these favorable results, a randomized, multicenter, double blind phase III trial comparing the efficacy of platinum/etoposide with or without ipilimumab in patients with newly diagnosed ES-SCLC, with OS as the primary endpoint, has completed accrual and results are anticipated (NCT01450761). There are ongoing early phase studies for patients with relapsed SCLC evaluating nivolumab, with and without ipilimumab (NCT01928394) and MEDI4736, the humanized IgG1κ monoclonal antibody directed against PDL1 (NCT01693562), for which we would anticipate favorable responses based on previous outcomes of these agents in patients with cancers that harbor increased mutational burden.
Moving Forward in SCLC
The pathogenesis of SCLC is driven by multiple aberrant pathways and mutations, leading to its unique biology and clinical features. Clinically meaningful progress has been slow in SCLC, although recent preclinical and clinical correlative analyses have pointed to a number of new targets of interest. Genomic and proteomic studies, as well as additional high throughput drug screening and pathway specific investigations, have led to clinical studies attempting to target MYC- and FGFR1-amplified SCLC and to disrupt DNA repair pathways to cause apoptosis. Further, mouse models have been instrumental at exploring the Hedgehog and Notch pathways, among others, leading to the development of additional trials. Ongoing studies in mouse models will allow us to further define the basic molecular and cellular changes in this disease, further fostering the development of novel therapeutic strategies. Importantly, immune checkpoint inhibitors may prove to be effective in this smoking-related disease.
However, there continues to be a critical need for a better understanding of this malignancy, and the mechanisms that lead to the shift from initial therapeutic sensitivity to ultimate therapeutic resistance. The necessity for accelerated progress in SCLC research and treatment recently has been recognized by the NCI, in response to the Recalcitrant Cancer Research Act of 2012 (105). This congressional bill charged the NCI with developing plans to accelerate progress in recalcitrant tumors, defined as those with 5-year survival rates of less than 20%. SCLC, along with pancreatic cancer, has been identified as an initial focus by the NCI.
During the summer of 2013, clinical, translational, and basic science investigators came together at the NCI to develop recommendations for how we might accelerate the pace of SCLC research and clinical progress. Several consensus recommendations were proposed to address the challenges facing those who study and treat SCLC. These included recognition of the need for (1) improved research tools for the study of SCLC (including collaborative efforts to increase the collection and quality of SCLC tumor tissue collection from treatment-naïve and refractory tumors, as well as the continued development of preclinical SCLC models); (2) high-quality molecular analysis of SCLC patient cohorts (including profiling of relapsed SCLC to investigate potential therapeutic vulnerabilities); (3) promotion of the most promising drug targets into high-quality, clinical trials; and (4) support for SCLC research and investigators both financially and through academic initiatives to create a community of SCLC investigators, medical professionals, advocates, and others to promote collaborations and career development within the field (24).
Multi-disciplinary and collaborative approaches across institutions with an emphasis on collecting adequate tissue from patients sequentially throughout their disease, with advances in technology to interrogate samples and translation of molecular findings into rational clinical trials, have the potential to advance the field. Recent discoveries based on these principles continue to inspire the next generation of innovative clinical trials for the disease.
Acknowledgments
The authors thank Allison Stewart, PhD, for her assistance with development of the section “Mouse models of SCLC.”
Grant Support: M.C. Pietanza and C.M. Rudin are supported, in part, the NCI of the NIH under award number P30CA008748 (through their institution). L.A. Byers is supported, in part, by the R. Lee Clark Fellow Award (supported by the Jeanne F. Shelby Scholarship Fund), the University of Texas MD Anderson Cancer Center Physician Scientist Award, the NCI Cancer Clinical Investigator Team Leadership Award, and the Sheikh Khalifa Bin Zayed Al Nahyan Institute for Personalized Cancer Therapy's Khalifa Scholars and Fellows Award. J.D. Minna is supported by the NCI of the NIH under award number P50CA70907 (SPORE in Lung Cancer).
Footnotes
Disclosure of Potential Conflicts of Interest: M.C. Pietanza reports receiving speakers bureau honoraria from Physicians' Education Resource (PER) and is a consultant/advisory board member for Celgene. L.A. Byers reports receiving commercial research grants from Astex Pharmaceuticals and Takeda, and is a consultant/advisory board member for AbbVie and Biomarin. C.M. Rudin reports receiving a commercial research grant from Biomarin and is a consultant/advisory board member for AbbVie, Boehringer Ingelheim, GlaxoSmithKline, and Merck. No potential conflicts of interest were disclosed by the other author.
Disclaimer: The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.American Cancer Society. Atlanta (GA): American Cancer Society; 2014. [cited 2015 Feb 8]. Cancer facts & figures 2014 [PDF on the Internet] Report No.: 500814. Available from: http://www.cancer.org/acs/groups/content/@research/documents/webcontent/acspc-042151.pdf. [Google Scholar]
- 2.Gaspar LE, McNamara EJ, Gay EG, Putnam JB, Crawford J, Herbst RS, et al. Small-cell lung cancer: prognostic factors and changing treatment over 15 years. Clin Lung Cancer. 2012;13:115–22. doi: 10.1016/j.cllc.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 3.Varghese AM, Zakowski MF, Yu HA, Won HH, Riely GJ, Krug LM, et al. Small-cell lung cancers in patients who never smoked cigarettes. J Thorac Oncol. 2014;9:892–6. doi: 10.1097/JTO.0000000000000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ou SH, Ziogas A, Zell JA. Prognostic factors for survival in extensive stage small cell lung cancer (ED-SCLC): the importance of smoking history, socioeconomic and marital statuses, and ethnicity. J Thorac Oncol. 2009;4:37–43. doi: 10.1097/JTO.0b013e31819140fb. [DOI] [PubMed] [Google Scholar]
- 5.Amini A, Byers LA, Welsh JW, Komaki RU. Progress in the management of limited-stage small cell lung cancer. Cancer. 2014;120:790–8. doi: 10.1002/cncr.28505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spiro SG, James LE, Rudd RM, Trask CW, Tobias JS, Snee M, et al. Early compared with late radiotherapy in combined modality treatment for limited disease small-cell lung cancer: a London Lung Cancer Group multicenter randomized clinical trial and meta-analysis. J Clin Oncol. 2006;24:3823–30. doi: 10.1200/JCO.2005.05.3181. [DOI] [PubMed] [Google Scholar]
- 7.Fried DB, Morris DE, Poole C, Rosenman JG, Halle JS, Detterbeck FC, et al. Systematic review evaluating the timing of thoracic radiation therapy in combined modality therapy for limited-stage small-cell lung cancer. J Clin Oncol. 2004;22:4837–45. doi: 10.1200/JCO.2004.01.178. [DOI] [PubMed] [Google Scholar]
- 8.De Ruysscher D, Pijls-Johannesma M, Bentzen SM, Minken A, Wanders R, Lutgens L, et al. Time between the first day of chemotherapy and the last day of chest radiation is the most important predictor of survival in limited-disease small-cell lung cancer. J Clin Oncol. 2006;24:1057–63. doi: 10.1200/JCO.2005.02.9793. [DOI] [PubMed] [Google Scholar]
- 9.De Ruysscher D, Pijls-Johannesma M, Vansteenkiste J, Kester A, Rutten I, Lambin P. Systematic review and meta-analysis of randomised, controlled trials of the timing of chest radiotherapy in patients with limited-stage, small-cell lung cancer. Ann Oncol. 2006;17:543–52. doi: 10.1093/annonc/mdj094. [DOI] [PubMed] [Google Scholar]
- 10.Pijls-Johannesma MC, De Ruysscher D, Lambin P, Rutten I, Vansteenkiste JF. Early versus late chest radiotherapy for limited stage small cell lung cancer. Cochrane Database Syst Rev. 2005;(1):CD004700. doi: 10.1002/14651858.CD004700.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huncharek M, McGarry R. A meta-analysis of the timing of chest irradiation in the combined modality treatment of limited-stage small cell lung cancer. Oncologist. 2004;9:665–72. doi: 10.1634/theoncologist.9-6-665. [DOI] [PubMed] [Google Scholar]
- 12.Turrisi AT, 3rd, Kim K, Blum R, Sause WT, Livingston RB, Komaki R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med. 1999;340:265–71. doi: 10.1056/NEJM199901283400403. [DOI] [PubMed] [Google Scholar]
- 13.Auperin A, Arriagada R, Pignon J, Le Pechoux C, Gregor A, Stephens RJ, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. N Engl J Med. 1999;341:476–84. doi: 10.1056/NEJM199908123410703. [DOI] [PubMed] [Google Scholar]
- 14.Slotman B, Faivre-Finn C, Kramer G, Rankin E, Snee M, Hatton M, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med. 2007;357:664–72. doi: 10.1056/NEJMoa071780. [DOI] [PubMed] [Google Scholar]
- 15.Simon GR, Turrisi A American College of Chest P. Management of small cell lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132:324S–39S. doi: 10.1378/chest.07-1385. [DOI] [PubMed] [Google Scholar]
- 16.von Pawel J, Schiller JH, Shepherd FA, Fields SZ, Kleisbauer JP, Chrysson NG, et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol. 1999;17:658–67. doi: 10.1200/JCO.1999.17.2.658. [DOI] [PubMed] [Google Scholar]
- 17.O'Brien ME, Ciuleanu TE, Tsekov H, Shparyk Y, Cucevia B, Juhasz G, et al. Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol. 2006;24:5441–7. doi: 10.1200/JCO.2006.06.5821. [DOI] [PubMed] [Google Scholar]
- 18.Eckardt JR, von Pawel J, Pujol JL, Papai Z, Quoix E, Ardizzoni A, et al. Phase III study of oral compared with intravenous topotecan as second-line therapy in small-cell lung cancer. J Clin Oncol. 2007;25:2086–92. doi: 10.1200/JCO.2006.08.3998. [DOI] [PubMed] [Google Scholar]
- 19.Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins GA, Shields TW, Keehn RJ. The solitary pulmonary nodule. Ten-year follow-up of veterans administration-armed forces cooperative study. Arch Surg. 1975;110:570–5. doi: 10.1001/archsurg.1975.01360110116019. [DOI] [PubMed] [Google Scholar]
- 21.Quoix E, Fraser R, Wolkove N, Finkelstein H, Kreisman H. Small cell lung cancer presenting as a solitary pulmonary nodule. Cancer. 1990;66:577–82. doi: 10.1002/1097-0142(19900801)66:3<577::aid-cncr2820660328>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 22.Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489:519–25. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511:543–50. doi: 10.1038/nature13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Cancer Institute. Bethesda (MD): National Cancer Institute; 2014. [cited 2015 Feb 8]. Scientific framework for small cell lung cancer (SCLC) [PDF on the Internet] Available from: http://deainfo.nci.nih.gov/advisory/ctac/workgroup/SCLC/SCLC%20Congressional%20Response.pdf. [Google Scholar]
- 25.Pleasance ED, Stephens PJ, O'Meara S, McBride DJ, Meynert A, Jones D, et al. A small-cell lung cancer genome with complex signatures of tobacco exposure. Nature. 2010;463:184–90. doi: 10.1038/nature08629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rudin CM, Durinck S, Stawiski EW, Poirier JT, Modrusan Z, Shames DS, et al. Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat Genet. 2012;44:1111–6. doi: 10.1038/ng.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peifer M, Fernandez-Cuesta L, Sos ML, George J, Seidel D, Kasper LH, et al. Integrative genome analyses identify key somatic driver mutations of small-cell lung cancer. Nat Genet. 2012;44:1104–10. doi: 10.1038/ng.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–8. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wistuba II, Gazdar AF, Minna JD. Molecular genetics of small cell lung carcinoma. Semin Oncol. 2001;28:3–13. [PubMed] [Google Scholar]
- 30.Mori N, Yokota J, Akiyama T, Sameshima Y, Okamoto A, Mizoguchi H, et al. Variable mutations of the RB gene in small-cell lung carcinoma. Oncogene. 1990;5:1713–7. [PubMed] [Google Scholar]
- 31.Arriola E, Canadas I, Arumi M, Rojo F, Rovira A, Albanell J. Genetic changes in small cell lung carcinoma. Clinic Transl Oncol. 2008;10:189–97. doi: 10.1007/s12094-008-0181-1. [DOI] [PubMed] [Google Scholar]
- 32.Riely GJ, Yu HA. EGFR: The paradigm of an oncogene-driven lung cancer. Clin Cancer Res. 2015;21:xxx–xxx. doi: 10.1158/1078-0432.CCR-14-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katayama R, Lovly CM, Shaw AT. Therapeutic targeting of anaplastic lymphoma kinase in lung cancer: a paradigm for precision cancer medicine. Clin Cancer Res. 2015;21:xxx–xxx. doi: 10.1158/1078-0432.CCR-14-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gazdar AF, Savage TK, Johnson JE, Berns A, Sage J, Linnoila RI, et al. The comparative pathology of genetically engineered mouse models for neuroendocrine carcinomas of the lung. J Thorac Oncol. 2015;10:553–64. doi: 10.1097/JTO.0000000000000459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McFadden DG, Papagiannakopoulos T, Taylor-Weiner A, Stewart C, Carter SL, Cibulskis K, et al. Genetic and clonal dissection of murine small cell lung carcinoma progression by genome sequencing. Cell. 2014;156:1298–311. doi: 10.1016/j.cell.2014.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cui M, Augert A, Rongione M, Conkrite K, Parazzoli S, Nikitin AY, et al. PTEN is a potent suppressor of small cell lung cancer. Mol Cancer Res. 2014;12:654–9. doi: 10.1158/1541-7786.MCR-13-0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jahchan NS, Dudley JT, Mazur PK, Flores N, Yang D, Palmerton A, et al. A drug repositioning approach identifies tricyclic antidepressants as inhibitors of small cell lung cancer and other neuroendocrine tumors. Cancer Discov. 2013;3:1364–77. doi: 10.1158/2159-8290.CD-13-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baetscher M, Schmidt E, Shimizu A, Leder P, Fishman MC. SV40 T antigen transforms calcitonin cells of the thyroid but not CGRP-containing neurons in transgenic mice. Oncogene. 1991;6:1133–8. [PubMed] [Google Scholar]
- 39.Magdaleno SM, Wang G, Mireles VL, Ray MK, Finegold MJ, DeMayo FJ. Cyclin-dependent kinase inhibitor expression in pulmonary Clara cells transformed with SV40 large T antigen in transgenic mice. Cell Growth Differ. 1997;8:145–55. [PubMed] [Google Scholar]
- 40.Kwak I, Tsai SY, DeMayo FJ. Genetically engineered mouse models for lung cancer. Annu Rev Physiol. 2004;66:647–63. doi: 10.1146/annurev.physiol.66.032102.134301. [DOI] [PubMed] [Google Scholar]
- 41.Meuwissen R, Linn SC, Linnoila RI, Zevenhoven J, Mooi WJ, Berns A. Induction of small cell lung cancer by somatic inactivation of both Trp53 and Rb1 in a conditional mouse model. Cancer Cell. 2003;4:181–9. doi: 10.1016/s1535-6108(03)00220-4. [DOI] [PubMed] [Google Scholar]
- 42.Song H, Yao E, Lin C, Gacayan R, Chen MH, Chuang PT. Functional characterization of pulmonary neuroendocrine cells in lung development, injury, and tumorigenesis. Proc Natl Acad Sci U S A. 2012;109:17531–6. doi: 10.1073/pnas.1207238109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sutherland KD, Proost N, Brouns I, Adriaensen D, Song JY, Berns A. Cell of origin of small cell lung cancer: inactivation of Trp53 and Rb1 in distinct cell types of adult mouse lung. Cancer Cell. 2011;19:754–64. doi: 10.1016/j.ccr.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 44.Schaffer BE, Park KS, Yiu G, Conklin JF, Lin C, Burkhart DL, et al. Loss of p130 accelerates tumor development in a mouse model for human small-cell lung carcinoma. Cancer Res. 2010;70:3877–83. doi: 10.1158/0008-5472.CAN-09-4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park KS, Martelotto LG, Peifer M, Sos ML, Karnezis AN, Mahjoub MR, et al. A crucial requirement for Hedgehog signaling in small cell lung cancer. Nat Med. 2011;17:1504–08. doi: 10.1038/nm.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Calbo J, van Montfort E, Proost N, van Drunen E, Beverloo HB, Meuwissen R, et al. A functional role for tumor cell heterogeneity in a mouse model of small cell lung cancer. Cancer Cell. 2011;19:244–56. doi: 10.1016/j.ccr.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 47.Daniel VC, Marchionni L, Hierman JS, Rhodes JT, Devereux WL, Rudin CM, et al. A primary xenograft model of small-cell lung cancer reveals irreversible changes in gene expression imposed by culture in vitro. Cancer Res. 2009;69:3364–73. doi: 10.1158/0008-5472.CAN-08-4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hodgkinson CL, Morrow CJ, Li Y, Metcalf RL, Rothwell DG, Trapani F, et al. Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. Nature Med. 2014;20:897–903. doi: 10.1038/nm.3600. [DOI] [PubMed] [Google Scholar]
- 49.Byers LA, Wang J, Nilsson MB, Fujimoto J, Saintigny P, Yordy J, et al. Proteomic profiling identifies dysregulated pathways in small cell lung cancer and novel therapeutic targets including PARP1. Cancer Discov. 2012;2:798–811. doi: 10.1158/2159-8290.CD-12-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Teicher BA. Perspective: Opportunities in recalcitrant, rare and neglected tumors. Oncol Rep. 2013;30:1030–4. doi: 10.3892/or.2013.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang J, Byers LA. Teaching an old dog new tricks: drug repositioning in small cell lung cancer. Cancer Discov. 2013;3:1333–5. doi: 10.1158/2159-8290.CD-13-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hwang DH, Sun H, Rodig SJ, Hornick JL, Sholl LM. Myc protein expression correlates with MYC amplification in small-cell lung carcinoma. Histopathology. 2014 Nov 19; doi: 10.1111/his.12622. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 53.Rudin CM, Poirier JT. MYC, MAX, and small cell lung cancer. Cancer Discov. 2014;4:273–4. doi: 10.1158/2159-8290.CD-14-0069. [DOI] [PubMed] [Google Scholar]
- 54.Sos ML, Dietlein F, Peifer M, Schottle J, Balke-Want H, Muller C, et al. A framework for identification of actionable cancer genome dependencies in small cell lung cancer. Proc Natl Acad Sci USA. 2012;109:17034–9. doi: 10.1073/pnas.1207310109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mertz JA, Conery AR, Bryant BM, Sandy P, Balasubramanian S, Mele DA, et al. Targeting MYC dependence in cancer by inhibiting BET bromodomains. Proc Natl Acad Sci U S A. 2011;108:16669–74. doi: 10.1073/pnas.1108190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brockmann M, Poon E, Berry T, Carstensen A, Deubzer HE, Rycak L, et al. Small molecule inhibitors of aurora-a induce proteasomal degradation of N-myc in childhood neuroblastoma. Cancer Cell. 2013;24:75–89. doi: 10.1016/j.ccr.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.den Hollander J, Rimpi S, Doherty JR, Rudelius M, Buck A, Hoellein A, et al. Aurora kinases A and B are up-regulated by Myc and are essential for maintenance of the malignant state. Blood. 2010;116:1498–505. doi: 10.1182/blood-2009-11-251074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu LY, Wood JL, Ye L, Minter-Dykhouse K, Saunders TL, Yu X, et al. Aurora A is essential for early embryonic development and tumor suppression. J Biol Chem. 2008;283:31785–90. doi: 10.1074/jbc.M805880200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vader G, Lens SM. The Aurora kinase family in cell division and cancer. Biochim Biophys Acta. 2008;1786:60–72. doi: 10.1016/j.bbcan.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 60.Hook KE, Garza SJ, Lira ME, Ching KA, Lee NV, Cao J, et al. An integrated genomic approach to identify predictive biomarkers of response to the aurora kinase inhibitor PF-03814735. Mol Cancer Ther. 2012;11:710–9. doi: 10.1158/1535-7163.MCT-11-0184. [DOI] [PubMed] [Google Scholar]
- 61.Melichar B, Adenis A, Havel L, Lockhart AC, Bennouna J, Schusterbauer C, et al. Phase (Ph) I/II study of investigational Aurora A kinase (AAK) inhibitor MLN8237 (alisertib): updated ph II results in patients (pts) with small cell lung cancer (SCLC), non-SCLC (NSCLC), breast cancer (BrC), head and neck squamos cell carcinoma (HNSCC), and gastroesophageal cancer (GE) J Clin Oncol. 2013;31(suppl; abstr 605) [Google Scholar]
- 62.Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683–92. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gregory PD, Wagner K, Horz W. Histone acetylation and chromatin remodeling. Exp Cell Res. 2001;265:195–202. doi: 10.1006/excr.2001.5187. [DOI] [PubMed] [Google Scholar]
- 64.Lane AA, Chabner BA. Histone deacetylase inhibitors in cancer therapy. J Clin Oncol. 2009;27:5459–68. doi: 10.1200/JCO.2009.22.1291. [DOI] [PubMed] [Google Scholar]
- 65.Bruzzese F, Rocco M, Castelli S, Di Gennaro E, Desideri A, Budillon A. Synergistic antitumor effect between vorinostat and topotecan in small cell lung cancer cells is mediated by generation of reactive oxygen species and DNA damage-induced apoptosis. Mol Cancer Ther. 2009;8:3075–87. doi: 10.1158/1535-7163.MCT-09-0254. [DOI] [PubMed] [Google Scholar]
- 66.Luchenko VL, Salcido CD, Zhang Y, Agama K, Komlodi-Pasztor E, Murphy RF, et al. Schedule-dependent synergy of histone deacetylase inhibitors with DNA damaging agents in small cell lung cancer. Cell Cycle. 2011;10:3119–28. doi: 10.4161/cc.10.18.17190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Romanski A, Schwarz K, Keller M, Wietbrauk S, Vogel A, Roos J, et al. Deacetylase inhibitors modulate proliferation and self-renewal properties of leukemic stem and progenitor cells. Cell Cycle. 2012;11:3219–26. doi: 10.4161/cc.21565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu Y, Voelter-Mahlknecht S, Mahlknecht U. The histone deacetylase inhibitor suberoylanilide hydroxamic acid down-regulates expression levels of Bcr-abl, c-Myc and HDAC3 in chronic myeloid leukemia cell lines. Int J Mol Med. 2005;15:169–72. [PubMed] [Google Scholar]
- 69.Seo SK, Jin HO, Woo SH, Kim YS, An S, Lee JH, et al. Histone deacetylase inhibitors sensitize human non-small cell lung cancer cells to ionizing radiation through acetyl p53-mediated c-myc down-regulation. J Thorac Oncol. 2011;6:1313–9. doi: 10.1097/JTO.0b013e318220caff. [DOI] [PubMed] [Google Scholar]
- 70.Esteller M, Herman JG. Generating mutations but providing chemosensitivity: the role of O6-methylguanine DNA methyltransferase in human cancer. Oncogene. 2004;23:1–8. doi: 10.1038/sj.onc.1207316. [DOI] [PubMed] [Google Scholar]
- 71.Toyooka S, Toyooka KO, Maruyama R, Virmani AK, Girard L, Miyajima K, et al. DNA methylation profiles of lung tumors. Mol Cancer Ther. 2001;1:61–7. [PubMed] [Google Scholar]
- 72.Gerson SL. Clinical relevance of MGMT in the treatment of cancer. J Clin Oncol. 2002;20:2388–99. doi: 10.1200/JCO.2002.06.110. [DOI] [PubMed] [Google Scholar]
- 73.Kalemkerian GP, Loo BW, Akerley W, Bogner P, Chow LQM, Doebele RC, et al. Fort Washington (PA): National Comprehensive Cancer Network; 2015. [cited 2015 Jan 22]. NCCN clinical practice guidelines in oncology: small cell lung cancer version I. 2015 [PDF on the Internet] Available from: http://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf. [Google Scholar]
- 74.Tamborini E, Bonadiman L, Negri T, Greco A, Staurengo S, Bidoli P, et al. Detection of overexpressed and phosphorylated wild-type kit receptor in surgical specimens of small cell lung cancer. Clin Cancer Res. 2004;10:8214–9. doi: 10.1158/1078-0432.CCR-04-1013. [DOI] [PubMed] [Google Scholar]
- 75.Wainberg ZA, Rafii S, Ramanathan RK, Mina LA, Byers LA, Chugh R, et al. Safety and antitumor activity of the PARP inhibitor BMN673 in a phase 1 trial recruiting metastatic small-cell lung cancer (SCLC) and germline BRCA-mutation carrier cancer patients. J Clin Oncol. 2014;32(suppl; abstr 7522):5s. [Google Scholar]
- 76.Liu H, Kho AT, Kohane IS, Sun Y. Predicting survival within the lung cancer histopathological hierarchy using a multi-scale genomic model of development. PLoS Med. 2006;3:e232. doi: 10.1371/journal.pmed.0030232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Park KS, Martelotto LG, Peifer M, Sos ML, Karnezis AN, Mahjoub MR, et al. A crucial requirement for Hedgehog signaling in small cell lung cancer. Nature Med. 2011;17:1504–8. doi: 10.1038/nm.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peacock CD, Watkins DN. Cancer stem cells and the ontogeny of lung cancer. J Clin Oncol. 2008;26:2883–9. doi: 10.1200/JCO.2007.15.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer. 2003;3:895–902. doi: 10.1038/nrc1232. [DOI] [PubMed] [Google Scholar]
- 80.Rubin LL, de Sauvage FJ. Targeting the Hedgehog pathway in cancer. Nat Rev Drug Discov. 2006;5:1026–33. doi: 10.1038/nrd2086. [DOI] [PubMed] [Google Scholar]
- 81.Malik B, Nie D. Cancer stem cells and resistance to chemo and radio therapy. Front Biosci (Elite Ed) 2012;4:2142–9. doi: 10.2741/531. [DOI] [PubMed] [Google Scholar]
- 82.Nguyen LV, Vanner R, Dirks P, Eaves CJ. Cancer stem cells: an evolving concept. Nat Rev Cancer. 2012;12:133–43. doi: 10.1038/nrc3184. [DOI] [PubMed] [Google Scholar]
- 83.Belani CP, Dahlberg SE, Rudin CM, Fleisher M, Chen HX, Takebe N, et al. Three-arm randomized phase II study of cisplatin and etoposide versus cisplatine and etoposide with either vismodegib or cixutumumab for patients with extensive stage-small cell lung cancer (ECOG 1508) J Clin Oncol. 2013;31(suppl; abstract 7508) [Google Scholar]
- 84.Takebe N, Nguyen D, Yang SX. Targeting notch signaling pathway in cancer: clinical development advances and challenges. Pharmacol Ther. 2014;141:140–9. doi: 10.1016/j.pharmthera.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yen WC, Fischer MM, Axelrod F, Bond C, Cain J, Cancilla B, et al. Targeting Notch signaling with a Notch2/Notch3 antagonist (tarextumab) inhibits tumor growth and decreases tumor initiating cell frequency. Clin Cancer Res. doi: 10.1158/1078-0432.CCR-14-2808. In press. [DOI] [PubMed] [Google Scholar]
- 86.Borges M, Linnoila RI, van de Velde HJ, Chen H, Nelkin BD, Mabry M, et al. An achaete-scute homologue essential for neuroendocrine differentiation in the lung. Nature. 1997;386:852–5. doi: 10.1038/386852a0. [DOI] [PubMed] [Google Scholar]
- 87.Jiang T, Collins BJ, Jin N, Watkins DN, Brock MV, Matsui W, et al. Achaete-scute complex homologue 1 regulates tumor-initiating capacity in human small cell lung cancer. Cancer Res. 2009;69:845–54. doi: 10.1158/0008-5472.CAN-08-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miki M, Ball DW, Linnoila RI. Insights into the achaete-scute homolog-1 gene (hASH1) in normal and neoplastic human lung. Lung Cancer. 2012;75:58–65. doi: 10.1016/j.lungcan.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 89.Augustyn A, Borromeo M, Wang T, Fujimoto J, Shao C, Dospoy PD, et al. ASCL1 is a lineage oncogene providing therapeutic targets for high-grade neuroendocrine lung cancers. Proc Natl Acad Sci U S A. 2014;111:14788–93. doi: 10.1073/pnas.1410419111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Osada H, Tatematsu Y, Yatabe Y, Horio Y, Takahashi T. ASH1 gene is a specific therapeutic target for lung cancers with neuroendocrine features. Cancer Res. 2005;65:10680–5. doi: 10.1158/0008-5472.CAN-05-1404. [DOI] [PubMed] [Google Scholar]
- 91.Graus F, Dalmou J, Rene R, Tora M, Malats N, Verschuuren JJ, et al. Anti-Hu antibodies in patients with small-cell lung cancer: association with complete response to therapy and improved survival. J Clin Oncol. 1997;15:2866–72. doi: 10.1200/JCO.1997.15.8.2866. [DOI] [PubMed] [Google Scholar]
- 92.Tanio Y, Watanabe M, Osaki T, Tachibana I, Kawase I, Kuritani T, et al. High sensitivity to peripheral blood lymphocytes and low HLA-class I antigen expression of small cell lung cancer cell lines with diverse chemo-radiosensitivity. Jpn J Cancer Res. 1992;83:736–45. doi: 10.1111/j.1349-7006.1992.tb01974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yazawa T, Kamma H, Fujiwara M, Matsui M, Horiguchi H, Satoh H, et al. Lack of class II transactivator causes severe deficiency of HLA-DR expression in small cell lung cancer. J Pathol. 1999;187:191–9. doi: 10.1002/(SICI)1096-9896(199901)187:2<191::AID-PATH206>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 94.Koyama K, Kagamu H, Miura S, Hiura T, Miyabayashi T, Itoh R, et al. Reciprocal CD4+ T-cell balance of effector CD62L low CD4+ and CD62L highCD25+ CD4+ regulatory T cells in small cell lung cancer reflects disease stage. Clin Cancer Res. 2008;14:6770–9. doi: 10.1158/1078-0432.CCR-08-1156. [DOI] [PubMed] [Google Scholar]
- 95.Schultheis AM, Scheel AH, Ozretic L, George J, Thomas RK, Hagemann T, et al. PD-L1 expression in small cell neuroendocrine carcinomas. Eur J Cancer. 2015;51:421–6. doi: 10.1016/j.ejca.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 96.Soria JC, Marabelle A, Brahmer JR, Gettinger S. Immune checkpoint modulation for non–small cell lung cancer. Clin Cancer Res. 2015;21:xxx–xxx. doi: 10.1158/1078-0432.CCR-14-2959. [DOI] [PubMed] [Google Scholar]
- 97.Hellmann M, Creelan B, Woo K, Sima C, Iams W, Antonia S, et al. Smoking history and response to nivolumab in patients with advanced NSCLCs. Ann Oncol. 2014;25:iv426–470. [Google Scholar]
- 98.Garon EB, Gandhi L, Rizvi N, Hui R, Balmanoukian AS, Patnaik A, et al. Antitumor activity of pembrolizumab (Pembro; MK-3475) and correlation with programmed death ligand (PD-L1) expression in a pooled analysis of patients with advanced NSCLC. Ann Oncol. 2014;25:v1–v41. [Google Scholar]
- 99.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–7. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rizvi NA, Mazieres J, Planchard D, Stinchcombe TE, Dy GK, Antonia SJ, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16:257–65. doi: 10.1016/S1470-2045(15)70054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gandara DR, Hammerman PS, Sos ML, Lara PN, Jr, Hirsch FR. Squamous cell lung cancer: from tumor genomics to cancer therapeutics. Clin Cancer Res. 2015;21:xxx–xxx. doi: 10.1158/1078-0432.CCR-14-3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rizvi NA, Hellman MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015 Mar 12; doi: 10.1126/science.aaa1348. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lynch TJ, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol. 2012;30:2046–54. doi: 10.1200/JCO.2011.38.4032. [DOI] [PubMed] [Google Scholar]
- 104.Reck M, Bondarenko I, Luft A, Serwatowski P, Barlesi F, Chacko R, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol. 2013;24:75–83. doi: 10.1093/annonc/mds213. [DOI] [PubMed] [Google Scholar]
- 105.Recalcitrant Cancer Research Act of 2012. 2012 Sep 19; Pub. L. No. 112-176, 112 Stat. 733. [Google Scholar]
- 106.Mattson K, Niiranen A, Pyrhonen S, Holsti LR, Holsti P, Kumpulainen E, et al. Natural interferon alfa as maintenance therapy for small cell lung cancer. Eur J Cancer. 1992;28A:1387–91. doi: 10.1016/0959-8049(92)90526-8. [DOI] [PubMed] [Google Scholar]
- 107.Prior C, Oroszy S, Oberaigner W, Schenk E, Kummer F, Aigner K, et al. Adjunctive interferon-alpha-2c in stage IIIB/IV small-cell lung cancer: a phase III trial. Eur Respir J. 1997;10:392–6. doi: 10.1183/09031936.97.10020392. [DOI] [PubMed] [Google Scholar]
- 108.Ruotsalainen TM, Halme M, Tamminen K, Szopinski J, Niiranen A, Pyrhonen S, et al. Concomitant chemotherapy and IFN-alpha for small cell lung cancer: a randomized multicenter phase III study. J Interferon Cytokine Res. 1999;19:253–9. doi: 10.1089/107999099314180. [DOI] [PubMed] [Google Scholar]
- 109.Kelly K, Crowley JJ, Bunn PA, Jr, Hazuka MB, Beasley K, Upchurch C, et al. Role of recombinant interferon alfa-2a maintenance in patients with limited-stage small-cell lung cancer responding to concurrent chemoradiation: a Southwest Oncology Group study. J Clin Oncol. 1995;13:2924–30. doi: 10.1200/JCO.1995.13.12.2924. [DOI] [PubMed] [Google Scholar]
- 110.Jett JR, Maksymiuk AW, Su JQ, Mailliard JA, Krook JE, Tschetter LK, et al. Phase III trial of recombinant interferon gamma in complete responders with small-cell lung cancer. J Clin Oncol. 1994;12:2321–6. doi: 10.1200/JCO.1994.12.11.2321. [DOI] [PubMed] [Google Scholar]
- 111.van Zandwijk N, Groen HJ, Postmus PE, Burghouts JT, ten Velde GP, Ardizzoni A, et al. Role of recombinant interferon-gamma maintenance in responding patients with small cell lung cancer. A randomised phase III study of the EORTC Lung Cancer Cooperative Group. Eur J Cancer. 1997;33:1759–66. doi: 10.1016/s0959-8049(97)00174-3. [DOI] [PubMed] [Google Scholar]
- 112.Clamon G, Herndon J, Perry MC, Ozer H, Kreisman H, Maher T, et al. Interleukin-2 activity in patients with extensive small-cell lung cancer: a phase II trial of Cancer and Leukemia Group B. J Natl Cancer Inst. 1993;85:316–20. doi: 10.1093/jnci/85.4.316. [DOI] [PubMed] [Google Scholar]
- 113.Shepherd FA, Giaccone G, Seymour L, Debruyne C, Bezjak A, Hirsh V, et al. Prospective, randomized, double-blind, placebo-controlled trial of marimastat after response to first-line chemotherapy in patients with small-cell lung cancer: a trial of the National Cancer Institute of Canada-Clinical Trials Group and the European Organization for Research and Treatment of Cancer. J Clin Oncol. 2002;20:4434–9. doi: 10.1200/JCO.2002.02.108. [DOI] [PubMed] [Google Scholar]
- 114.Rigas JR, Denham CA, Rinaldi DA, Moore TD, Smith JW, Winston RD, et al. Randomized placebo-controlled trials of the matrix metalloproteinase inhibitor BAY12-9566 as adjuvant therapy for patients with small cell and non-small cell lung cancer. J Clin Oncol. 2003;22(suppl; abstr 2525) [Google Scholar]
- 115.Dy GK, Miller AA, Mandrekar SJ, Aubry MC, Langdon RM, Jr, Morton RF, et al. A phase II trial of imatinib (ST1571) in patients with c-kit expressing relapsed small-cell lung cancer: a CALGB and NCCTG study. Ann Oncol. 2005;16:1811–6. doi: 10.1093/annonc/mdi365. [DOI] [PubMed] [Google Scholar]
- 116.Johnson BE, Fischer T, Fischer B, Dunlop D, Rischin D, Silberman S, et al. Phase II study of imatinib in patients with small cell lung cancer. Clin Cancer Res. 2003;9:5880–7. [PubMed] [Google Scholar]
- 117.Krug LM, Crapanzano JP, Azzoli CG, Miller VA, Rizvi N, Gomez J, et al. Imatinib mesylate lacks activity in small cell lung carcinoma expressing c-kit protein: a phase II clinical trial. Cancer. 2005;103:2128–31. doi: 10.1002/cncr.21000. [DOI] [PubMed] [Google Scholar]
- 118.Pandya KJ, Levy DE, Hidalgo M, Cohen RB, Lee MW, Schiller JH, et al. A randomized, phase II ECOG trial of two dose levels of temsirolimus (CCI-779) in patients with extensive stage small cell lung cancer in remission after induction chemotherapy. A preliminary report. J Clin Oncol. 2005;23(suppl; abstr 7005):16s. doi: 10.1097/JTO.0b013e318155a439. [DOI] [PubMed] [Google Scholar]
- 119.Tarhini A, Kotsakis A, Gooding W, Shuai Y, Petro D, Friedland D, et al. Phase II study of everolimus (RAD001) in previously treated small cell lung cancer. Clin Cancer Res. 2010;16:5900–7. doi: 10.1158/1078-0432.CCR-10-0802. [DOI] [PubMed] [Google Scholar]
- 120.Heymach JV, Johnson DH, Khuri FR, Safran H, Schlabach LL, Yunus F, et al. Phase II study of the farnesyl transferase inhibitor R115777 in patients with sensitive relapse small-cell lung cancer. Ann Oncol. 2004;15:1187–93. doi: 10.1093/annonc/mdh315. [DOI] [PubMed] [Google Scholar]
- 121.Rudin CM, Salgia R, Wang X, Hodgson LD, Masters GA, Green M, et al. Randomized phase II Study of carboplatin and etoposide with or without the bcl-2 antisense oligonucleotide oblimersen for extensive-stage small-cell lung cancer: CALGB 30103. J Clin Oncol. 2008;26:870–6. doi: 10.1200/JCO.2007.14.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rudin CM, Hann CL, Garon EB, Ribeiro de Oliveira M, Bonomi PD, Camidge DR, et al. Phase II study of single-agent navitoclax (ABT-263) and biomarker correlates in patients with relapsed small cell lung cancer. Clin Cancer Res. 2012;18:3163–9. doi: 10.1158/1078-0432.CCR-11-3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Paik PK, Rudin CM, Pietanza MC, Brown A, Rizvi NA, Takebe N, et al. A phase II study of obatoclax mesylate, a Bcl-2 antagonist, plus topotecan in relapsed small cell lung cancer. Lung Cancer. 2011;74:481–5. doi: 10.1016/j.lungcan.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Langer CJ, Albert I, Kovacs P, Blakely LJ, Pajkos G, Petrov P, et al. A randomized phase II study of carboplatin and etoposide with or without pan-BCL-2 antagonist obatoclax in extensive-stage small cell lung cancer. J Clin Oncol. 2011;29(suppl; abstr 7001) [Google Scholar]
- 125.Lara PN, Jr, Chansky K, Davies AM, Franklin WA, Gumerlock PH, Guaglianone PP, et al. Bortezomib (PS-341) in relapsed or refractory extensive stage small cell lung cancer: a Southwest Oncology Group phase II trial (S0327) J Thorac Oncol. 2006;1:996–1001. [PubMed] [Google Scholar]
- 126.Giaccone G, Debruyne C, Felip E, Chapman PB, Grant SC, Millward M, et al. Phase III study of adjuvant vaccination with Bec2/bacille Calmette-Guerin in responding patients with limited-disease small-cell lung cancer (European Organisation for Research and Treatment of Cancer 08971-08971B; Silva Study) J Clin Oncol. 2005;23:6854–64. doi: 10.1200/JCO.2005.17.186. [DOI] [PubMed] [Google Scholar]
- 127.Pujol JL, Breton JL, Gervais R, Tanguy ML, Quoix E, David P, et al. Phase III double-blind, placebo-controlled study of thalidomide in extensive-disease small-cell lung cancer after response to chemotherapy: an intergroup study FNCLCC cleo04 IFCT 00-01. J Clin Oncol. 2007;25:3945–51. doi: 10.1200/JCO.2007.11.8109. [DOI] [PubMed] [Google Scholar]
- 128.Lee SM, Woll PJ, Rudd R, Ferry D, O'Brien M, Middleton G, et al. Anti-angiogenic therapy using thalidomide combined with chemotherapy in small cell lung cancer: a randomized, double-blind, placebo-controlled trial. J Natl Cancer Inst. 2009;101:1049–57. doi: 10.1093/jnci/djp200. [DOI] [PubMed] [Google Scholar]
- 129.Arnold AM, Seymour L, Smylie M, Ding K, Ung Y, Findlay B, et al. Phase II study of vandetanib or placebo in small-cell lung cancer patients after complete or partial response to induction chemotherapy with or without radiation therapy: National Cancer Institute of Canada Clinical Trials Group Study BR.20. J Clin Oncol. 2007;25:4278–84. doi: 10.1200/JCO.2007.12.3083. [DOI] [PubMed] [Google Scholar]
- 130.Gitlitz BJ, Moon J, Glisson BS, Reimers HJ, Bury MJ, Floyd JD, et al. Sorafenib in platinum-treated patients with extensive stage small cell lung cancer: a Southwest Oncology Group (SWOG 0435) phase II trial. J Thorac Oncol. 2010;5:1835–40. doi: 10.1097/JTO.0b013e3181f0bd78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ramalingam SS, Belani CP, Mack PC, Vokes EE, Longmate J, Govindan R, et al. Phase II study of cediranib (AZD 2171), an inhibitor of the vascular endothelial growth factor receptor, for second-line therapy of small cell lung cancer (National Cancer Institute #7097) J Thorac Oncol. 2010;5:1279–84. doi: 10.1097/JTO.0b013e3181e2fcb0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Heymach J, Glisson BS, Doebele RC, Huang C, Gandara DR, Le Scouiller S, et al. Phase I open-label study of cediranib plus etoposide and cisplatin as first-line therapy for patients with small cell lung cancer or lung neuroendocrine cancer. J Clin Oncol. 2010;28(suppl: abstr 7050):15s. doi: 10.1016/j.cllc.2024.08.015. [DOI] [PubMed] [Google Scholar]
- 133.Horn L, Dahlberg SE, Sandler AB, Dowlati A, Moore DF, Murren JR, et al. Phase II study of cisplatin plus etoposide and bevacizumab for previously untreated, extensive-stage small-cell lung cancer: Eastern Cooperative Oncology Group Study E3501. J Clin Oncol. 2009;27:6006–11. doi: 10.1200/JCO.2009.23.7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ready N, Dudek AZ, Wang XF, Graziano SL, Green MR, Vokes EE. CALGB 30306: A phase II study of cisplatin, irinotecan and bevacizumab for untreated extensive stage small cell lung cancer. J Clin Oncol. 2007;25(suppl; abstr 7563) doi: 10.1200/JCO.2011.35.6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Spigel DR, Greco FA, Zubkus JD, Murphy PB, Saez RA, Farley C, et al. Phase II trial of irinotecan, carboplatin, and bevacizumab in the treatment of patients with extensive-stage small-cell lung cancer. J Thorac Oncol. 2009;4:1555–60. doi: 10.1097/JTO.0b013e3181bbc540. [DOI] [PubMed] [Google Scholar]
- 136.Spigel DR, Townley PM, Waterhouse DM, Fang L, Adiguzel I, Huang JE, et al. Randomized phase II study of bevacizumab in combination with chemotherapy in previously untreated extensive-stage small-cell lung cancer: results from the SALUTE trial. J Clin Oncol. 2011;29:2215–22. doi: 10.1200/JCO.2010.29.3423. [DOI] [PubMed] [Google Scholar]
- 137.Ready N, Pang H, Gu L, Otterson GA, Thomas SP, Miller AA, et al. Chemotherapy with or without maintenance sunitinib for untreated extensive-stage small cell lung cancer: A randomized, double-blind, placebo controlled phase II study-CALGB 30504 (ALLIANCE) J Clin Oncol. 2015 Mar 2; doi: 10.1200/JCO.2014.57.3105. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.DeVita VT Jr, Lawrence TS, Rosenberg SA, editors. DeVita, Hellman, and Rosenberg's cancer: principles & practice of oncology. 10th. Philadelphia: Wolters Kluwer; 2014. [Google Scholar]